Abstract
Chorioretinal folds (CRFs) are undulations of the choroid and overlying Bruch’s membrane, retinal pigment epithelium and neurosensory retina. CRFs represent a clinical sign that is mandatory to investigate assuming their association with several ocular and extra-ocular disorders. Recent advances in retinal imaging have improved the characterization of CRFs. More importantly, retinal imaging may be useful to detect ocular complications secondary to chronic CRFs, including the development of choroidal neovascularization.
Keywords: chorioretinal folds, maculopathy, choroid, retina
Introduction
Chorioretinal folds (CRFs) are undulations of the choroid, Bruch’s membrane (BM), retinal pigment epithelium (RPE), and the overlying neurosensory retina.
CRFs were first described by Nettleship1 in 1884 in a patient with papilledema secondary to an intracranial mass. The term “choroidal folds” was successively employed by Norton2 in 1968. This term was subsequently modified by Gass3 in “chorioretinal folds” as this term was suggested to better describe the anatomical alterations occurring in this disorder. Since their first description, many other diseases have been ascribed to cause CRFs such as choroidal or orbital tumors, infections, immunologic disorder, high hyperopia, thyroid eye disease, posterior scleritis, uveal effusion syndrome, uveitis, ocular hypotony, and age-related macular degeneration (AMD).
Pathogenesis
Many authors have tried to characterize the pathogenesis of CRFs. In details, it was speculated that CRFs are secondary to a congestion of the choroid,4 this causing changes in the shape of BM and RPE.5 In 1989, Friberg6 proposed that CRFs occur because of the stress-and-strain relationship between choroid and sclera, resulting in a buckling force affecting choroid from either scleral thickening or shrinkage.
Diagnosis
Chorioretinal folds are usually diagnosed during routine ophthalmological examinations in patients without symptoms or undergoing visits because of other disorders. Less commonly, CRFs may cause metamorphopsia, photopsia or vision changes and thus represent the “primum movens” bringing patients to the ophthalmology attention.
At the fundoscopic examination, CRFs usually appear as alternating yellow and dark lines, that are often arranged in parallel. Based on their arrangement, CRF may be classified in horizontal, oblique, vertical, radiating, and concentric.7
Fundus fluorescein angiography (FA) has been historically considered as one of the most sensitive imaging tools for the diagnosis of CRFs. The CRFs’ characteristics on FA imaging were first described by Norton2 as alternating hypofluorescent and hyperfluorescent bands. In details, hyperfluorescent bands are due to the rarefaction of RPE at the CRF peak. Conversely, hypofluorescent bands are secondary to the redundancy of RPE within the CRFs’ pits. Uniformly, CRFs are displayed as alternating hyperautofluorescent and hypoautofluorescent bands on fundus autofluorescence (FAF) imaging (Figure 1).8
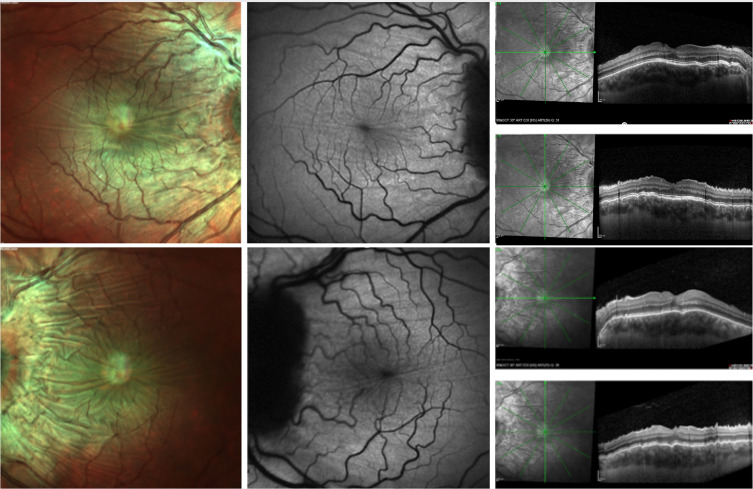
Figure 1.
Multimodal imaging from a patient with chorioretinal folds secondary to idiopathic intracranial hypertension. MultiColor fundus images (left) of the right (above) and left (below) eyes demonstrate undulations of the retinal pigment epithelium (RPE) within the macula and around the optic nerve. Blue-fundus autofluorescence (BAF) images (middle) show CRFs as alternating hypoautofluorescent and hyperautofluorescent bands. Structural optical coherence tomography (OCT) images (right) confirm undulations of retina, RPE and choroid that are easier to detect in the perpendicular scans.
