Abstract
Exosomes are a subset of tiny extracellular vesicles manufactured by all cells and are present in all body fluids. They are produced actively in tumor cells, which are released and utilized to facilitate tumor growth. Their characteristics enable them to assist major cancer hallmarks, leveraged by cancer cells in fostering cancer growth and spread while implementing ways to escape elimination from the host environment. This review updates on the latest progress on the roles of cancer-derived exosomes, of 30–100 nm in size, in deregulating paracrine trafficking in the tumor microenvironment and circulation. Thus, exosomes are being exploited in diagnostic biomarker development, with its potential in clinical applications as therapeutic targets utilized in exosome‐based nanoparticle drug delivery strategies for cancer therapy. Ongoing studies were retrieved from PubMed® and Scopus database and ClinicalTrials.gov registry for review, highlighting how cancer cells from entirely different cell lines rely on genetic information carried by their exosomes for homotypic and heterotypic intercellular communications in the microenvironment to favor proliferation and invasion, while establishing a pre-metastatic niche in welcoming cancer cells’ arrival. We will elaborate on the trafficking of tumor-derived exosomes in fostering cancer proliferation, invasion, and metastasis in hematopoietic (leukemia and myeloma), epithelial (breast cancer), and mesenchymal (soft tissue sarcoma and osteosarcoma) cancers. Cancer-derived exosomal trafficking is observed in several types of liquid or solid tumors, confirming their role as cancer hallmark enabler. Their enriched genetic signals arising from their characteristic DNA, RNA, microRNA, and lncRNA, along with specific gene expression profiles, protein, or lipid composition carried by the exosomal cargo shed into blood, saliva, urine, ascites, and cervicovaginal lavage, are being studied as a diagnostic, prognostic, or predictive cancer biomarker. We reveal the latest research efforts in exploiting the use of nanoparticles to improve the overall cancer diagnostic capability in the clinic.
Keywords: tumor-released exosomes, carcinoma-associated fibroblasts, exosome cargo, exosome-induced chemoresistance, hallmarks of cancer, tumor-stromal communications
Introduction
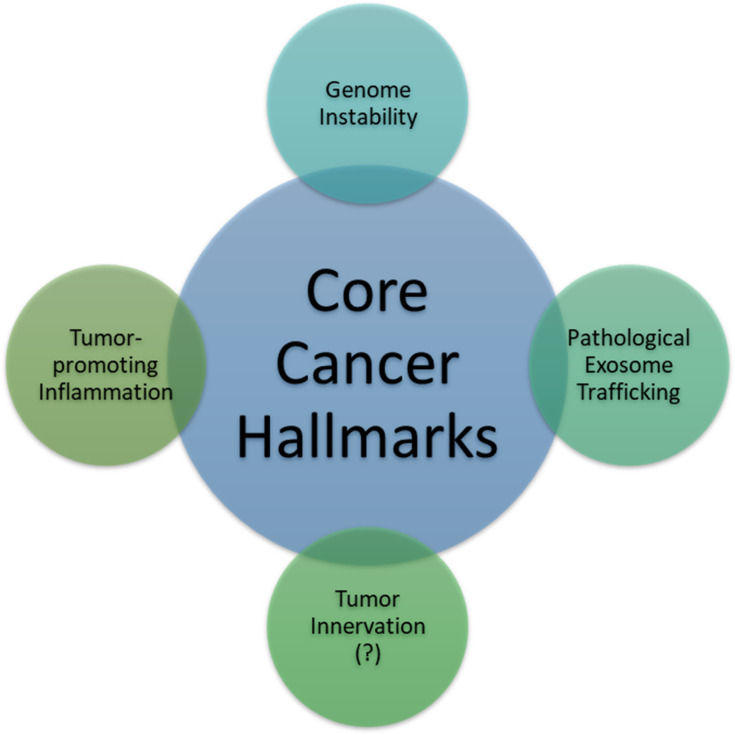
There exist six well-researched cancer hallmarks, and they are as follows: (1) sustaining proliferative signaling, (2) evading growth suppressors, (3) enabling replicative immortality, (4) activating invasion and metastasis, (5) inducing angiogenesis, and (6) resisting cell death. However, two emerging hallmarks were identified and added by Hanahan and Weinberg:1,2 (7) deregulating cellular metabolism and (8) avoiding immune destruction. Cancer cells typically acquire these core capabilities through sustaining selective pressures and adopting alterations in specific and ubiquitous cellular function, defined as “enabling characteristics,” to permit cancer hallmark capabilities. Enabling characteristics, which foster cancer growth and spread, are typically adopted by cancer cells to survive, proliferate, and escape elimination by the host environment. In the next-generation review by Hanahan and Weinberg on cancer hallmarks’ molecular mechanisms, they proposed two enabling characteristics that are present in cancer cells across the board, which are genome instability/mutation and tumor-promoting inflammation.1
In this review, we propose pathological trafficking of genetic materials carried by cancer-derived exosomes (CDE) cargo as a new enabling characteristic that fits the proposed criteria (Table 1). This CDE cargo phenomenon has been demonstrated to be present in virtually all kinds of cancer cell types, fostering the core hallmarks of cancer by effective homotypic or heterotypic intercellular (tumor-to-microenvironment) communications, which facilitate cancer invasion and metastasis as well as evasion from immune destruction. The second part of the review is dedicated to the updated status of CDE being evaluated as a valid diagnostic cancer biomarker in human studies.
Table 1.
Criteria for Classification as a Hallmark of Cancer or an Enabling Characteristic, According to Hanahan and Weinberg1,2
| Hallmark of Cancer |
|
|
|
|
| Enabling characteristic |
|
|
|
|
This study aims to present a comprehensive overview of the roles of CDE in cancer progression and development, assimilated from the current literature and translational cancer research to stimulate cancer biologists, scientists, and oncologists who are interested in the involvement of CDE in cancer pathogenesis, cancer microenvironment, molecular mechanisms on cancer progression and who plan to apply the knowledge in developing more effective diagnostic strategies for various types of cancer. Ongoing research studies evaluating CDE as a cancer biomarker registered in the ClinicalTrials.gov were selected to enrich the discussion on the clinical application of CDE as a biological cancer marker and to indicate future opportunities for cross-disciplinary collaborations.
Literature Search
To ensure a comprehensive and unbiased literature review, we performed both electronic and manual literature search in the PubMed® and Scopus database to retrieve relevant original articles. We leveraged the use of PubMed Advanced Search Builder, Medical Subject Headings (MeSH), and Boolean logic to add terms or combine search terms using connector words, such as AND, OR, or NOT, as well as truncate terms. We used a controlled vocabulary to produce highly relevant search results. The search terms included exosome, exosomal cargo, cancer-derived or tumor-derived, cancer biology, proteasome, and cancer biology. Subsequently, we surveyed the ClinicalTrials.gov registry for clinical studies conducted in the United States and around the world.
Cancer-Derived Exosomes in Cancer Biology
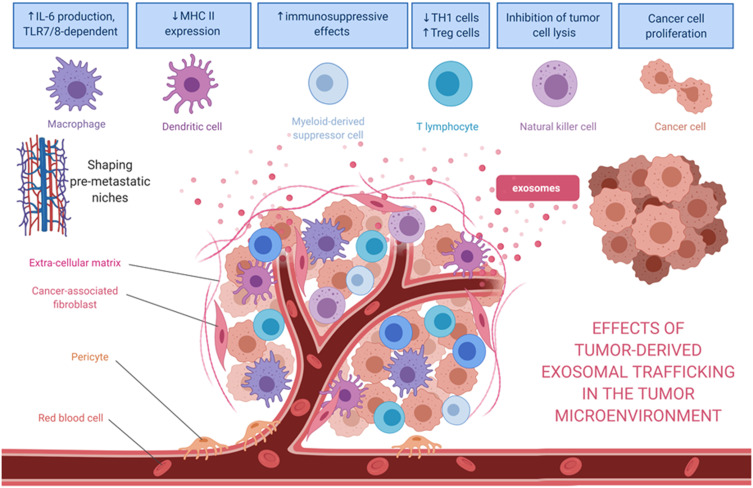
The tumor microenvironment (TME) surrounding cancer cells is identified to be comprised of cancer-associated fibroblasts, blood vessels, nerve fibers, immune cells, other stromal cells, and extracellular vesicles containing various kinds of genetic signals. Considering all are functional in anticancerous immunosuppressive cells, the TME is known to create a milieu that prevents the free spread of the malignant cells.3 The cancer cells, however, communicate with the neighboring stromal and immune cells, promoting immune evasion, and could also activate angiogenesis, tumor innervation, and epithelial-mesenchymal transition (EMT), in order to facilitate neoplastic growth. Recently, a phenomenon involving tumor-infiltrating innervation in the TME has also been proposed as a prerequisite for cancer cells of many types such as in prostate, gastric, pancreatic, and rectal cancers.4–6 Tumors are capable of recruiting nerves via the release of neurotrophic factors and axonal guidance molecules, and, with the contribution of CDEs, induction of axonogenesis is initiated, whereby the communication between the tumor and potentially innervating nerves work in concert to promote tumor innervation.4 Thus, it has been proposed that tumor innervation with neurite outgrowth (axonogenesis), just like angiogenesis, might be considered a new emerging hallmark of cancer.7–13 Although research has demonstrated that angiogenesis is frequently associated with axonogenesis, more studies are eagerly required to elucidate the roles of tumor-infiltrating innervation in tumor initiation, growth, and spread for this phenomenon to be widely accepted as a hallmark of cancer. Recent studies were able to confirm that the neurite recruitment/outgrowth and tumor innervation were promoted by the release of exosomes in the head and neck squamous cell carcinomas model5 and human papillomavirus-positive uterine cervical cancer cell lines.6
The name “tumor-derived exosomes” was coined in 1981, and this phenomenon has received extensive research attention over the past decade.14 Exosomes are extracellular, membranous, cup-shaped microvesicles 30 to 100 nm in size, which are produced by most types of cells.15–17 They originate from intracellular multivesicular bodies and are released by exocytosis into the extracellular microenvironment.18 Exosome typically consists of a variety of genetic messengers such as DNA, mRNA, microRNA, cytosolic proteins, and lipids.19–23 Exosomal markers such as tetraspanin proteins CD63, CD9, and CD81 allow sorting, selective recruitment, capturing, or profiling of CDEs.24,25 Once the recipient cells internalize tumor-derived exosomes, the ensuing biological response is determined explicitly by the dedicated trafficking routes, the exosomal internalization pathway, and the complex surface molecules on the membrane of both the extracellular vesicle and the recipient cell. With the advent of theranostic nanotechnologies such as differential ultracentrifugation, nanofluidic technology, and the exosome total isolation chip (ExoTIC), a size-based extracellular vesicle isolation apparatus, researchers nowadays are now able to capture nano-sized CDE for further analyses.26–28 The latest biosensing technologies, such as afterglow sensors with aptamer-based signal amplification, improve the limit of detection (LOD) that is nearly two orders of magnitude lower than that of fluorescence methods.29 With the advent of these sensitive biosensors, the LOD can practically be improved to 102 exosomes per milliliter.
