Abstract
Background
Microsatellite instability (MSI) has been a hot topic in cancer research. Determining MSI status greatly aids tumor prognosis and treatment plans. However, MSI data for Asian cancer patients with prognostic information are scarce. Here, our aim was to clarify MSI status and its prognostic value in a large Chinese cohort with different tumors.
Patients and Methods
Tissue samples from 600 Chinese cases, including 150 endometrial cancers, 150 colorectal cancers, 150 liver cancers and 150 gastric cancers, were used for IHC and MSI examinations. Two mononucleotide and three dinucleotide markers were used to analyze MSI status.
Results
In total,17.3% (26/150) of endometrial cancer patients showed positive MSI,10.0% (15/150) in colorectal cancer, 2.7% (4/150) in liver cancer, and 2.7% (4/150) in gastric cancer. Tumor location (P < 0.001 for colorectal cancer) and clinical stage (P =0.038 for gastric cancer) showed significant correlations with MSI status in gastrointestinal carcinogenesis. The mismatch repair (MMR) deficiency was observed in 20 colorectal cases (13.3%) and was significantly more frequent in the MSI-positive group (P < 0.001). Interestingly, the prevalence of MSI-H was mostly occurred in early-stage tumors, and none was in late stage (stage IV). Meanwhile, low clinicopathological stage had significant correlation with longer survival in multiple cancers here.
Conclusion
The incidence of microsatellite instability varies among different cancer types. And the prevalence of MSI-H mostly occurred early clinicopathological stage. In addition, our study provided a large Asian cohort screened by five loci PCR method and significantly increased knowledge on the prognostic significance of MSI in Asia.
Keywords: microsatellite instability, endometrial cancer, colorectal cancer, liver cancer, gastric cancer
Introduction
Microsatellite instability (MSI) is frequently involved in the carcinogenesis of various tumors,1,2 which defines the typical genomic phenotype of DNA repeat region insertion and deletion mutations.3 MSI tumors accumulate mutations due to mismatch repair (MMR) defects, which is a DNA repair system designed to correct errors caused by polymerases during replication.4 The MMR system is composed of a set of proteins that interact as heterodimers to sense and repair unpaired bases and small loops formed by insertions or deletions.5 These components are encoded by 8 genes, including hMSH2, hMSH3, hMSH5, hMSH6, hMLH1, hPMS1 (hMLH2), hMLH3, hPMS2 (hMLH4).6–8 In the absence of a functional MMR system, the insertion-deletion mutation was not repaired, resulting in a new allele with a length change.9 The variation of microsatellite repetition sequence length is called MSI. Of these, MSI is typically associated with colorectal cancer and has clear clinical significance, although has been also found in other diverse cancer types.10−12 Moreover, recent studies have shown that MSI may be a viable marker for immunological checkpoint blockade therapy. Determining MSI status greatly aids tumor prognosis and treatment plans and plays a critical role in immunotherapies.13 These observations emphasize the need for a more comprehensive and profound understanding of MSI.
In addition, microsatellite instability varies widely among different races and tumor types. However, prognostic factors in Caucasians may not be applicable to Asians, since known CRC survival rates for example, vary among major ethnic groups.14,15 Despite the adjustment of confounding variables such as age, grade, histology, and socioeconomic status, these differences still exist, suggesting that biological factors may be the cause of differences in survival. Whether the prognostic significance of MSI tumors in Caucasians is applicable to Asians remains to be seen, as there are few related studies.16,17 Thus, we presented a large report adding to the field on the prevalence of microsatellite instability in a large Asian cohort with different tumors including endometrial cancer, colorectal cancer, liver cancer and gastric cancer, and assess its prognostic value.
Patients and Methods
Patients and Samples
We analyzed tissue samples from 600 patients (150 endometrial cancer patients, 150 colorectal cancer patients, 150 liver cancer patients and 150 gastric cancer patients) with sporadic surgical resection during the period from October 2011 to November 2017 at the First Affiliated Hospital of Zhengzhou University. The average age of the patients was 56.5 years. And none had a known genetic disease. All specimens were collected for MSI analysis with the informed consent of the patient. The study was approved by Institutional Review Board of the First Affiliated Hospital of Zhengzhou University (approval number: 2019-KY-423) and was conducted according to the ethical standards of the Helsinki Declaration.