Indocyanine green angiography (ICGA) findings in CRFs were fully described by Haruyama et al.9 In CRFs’ cases secondary to orbital tumor, the authors described the presence of choroidal venous tortuousness, as well as dilatation and loop formation in choroidal vascularization with a delay in the colorant filling of the choroidal vessels. Moreover, in cases of CRFs with associated posterior scleritis, the authors detected the presence of delayed choroidal filling, presence of multiple patches of hypofluorescence, and choroidal venous dilatation. Furthermore, the authors were not able to identify specific ICGA findings in idiopathic choroidal folds and AMD-associated CRFs.
Structural optical coherence tomography (OCT) is currently considered as the most specific imaging tool in the diagnosis and characterization of CRFs. Importantly, OCT has granted an improved differential diagnosis between CRF and retinal folds, that are secondary to other causes (eg epiretinal membrane).10–12 On OCT images, CRFs appear as typical undulation of the retina, RPE, and choroid that are easier to detect on perpendicular scans (Figure 1).13
More recently, OCT angiography (OCTA) characteristics of CRFs have been described in 3 affected eyes.14 Interestingly, the authors identified linear signal decreases in the choriocapillaris perfusion that were topographically associated with the folds (Figure 2). Based on these results, the authors hypothesized that choroidal swelling may cause a stretching of the choriocapillaris and a consequent reduction in perfusion.
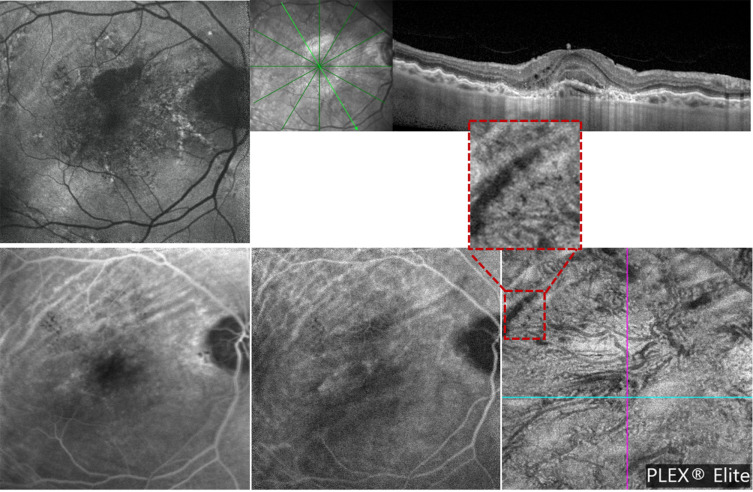
Figure 2.
Multimodal imaging from a patient with unilateral chorioretinal folds associated with macular neovascularization in right eye. (Row above): (Left) blue fundus autofluorescence (BAF) image shows linear folds at the macula, easier to detect in the superior region. Structural optical coherence tomography (OCT) image (right) confirms the presence of CFRs. (Row below): The late fluorescein angiography (FA - left) and intermediate indocyanine green angiography (ICGA - middle) images show typical alternating hypofluorescent and hyperfluorescent bands. The optical coherence tomography angiography (OCTA - right) image shows a transversal line of signal reduction corresponding to vascular rarefaction at the choriocapillaris layer.
Causes of CRFs
Chorioretinal folds may be secondary to ocular and extra-ocular causes.
In 1993, Leahey et al7 reviewed 54 patients with CRFs and identified both bilateral and unilateral cases (56% and 44%, respectively). Causes of CRFs were differently represented in unilateral and bilateral cases. Causes as thyroid eye disease, uveitis and choroiditis were more commonly found in bilateral CRFs. Conversely, ocular tumors and trauma were frequent causes of unilateral CRFs. Finally, posterior scleritis, AMD, hypotony and hyperopia were causes of both unilateral and bilateral CRFs.
Ocular Causes
Hyperopia has been identified as one of the main causes of CRFs’ formation, as suggested by three important studies with large cohorts.7,15,16 In details, hyperopia was identified as the cause of CRFs in 13% to 26% of the total cases, this percentage ranging on the basis of the study cohort.7,15,16 Of note, while CRFs may be secondary to primary hyperopia, CRFs may also occur in cases of acquired hyperopia (see below for details).17
Central serous chorioretinopathy (CSC) has been recently described as an additional cause of CRFs.18 In the latter study, the authors described a case series of six patients with CSC and CRFs. Importantly, the whole cohort of six eyes had hyperopia. Therefore, the authors concluded that the presence of chorioretinal folds in hyperopic CSC eyes could be associated with an excessive thickness of the choroid occurring in short eyes. Similarly, Corvi et al19 described a case series of patients with CRFs and choroidal vessel dilatation and hyperpermeability. The latter report further suggests the association between CSC – more generally with the pachychoroid spectrum – and the development of CRFs.