These exosomes, particularly those that are tumor-derived, act as signal transducers or messengers in the cell-cell communication.5,30–33 The recipient cells respond to the exosomal contents (such as microRNA) by changing their phenotypes. microRNAs are considered an evolutionarily conserved family of molecules that bind to complementary sequences in the 3ʹ-untranslated region (3ʹUTR) of their target mRNAs, post-transcriptionally repressing gene expression.34 It has been demonstrated that in high-grade bladder cancer cell line, TCC-SUP, for example, exosomes promoted angiogenesis and migration of both cancer and endothelial cells.35 In another study in prostate cancer, the malicious CDEs induced differentiation of the stromal mesenchymal stem cells toward alpha-smooth muscle actin-positive myofibroblasts, which secreted high levels of proangiogenic VEGF-A, pro-invasive HGF, MMP-1, MMP−3, and MMP−13.36
The role of CDEs as characteristic enablers of cancer hallmarks to facilitate organ-specific metastasis has been demonstrated by the proof-of-principle study conducted by Hoshino et al,37 In their peripheral blood study of mouse and human cell lines, they claimed that during the metastatic cascade, organ-specific metastasis took place not by a random process but by somewhat predictable and trackable events. This happened through distinct integrin expression patterns contained in the CDEs, a phenomenon that now elucidates the mechanism of specific cancer organotropism adequately. The exosomal integrin αvβ5 was associated with hepatic metastasis, while exosomal integrins α6β4 and α6β1 were linked to lung metastasis.37
In the following sections, we will use three different cancer types: hematopoietic, epithelial, and sarcomatous malignancies (leukemia/myeloma, breast cancer, soft tissue sarcoma, and osteosarcoma) to prove, using compelling evidence, that cancer cells across the board leverage the pathological trafficking of exosomes to promote neoplastic growth, facilitate cancer spread through tumor-stromal interaction, and evade destruction by the host (Figure 1).
Figure 1.
Two established enabling characteristics (genome instability or mutation and tumor-promoting inflammation): one investigating feature (tumor innervation) and one hitherto proposed enabling characteristic, that is, pathological exosome trafficking. Enabling characteristics are defined as the capabilities possessed by most cancer types to foster and/or expedite the acquisition of one or some core hallmarks of cancer.
Exosomal Trafficking in Leukemia Pathogenesis
Although leukemia can reach every part of the host body through the ever-reaching blood vessels, recent research has reported that leukemic cells also employ paracrine exosome trafficking to achieve leukemogenesis, maintain leukemic persistence by shaping the leukemic niche and its progression, suppress hematopoiesis, modify anti-leukemic immunity, and evade destruction by chemotherapy. Table 2 illustrates these aspects taking acute myeloid leukemia (AML) as an example.
Table 2.
Paracrine Exosome Trafficking Employed by Acute Myeloid Leukemia (AML) as an Example and Its Specific Functional Outcomes
| Leukemogenesis | Immunosuppression | Suppression of Hematopoiesis | Chemotherapy Resistance | Leukemic Persistence/Progression |
|---|---|---|---|---|
| AML-derived bone marrow mesenchymal stromal cells release exosomes that can affect gene regulatory networks.45 | AML-derived exosomes carry immunosuppressive molecules responsible for immune cell deregulation.46,47 | Blast-derived exosomes remodel the bone marrow niche into a leukemia growth-permissive microenvironment.48,49 | Blast-derived exosomes propel bone marrow stromal cells to generate IL-8, which regulates chemo- cytotoxicity.50 AML cells secrete VEGF/VEGFR-containing exosomes that induce glycolysis in endothelial cells, leading to vascular remodeling and chemoresistance.51 |
May have a broader role in shaping the leukemic niche.52–54 |
Patients with chronic lymphocytic leukemia (CLL) have been identified to have decreased T-cell immunity. A recent study showed that CLL induced myeloid-derived suppressor cells (MDSCs), which, in turn, suppressed T-cell activation and induced suppressive regulatory T cells (Treg) through exosomal miR-155 transfer.38,39 This exosome-mediated transfer of microRNAs to monocytes could significantly contribute to CLL-related immune escape via PD-L1 expression. Colleagues from the University of Liverpool verified that CLL-derived exosomes encapsulate small RNAs, and the encapsulated microRNA miR-202-3p enhanced the expression of a Hedgehog signaling intermediate.40 An enriching body of evidence shows that the TME created by the bone marrow significantly favors the survival, growth, and proliferation of leukemic cells. For example, CLL leukemic cells can establish their favorable leukemic niche in the TME. Paggetti et al have discovered that CLL-derived exosomes could affect bone marrow stromal cells in adopting a cancer-associated fibroblast phenotype, which would contribute to a tumor-supportive microenvironment.41 In the chronic myeloid leukemia (CML) model, CML-derived exosomal paracrine trafficking stimulated bone marrow stromal cells to produce interleukin (IL)-8.42,43 Further, another study demonstrated that exosomes released from CML cells affect the endothelium directly to modify the neovascularization process.44
Exosomal Trafficking in Multiple Myeloma (MM) Pathogenesis
In a murine MM model, the myeloma exosomes were identified to have a proangiogenic function to enhance the viability of bone marrow endothelial cells; besides, an in vivo experiment demonstrated that these exosomes increased the presence of bone marrow MDSCs and changed their subsets to a more tumorigenic profile.55 MM-derived exosomes could modify the bone marrow microenvironment to facilitate myeloma progression. Conversely, the bone marrow stromal cells could, reciprocally, also release certain exosomes to be taken up by MDSCs through the STAT3 and STAT1 pathways, which leads to increased immunosuppression, thereby inducing MM expansion.56 Initially, investigators from the Dana-Farber Cancer Institute demonstrated that there were significant differences in microRNA profiling between normal and bone marrow mesenchymal stromal cell-derived exosomes in MM.57 A recent study in patients with MM using small RNA sequencing of circulating exosomes from ten patient samples confirms that microRNAs are the most predominant small RNAs present in MM exosomes.58 Meanwhile, investigators from the Karolinska Institute have examined the human bone marrow stromal cell line L88 and verified that caspase-3 is activated by the stroma cell–released exosomes, which can cleave the anti-apoptotic protein Bcl-xL, localized on the outer exosomal membrane. Through the cleavage of Bcl-xL, these exosomes could then be internalized using plasma cell myeloma, which led to their increased proliferation.59
Finally, in another study performed at Tokyo Medical University, researchers established a hypoxia-resistant MM cell model to mimic the in vivo hypoxic microenvironment induced by the rapid proliferation of MM in the bone marrow. Their experiment showed that under normoxic or acute hypoxic settings, the hypoxia-resistant MM cells produced more exosomes than the parental cells, and the major functional protein in the exosomal cargo was identified to be miR-135b. This protein directly suppressed factor-inhibiting hypoxia-inducible factor 1 (FIH-1) in bone marrow endothelial cells.60 Hence, further studies are needed to test if miR-135b could be used as a target for therapeutically avert angiogenesis in MM.
Exosomal Trafficking in Breast Cancer Pathogenesis
A study using plasma exosomal microRNAs as a diagnostic biomarker in breast cancer patients demonstrated that these molecules have outstanding power to distinguish breast cancer patients from normal counterparts. Zhai et al used a nucleic acid-functionalized Au nanoflare probe, which are known to have the ability to directly enter plasma exosomes and generate quantitative fluorescent signals for successful in situ detection of exosome-located microRNA-1246. At its best cutoff point, the in situ detection of the exosomal miRNA-1246 in the peripheral blood was able to distinguish 46 breast cancer patients from 28 healthy controls with 100% sensitivity and 93% specificity.61 Another clinical study on the exosomal microRNA signatures of 20 healthy women and 435 breast cancer patients discovered that 10 miRNAs in the entire breast cancer patient cohort, 13 in the HER2-positive subgroup (211 patients), and 17 in the triple-negative subgroup (224 patients) were significantly deregulated in comparison to those in healthy women, indicating different underlying aspects of cancer biology in different breast cancer types.62 These different exosomal microRNA signatures are associated with the clinicopathological features of each subgroup. In addition, exosomes that are released by breast cancer cells could modify TME through direct suppression of T-cell proliferation and inhibition of NK cell cytotoxicity, thus dampening the anticancer immune response in pre-metastatic organs.63
Another hallmark of cancer is the transfer of chemoresistant or hormone-resistant propensity from breast cancer stem cells to the daughter cells, explored in the study of Santos et al, who demonstrated that miR-155 was upregulated in breast cancer stem cells and chemoresistant cells and was involved in the EMT. An enrichment in miR-155 was noted in exosomes isolated from stem-like breast cancer stem and chemoresistant cells. Moreover, the experiments demonstrated the capability of the horizontal transfer of miR-155 from the chemoresistant cells’ exosomal cargo to the recipient sensitive cells.64 This study supports the presence of exosome-mediated chemoresistance and EMT in refractory cancer. Estrogen receptor (ER)-positive cancers are found to transition from an endocrine sensitive/dormant state to a resistant one, acquiring host mitochondrial DNA, which promoted oxidative phosphorylation (OXPHOS) and signaled the transition from metabolic quiescence toward hormonal therapy resistance.65 Further, functional studies have identified cancer-associated fibroblast-derived extracellular vesicles containing whole genomic mitochondrial DNA in patients and xenograft models.