Pathological Assessment and Immunohistochemical Study
Pathological examinations were carried out based on the World Health Organization classification criteria. The degree of differentiation of tumor cells was determined by the proportion of the glandular formation region to the high power region. Less than 50% was defined as poorly differentiated carcinomas, over 95% was well differentiated carcinomas, and between the two was diagnosed moderately differentiated carcinomas. The diagnosis was made based on the predominant cell type when two different cell types are mixed. Lauren’s classification of gastric cancer was also analyzed including intestinal, diffuse and mixed type.
For the analysis of MMR proteins, immunohistochemistry staining for MLH1, MSH2, PMS2, and MSH6 was performed. These MMR proteins, as a group of nuclear enzymes, were detected in the nucleus. MMR was considered positive if the tumor cell nucleus was positive. Negative staining was defined as all loss of tumor epithelium.
Analysis for Microsatellite Instability
DNA preparation was performed using a QIAamp DNA FFPE Tissue kit (Qiagen Inc.), which were dissected under a microscope from the same paraffin tissues as those used in IHC. According to the National Cancer Institute (NCI), two mononucleotide repeats (BAT25 and BAT26) and three dinucleotide repeats (D5S346, D2S123, and D17S250), as the standard sites, were tested for MSI. MSI results were evaluated with a control of non-tumor tissues. MSI-positive tumors were designated a detection of instability in at least two of the five MSI markers. And a low incidence of MSI (MSI-L) and microsatellite stability (MSS) were defined as MSI-negative group, which was assessed by a detection of instability in one marker or no definite evidence of MSI.
Statistical Analyses
SPSS v25 (IBM Corporation, NY, USA) was used to perform statistical analysis. The statistical significance level was defined as two-sided P<0.05. Continuous variables were compared by t-test and presented as mean± SD. Overall survival (OS) was calculated from the date of the operation and death for any cause or last follow-up (censored patient). Patients who were still alive were censored on their date of last follow-up per chart review. The cut-off date for analysis was July 1st, 2019. Survival curves were analyzed via Kaplan–Meier method.
Results
Characteristics of the Subjects
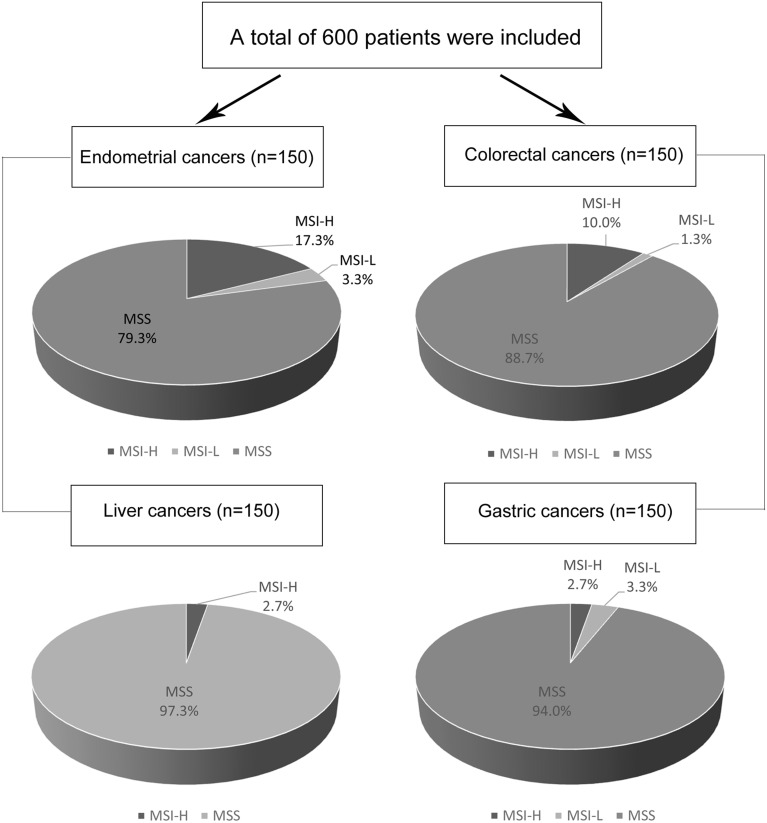
A total of 600 Chinese patients with sporadic surgical resection at the First Affiliated Hospital of Zhengzhou University during the period from October 2011 to November 2017, histologically confirmed endometrial cancer, colorectal cancer, liver cancer and gastric cancer, respectively, were included (Figure 1). The clinicopathological features are shown in Table 1. Of these, 308 patients were male and 292 were female. The median age at diagnosis was 56.5 years. The number of patients with stage I–IV disease were 202 (33.7%), 130 (21.7%), 213 (35.5%) and 55 (9.2%), respectively. MSI status can be evaluated the file FFPE tissue sections of all patients.