Macular neovascularization (MNV)20 represents a well-recognized cause of CRFs. MNV may be secondary to different disorders, including AMD.21 The association between CRFs and MNV was first reported by Gass,3 who provided a detailed description of the radial-shaped pattern of MNV-associated CRFs. This pattern of CRFs may be typical in type 1 (or “occult”) MNV and, in the pre-imaging era, CRFs could represent the first sign in presence of MNV. Notably, MNV-associated CRFs were speculated to occur because of the contraction of a fibrovascular membrane adherent to Bruch’s membrane, this resulting in a puckering of the underlying choroid and a series of CRFs spreading outward.3 (Figure 2)
Scleritis is an inflammatory disorder involving the sclera. In cases of involvement of the sclera posteriorly to the insertion of the rectus muscles, the term “posterior scleritis” is usually employed. This form of scleritis has been reported in 2 to 12% of all cases of scleritis.22 Of note, posterior scleritis is usually suspected in presence of symptoms such as periocular pain, blurred vision, and/or headache.23 Common reported signs include conjunctival chemosis and conjunctival hyperemia.23 Posterior scleritis may be associated with systemic inflammatory conditions including rheumatoid arthritis and Wegener’s granulomatosis.23 Also, posterior scleritis may be associated with infectious diseases.24 CRFs may be associated with posterior scleritis as this complication may occur in 28.1 to 35.0% of all cases.25–27 Ocular ultrasound should be performed in suspect of posterior scleritis as this examination may display a diffuse or nodular thickening of the sclera, often in presence of retrobulbar and perineural fluid determining the pathognomonic “T-sign”.28 Resolution of CRFs have been reported in several case reports after resolution of scleritis.24,29 These findings confirm the stressing role of a thickened sclera to the overlying layers.
Hypotony consists in a transient or permanent reduction in intraocular pressure (IOP) under 5 mmHg.30 Importantly, it may be silent or may be associated with visual loss.31 Hypotony may be associated with a reduction in aqueous fluid production such as in cases of iridocyclitis and ciliochoroidal detachment32–34 or with an increase in aqueous outflow such as in cases with glaucoma filtering surgery35,36 and other surgical procedures (eg vitreoretinal surgeries, corneal transplant, strabismus surgeries, medial canthopexy). The incidence of hypotony after glaucoma surgery has been reported in 1.3% to 20.0% cases according to different reports. Noteworthy, the use of antimetabolites during filtration surgery, that is aimed at reducing the occurrence of scar,37 has increased the rate of hypotony.37,38 Hypotony is an intraoperative and postoperative complication of pars plana vitrectomy. Intraoperatively, the incidence of hypotony can be avoided by limiting the leaks trough sclerotomy thanks to cauterization, subconjunctival ophthalmic viscoelastic device (OVD) injection, tissue glue and wound suturing. The postoperative incidence of ocular hypotony is influenced by the underlying pathology. In details, proliferative vitreoretinopathy and inflammatory disorders have a substantial risk of postoperative hypotony.39 In posterior lamellar keratoplasty surgery, hypotony is more a risk factor for graft dislocation than a postoperatively complication,40,41 mainly in eyes with prior glaucoma surgeries.40 Hypotony may clinically result in different complications, including keratopathy, cataract formation, optic nerve edema, irregular astigmatism, choroidal detachment, and hypotony-related maculopathy.42–44 CRFs, papilledema and vascular tortuosity are the main features of hypotony-related maculopathy, as described by Dellaporta in 1953.42–44 In ocular hypotony, CRFs seem to be secondary to choroidal thickening.6
Extra-Ocular Causes
CRFs may also develop in cases of idiopathic or secondary intracranial hypertension. Importantly, CRFs may be the unique sign of idiopathic intracranial hypertension, even in the absence of papilledema. Folds are thought to be secondary to flattening of the posterior pole and dilatation of the optic nerve sheath. It must be emphasized that several papers have reported this association and, therefore, cases of CRFs should be investigated for the presence of idiopathic hypertension.45,46 In these cases, a neuroimaging follow-up should be performed.47 In absence of imaging suggestive for hypertension, lumbar puncture should be considered in order to further investigate the presence of idiopathic intracranial hypertension.48 Remarkably, Kupersmith et al reported the effect of treatment with acetazolamide49 on CRFs in patient with idiopathic intracranial hypertension.50 The authors did not find significant differences between placebo and treatment groups in terms of CRF prevalence at 6 months. Furthermore, CRFs tend to persist irrespective of the degree of papilledema.