In a breast cancer cell line, recipient cells treated with exosomes from stemness-related breast cancer CXCR4-positive cells showed an increase in the same oncogenic abilities.66 This experiment has also demonstrated that inoculating exosomes derived from CXCR4-positive cells into immunocompromised mice can stimulate primary tumor proliferation and metastatic potential. The same investigators also discovered a “stemness and metastatic” signature in the exosomes of patients with worse prognoses after comparing exosomal nucleic acid contents.66
Exosomal Trafficking in the Pathogenesis of Soft Tissue Sarcoma
In 2013, for the first time, a study has showed that exosome-mediated pathogenesis, similar to epithelial carcinoma and hematopoietic malignancy, was also present in Ewing sarcoma.67 Microarray analysis of exosomes shed by the Ewing sarcoma cell line revealed that their exosomal content shared a transcriptional signature potentially involved in the modification of the surrounding microenvironment via G-protein-coupled signaling, neurotransmitter signaling, and stemness.67
A recently published study used both patient plasma samples and cell lines to demonstrate that liposarcoma cells secreted miR-25-3p and miR-92a-3p in exosomes. Subsequently, it stimulated the secretion of the proinflammatory cytokine, interleukin (IL)-6, in tumor-associated macrophages through a TLR7/8-dependent mechanism, which can ultimately cause liposarcoma progression.68 In another study using patient-derived Ewing sarcoma cells, miR-34a, an inhibitor of Notch-NFκB signaling, was enriched and secreted through exosomes shed by CD99-silenced (by small interfering RNA) cells.69 CD99 has been identified as a cell surface molecule involved in cell differentiation, migration, and death. In Ewing sarcoma cells, it is pro-oncogenic due to its effect on the prevention of NFκB-mediated neural differentiation and is continuously present at high levels. The horizontal transfer of miR-34a through exosomes to recipient cells enhanced neural differentiation in Ewing sarcoma.69
Moreover, another study demonstrated that the membrane-type 1 matrix metalloproteinase (MT1-MMP, MMP14) was released by exosomes of cultured human fibrosarcoma (HT-1080) cells.70 MT1-MMP is a crucial metalloproteinase that facilitates tumor invasion by remodeling the extracellular matrix. Pathological sarcomatous exosomal trafficking carrying MT1-MMP could be detrimental to the host by providing a favorable microenvironment for sarcoma.
Exosomal Trafficking in Osteosarcoma Pathogenesis
Osteosarcomas are known as malignant mesenchymal-derived bone tumors and the most common bone cancers in children and adolescents. Emerging evidence has also shed light on the exosomal trafficking employed by osteosarcoma cells to shape its supporting TME and facilitate growth, as well as hematogenous spread. Among the specific exosomal contents, miR-148a and miR-21-5p are known to help shape the TME.71 The microRNA, miR-21, is a common oncological molecule taking part in the pathogenesis of various types of malignancies.71–80 Take esophageal cancer as an example; it has been demonstrated in a human esophageal carcinomas cell line co-cultivation experiment that miR-21 in the CDE shuttled from donor cells significantly promoted the migration and invasion capability of recipient cells by activating c-Jun N-terminal kinase signaling pathway.74 A recent multi-omics study observed that the progression from localized to metastatic osteosarcoma was accompanied by an elevation of the levels of urokinase plasminogen activator (uPA) and uPA receptor in the metastatic cells’ exosomal cargo.81 The impact of abundant miR-25-3p in the liposarcoma-derived exosomes on the surrounding microenvironment was similar to what was observed in osteosarcoma cases.82 Jerez et al conducted a gene ontology analysis of predicted targets for the miRNAs present in osteosarcoma-derived extracellular vesicles. Their bioinformatics analysis indicated that miRNAs derived from osteosarcoma cell lines might regulate metastatic potential by inhibiting a network of genes involved in apoptosis and cell adhesion.83 Further research is needed to provide more evidence on the details and importance of exosomal trafficking in osteosarcoma pathogenesis and to determine it as a core hallmark of cancer.
Other Molecules (Proteins, Enzymes, Receptors, Ligands, or Signaling Molecules) That Can Exert Neoplastic Functional Activities Carried by Exosomes
Certainly, exosomal cargoes are not limited by miRNAs only but also by a lot of other candidate molecules such as proteins, lipids, enzymes, signaling molecules, which can exert their functional activities far from the exosome-producing cells.84 Table 3 demonstrates that CDE cargoes could contain basically any purpose-built loaded nano-molecules for its ultimately release from the parental cells. It is evident that horizontal or paracrine transfer of these molecules, when received by the specific recipient cells in the TME or any distant metastatic niches, could facilitate the progression, invasion, and metastatic spread of cancer cells. Some of these exosomal molecules have the potential to serve as valid biomarkers, and, thus, there should be worthwhile testing for cancer detection and/or diagnosis.
Table 3.
Representative Exosomal Cargoes Other Than miRNAs (Such as Proteins, Lipids, Signaling Molecules, DNA, Mitochondrial DNAs, circRNAs, lncRNAs, Integrin, and Enzyme), Which are Involved in Cancer Progression or in the Interplay in Anticancer Immunity or Served as a Biomarker in Various Cancer Types
| Exosomal Cargo | Cancer Type | Functions (Mechanism)/Usage | Measurement | Reference (First Author/Year Published) |
|---|---|---|---|---|
| Proteins associated with cell adhesion, extracellular matrix, and some signaling molecules (EGFR, GRB2, and SRC) | KRAS-activated or EGFR-activated NSCLC/two cell lines | Abundance differences in exosomal protein cargo detected between two NSCLC cell lines and non-cancer cell lines | Triple SILAC quantitative proteomic strategy | Clark, D.J./201685 |
| Exosome proteome | Four epithelial ovarian cancer cell lines | Signaling biology and biomarker discovery | Mass spectrometry-based proteomics | Sinha, A./201486 |
| Proteins | Several mouse breast tumor lines with a different metastatic propensity | Protein cargo varies significantly between nonmetastatic and metastatic cell-derived exosomes | Comparative proteomic analysis | Gangoda, L./201787 |
| EGF-like repeats and discoidin I-like domain-3 (EDIL-3) protein | Human bladder cancer cell lines and urine of patients with high-grade bladder cancer | Facilitates bladder cancer progression; potential for therapeutic target | Mass spectrometry analysis | Beckham, C. J./201435 |
| Epithelial cell adhesion molecule (EpCAM) glycoprotein | Pancreatic ductal carcinoma (patients) | Liquid biopsy for EpCAM quantification as prognostication | High sensitivity enzyme linked immunoassay (ELISA) | Giampieri, R./201988 |
| Myoferlin | Breast and pancreatic cancer cell lines | Promotes cancer cell migration and invasion | Proteomic analysis | Blomme, A./201689 |
| Exosomal lipid profiles | Lung cancer/patients’ plasma | Lipid profiles successfully distinguish early-stage lung cancer from healthy subjects | Ultrahigh-resolution Fourier transform mass spectrometry (UHR-FTMS) | Fan, T.W.M./201890 |
| A total of 162 lipids such as diacylglycerol, triacylglycerol, and phosphatidylglycerol | Urinary exosomes from prostate cancer patients | Potentially be used as a prostate cancer biomarker | Flow field-flow fractionation and nanoflow liquid chromatography-tandem mass spectrometry | Yang, J.S./201791 |
| 27-Hydroxycholesterol | ER+ breast cancer cell line (MCF-7) | Possibility of diagnostic value | Capillary liquid chromatography-mass spectrometry | Roberg-Larsen, H./201792 |
| Lipid composition of urinary exosomes (phosphatidylserine 18:1/18:1, phosphatidylserine 18:0–18:2, and lactosylceramide d18:1/16:0) | Prostate cancer patients | Prostate cancer urinary biomarkers | High-throughput mass spectrometry quantitative lipidomic analysis | Skotland, T./201793 |
| Esophageal cancer related gene-4 (ECRG4) mRNA (tumor suppressor) | Oral squamous cell carcinoma patients | Suppresses cell proliferation and inhibits cancerous growth | Ultracentrifugation method | Mao, L./201894 |
| The signaling molecule, Wnt5b | Lung adenocarcinoma cells (A549) | Promotes cancer cell migration and proliferation | MALDI mass spectrometry and electrospray ionization mass spectrometry | Harada, T./201795 |
| Double-stranded DNA | Chronic myeloid leukemia (K-562), colorectal carcinoma (HCT116), and murine melanoma (B16-F10) cell lines | Novel potential biomarker for cancer detection as a surrogate for tumor tissues | dsDNA-specific shrimp DNase and atomic force microscopy (AFM) | Thakur, B. K./201421 |
| Tumor cell-derived DNA | Murine breast cancer cell line E0771 post topotecan treated | Activate dendritic cells via STING signaling | Purified DNA was stained with SYBR Gold following agarose gel electrophoresis and visualized with a UV transilluminator | Kitai, Y./201796 |
| Mitochondrial DNA | ER+ breast cancer xenograft from metastatic hormonal therapy-resistant patient | Promotes exit from dormancy of therapy-induced cancer stem-like cells | Whole-mtDNA amplification and sequencing assays | Sansone, P./201765 |
| Circular RNAs (circRNAs) | Liver cancer cells (MHCC-LM3) | circRNAs able to bind to miRNA; exosome-based cancer biomarkers | RNA-seq analyses | Li, Y./201522 |
| Long noncoding (lnc) RNA ZFAS1 | Gastric cancer cell lines | Enhances gastric cancer cell proliferation and migration | Transmission electron microscopy, Nanoparticle Tracking Analysis (NTA), and Western blot. | Pan, L./201797 |
| Alphavbeta3 integrin | Prostate metastatic PC3 and CWR22Pc cancer cells | Promotes a migratory and metastatic phenotype | Nanoparticle Tracking Analysis; BCA followed by immunoblotting | Singh, A./201698 |
| GSTP1 in exosomes from patient’s serum | Anthracycline/taxane-based neoadjuvant chemotherapy-treated breast cancer | Confers drug resistance | Confocal microscopy images; Western blot analyses | Yang, S. J./201799 |
In summary, it is evident that cancer cells from entirely different lineages, such as those from leukemia to osteosarcoma, rely on their exosomes to carry the genetic information for homotypic and heterotypic intercellular communications in the TME (Figure 2). This communication creates a favorable environment for cell proliferation and invasion and further establishes a pre-metastatic niche that is readily welcoming for the arrival of cancer cells when they carry the correct form of exosomal integrins. Therefore, these CDEs and their pathological trafficking capabilities should be considered as an emerging enabling characteristic for the well-established hallmarks of cancer.