Figure 1.
Study flow. A total of 600 patients were included. 150 endometrial cancers, 150 colorectal cancers, 150 liver cancers and 150 gastric cancers were analyzed. MSI-L, low incidence of microsatellite instability; MSI-H, high incidence of microsatellite instability; MSS, microsatellite stability.
Table 1.
The Clinicopathological Characteristics of MSI-Negative and MSI-Positive Cancers
| Characteristics | No. of Patients (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endometrial Cancers (n=150) | Colorectal Cancers (n=150) | Liver Cancers (n=150) | Gastric Cancers(n=150) | ||||||||||
| All (n=600) | MSI-Negative (n=124) | MSI-Positive (n=26) | p | MSI-Negative (n=135) | MSI-Positive (n=15) | p | MSI-Negative (n=146) | MSI-Positive (n=4) | p | MSI-Negative (n=146) | MSI-Positive (n=4) | p | |
| Age, yr | 0.495 | 0.736 | 0.467 | 0.062 | |||||||||
| Median | 56.5±10.9 | 55.3±9.5 | 54.2±8.8 | 59.5±12.2 | 55.4±16.9 | 54.3±9.0 | 49.3±7.1 | 57.5±11.7 | 65.0±2.9 | ||||
| <65 | 455 (75.8) | 103 (83.1) | 23 (88.5) | 84 (62.2) | 10 (66.7) | 129 (88.4) | 4 (100.0) | 101 (69.2) | 1 (25.0) | ||||
| ≥65 | 145 (24.2) | 21 (16.9) | 3 (11.5) | 51 (37.8) | 5 (33.3) | 17 (11.6) | 0 (0.0) | 45 (30.8) | 3 (75.0) | ||||
| Gender | / | 0.252 | 0.376 | 0.415 | |||||||||
| Male | 308 (51.3) | / | / | 69 (51.1) | 10 (66.7) | 122 (83.6) | 4 (100.0) | 101 (69.2) | 2 (50.0) | ||||
| Female | 292 (48.7) | 124 (100.0) | 26 (100.0) | 66 (48.9) | 5 (33.3) | 24 (16.4) | 0 (0.0) | 45 (30.8) | 2 (50.0) | ||||
| Location | <0.001 | ||||||||||||
| Colon | / | / | / | 13 (9.6) | 7 (46.7) | / | / | / | / | ||||
| Left | / | / | / | 4 (3.0) | 2 (13.3) | / | / | / | / | ||||
| Right | / | / | / | 21 (15.6) | 5 (33.3) | / | / | / | / | ||||
| RS junction | / | / | / | 10 (7.4) | 0 (0.0) | / | / | / | / | ||||
| Rectum | / | / | / | 87 (64.4) | 1 (6.7) | / | / | / | / | ||||
| Clinical stage | 0.559 | 0.354 | 0.601 | 0.038 | |||||||||
| I/II | 332 (55.3) | 110 (88.7) | 23 (88.5) | 68 (50.4) | 10 (66.7) | 76 (52.1) | 1 (25.0) | 41 (28.1) | 3 (75.0) | ||||
| III/IV | 268 (44.7) | 14 (11.3) | 3 (11.5) | 67 (49.6) | 5 (33.3) | 70 (47.9) | 3 (75.0) | 105 (71.9) | 1 (25.0) | ||||
| Histological type | 0.012 | 0.705 | |||||||||||
| Mucinous | / | / | / | 25 (18.5) | 7 (46.7) | / | / | 5 (3.5) | 0 (0.0) | ||||
| Nonmucinous | / | / | / | 110(81.5) | 8 (53.3) | / | / | 139 (96.5) | 4 (100.0) | ||||
| IHC for MMR protein | |||||||||||||
| Loss of MLH1 | / | / | / | 0 (0.0) | 5 (33.3) | <0.001 | / | / | / | / | |||
| Loss of MSH2 | / | / | / | 0 (0.0) | 3 (20.0) | <0.001 | / | / | / | / | |||
| Loss of MSH6 | / | / | / | 1 (0.7) | 4 (26.7) | <0.001 | / | / | / | / | |||
| Loss of PMS2 | / | / | / | 1 (0.7) | 6 (40.0) | <0.001 | / | / | / | / | |||
Abbreviations: IHC, immunohistochemistry; MMR, mismatch repair; MSI, microsatellite instability.