Orbital compression represents another cause of CRFs. Friberg6 described the occurrence of CRFs in patients with orbital tumor. In details, intraconal tumors may displace the optic nerve and the latter may thus compress the choroid and eventually result in CRFs’ formation. Conversely, extraconal tumors may directly compress the globe and cause CRFs. Orbital plasmacytoma,51 enlarged lacrimal gland caused by dacryoadenitis,52 frontoethmoidal mucocele,53 meningioma,54,55 cavernous hemangioma,56 meningocele,46 sinus transverse thrombosis,46 orbital pseudotumor5,46 are some of the cases reported in the last years. Resolution of CRFs after surgical or medical therapy in orbital masses are unpredictable. In fact, Yeung et al54 reported a complete resolution of CRFs after surgical removal of meningioma. On contrary, in case of surgical removal of parasellar meningioma, Taban et al55 reported the persistence of CRFs at the one year follow-up.
CRFs may also occur in patients with thyroid eye disease. (TED or Graves’ ophthalmopathy). This is an autoimmune disorder of the retrobulbar tissue associated with Graves’ disease. The ophthalmic disease is present in up to 50% of all cases of Grave’s disease and may have mild to severe manifestations,57–60 including CRFs.61–66 Mechanisms involved in CRFs’ development include exophthalmos, extraocular muscle expansion, and orbital hypertension. Recently, Tran et al67 reported on 10 patients (17 eyes) with unilateral or bilateral CRFs secondary to thyroid eye disease. Importantly, treatment of TED resulted in vision improvement in 53% of patients (10/17) and was not correlated with CRFs’ resolution or persistence. In agreement, 70% of patients had persistence of CRFs after TED treatment with a mean±SD follow-up of 24.7±23.7 months.
Idiopathic CRFs
Cases without a specific etiology are classified as idiopathic. Recent reports have suggested that idiopathic CRFs represent a percentage of 14–15% of all cases.7,15,68 Of note, Olsen et al68 speculated that a number of idiopathic CRFs may be actually secondary to silent scleritis, assuming that these patients with idiopathic CRFs are frequently affected by systemic autoimmune disorders.
CRF-Related Maculopathy
Presence of CRFs may eventually result in macular alterations, including ruptures of Bruch’s membrane (similar to angioid streaks),6 RPE atrophy,8 and development of choroidal neovascularization (CNV).68 Presence of one of these complications characterizes the CRF-related maculopathy.
The CRF-related maculopathy was characterized by Olsen et al68 who described three stages of this maculopathy on the basis of fluorescein angiography (FA) findings. The stage 3 is characterized by late stippled leakage, this FA pattern resembling presence of type 1 CNV. Assuming that type 1 CNV may characterize the CRF-related maculopathy (in approximately 10% of cases), the differential diagnosis between CNV and stage 3 maculopathy may be challenging as in both cases FA may display the presence of leakage. In these cases, structural OCT may be helpful in order to detect the presence of CNV. Choroidal neovascularizations in CRF-related maculopathy may be treated with anti-VEGF intravitreal injections.19 Conversely, the stage 3 CRF-related maculopathy, that also may be characterized by subretinal or intraretinal fluid, even in the absence of CNV, is less responsive to anti-VEGF injections.19 In these cases, the intravitreal injection of dexamethasone may be effective.19
Conclusions
Chorioretinal folds represent a quite frequent disease of the posterior eye. This disease should not be underestimated by ophthalmologists, as different ocular and extra-ocular causes may result in CRFs’ formation. Importantly, the presence of CRFs may eventually result in a secondary maculopathy, that must be recognized and appropriately treated.