Figure 2.
Effects of tumor-derived exosomes and their horizontal paracrine trafficking to impact on the tumor microenvironment. For example, breast cancer-derived exosomes modify the TME through the suppression of T-cell proliferation and NK cell cytotoxicity. Also, the exosomal content (eg, miR-1246 or miR-155) might contribute to the chemoresistance or hormone-resistance in tumor cells. Exosomes secreted by liposarcoma cells containing miR-25-3p and miR-92a-3p have been found to stimulate IL-6 secretion in tumor-associated macrophages, leading to liposarcoma progression. miR-34a in the released exosomes enhances the neural differentiation of Ewing sarcoma. Myeloma-derived exosomes could modify the microenvironment, affecting various recipient cells such as bone marrow endothelial cells or myeloid‐derived suppressor cells. In the case of myeloma, these exosomal cargoes include miR-135b, miR18a, and let7b. After being internalized by recipient cells, miRs could bind to their target genes and trigger numerous pathways to facilitate tumor progression.
Cancer-Derived Exosomes in Biomarker Development
The first half of the review has exemplified the role of exosome in specific cancer types. Shifting gear to discuss the potential clinical application, this review will examine and discuss how we can develop cancer biomarkers based on characteristics such as exosomal cargo contents, detection methods, and localization of these exosomes in peripheral blood, pleural effusions, ascites, or urine. Further clinical studies are eagerly awaited to establish and validate the usefulness of specific CDE biomarkers in the different clinical setting during the cancer management. For example, in breast cancer, we can leverage the characteristics of exosomal microRNA signatures and exosomal nucleic acid contents in assisting in breast cancer subtyping or discovering a stemness and metastatic signature, as mentioned previously. Similarly, in the case of soft tissue sarcoma, in recognition of the role of exosomal cargo transcriptional signatures or microRNA profiling including miR-25-3p, miR-92a-3p, miR-34a, MT1-MMP, and MMP-14 playing in the promotion of sarcoma progression, remodeling extra-sarcomatous matrix to facilitate tumor invasion, and establishing a favorable TME for sarcoma growth, we can develop a practical analysis to investigate these soft tissue sarcoma-derived exosomes in assisting diagnosis or monitoring of disease along with the treatment milestones for a patient.
Although the technology for developing exosome-encapsulated therapeutics as targeted drug delivery is still in infancy, with the help of improving detection methods, rapid application of the analytic tests for specific exosomal cargoes for diagnostic purposes has become feasible, facilitating exosomal biomarker development. Several characteristics of CDEs, previously discussed in the cancer biology section, such as analyzable cancer-specific and stage-specific genetic contents in the cargo of CDEs, allow us to capture, profile and quantify using the current nanoanalytical technology. The phenomenon of pathological exosomal trafficking during cancer development and progression can be utilized in cancer diagnosis, prognostication, and treatment strategies. Studies have demonstrated that CDEs containing enriched genetic signals involved in cancer initiation and progression are shed by cancer cells into the blood, saliva, urine, ascites, and even cervicovaginal lavage. In clinical oncology, a cancer biomarker can be used for a diagnostic purpose, for example, in differentiating cancer from the non-cancer conditions. It can also be used for disease monitoring during antineoplastic therapy or follow-ups, for prognosticating a patient’s survival, and for predicting a tumor response after anticancer treatment.
Current sophisticated purification techniques offer an opportunity to utilize isolated exosomal cargoes to assist in differentiating the type of cancer and high tumor grade from low-grade cancer.61,75,100–102 In a recent study, using a urinary exosome 3-gene signature obtained from the ExoDx Prostate IntelliScore urine exosome assay, the investigators can differentiate high-grade (Gleason’s score > 7) vs low-grade prostate cancer and benign prostatic hyperplasia.100 This noninvasive urine testing implies that many unnecessary invasive transrectal biopsies could be avoided. As was aforementioned in the review, in patients with multiple myeloma, serum exosomal miRNAs could add to the risk stratification in identifying newly diagnosed multiple myeloma with particularly poor outcomes.58 Table 3 presents the select representations of exosomal cargo other than miRNAs, namely, proteins, lipids, signaling molecules, DNA, mitochondrial DNAs, circRNAs, lncRNAs, integrin, and enzyme, which potentially serve as a biomarker in various cancer types. Laboratory analytic methods to measure these contents in the research included triple SILAC quantitative proteomic analysis, mass spectrometry (MS)-based proteomic assays, lectin blotting, NP-HPLC analysis, ultrahigh-resolution Fourier transform MS, shotgun and targeted molecular quantitative lipidomic assays, capillary liquid chromatography-MS, MALDI MS, electrospray ionization MS, dsDNA-specific shrimp DNase and atomic force microscopy, RNA-seq analysis, transmission electron microscopy, nanoparticle tracking analysis, etc., depending on the study design (Table 3).
One of the advantages of investigating the CDEs as either a diagnostic, prognostic, or predictive biomarker is that physicians can obtain a specimen for CDE testing from a patient via relatively noninvasive methods. The shed CDEs into body secretion or discharges such as saliva, ascites, and cervicovaginal lavage can now be noninvasively or microinvasively assessed. In the past 5 years, clinical studies on exploiting CDEs as a clinical biomarker reported some promising results in various types of cancer (Table 4). The CDE cargo tested include lncRNAs, microRNAs, exosomal phosphatidylserine, urinary 3-gene expression profile, shuttle RNA pattern, RNA cargo, exosomal cancer stem cell-like marker CD133, exosomal EpCAM protein, and exosomal glutathione S-transferase P1 (Table 4).
Table 4.
Published Clinical Studies on Exploiting Exosomes as Diagnostic, Prognostic, or Predictive Biomarkers in Various Types of Cancer
| Biomarker Function | Exosomes/Exosomal Cargo Content | Specimen Origin | Cancer Type | Utilization | References (First Author, Year) |
|---|---|---|---|---|---|
| DIAGNOSTIC | |||||
| Exosomal lncRNAs | Cervicovaginal lavage | Uterine cervix | Differentiate cervical cancer from normal controls | Zhang J, 2016103 | |
| ExomiR-1246 | Serum | Breast | 100% sensitivity and 92.9% specificity | Zhai LY, 201861 | |
| miRNAs from the miR-106a-363 cluster | Plasma and serum | Breast | Serve as potential diagnostic biomarkers | Li M, 2018102 | |
| Exosomal phosphatidylserine | Serum | Ovary | AUC of 1.0 for predicting malignant against normal | Lea J, 2017104 | |
| Urinary 3-gene expression assay | Urine | Men with elevated PSA levels | Discriminate between Gleason score (GS)7 and GS6 prostate cancer and benign disease on initial biopsy | McKiernan J, 2016;100 McKiernan J, 2018105 | |
| Exosomal shuttle RNA pattern | Urine | Clear cell renal cell carcinoma | Provide a noninvasive test to diagnose clear cell RCC | De Palma G, 2016106 | |
| Exosomal miR-25-3p | Serum | Osteosarcoma | Reflect tumor burden | Fujiwara T, 2017;82 Yoshida A, 2018107 | |
| Exosomal lncRNA PRINS | Serum | Multiple myeloma or monoclonal gammopathies | Differentiate from healthy donors: sensitivity 84.9% and specificity 83.3% | Sedlarikova L, 2018108 | |
| Tumor-derived exosomal miRNAs | Plasma | Early-stage NSCLC | Differentiate adenocarcinoma from squamous cell carcinoma | Jin X, 2017109 | |
| Exosomal microRNA-191, - 21, −451a | Serum | Pancreas | Differentiate cancer and IPMN from normal | Goto T, 201872 | |
| Exosomal RNA cargo | Serum | Pancreas | Differentiate cancer from healthy controls in blinded studies | Ko J, 201727 | |
| Exosomal miRNA-21 and miRNA-181a-5p | Serum | Thyroid | Differentiate follicular from papillary thyroid cancer | Samsonov R, 2016101 | |
| Phosphatidylserine-expressing CDEs | Blood of tumor-bearing mice | Early-stage malignancies | Detect very early-stage cancers before clinical evidence of disease in four mouse models. | Sharma R, 2017110 | |
| PROGNOSTIC | |||||
| Exosomal miR-21 | Serum | Pediatric hepatoblastoma | Predict event-free survival | Liu W, 201675 | |
| Exosomal cancer stem cell-like marker CD133 | Ascites | Pancreas | Western blot revealed enhanced expression of CD133 in exosomes from pancreatic cancer patients | Sakaue, T. 2019111 | |
| Exosomal miR-638 | Serum | Hepatocellular carcinoma | Lower levels of serum exosomal miR-638 associates with poor overall survival | Shi M, 2018112 | |
| PREDICTIVE | |||||
| Chimeric GOLM1-NAA35 RNA | Saliva | Esophageal squamous cell carcinoma | Changes in chimeric RNA levels predict PFS after chemoradiation. | Lin Y 2019113 | |
| Exosomal EpCAM protein | Plasma | Pancreas (on palliative chemotherapy) | Exosomal EpCAM increase during treatment was associated with better PFS | Giampieri, R. 201988 | |
| Exosomal glutathione S-transferase P1 | Serum | Breast (on neoadjuvant anthracycline/taxane) | GSTP1 from the PD/SD group was significantly higher than those in the PR/CR group | Yang SJ 201799 | |
Abbreviations: AUC, area under the curve; ExomiR, exosomal microRNA; lncRNAs, long noncoding RNAs; IPMN, intraductal papillary mucinous neoplasm; NSCLC, non-small cell lung cancer; PSA, prostate-specific antigen; PFS, progression-free survival.