Clinicopathologic Characteristics According to MSI Status
The MSI status was evaluated with the above five-site panel. Of these, the incidence of the five loci instability is also different among these cancers. Two mononucleotide sites, BAT-25 and BAT-26 seem to be more prone to instability, especially in MSI-positive endometrial (80.8% and 96.2%) and colorectal cancers (93.3% and 93.3%). And D17S250 instability was infrequent except the one in MSI-positive liver cancer patients (50%, 2/4) (Supplementary Table 1). In addition, a total of 49 cases (8.2%) showed a high frequency of MSI (MSI-H) having MSI at 2 or more loci in this study. Interestingly, MSI-H was more common in early-stage tumors, and none in late-stage tumors (Supplementary Table 2).
Of 150 endometrial cancer patients, 17.3% showed MSI-H (26/150), 3.3% showed MSI-L (5/150) and 79.3% showed MSS (119/150). There was no obvious correlation between MSI status and these clinicopathological factors including age and clinical stage. Of 150 colorectal cancer patients, 10.0% showed MSI-H (15/150), 1.3% showed MSI-L (2/150) and 88.7% showed MSS (133/150). A proximal location and histological type (Mucinous or not) showed significant correlations with MSI status (p<0.001 and 0.012). And MMR proteins deletions occurred more frequently in the MSI-positive group, 5 (33.3%) vs 0 (0.0%) for MLH1 deletion, 3 (20.0%) vs 0 (0%) for MSH2 deletion, 4 (26.7%) vs 1 (0.7%) for MSH6 deletion, 6 (40.0%) vs 1 (0.7%) for PMS2 deletion (P < 0.001, respectively). Details of the MMR proteins are presented in Table 1. Of 150 liver cancer patients, 2.7% showed MSI-H (4/150) and 97.3% showed MSS (146/150). And of 150 gastric cancer patients, 2.7% showed MSI-H (4/150), 3.3% showed MSI-L (5/150) and 94.0% showed MSS (141/150). Interestingly, the pathologic stage was significantly associated with MSI status (p=0.038), MSI-H occurs more frequently in low-stage tumors. In addition, the MSI status of these four cancers from the TCGA database was analyzed and compared with our data (Table 2). The prevalence of MSI-H was lower in our cohort except for liver cancer.
Table 2.
Prevalence of Microsatellite Instability (MSI) in Our Cohort and TCGA
| Cancer Types | No. of Cases (%) | |
|---|---|---|
| Data of This Cohort | Data of TCGA | |
| Endometrial cancers | 17.3% (26/150) | 31.4% (170/542) |
| Colorectal cancers | 10.0% (15/150) | 19.7% (85/431) |
| Liver cancers | 2.7% (4/150) | 0.8% (3/375) |
| Gastric cancers | 2.7% (4/150) | 19.1% (84/440) |
Abbreviation: TCGA, The Cancer Genome Atlas.