Disclosure
Enrico Borrelli is advisor for: Zeiss (Dublin, USA), Centervue (Padua, Italy). Riccardo Sacconi is advisor for: Zeiss (Dublin, USA), Novartis (Basel, Switzerland); reports personal fees from Zeiss and Novartis, outside the submitted work. Francesco Bandello is a consultant for: Alcon (Fort Worth,Texas,USA), Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California,USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, New York, USA), Genentech (San Francisco, California, USA), Hoffmann-La-Roche (Basel, Switzerland), Novagali Pharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee,Belgium), Zeiss (Dublin, USA); reports personal fees from ALLERGAN, outside the submitted work. Giuseppe Querques is a consultant for: Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California, USA), Amgen (Thousand Oaks,USA), Bayer Shering-Pharma (Berlin, Germany), Heidelberg (Germany), KBH (Chengdu; China), LEH Pharma (London, UK), Lumithera (Poulsbo; USA), Novartis (Basel, Switzerland), Sandoz (Berlin, Germany), Sifi (Catania, Italy), Sooft-Fidea (Abano, Italy), Zeiss (Dublin, USA). The aforementioned authors report no other potential conflicts of interest for this work. The other authors have no disclosures.
References
- 1.Nettleship E. Peculiar lines in the choroid in a case of postpapillitic atrophy. Trans Ophthalmol Soc UK. 1884;4:167–168. [Google Scholar]
- 2.Norton E. Section of ophthalmology lang lecture a characteristic fluorescein angiographic pattern in choroidal folds. 1969;62:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gass JDM. Radial chorioretinal folds: a sign of choroidal neovascularization. Arch Ophthalmol. 1981;99:1016–1018. doi: 10.1001/archopht.1981.03930011016006 [DOI] [PubMed] [Google Scholar]
- 4.Newell FW. Choroidal folds. The seventh Harry Searls Gradle Memorial Lecture. Am J Ophthalmol. 1973;75(6):930–942. doi: 10.1016/0002-9394(73)91080-5 [DOI] [PubMed] [Google Scholar]
- 5.Bullock JD, Egbert PR. The origin of choroidal folds a clinical, histopathological, and experimental study. Doc Ophthalmol. 1974;37(2):261–293. doi: 10.1007/BF00147262 [DOI] [PubMed] [Google Scholar]
- 6.Friberg TR. The etiology of choroidal folds - a biomechanical explanation. Graefe’s Arch Clin Exp Ophthalmol. 1989;227(5):459–464. doi: 10.1007/BF02172899 [DOI] [PubMed] [Google Scholar]
- 7.Leahey AB, Brucker AJ, Wyszynski RE, et al. Chorioretinal folds: a comparison of unilateral and bilateral cases. Arch Ophthalmol. 1993;111:357–359. doi: 10.1001/archopht.1993.01090030075042 [DOI] [PubMed] [Google Scholar]
- 8.Fine HF, Cunningham ET, Kim E, et al. Autofluorescence imaging findings in long-standing chorioretinal folds. Retin Cases Brief Rep. 2009;3:137–139. doi: 10.1097/ICB.0b013e3181679f91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haruyama M, Yuzawa M. Angiographic findings of chorioretinal folds. Jpn J Ophthalmol. 2001;5155. [DOI] [PubMed] [Google Scholar]
- 10.Nishina S, Suzuki Y, Yokoi T, et al. Clinical features of congenital retinal folds. Am J Ophthalmol. 2012;153(1):81–87.e1. doi: 10.1016/j.ajo.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 11.Heimann H, Bopp S. Retinal folds following retinal detachment surgery. Ophthalmologica. 2011;226(s1):18–26. doi: 10.1159/000328380 [DOI] [PubMed] [Google Scholar]
- 12.Kupersmith MJ, Sibony PA, Dave S. Nonarteritic anterior ischemic optic neuropathy induced retinal folds and deformations. Investig Ophthalmol Vis Sci. 2017;58(10):4286. doi: 10.1167/iovs.17-22140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giuffrè G, Distefano MG. Optical coherence tomography of chorioretinal and choroidal folds. Acta Ophthalmol Scand. 2007;85:333–336. doi: 10.1111/j.1600-0420.2006.00799.x [DOI] [PubMed] [Google Scholar]