As of this writing, there have been several dozens of prospective observational studies being carried out to investigate the role of specific exosomal cargo as a cancer biomarker in various cancers and their diagnostic performance in a particular clinical setting (Table 5).
Table 5.
Ongoing Human Studies Investigating Exosomes as a Biomarker in Various Types of Cancer, as Registered in ClinicalTrials.gov
| ClinicalTrials.gov ID | Phase of Study | Cancer Type and Setting | Objectives | Outcome Measures |
|---|---|---|---|---|
| Lung Cancer | ||||
| NCT03542253 | Observational (Observ.) | Early lung cancer | Combined diagnosis of computerized tomography and exosome | Exosomal micro-A was highly expressed in early-stage lung cancer tissues |
| NCT02890849 | Prospect. cohort observ. | Non-small cell lung cancer | Consistency analysis of PD-L1 in cancer tissue and plasma exosome | Match rate of PD-L1 protein expression in cancer tissue and PD-L1 mRNA expression in exosome |
| NCT02921854 | Prospect. cohort observ. | Non-small cell lung cancer after radiotherapy and chemotherapy | Detection of circulating biomarkers of immunogenic cell death (ICD) | Research to see if exosomal markers of anti-tumor immunity can be detected in the serum |
| Breast Cancer | ||||
| NCT04288141 | Prospect. cohort observ. | HER2+ breast cancer on HER2 targeted therapies | Measure the expression of the HER2-HER3 dimer in the blood (exosomes) | Compare HER2 expression in blood exosomes by protein detection assays; correlate with change in HER2-HER3 dimer expression after HER2-directed therapy |
| NCT03974204 | Multicenter prospective single-arm observ. | Breast cancer patients suspected of leptomeningeal metastasis | Analyses of exosomes in the cerebrospinal fluid | Evaluate the use of proteomic profiles issued from cerebrospinal fluid exosomes |
| Gastrointestinal Cancer | ||||
| NCT01779583 | Prospect. case-control | Advanced gastric cancer on first-line chemotherapy | Circulating exosomes as potential prognostic and predictive biomarkers | Characterization of the molecular profile in cancer-derived exosomes |
| NCT03581435 | Prospect. case-control | Gallbladder carcinoma | Study of circulating exosome proteomics | Proteomics studies will be done in both tumor tissue and the circulating exosome |
| NCT03102268 | Prospect. cohort observ. | Cholangiocarcinoma patients without any anticancer therapy | Characterization of the noncoding RNAs in cancer-derived exosomes | Correlation of exosomes-derived ncRNAs and time-to-event end-points |
| NCT02393703 | Prospect. cohort observ. | Pancreatic cancer | Exosome-mediated intercellular signaling | Prospective cohort; exosomes purification for downstream proteomic and RNA sequencing |
| NCT03821909 | Prospect. cohort observ. | Patients suspected to have pancreatic masses undergoing diagnostic workup | Endoscopic ultrasound-guided portal venous blood sampling | Compare the expression of specific exosomal mRNA markers between portal venous and peripheral blood |
| NCT03874559 | Prospect. cohort observ. | Locally advanced rectal cancer on neoadjuvant chemoradiation | Characterize exosomal biomarker levels | Compare rates of exosomal expression before during and after chemoradiation therapy with pathological response rates |
| Genitourinary Tract Cancer | ||||
| NCT04053855 | Prospect. cohort observ. | Clear cell renal cell carcinoma post partial or total nephrectomy | Evaluation of urinary exosomes | Study CD9+ and CA9+ exosomes by electron microscopy technique |
| NCT04155359 | Prospect. cohort observ. | Urinary bladder cancer (UBC) in participants presenting with hematuria and another cohort of UBC patients | To establish the performance characteristics of a urine exosome-based diagnostic test to identify bladder cancer | Results compared to that of cystoscopy |
| NCT04357717 | Prospect. cohort observ. | Elevated PSA between 2 and 10 ng/mL and at least one prior negative prostate biopsy | Correlation of the ExoDx Prostate test results with the outcome of prostate biopsies in a prior negative repeat biopsy patient cohort | Clinical Evaluation of ExoDx™ Prostate (IntelliScore) |
| NCT03911999 | Prospect. cohort observ. | Prostate cancer | Exosomal microRNA in predicting the aggressiveness of prostate cancer in Hong Kong Chinese patients | To compare the differences in miRNA expression |
| NCT03694483 | Prospect. case-control | Prostate cancer | Genetic analysis for the detection of prostasomes | Determine the sensitivity and specificity of the prostasome purification methodology |
| NCT02702856 | Prospective (Prospect.) cohort observ. | Prostate cancer (for first-time biopsy patients in the PSA Gray zone of 2.0–10 ng/mL) | Validation of a urinary exosome gene signature in men suspicious of prostate cancer | Correlate signature with the presence or absence of high-grade prostate cancer biopsy |
| NCT03236688 | Prospect. cohort observ. | Advanced metastatic castrate-resistant prostate cancer | Detection of ARv7 splice variant transcripts from exosomes in circulation | Correlate ARv7 status with PSA response and correlate non-Arv7 with clinical outcomes |
| NCT04167722 | Prospect. case-control | Prostate cancer status post robotic radical prostatectomy | Understanding the role of exosomal communication in lean vs obese patients | Collecting prostate and fat tissue from radical prostatectomy participants for culture |
| Sarcoma | ||||
| NCT03800121 | Prospect. cohort observ. | Soft tissue sarcoma | Study of exosomes in monitoring patients with sarcoma | To quantify circulating exosomes and analyze their protein and RNA content |
| NCT03108677 | Prospect. case-control | Primary High-Grade Osteosarcoma | Study if the profile of circulating exosomal RNA can be used as a biomarker for lung metastases | Differences in the levels and profiles of circulating exosome RNA from patients with or without lung metastasis |
| Hematological Cancer | ||||
| NCT03985696 | Prospect. cohort observ. | Aggressive non-Hodgkin B-cell lymphoma (B-NHL) | Evaluation of peripheral exosomes can be used as novel biomarkers in B-NHL | Evaluate if a high expression of CD20 and PD-L1 on exosomes may allow tumor cells to evade immunotherapy |
| Other Types of Cancer | ||||
| NCT02862470 | Prospect. cohort observ. | Newly diagnosed thyroid papillary, follicular and anaplastic thyroid cancer | Pilot prognostic study via urine exosomal biomarkers | Collect urine samples before an operation, immediately after surgery, postoperative 3, 6, and 12 months. |
| NCT02147418 | Prospect. case-control | Human papillomavirus (HPV)-positive oropharyngeal squamous cell carcinoma (OPSCC) | Exosome testing as a screening modality for HPV-positive OPSCC | To develop a new test that can detect specific HPV proteins in the blood or saliva to help improve detection of OPSCC |
| NCT03738319 | Prospect. case-control | High-grade serous ovarian carcinoma (HGSOC) | Analyze the expression of miRNA and lncRNA by next-generation sequencing | Candidate miRNA/lncRNA will be validated as a biomarker for the detection and prognosis of HGSOC |
| NCT03895216 | Prospect. cohort observ. | Cancer patients with bone metastases | To identify deregulated miRNAs within the circulating exosomes | Changes in miRNAs content of circulating tumor exosomes |
Rapidly evolving nanotechnologies provide an opportunity to exploit and engineer exosomes for therapeutic purposes, which is gradually becoming a new class of cell-free nanomedicine. Therapeutic blockade of the exosome biogenesis to halt cancer progression at specific stages of the disease could be enticing in the development of cancer therapeutics.80,114,115 The potential application of responsive exosome nano‐bioconjugates for cancer therapy has also been confirmed in a recent study; the nano‐bioconjugates can actively target tumors through the specific recognition on the surface of tumor cell and abolished signaling and improved phagocytosis of macrophages.116 There are growing interests in investigating engineered exosomes as potential therapeutic vehicles or an active drug delivery system.117–123 Making use of the exosomal organotropic characteristics, exosomes loaded with therapeutic compounds could be employed to target a recipient cell to carry out gene therapy selectively.