Clinical Outcomes
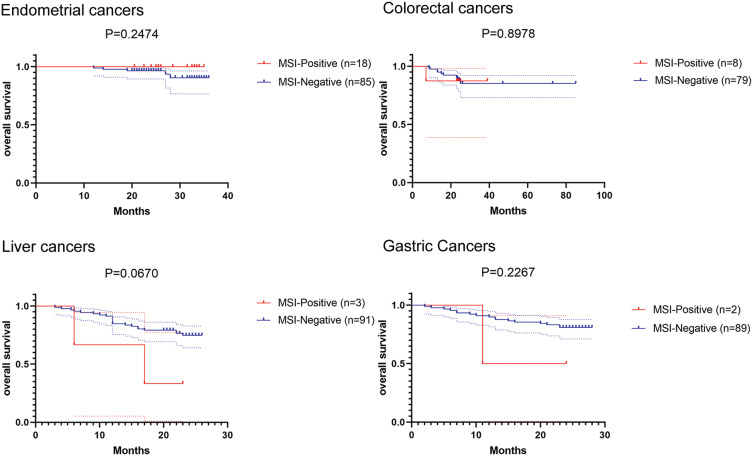
All patients received at least one follow-up call or visit. Of the 600 patients, 375 (62.5%) were analyzed for survival, and the longest follow-up was 85 months. Fifty-eight of 375 deaths (16.0%) occurred during follow up, including 5 of 103 deaths in endometrial cancers (4.8%), 11 of 87 deaths in colorectal cancers (12.6%), 24 of 94 deaths in liver cancers (25.5%) and 18 of 91 in gastric cancers (19.8%). For MSI status, 4 of 31 deaths (12.9%) in MSI-positive patients and 54 of 344 deaths (15.7%) in MSI-negative patients. And MSI-positive patients have no trend to have a longer OS than MSI-negative patients in these tumors. The survival curve is shown in Figure 2.
Figure 2.
The Kaplan–Meier curve illustrates overall survival (OS) in the groups that were negative and positive for the MSI (microsatellite instability).
Univariate analysis revealed that age <65 years (median OS for age <65 vs ≥65 years, undefined vs undefined; hazard ratio (HR), 7.609 [95% CI, 0.716–80.87], p=0.009) and low pathologic stage (median OS for stage I/II vs stage III/IV, undefined vs undefined; HR, 5.300 [95% CI, 0.328–85.62], p=0.041) were correlated with prolonged survival in endometrial cancers. For colorectal cancers, the pathologic stage showed significant correlations with overall survival (p<0.001, respectively). However, there was no longer significant association in multivariable analysis. Interestingly, high pathologic stage was significantly associated with poor OS in both univariate (median OS for stage I/II vs stage III/IV, undefined vs undefined; HR, 3.040 [95% CI, 1.359–6.801], p=0.008) and multivariate analysis adjusted with age, gender and MSI status (HR, 2.931 [95% CI, 1.181–7.274], p=0.020) in liver cancer patients. Similarly, age, gender, clinical stage and MSI status were included for analysis in gastric cancers. Unfortunately, the results were not statistically significant (Table 3).
Table 3.
Univariate and Multivariate Analyses of Prognostic Factors in Patients
| Variables | Cancer Type | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age<65 y vs ≥65 y | Endometrial Cancers | 7.609(0.716–80.87) | 0.009 | 2.890E+5(0.000–1.330E+139) | 0.936 |
| Colorectal Cancers | 0.857(0.257–2.863) | 0.805 | 0.893(0.251–3.174) | 0.861 | |
| Liver Cancers | 0.805(0.214–3.027) | 0.766 | 0.859(0.197–3.739) | 0.840 | |
| Gastric Cancers | 1.960(0.738–5.206) | 0.145 | 1.936(0.727–5.154) | 0.186 | |
| Male vs Female | Endometrial Cancers | / | / | / | / |
| Colorectal Cancers | 0.723(0.220–2.378) | 0.602 | 0.578(0.165–2.027) | 0.392 | |
| Liver Cancers | 0.858(0.309–2.383) | 0.777 | 1.035(0.346–3.101) | 0.951 | |
| Gastric Cancers | 1.625(0.608–4.340) | 0.300 | 1.854(0.711–4.832) | 0.206 | |
| Stage I/II vs stage III/IV | Endometrial Cancers | 5.300(0.328–85.62) | 0.041 | 3.052E+5(0.000–1.406E+139) | 0.936 |
| Colorectal Cancers | / | <0.001 | 2.513E+5(0.000–9.400E+120) | 0.927 | |
| Liver Cancers | 3.040(1.359–6.801) | 0.008 | 2.896(1.177–7.126) | 0.021 | |
| Gastric Cancers | 1.653(0.615–4.448) | 0.369 | 1.976(0.566–6.907) | 0.286 | |
| MSI-negative vs MSI-positive | Endometrial Cancers | 0.000(−1.000- −1.000) | 0.247 | 0.000(0.000–2.150E+127) | 0.949 |
| Colorectal Cancers | 1.144(0.130–10.05) | 0.898 | 1.046(0.124–8.815) | 0.967 | |
| Liver Cancers | 3.510(0.274–44.97) | 0.067 | 2.325(0.526–10.281) | 0.266 | |
| Gastric Cancers | 3.230(0.990–105.4) | 0.227 | 3.701(0.357–38.369) | 0.273 | |
Abbreviations: CI, confidence interval; HR, hazard radio; MSI, microsatellite instability.