- 14.Del Turco C, Rabiolo A, Carnevali A, et al. Optical coherence tomography angiography features of chorioretinal folds: a case series. Eur J Ophthalmol. 2017;27:e35–e38. doi: 10.5301/ejo.5000872 [DOI] [PubMed] [Google Scholar]
- 15.Cangemi FE, Trempe CL, Walsh JB. Choroidal folds. Am J Ophthalmol. 1978;86:380–387. doi: 10.1016/0002-9394(78)90243-X [DOI] [PubMed] [Google Scholar]
- 16.Byrne SF, Atta HR Findings of Standardized Echography for. [DOI] [PubMed]
- 17.Kalina RE, Mills RP. Acquired hyperopia with choroidal folds. Ophthalmology. 1980;7(1):44–50 doi: 10.1016/S0161-6420(80)35279-2 [DOI] [PubMed] [Google Scholar]
- 18.Cohen SY, Ducos de Lahitte G, Gaudric A, et al. Chorioretinal folds in patients with central serous chorioretinopathy. Retin Cases Brief Rep. 2019. doi: 10.1097/ICB.0000000000000944 [DOI] [PubMed] [Google Scholar]
- 19.Corvi F, Capuano V, Benatti L, et al. Atypical presentation of chorioretinal folds-related maculopathy. Optom Vis Sci. 2016;93:1304–1314. doi: 10.1097/OPX.0000000000000953 [DOI] [PubMed] [Google Scholar]
- 20.Spaide RF, Jaffe GJ, Sarraf D, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127(5):616–636. doi: 10.1016/j.ophtha.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrelli E, Sarraf D, Freund KB, et al. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog Retin Eye Res. 2018;67:30–55. doi: 10.1016/j.preteyeres.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 22.Benson WE. Posterior scleritis. Surv Ophthalmol. 1988;32(5):297–316. doi: 10.1016/0039-6257(88)90093-8 [DOI] [PubMed] [Google Scholar]
- 23.McCluskey PJ, Watson PG, Lightman S, et al. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology. 1999;106(12):2380–2386. doi: 10.1016/S0161-6420(99)90543-2 [DOI] [PubMed] [Google Scholar]
- 24.Krist D, Wenkel H. Posterior scleritis associated with Borrelia burgdorferi (Lyme disease) infection. Ophthalmology. 2002;109(1):143–145. doi: 10.1016/S0161-6420(01)00868-5 [DOI] [PubMed] [Google Scholar]
- 25.Lavric A, Gonzalez-Lopez JJ, Majumder PD, et al. Posterior scleritis: analysis of epidemiology, clinical factors, and risk of recurrence in a cohort of 114 patients. Ocul Immunol Inflamm. 2016;24(1):6–15. doi: 10.3109/09273948.2015.1005240 [DOI] [PubMed] [Google Scholar]
- 26.Agrawal R, Lavric A, Restori M, et al. Nodular posterior scleritis: clinico-sonographic characteristics and proposed diagnostic criteria. Retina. 2016;36(2):392–401. doi: 10.1097/IAE.0000000000000699 [DOI] [PubMed] [Google Scholar]
- 27.Kumar A, Ghose A, Biswas J, et al. Clinical profile of patients with posterior scleritis: a report from Eastern India. Indian J Ophthalmol. 2018;66(8):1109. doi: 10.4103/ijo.IJO_121_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shields RA, Schachar IH. Posterior scleritis. Ophthal Surg Lasers Imag Retina. 2019;50(10):660. doi: 10.3928/23258160-20191009-11 [DOI] [PubMed] [Google Scholar]
- 29.Yanagida C, Usui Y, Sakai JI, et al. An unusual case of Behcet disease with posterior scleritis: a case report. Medicine (Baltimore). 2019;98:e16886. doi: 10.1097/MD.0000000000016886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew DJ, McKay BR, Basilious A, et al. Adherence to world glaucoma association guidelines for surgical trials in the era of microinvasive glaucoma surgeries. Ophthalmol Glaucoma. 2019;2(2):78–85. doi: 10.1016/j.ogla.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 31.Abbas A, Agrawal P, King AJ. Exploring literature-based definitions of hypotony following glaucoma filtration surgery and the impact on clinical outcomes. Acta Ophthalmol. 2018;96(3):e285–e289. doi: 10.1111/aos.13601 [DOI] [PubMed] [Google Scholar]
- 32.Kato T, Hayasaka S, Nagaki Y, et al. Management of traumatic cyclodialysis cleft associated with ocular hypotony. Ophthalmic Surg Lasers. 1999. doi: 10.3928/1542-8877-19990601-10 [DOI] [PubMed] [Google Scholar]