The following examples shall illustrate how the application of exosomal engineering technology may enhance cancer therapeutics. Targeting the immune cells in the TME as an adjunct of anticancer treatment has been becoming a hot research area. In the application of nanomedicine, various forms of nanoparticles-bioconjugate exosomes have been synthesized and tested to target specific immune cells in the acidic TME. Recent research has demonstrated that anti-tumoral M1 macrophages-derived exosomes conjugated with CD47 and SIRPα antibodies effectively reprogrammed the macrophages from M2 to M1 phenotype in the TME.116 In another study in the living mice, cancer-associated fibroblasts in the TME can be specifically targeted by activated fibroblasts whose cell membrane was coated with semiconducting polymer nanoagents aiming to enhance multimodal cancer theranostics.124
MicroRNA-21 is a well-known microRNA that overexpresses in almost all cancer types, where its upregulation promotes cell proliferation, invasion, and metastasis.69–78 MiR-21 derived from the exosomes of MSCs regulates the death and differentiation of neurons in patients with spinal cord injury. Recent efforts involve utilizing an exosomal transfer of miRNAs or anti-miRNAs to tumor cells as a new approach for the therapeutic application of miRNAs to combat the most aggressive form of glioma, glioblastoma multiforme. Monfared and coworkers recently attempted to down-regulate miR-21 expression in glioma cell lines, U87-MG and C6, and rat glioblastoma models treated with miR-21-sponge exosomes and demonstrated a decline in tumor cell proliferation, a dramatic enhancement of apoptotic rate, and a significant reduction in tumor volume.125
Conclusion
Cancer-derived exosomal trafficking is observed in almost all types of liquid or solid tumors, including leukemia, soft tissue sarcoma, and osteosarcoma, which supports its role as an enabling characteristic for cancer hallmarks. The cargoes carried by CDEs contain enriched genetic signals in the form of DNA, RNA, microRNA, lncRNA, protein, lipid composition, or specific gene expression profiles, which are shed into blood, saliva, urine, effusions, ascites, and cervicovaginal lavage. There are a growing number of studies that investigate CDE as either a diagnostic, prognostic, or predictive nano-biomarker in various kinds of cancer. Out of the published clinical studies on exploiting CDE as a cancer biomarker, 70% of them were looking at the CDE as a diagnostic biomarker. In contrast, the rest of the studies were testing the role of CDE as a prognostic or predictive biomarker. Not surprisingly, only a few of them have reached the state of validation trials. In the near future, we shall expect to see more prospective clinical trials to validate the performance of these nanoparticle biomarkers aiming to improve the overall cancer diagnostic capability in the clinic.
Acknowledgments
We are grateful that part of this review has been critically reviewed by Prof. Ed Harlow of Harvard Medical School, Boston, in the High-Impact Cancer Research: Cancer Biology and Therapeutics program, where Prof. Robert Weinberg was also a faculty member. A significant amount of time was dedicated to the hallmarks of cancer. The authors of this review took part in the program as students and this review is a result of these activities.
Funding Statement
This work received no external fundings.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article and revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 3.Xu B, Wang T. Intimate cross-talk between cancer cells and the tumor microenvironment of B-cell lymphomas: the key role of exosomes. Tumour Biol. 2017;39(6):1010428317706227. doi: 10.1177/1010428317706227 [DOI] [PubMed] [Google Scholar]
- 4.Vermeer PD. Exosomal induction of tumor innervation. Cancer Res. 2019;79(14):3529–3535. doi: 10.1158/0008-5472.CAN-18-3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madeo M, Colbert PL, Vermeer DW, et al. Cancer exosomes induce tumor innervation. Nat Commun. 2018;9(1):4284. doi: 10.1038/s41467-018-06640-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucido CT, Wynja E, Madeo M, et al. Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecol Oncol. 2019. doi: 10.1016/j.ygyno.2019.04.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosoi J, Murphy GF, Egan CL, et al. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363(6425):159–163. doi: 10.1038/363159a0 [DOI] [PubMed] [Google Scholar]
- 8.Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. doi: 10.1126/science.1236361 [DOI] [PubMed] [Google Scholar]
- 9.Dubeykovskaya Z, Si Y, Chen X, et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat Commun. 2016;7:10517. doi: 10.1038/ncomms10517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayakawa Y, Sakitani K, Konishi M, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31(1):21–34. doi: 10.1016/j.ccell.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monje M. Settling a nervous stomach: the neural regulation of enteric cancer. Cancer Cell. 2017;31(1):1–2. doi: 10.1016/j.ccell.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 12.Zahalka AH, Arnal-Estape A, Maryanovich M, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358(6361):321–326. doi: 10.1126/science.aah5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renz BW, Takahashi R, Tanaka T, et al. beta2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33(1):75–90.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645(1):63–70. doi: 10.1016/0005-2736(81)90512-5 [DOI] [PubMed] [Google Scholar]
- 15.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855 [DOI] [PubMed] [Google Scholar]
- 16.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 17.Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379(10):958–966. doi: 10.1056/NEJMra1704286 [DOI] [PubMed] [Google Scholar]
- 18.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simona F, Laura S, Simona T, Riccardo A. Contribution of proteomics to understanding the role of tumor-derived exosomes in cancer progression: state of the art and new perspectives. Proteomics. 2013;13(10–11):1581–1594. doi: 10.1002/pmic.201200398 [DOI] [PubMed] [Google Scholar]
- 20.Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci. 2013;14(7):14240–14269. doi: 10.3390/ijms140714240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakur BK, Zhang H, Becker A, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766–769. doi: 10.1038/cr.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Zheng Q, Bao C, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi: 10.1038/cr.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalluri R, LeBleu VS. Discovery of double-stranded genomic DNA in circulating exosomes. Cold Spring Harb Symp Quant Biol. 2016;81:275–280. doi: 10.1101/sqb.2016.81.030932 [DOI] [PubMed] [Google Scholar]
- 24.Bilen MA, Pan T, Lee YC, et al. Proteomics profiling of exosomes from primary mouse osteoblasts under proliferation versus mineralization conditions and characterization of their uptake into prostate cancer cells. J Proteome Res. 2017;16(8):2709–2728. doi: 10.1021/acs.jproteome.6b00981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khushman M, Bhardwaj A, Patel GK, et al. Exosomal markers (CD63 and CD9) expression pattern using immunohistochemistry in resected malignant and nonmalignant pancreatic specimens. Pancreas. 2017;46(6):782–788. doi: 10.1097/MPA.0000000000000847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizutani K, Terazawa R, Kameyama K, et al. Isolation of prostate cancer-related exosomes. Anticancer Res. 2014;34(7):3419–3423. [PubMed] [Google Scholar]
- 27.Ko J, Bhagwat N, Yee SS, et al. Combining machine learning and nanofluidic technology to diagnose pancreatic cancer using exosomes. ACS Nano. 2017;11(11):11182–11193. doi: 10.1021/acsnano.7b05503 [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Vermesh O, Mani V, et al. The exosome total isolation chip. ACS Nano. 2017;11(11):10712–10723. doi: 10.1021/acsnano.7b04878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyu Y, Cui D, Huang J, Fan W, Miao Y, Pu K. Near-infrared afterglow semiconducting nano-polycomplexes for the multiplex differentiation of cancer exosomes. Angew Chem Int Ed Engl. 2019;58(15):4983–4987. doi: 10.1002/anie.201900092 [DOI] [PubMed] [Google Scholar]
- 30.Dioufa N, Clark AM, Ma B, Beckwitt CH, Wells A. Bi-directional exosome-driven intercommunication between the hepatic niche and cancer cells. Mol Cancer. 2017;16(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–294. doi: 10.1016/j.jconrel.2015.06.029 [DOI] [PubMed] [Google Scholar]
- 32.Sung BH, Weaver AM. Exosome secretion promotes chemotaxis of cancer cells. Cell Adh Migr. 2017;11(2):187–195. doi: 10.1080/19336918.2016.1273307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bobrie A, Thery C. Exosomes and communication between tumours and the immune system: are all exosomes equal? Biochem Soc Trans. 2013;41(1):263–267. doi: 10.1042/BST20120245 [DOI] [PubMed] [Google Scholar]
- 34.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beckham CJ, Olsen J, Yin PN, et al. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol. 2014;192(2):583–592. doi: 10.1016/j.juro.2014.02.035 [DOI] [PubMed] [Google Scholar]
- 36.Chowdhury R, Webber JP, Gurney M, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget. 2015;6(2):715–731. doi: 10.18632/oncotarget.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruns H, Bottcher M, Qorraj M, et al. CLL-cell-mediated MDSC induction by exosomal miR-155 transfer is disrupted by vitamin D. Leukemia. 2017;31(4):985–988. doi: 10.1038/leu.2016.378 [DOI] [PubMed] [Google Scholar]
- 39.Jitschin R, Braun M, Buttner M, et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. 2014;124(5):750–760. doi: 10.1182/blood-2013-12-546416 [DOI] [PubMed] [Google Scholar]