Discussion
Tumor MSI status has become an important molecular marker for judging tumor prognosis and treatment. However, MSI studies for some cancers in East Asian races have been limited. In this study, we clarify MSI status and its prognostic value in a Chinese cohort of 600 patients with different tumors. A total of 49 (8.2%, 49/600) patients were identified as positive for MSI by 5 sites PCR–based screening. Of these, endometrial cancer patients presented the highest prevalence (26/150=17.3%), closely followed by colorectal cancers (15/150=10%), while gastric cancers had a lower rate here similar to liver cancers (4/150=2.7%). The ranking of rate is consistent with that reported in the previous literature. Of which, endometrial carcinoma, colorectal adenocarcinoma and gastric adenocarcinoma rank in the top 3 in the prevalence of MSI-H in 39 cancer types.18 Respectively, MSI-H varies from 17.00% to 31.37% in endometrial carcinoma, 6.00–19.72% in colon adenocarcinoma, and 9.00–19.09% in stomach adenocarcinoma. The prevalence of MSI-H in liver cancers was a little bit lower, about 0.80–3.00%.19 Our data show that the incidence of MSI in East Asian is lower than that of Caucasians, possibly due to ethnic differences, just as prognostic factors for Caucasians may not be applicable to Asians.14,15 Moreover, the difference in specificity and sensitivity of screening methods may also lead to the difference in morbidity.20 These suggest that our results were roughly consistent with previous reports and provided a large five-loci PCR screened Asian cohort for MSI in Asia.
In addition, the MSI status of all 600 patients was determined by the NCI panel PCR amplification method described above.10,21 Five loci including two mononucleotide repeats (BAT25 and BAT26) and three dinucleotide repeats (D5S346, D2S123, and D17S250) were tested for MSI. And the most instable sites were still concentrated in BAT-25 and BAT-26, especially in endometrial and colorectal cancers, where the incidence of instability was more than 90%, reinforcing previous reports.20 Brennetot et al22 also indicated that BAT-26 and BAT-25 markers can accurately identify MSI-H tumors before screening for unstable target gene mutations without pre-concentration of tumor cells, and indicate which samples need further purification. Therefore, BAT-26 and BAT-25 may be used to determine the MSI status of human tumors without the need for expensive and time-consuming population screening or sample purification methods. The incidence of other three dinucleotide repeats sites is lower, especially the locus of D17S250. Interestingly, however, the incidence of D17S250 instability in MSI-positive liver cancers in our cohort was 50%, although there were only 4 MSI-positive HCC patients overall. This may suggest that the instability of microsatellite loci is significantly different in different cancers, which needs to be demonstrated by a larger study cohort. And this may also suggest Asian characteristic. Moreover, since MSI-L occurs mostly at the dinucleotide site, the detection rate of MSI-L at simple mononucleotide site panels is low.21,23 Therefore, it seems more reasonable using the NCI panel in our cohort to avoid the effect of detecting simple mononucleotide sites on the overall evaluation of MSI status in patients with other cancers. The similar conclusion has been also pointed out in previous studies, which have suggested that NCI panel is more suitable for East Asian populations.20
The prevalence of MSI-H in our cohorts was mostly derived from tissues of early-stage tumors. Here, our study showed that 37 of 49 were in early clinicopathological stage (I/II) for MSI-positive patients (75.5%), and none was in late-stage (stage IV). Similar results were also reported previously,24–26 MSI positivity (or Mismatch repair deficiency) occurs mostly in the early stages of a variety of tumors, including endometrial, gastric, colorectal, and liver cancers. And a higher incidence of Mismatch repair deficiency (dMMR) was shown in early-stage CRC than that in metastatic CRC.27 In our cohort, it is interesting to note that MSI status has no prognostic significance for overall survival. Our data suggested low clinicopathological stage were associated with longer survival in multiple cancers, which could serve as a support for previous studies. Most previous results indicated that the prognostic and predictive values of MMR are different in early-stage and late-stage. In early-stage (II/III), dMMR as a positive prognostic factor was significantly associated with longer survival rather than late-stage (IV).24,27–29 For example, MSI-H has a positive predictive value in early-stage CRC patients without adjuvant chemotherapy and plays a negative prognostic predictive role for resected CRC patients with adjuvant fluorouracil-based chemotherapy.30 In addition, the predictive value of dMMR was also investigated in other tumors including gastric cancer, endometrial cancer and melanoma.31–33 And its prognostic value for different types of cancer is different. Researches on these tumors were rare and limited, so our data, such as liver cancer, can be used as a supplement to evidence of prognostic value. Analysis of MSI status and their functional roles across tumor types will teach us how to extend therapies effective in one cancer type to others.