- 33.Toris CB, Pederson JE. Aqueous humor dynamics in experimental iridocyclitis. Investig Ophthalmol Vis Sci. 1987;28(3):477–81. [PubMed] [Google Scholar]
- 34.Fannin LA, Schiffman JC, Budenz DL. Risk factors for hypotony maculopathy. Ophthalmology. 2003;110(6):1185–1191. doi: 10.1016/S0161-6420(03)00227-6 [DOI] [PubMed] [Google Scholar]
- 35.Costa VP, Wilson RP, Moster MR, et al. Hypotony maculopathy following the use of topical mitomycin C in glaucoma filtration surgery. Ophthalmic Surg. 1993;24(6):389. [PubMed] [Google Scholar]
- 36.Rasheed ES. Initial trabeculectomy with intraoperative mitomycin-C application in primary glaucomas. Ophthalmic Surg Lasers. 1999;30(5):360–366. doi: 10.3928/1542-8877-19990501-07 [DOI] [PubMed] [Google Scholar]
- 37.Suner IJ, Greenfield DS, Miller MP, et al. Hypotony maculopathy after filtering surgery with mitomycin C: incidence and treatment. Ophthalmology. 1997;104:207–215. doi: 10.1016/S0161-6420(97)30332-7 [DOI] [PubMed] [Google Scholar]
- 38.Mietz H, Jacobi PC, Krieglstein GK. Intraoperative episcleral versus postoperative topical application of mitomycin-C for trabeculectomies. Ophthalmology. 2002;109(7):1343–1349. doi: 10.1016/S0161-6420(02)01101-6 [DOI] [PubMed] [Google Scholar]
- 39.Henderer JD, Budenz DL, Flynn HW, et al. Elevated intraocular pressure and hypotony following silicone oil retinal tamponade for complex retinal detachment: incidence and risk factors. Arch Ophthalmol. 1999;117(2):189–195. doi: 10.1001/archopht.117.2.189 [DOI] [PubMed] [Google Scholar]
- 40.Goshe JM, Terry MA, Li JY, et al. Graft dislocation and hypotony after Descemet’s stripping automated endothelial keratoplasty in patients with previous glaucoma surgery. Ophthalmology. 2012;119:1130–1133. doi: 10.1016/j.ophtha.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 41.Lavy I, Liarakos VS, Verdijk RM, et al. Outcome and histopathology of secondary penetrating keratoplasty graft failure managed by descemet membrane endothelial keratoplasty. Cornea. 2017;36(7):777–784. doi: 10.1097/ICO.0000000000001180 [DOI] [PubMed] [Google Scholar]
- 42.Camp AS, Weinreb RN. Hypotony keratopathy following trabeculectomy. J Glaucoma. 2020. doi: 10.1097/IJG.0000000000001425 [DOI] [PubMed] [Google Scholar]
- 43.Sugar HS. Postoperative cataract in successfully filtering glaucomatous eyes. Am J Ophthalmol. 1970;69(5):740–746. doi: 10.1016/0002-9394(70)93414-8 [DOI] [PubMed] [Google Scholar]
- 44.Dellaporta A. Fundus changes in postoperative hypotony. Am J Ophthalmol. 1955;40(6):781–785. doi: 10.1016/0002-9394(55)91105-3 [DOI] [PubMed] [Google Scholar]
- 45.Lavinsky J, Lavinsky D, Lavinsky F, et al. Acquired choroidal folds: a sign of idiopathic intracranial hypertension. Graefe’s Arch Clin Exp Ophthalmol. 2007;245:883–888. doi: 10.1007/s00417-006-0455-7 [DOI] [PubMed] [Google Scholar]
- 46.Griebel SR, Kosmorsky GS. Choroidal folds associated with increased intracranial pressure. Am J Ophthalmol. 2000;129(4):513–516. doi: 10.1016/S0002-9394(99)00412-2 [DOI] [PubMed] [Google Scholar]
- 47.Kwee RM, Kwee TC. Systematic review and meta-analysis of MRI signs for diagnosis of idiopathic intracranial hypertension. Eur J Radiol. 2019;116:106–115. doi: 10.1016/j.ejrad.2019.04.023 [DOI] [PubMed] [Google Scholar]
- 48.Musetti D, Nicolò M, Bagnis A, et al. Chorioretinal folds: associated disorders and a related maculopathy. Am J Ophthalmol. 2014;158:409. doi: 10.1016/j.ajo.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 49.Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014;311:1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kupersmith MJ, Sibony PA, Feldon SE, et al. The effect of treatment of idiopathic intracranial hypertension on prevalence of retinal and choroidal folds. Am J Ophthalmol. 2017;176:77–86. doi: 10.1016/j.ajo.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poon JS, Vahdani K, Booth AP. Chorioretinal folds in a patient with multiple myeloma treated with stem cell transplant. Eur J Ophthalmol. 2017;27(2):e46–e49. doi: 10.5301/ejo.5000928 [DOI] [PubMed] [Google Scholar]