- 40.Farahani M, Rubbi C, Liu L, Slupsky JR, Kalakonda N. CLL exosomes modulate the transcriptome and behaviour of recipient stromal cells and are selectively enriched in miR-202-3p. PLoS One. 2015;10(10):e0141429. doi: 10.1371/journal.pone.0141429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paggetti J, Haderk F, Seiffert M, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126(9):1106–1117. doi: 10.1182/blood-2014-12-618025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corrado C, Raimondo S, Saieva L, Flugy AM, De Leo G, Alessandro R. Exosome-mediated crosstalk between chronic myelogenous leukemia cells and human bone marrow stromal cells triggers an interleukin 8-dependent survival of leukemia cells. Cancer Lett. 2014;348(1–2):71–76. doi: 10.1016/j.canlet.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 43.Gao X, Wan Z, Wei M, et al. Chronic myelogenous leukemia cells remodel the bone marrow niche via exosome-mediated transfer of miR-320. Theranostics. 2019;9(19):5642–5656. doi: 10.7150/thno.34813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taverna S, Flugy A, Saieva L, et al. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2012;130(9):2033–2043. doi: 10.1002/ijc.26217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrera-Ramirez J, Lavoie JR, Maganti HB, et al. Micro-RNA profiling of exosomes from marrow-derived mesenchymal stromal cells in patients with acute myeloid leukemia: implications in leukemogenesis. Stem Cell Rev Rep. 2017;13(6):817–825. doi: 10.1007/s12015-017-9762-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong CS, Danet-Desnoyers G, Shan X, Sharma P, Whiteside TL, Boyiadzis M. Human acute myeloid leukemia blast-derived exosomes in patient-derived xenograft mice mediate immune suppression. Exp Hematol. 2019;76:60–66.e62. [DOI] [PubMed] [Google Scholar]
- 47.Hong CS, Sharma P, Yerneni SS, et al. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci Rep. 2017;7(1):14684. doi: 10.1038/s41598-017-14661-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyiadzis M, Whiteside TL. Exosomes in acute myeloid leukemia inhibit hematopoiesis. Curr Opin Hematol. 2018;25(4):279–284. doi: 10.1097/MOH.0000000000000439 [DOI] [PubMed] [Google Scholar]
- 49.Hornick NI, Doron B, Abdelhamed S, et al. AML suppresses hematopoiesis by releasing exosomes that contain microRNAs targeting c-MYB. Sci Signal. 2016;9(444):ra88. doi: 10.1126/scisignal.aaf2797 [DOI] [PubMed] [Google Scholar]
- 50.Chen T, Zhang G, Kong L, Xu S, Wang Y, Dong M. Leukemia-derived exosomes induced IL-8 production in bone marrow stromal cells to protect the leukemia cells against chemotherapy. Life Sci. 2019;221:187–195. doi: 10.1016/j.lfs.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 51.Wang B, Wang X, Hou D, et al. Exosomes derived from acute myeloid leukemia cells promote chemoresistance by enhancing glycolysis-mediated vascular remodeling. J Cell Physiol. 2019;234(7):10602–10614. doi: 10.1002/jcp.27735 [DOI] [PubMed] [Google Scholar]
- 52.Huan J, Hornick NI, Goloviznina NA, et al. Coordinate regulation of residual bone marrow function by paracrine trafficking of AML exosomes. Leukemia. 2015;29(12):2285–2295. doi: 10.1038/leu.2015.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Javidi-Sharifi N, Martinez J, English I, et al. FGF2-FGFR1 signaling regulates release of leukemia-protective exosomes from bone marrow stromal cells. eLife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar B, Garcia M, Weng L, et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia. 2018;32(3):575–587. doi: 10.1038/leu.2017.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, De Veirman K, Faict S, et al. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J Pathol. 2016;239(2):162–173. doi: 10.1002/path.4712 [DOI] [PubMed] [Google Scholar]
- 56.Wang J, De Veirman K, De Beule N, et al. The bone marrow microenvironment enhances multiple myeloma progression by exosome-mediated activation of myeloid-derived suppressor cells. Oncotarget. 2015;6(41):43992–44004. doi: 10.18632/oncotarget.6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123(4):1542–1555. doi: 10.1172/JCI66517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manier S, Liu CJ, Avet-Loiseau H, et al. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood. 2017;129(17):2429–2436. doi: 10.1182/blood-2016-09-742296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vardaki I, Sanchez C, Fonseca P, et al. Caspase-3-dependent cleavage of Bcl-xL in the stroma exosomes is required for their uptake by hematological malignant cells. Blood. 2016;128(23):2655–2665. doi: 10.1182/blood-2016-05-715961 [DOI] [PubMed] [Google Scholar]
- 60.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124(25):3748–3757. doi: 10.1182/blood-2014-05-576116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhai LY, Li MX, Pan WL, et al. In situ detection of plasma exosomal microRNA-1246 for breast cancer diagnostics by a au nanoflare probe. ACS Appl Mater Interfaces. 2018;10(46):39478–39486. doi: 10.1021/acsami.8b12725 [DOI] [PubMed] [Google Scholar]
- 62.Stevic I, Muller V, Weber K, et al. Specific microRNA signatures in exosomes of triple-negative and HER2-positive breast cancer patients undergoing neoadjuvant therapy within the GeparSixto trial. BMC Med. 2018;16(1):179. doi: 10.1186/s12916-018-1163-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wen SW, Sceneay J, Lima LG, et al. The biodistribution and immune suppressive effects of breast cancer-derived exosomes. Cancer Res. 2016;76(23):6816–6827. doi: 10.1158/0008-5472.CAN-16-0868 [DOI] [PubMed] [Google Scholar]
- 64.Santos JC, Lima NDS, Sarian LO, Matheu A, Ribeiro ML, Derchain SFM. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci Rep. 2018;8(1):829. doi: 10.1038/s41598-018-19339-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sansone P, Savini C, Kurelac I, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A. 2017;114(43):E9066–E9075. doi: 10.1073/pnas.1704862114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez M, Silva J, Herrera A, et al. Exosomes enriched in stemness/metastatic-related mRNAS promote oncogenic potential in breast cancer. Oncotarget. 2015;6(38):40575–40587. doi: 10.18632/oncotarget.5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller IV, Raposo G, Welsch U, et al. First identification of Ewing’s sarcoma-derived extracellular vesicles and exploration of their biological and potential diagnostic implications. Biol Cell. 2013;105(7):289–303. doi: 10.1111/boc.201200086 [DOI] [PubMed] [Google Scholar]
- 68.Casadei L, Calore F, Creighton CJ, et al. Exosome-Derived miR-25-3p and miR-92a-3p Stimulate Liposarcoma Progression. Cancer Res. 2017;77(14):3846–3856. doi: 10.1158/0008-5472.CAN-16-2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ventura S, Aryee DN, Felicetti F, et al. CD99 regulates neural differentiation of Ewing sarcoma cells through miR-34a-Notch-mediated control of NF-kappaB signaling. Oncogene. 2016;35(30):3944–3954. doi: 10.1038/onc.2015.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem. 2008;105(5):1211–1218. doi: 10.1002/jcb.21923 [DOI] [PubMed] [Google Scholar]
- 71.Raimondi L, De Luca A, Gallo A, et al. Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis. 2019. [DOI] [PubMed] [Google Scholar]
- 72.Goto T, Fujiya M, Konishi H, et al. An elevated expression of serum exosomal microRNA-191, - 21, −451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer. 2018;18(1):116. doi: 10.1186/s12885-018-4006-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng P, Chen L, Yuan X, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):53. doi: 10.1186/s13046-017-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ragusa M, Barbagallo C, Statello L, et al. miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: pathological and diagnostic implications. Cancer Biol Ther. 2015;16(9):1387–1396. doi: 10.1080/15384047.2015.1046021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu W, Chen S, Liu B. Diagnostic and prognostic values of serum exosomal microRNA-21 in children with hepatoblastoma: a Chinese population-based study. Pediatr Surg Int. 2016;32(11):1059–1065. doi: 10.1007/s00383-016-3960-8 [DOI] [PubMed] [Google Scholar]
- 76.Liao J, Liu R, Shi YJ, Yin LH, Pu YP. Exosome-shuttling microRNA-21 promotes cell migration and invasion-targeting PDCD4 in esophageal cancer. Int J Oncol. 2016;48(6):2567–2579. doi: 10.3892/ijo.2016.3453 [DOI] [PubMed] [Google Scholar]
- 77.Hannafon BN, Trigoso YD, Calloway CL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18(1):90. doi: 10.1186/s13058-016-0753-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Foj L, Ferrer F, Serra M, et al. Exosomal and non-exosomal urinary miRNAs in prostate cancer detection and prognosis. Prostate. 2017;77(6):573–583. doi: 10.1002/pros.23295 [DOI] [PubMed] [Google Scholar]
- 79.Bhome R, Goh RW, Bullock MD, et al. Exosomal microRNAs derived from colorectal cancer-associated fibroblasts: role in driving cancer progression. Aging. 2017;9(12):2666–2694. doi: 10.18632/aging.101355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bahrami A, Aledavood A, Anvari K, et al. The prognostic and therapeutic application of microRNAs in breast cancer: tissue and circulating microRNAs. J Cell Physiol. 2018;233(2):774–786. doi: 10.1002/jcp.25813 [DOI] [PubMed] [Google Scholar]
- 81.Endo-Munoz L, Cai N, Cumming A, et al. Progression of osteosarcoma from a non-metastatic to a metastatic phenotype is causally associated with activation of an autocrine and paracrine uPA axis. PLoS One. 2015;10(8):e0133592. doi: 10.1371/journal.pone.0133592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fujiwara T, Uotani K, Yoshida A, et al. Clinical significance of circulating miR-25-3p as a novel diagnostic and prognostic biomarker in osteosarcoma. Oncotarget. 2017;8(20):33375–33392. doi: 10.18632/oncotarget.16498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jerez S, Araya H, Hevia D, et al. Extracellular vesicles from osteosarcoma cell lines contain miRNAs associated with cell adhesion and apoptosis. Gene. 2019;710:246–257. doi: 10.1016/j.gene.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu G, Zhang J, Zhao Q, et al. Molecularly engineered macrophage-derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew Chem Int Ed Engl. 2020;59(10):4068–4074. doi: 10.1002/anie.201913700 [DOI] [PubMed] [Google Scholar]