Conclusions
Our study highlights that the incidence of microsatellite instability varies among different cancers. The prevalence of MSI-H was mostly occurred early clinicopathological stage. And low clinicopathological stage had significant correlation with longer survival in multiple cancers here. Moreover, our study provided a large MSI screening for Asian cohort and significantly increase knowledge on the prognostic significance of MSI in Asia. Nonetheless, the main limitation of this study is that IHC for MMR protein was performed only for colorectal cancers and not for others so further investigation is needed.
Acknowledgments
The authors thank Shanghai Tongshu Biotechnology Co., Ltd. for technical support.
Funding Statement
This work was supported by grant from National Natural Science Foundation of China (No. 81272371 and No.U1904148), the National Science and Technology Major Project of China (No. 2018ZX10302205), Zhengzhou Major Project for Collaborative Innovation (Zhengzhou University, No. 18XTZX12007), and the Youth Innovation Fund of The First Affiliated Hospital of Zhengzhou University to Wei-Wei Wang.
Ethics Approval and Informed Consent
All specimens were collected for MSI analysis with the informed consent of the patient. The study was approved by Institutional Review Board of the First Affiliated Hospital of Zhengzhou University (approval number: 2019-KY-423) and was conducted according to the ethical standards of the Helsinki Declaration.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer. 2015;15(3):181–194. doi: 10.1038/nrc3878 [DOI] [PubMed] [Google Scholar]
- 2.Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22(11):1342–1350. doi: 10.1038/nm.4191 [DOI] [PubMed] [Google Scholar]
- 3.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–819. doi: 10.1126/science.8484122 [DOI] [PubMed] [Google Scholar]
- 4.Goumard C, Desbois-Mouthon C, Wendum D, et al. Low levels of microsatellite instability at simple repeated sequences commonly occur in human hepatocellular carcinoma. Cancer Genomics Proteomics. 2017;14(5):329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pecina-Slaus N, Kafka A, Salamon I, Bukovac A. Mismatch repair pathway, genome stability and cancer. Front Mol Biosci. 2020;7:122. doi: 10.3389/fmolb.2020.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amaral-Silva GK, Martins MD, Pontes HA, et al. Mismatch repair system proteins in oral benign and malignant lesions. J Oral Pathol Med. 2017;46(4):241–245. doi: 10.1111/jop.12484 [DOI] [PubMed] [Google Scholar]
- 7.Clark N, Wu X, Her C. MutS homologues hMSH4 and hMSH5: genetic variations, functions, and implications in human diseases. Curr Genomics. 2013;14(2):81–90. doi: 10.2174/1389202911314020002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipkin SM, Wang V, Jacoby R, et al. MLH3: a DNA mismatch repair gene associated with mammalian microsatellite instability. Nat Genet. 2000;24(1):27–35. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt MHM, Pearson CE. Disease-associated repeat instability and mismatch repair. DNA Repair (Amst). 2016;38:117–126. doi: 10.1016/j.dnarep.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 10.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer—the stable evidence. Nat Rev Clin Oncol. 2010;7(3):153–162. doi: 10.1038/nrclinonc.2009.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arulananda S, Thapa B, Walkiewicz M, et al. Mismatch repair protein defects and microsatellite instability in malignant pleural mesothelioma. J Thorac Oncol. 2018;13(10):1588–1594. doi: 10.1016/j.jtho.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 12.Haron NH, Mohamad Hanif EA, Abdul Manaf MR, et al. Microsatellite instability and altered expressions of MLH1 and MSH2 in gastric cancer. Asian Pac J Cancer Prev. 2019;20(2):509–517. doi: 10.31557/APJCP.2019.20.2.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Xu J, Li L, Mu X, Wang Y, Li X. Evaluation of a fully automated idylla test system for microsatellite instability in colorectal cancer. Clin Colorectal Cancer. 2019;18(4):e316–e323. doi: 10.1016/j.clcc.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 14.Lai Y, Wang C, Civan JM, et al. Effects of cancer stage and treatment differences on racial disparities in survival from colon cancer: a United States population-based study. Gastroenterology. 2016;150(5):1135–1146. doi: 10.1053/j.gastro.2016.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1950–1962. doi: 10.1158/1055-9965.EPI-07-2774 [DOI] [PubMed] [Google Scholar]