- 52.Kurokawa T, Hamano H, Muraki T, et al. Immunoglobulin G4-related dacyroadenitis presenting as bilateral chorioretinal folds from severely enlarged lacrimal glands. Am J Ophthalmol Case Rep. 2018;9:88–92. doi: 10.1016/j.ajoc.2018.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leventer DB, Linberg JV, Ellis B. Frontoethmoidal mucoceles causing bilateral chorioretinal folds. Arch Ophthalmol. 2001;119:922–923. doi: 10.1001/archopht.119.6.922 [DOI] [PubMed] [Google Scholar]
- 54.Yeung L, Lai CC, Chen TL, et al. Chorioretinal folds associated with a meningioma. Chang Gung Med J. 2005;28:575–580. [PubMed] [Google Scholar]
- 55.Taban M, Kosmorsky GS, Singh AD, et al. Choroidal folds secondary to parasellar meningioma [41]. Eye. 2007;21(1):147–150. doi: 10.1038/sj.eye.6702479 [DOI] [PubMed] [Google Scholar]
- 56.Zbiba W, Elleuch IE, Sayadi S, et al. Multimodal imaging in a case of a congenital retinal macrovessel associated with a retinal cavernous hemangioma: a case report. BMC Ophthalmol. 2020;20:1–7. doi: 10.1186/s12886-020-1326-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stan MN, Garrity JA, Bahn RS. The evaluation and treatment of graves ophthalmopathy. Med Clin North Am. 2012;96(2):311–328. doi: 10.1016/j.mcna.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park J, Baek S. Dry eye syndrome in thyroid eye disease patients: the role of increased incomplete blinking and Meibomian gland loss. Acta Ophthalmol. 2019;97(5). doi: 10.1111/aos.14000 [DOI] [PubMed] [Google Scholar]
- 59.Heinz C, Eckstein A, Steuhl KP, et al. Amniotic membrane transplantation for reconstruction of corneal ulcer in Graves ophthalmopathy. Cornea. 2004;23(5):524–526. doi: 10.1097/01.ico.0000114128.63670.e2 [DOI] [PubMed] [Google Scholar]
- 60.Gold KG, Scofield S, Isaacson SR, et al. Orbital radiotherapy combined with corticosteroid treatment for thyroid eye disease-compressive optic neuropathy. Ophthal Plast Reconstr Surg. 2018;34(2):172–177. doi: 10.1097/IOP.0000000000001003 [DOI] [PubMed] [Google Scholar]
- 61.Kowal L, Georgievski Z. Choroidal folds in Graves’ ophthalmopathy. Aust N Z J Ophthalmol. 1994;22:216. doi: 10.1111/j.1442-9071.1994.tb01721.x [DOI] [PubMed] [Google Scholar]
- 62.Gupta D, Slabaugh M, Francis CE. Delayed correction of hypotony maculopathy in a patient with glaucoma and thyroid-related orbitopathy. Case Rep Ophthalmol. 2015;6(3):356–360. doi: 10.1159/000441121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jorge R, Scott IU, Akaishi PMS, et al. Resolution of choroidal folds and improvement in visual acuity after orbital decompression for graves orbitopathy. Retina. 2003;23(4):563–565. doi: 10.1097/00006982-200308000-00025 [DOI] [PubMed] [Google Scholar]
- 64.Rose GE. Postural visual obscurations in patients with inactive thyroid eye disease; a variant of ‘hydraulic’ disease. Eye. 2006;2006. doi: 10.1038/sj.eye.6702381. [DOI] [PubMed] [Google Scholar]
- 65.Vahdani K, Rose GE. Chorioretinal folds in thyroid eye disease. Ophthalmology. 2019;126(8):1106. doi: 10.1016/j.ophtha.2019.04.045 [DOI] [PubMed] [Google Scholar]
- 66.Roelofs KA, Eliott D, Freitag SK. Central retinal vein occlusion with chorioretinal folds secondary to active thyroid eye disease. Ophthalmology. 2018;125(10):1645. doi: 10.1016/j.ophtha.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 67.Tran AQ, Zhang-Nunes SX, Cahill K, et al. Thyroid eye disease with choroidal folds. Orbit (London). 2020;00:1–9. [DOI] [PubMed] [Google Scholar]
- 68.Olsen TW, Palejwala NV, Lee LB, et al. Chorioretinal folds: associated disorders and a related maculopathy. Am J Ophthalmol. 2014;157:1038–1047.e1. doi: 10.1016/j.ajo.2014.02.021 [DOI] [PubMed] [Google Scholar]