- 85.Clark DJ, Fondrie WE, Yang A, Mao L. Triple SILAC quantitative proteomic analysis reveals differential abundance of cell signaling proteins between normal and lung cancer-derived exosomes. J Proteomics. 2016;133:161–169. doi: 10.1016/j.jprot.2015.12.023 [DOI] [PubMed] [Google Scholar]
- 86.Sinha A, Ignatchenko V, Ignatchenko A, Mejia-Guerrero S, Kislinger T. In-depth proteomic analyses of ovarian cancer cell line exosomes reveals differential enrichment of functional categories compared to the NCI 60 proteome. Biochem Biophys Res Commun. 2014;445(4):694–701. doi: 10.1016/j.bbrc.2013.12.070 [DOI] [PubMed] [Google Scholar]
- 87.Gangoda L, Liem M, Ang CS, et al. Proteomic profiling of exosomes secreted by breast cancer cells with varying metastatic potential. Proteomics. 2017;17:23–24. doi: 10.1002/pmic.201600370 [DOI] [PubMed] [Google Scholar]
- 88.Giampieri R, Piva F, Occhipinti G, et al. Clinical impact of different exosomes’ protein expression in pancreatic ductal carcinoma patients treated with standard first line palliative chemotherapy. PLoS One. 2019;14(5):e0215990. doi: 10.1371/journal.pone.0215990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blomme A, Fahmy K, Peulen O, et al. Myoferlin is a novel exosomal protein and functional regulator of cancer-derived exosomes. Oncotarget. 2016;7(50):83669–83683. doi: 10.18632/oncotarget.13276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fan TWM, Zhang X, Wang C, et al. Exosomal lipids for classifying early and late stage non-small cell lung cancer. Anal Chim Acta. 2018;1037:256–264. doi: 10.1016/j.aca.2018.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang JS, Lee JC, Byeon SK, Rha KH, Moon MH. Size dependent lipidomic analysis of urinary exosomes from patients with prostate cancer by flow field-flow fractionation and nanoflow liquid chromatography-tandem mass spectrometry. Anal Chem. 2017;89(4):2488–2496. [DOI] [PubMed] [Google Scholar]
- 92.Roberg-Larsen H, Lund K, Seterdal KE, et al. Mass spectrometric detection of 27-hydroxycholesterol in breast cancer exosomes. J Steroid Biochem Mol Biol. 2017;169:22–28. doi: 10.1016/j.jsbmb.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 93.Skotland T, Ekroos K, Kauhanen D, et al. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017;70:122–132. doi: 10.1016/j.ejca.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 94.Mao L, Li X, Gong S, et al. Serum exosomes contain ECRG4 mRNA that suppresses tumor growth via inhibition of genes involved in inflammation, cell proliferation, and angiogenesis. Cancer Gene Ther. 2018;25(9–10):248–259. doi: 10.1038/s41417-018-0032-3 [DOI] [PubMed] [Google Scholar]
- 95.Harada T, Yamamoto H, Kishida S, et al. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. 2017;108(1):42–52. doi: 10.1111/cas.13109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kitai Y, Kawasaki T, Sueyoshi T, et al. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. J Immunol. 2017;198(4):1649–1659. doi: 10.4049/jimmunol.1601694 [DOI] [PubMed] [Google Scholar]
- 97.Pan L, Liang W, Fu M, et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143(6):991–1004. doi: 10.1007/s00432-017-2361-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh A, Fedele C, Lu H, Nevalainen MT, Keen JH, Languino LR. Exosome-mediated transfer of alphavbeta3 integrin from tumorigenic to nontumorigenic cells promotes a migratory phenotype. Mol Cancer Res. 2016;14(11):1136–1146. doi: 10.1158/1541-7786.MCR-16-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang SJ, Wang DD, Li J, et al. Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer. Gene. 2017;623:5–14. doi: 10.1016/j.gene.2017.04.031 [DOI] [PubMed] [Google Scholar]
- 100.McKiernan J, Donovan MJ, O’Neill V, et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016;2(7):882–889. doi: 10.1001/jamaoncol.2016.0097 [DOI] [PubMed] [Google Scholar]
- 101.Samsonov R, Burdakov V, Shtam T, et al. Plasma exosomal miR-21 and miR-181a differentiates follicular from papillary thyroid cancer. Tumour Biol. 2016;37(9):12011–12021. doi: 10.1007/s13277-016-5065-3 [DOI] [PubMed] [Google Scholar]
- 102.Li M, Zhou Y, Xia T, et al. Circulating microRNAs from the miR-106a-363 cluster on chromosome X as novel diagnostic biomarkers for breast cancer. Breast Cancer Res Treat. 2018;170(2):257–270. doi: 10.1007/s10549-018-4757-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang J, Liu SC, Luo XH, et al. Exosomal long noncoding RNAs are differentially expressed in the cervicovaginal lavage samples of cervical cancer patients. J Clin Lab Anal. 2016;30(6):1116–1121. doi: 10.1002/jcla.21990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lea J, Sharma R, Yang F, Zhu H, Ward ES, Schroit AJ. Detection of phosphatidylserine-positive exosomes as a diagnostic marker for ovarian malignancies: a proof of concept study. Oncotarget. 2017;8(9):14395–14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McKiernan J, Donovan MJ, Margolis E, et al. A prospective adaptive utility trial to validate performance of a novel urine exosome gene expression assay to predict high-grade prostate cancer in patients with prostate-specific antigen 2-10ng/mL at initial biopsy. Eur Urol. 2018;74(6):731–738. doi: 10.1016/j.eururo.2018.08.019 [DOI] [PubMed] [Google Scholar]
- 106.De Palma G, Sallustio F, Curci C, et al. The three-gene signature in urinary extracellular vesicles from patients with clear cell renal cell carcinoma. J Cancer. 2016;7(14):1960–1967. doi: 10.7150/jca.16123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoshida A, Fujiwara T, Uotani K, et al. Clinical and functional significance of intracellular and extracellular microRNA-25-3p in Osteosarcoma. Acta Med Okayama. 2018;72(2):165–174. [DOI] [PubMed] [Google Scholar]
- 108.Sedlarikova L, Bollova B, Radova L, et al. Circulating exosomal long noncoding RNA PRINS-First findings in monoclonal gammopathies. Hematol Oncol. 2018;36(5):786–791. doi: 10.1002/hon.2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jin X, Chen Y, Chen H, et al. Evaluation of tumor-derived exosomal miRNA as potential diagnostic biomarkers for early-stage non-small cell lung cancer using next-generation sequencing. Clin Cancer Res. 2017;23(17):5311–5319. doi: 10.1158/1078-0432.CCR-17-0577 [DOI] [PubMed] [Google Scholar]
- 110.Sharma R, Huang X, Brekken RA, Schroit AJ. Detection of phosphatidylserine-positive exosomes for the diagnosis of early-stage malignancies. Br J Cancer. 2017;117(4):545–552. doi: 10.1038/bjc.2017.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sakaue T, Koga H, Iwamoto H, et al. Glycosylation of ascites-derived exosomal CD133: a potential prognostic biomarker in patients with advanced pancreatic cancer. Med Mol Morphol. 2019;52(4):198–208. doi: 10.1007/s00795-019-00218-5 [DOI] [PubMed] [Google Scholar]
- 112.Shi M, Jiang Y, Yang L, Yan S, Wang YG, Lu XJ. Decreased levels of serum exosomal miR-638 predict poor prognosis in hepatocellular carcinoma. J Cell Biochem. 2018;119(6):4711–4716. doi: 10.1002/jcb.26650 [DOI] [PubMed] [Google Scholar]
- 113.Lin Y, Dong H, Deng W, et al. Evaluation of salivary exosomal chimeric GOLM1-NAA35 RNA as a potential biomarker in esophageal carcinoma. Clin Cancer Res. 2019;25(10):3035–3045. doi: 10.1158/1078-0432.CCR-18-3169 [DOI] [PubMed] [Google Scholar]
- 114.Sung JS, Kang CW, Kang S, et al. ITGB4-mediated metabolic reprogramming of cancer-associated fibroblasts. Oncogene. 2019. [DOI] [PubMed] [Google Scholar]
- 115.Pando A, Reagan JL, Quesenberry P, Fast LD. Extracellular vesicles in leukemia. Leuk Res. 2018;64:52–60. doi: 10.1016/j.leukres.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 116.Nie W, Wu G, Zhang J, et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl. 2020;59(5):2018–2022. doi: 10.1002/anie.201912524 [DOI] [PubMed] [Google Scholar]
- 117.Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheng Q, Shi X, Han M, Smbatyan G, Lenz HJ, Zhang Y. Reprogramming exosomes as nanoscale controllers of cellular immunity. J Am Chem Soc. 2018;140(48):16413–16417. doi: 10.1021/jacs.8b10047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gomari H, Forouzandeh Moghadam M, Soleimani M, Ghavami M, Khodashenas S. Targeted delivery of doxorubicin to HER2 positive tumor models. Int J Nanomedicine. 2019;14:5679–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gorji-Bahri G, Hashemi A, Moghimi HR. ExomiRs: a novel strategy in cancer diagnosis and therapy. Curr Gene Ther. 2018;18(6):336–350. doi: 10.2174/1566523218666181017163204 [DOI] [PubMed] [Google Scholar]
- 121.Kumar S, Michael IJ, Park J, Granick S, Cho YK. Cloaked exosomes: biocompatible, durable, and degradable encapsulation. Small. 2018;14(34):e1802052. doi: 10.1002/smll.201802052 [DOI] [PubMed] [Google Scholar]
- 122.Vazquez-Rios AJ, Molina-Crespo A, Bouzo BL, Lopez-Lopez R, Moreno-Bueno G, de la Fuente M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J Nanobiotechnology. 2019;17(1):85. doi: 10.1186/s12951-019-0517-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu M, Gai C, Li Z, et al. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019;110(10):3173–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li J, Zhen X, Lyu Y, Jiang Y, Huang J, Pu K. Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano. 2018;12(8):8520–8530. doi: 10.1021/acsnano.8b04066 [DOI] [PubMed] [Google Scholar]
- 125.Monfared H, Jahangard Y, Nikkhah M, Mirnajafi-Zadeh J, Mowla SJ. Potential therapeutic effects of exosomes packed with a miR-21-sponge construct in a rat model of glioblastoma. Front Oncol. 2019;9:782. doi: 10.3389/fonc.2019.00782 [DOI] [PMC free article] [PubMed] [Google Scholar]