- 16.Jung SH, Kim SH, Kim JH. Prognostic impact of microsatellite instability in colorectal cancer presenting with mucinous, signet-ring, and poorly differentiated cells. Ann Coloproctol. 2016;32(2):58–65. doi: 10.3393/ac.2016.32.2.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin US, Cho SS, Moon SM, et al. Is microsatellite instability really a good prognostic factor of colorectal cancer? Ann Coloproctol. 2014;30(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precision Oncol. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12(1):54. doi: 10.1186/s13045-019-0738-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng J, Huang B, Nie X, Zhu Y, Han N, Li Y. The clinicopathological features and prognosis of tumor MSI in East Asian colorectal cancer patients using NCI panel. Fut Oncol. 2018;14(14):1355–1364. doi: 10.2217/fon-2017-0662 [DOI] [PubMed] [Google Scholar]
- 21.Perucho M, Boland CR et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res., 58: 5248-5257, 1998. Cancer Res. 1999;59(1):249–256. [PubMed] [Google Scholar]
- 22.Brennetot C, Buhard O, Jourdan F, Flejou JF, Duval A, Hamelin R. Mononucleotide repeats BAT-26 and BAT-25 accurately detect MSI-H tumors and predict tumor content: implications for population screening. Int J Cancer. 2005;113(3):446–450. doi: 10.1002/ijc.20586 [DOI] [PubMed] [Google Scholar]
- 23.Xicola RM, Llor X, Pons E, et al. Performance of different microsatellite marker panels for detection of mismatch repair-deficient colorectal tumors. J Natl Cancer Inst. 2007;99(3):244–252. doi: 10.1093/jnci/djk033 [DOI] [PubMed] [Google Scholar]
- 24.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao X, Dong D, He W, et al. Mismatch repair deficiency is associated with MSI phenotype, increased tumor-infiltrating lymphocytes and PD-L1 expression in immune cells in ovarian cancer. Gynecol Oncol. 2018;149(1):146–154. doi: 10.1016/j.ygyno.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 26.Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol. 2014;25(5):1032–1038. doi: 10.1093/annonc/mdu100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20(20):5322–5330. doi: 10.1158/1078-0432.CCR-14-0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Z, Sanhueza CT, Johnson B, et al. Outcome of mismatch repair-deficient metastatic colorectal cancer: the mayo clinic experience. Oncologist. 2018;23(9):1083–1091. doi: 10.1634/theoncologist.2017-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46(15):2788–2798. doi: 10.1016/j.ejca.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 30.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–257. doi: 10.1056/NEJMoa022289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang WL, Chang SC, Lan YT, et al. Microsatellite instability is associated with a better prognosis for gastric cancer patients after curative surgery. World J Surg. 2012;36(9):2131–2138. doi: 10.1007/s00268-012-1652-7 [DOI] [PubMed] [Google Scholar]
- 32.Kato M, Takano M, Miyamoto M, et al. DNA mismatch repair-related protein loss as a prognostic factor in endometrial cancers. J Gynecol Oncol. 2015;26(1):40–45. doi: 10.3802/jgo.2015.26.1.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvino E, Passarelli F, Cannavo E, et al. High expression of the mismatch repair protein MSH6 is associated with poor patient survival in melanoma. Am J Clin Pathol. 2014;142(1):121–132. doi: 10.1309/AJCPCX2D9YULBBLG [DOI] [PubMed] [Google Scholar]