Abstract
Background
Elderly patients with diffuse large B-cell lymphoma (DLBCL) present with poor clinical outcome and intolerance to intensive chemotherapy. Histone deacetylase inhibitors (HDACIs) show anti-lymphoma activities and can be applied to treat DLBCL. This study aimed to evaluate efficacy and safety of oral HDACI tucidinostat (formerly known as chidamide) plus R-CHOP (CR-CHOP) in elderly patients with newly diagnosed DLBCL (International Prognostic Index ≥ 2).
Results
Among 49 patients, the complete response rate was 86%, with overall response rate achieving 94%. The 2-year progression survival (PFS) and overall survival (OS) rates were 68% (95% CI 52–79) and 83% (95% CI 68–91). Comparing with historical control (NCT01852435), the 2-year PFS and OS rates of double-expressor lymphoma phenotype (DEL) were improved, and negative prognostic effect of histone acetyltransferases CREBBP/EP300 mutations was also mitigated by CR-CHOP. Grade 3–4 neutropenia was reported in 171, grade 3–4 thrombocytopenia in 27, and grade 3 anemia in 11 of 283 cycles. No grade 4 non-hematological adverse event was reported.
Conclusion
CR-CHOP is effective and safe in elderly patients with newly diagnosed DLBCL. Relevance of DEL phenotype and molecular biomarkers on CR-CHOP response warrants further investigation in DLBCL.
Trial registration ClinicalTrial.gov, NCT02753647. Registered on April 28, 2016.
Keywords: Diffuse large B-cell lymphoma, Double expressor lymphoma, Histone deacetylase inhibitor, Tucidinostat, CREBBP/EP300
Background
Diffuse large B-cell lymphoma (DLBCL) represents the most common subtype of non-Hodgkin’s lymphoma and is heterogeneous in clinical, immunophenotypic, and molecular features. More than 50% of DLBCL are elderly patients older than 60 years at diagnosis [1] and have advanced stage disease, intermediate- to high-risk International Prognostic Index (IPI) and adverse clinical outcome [2–4]. The progression-free survival (PFS) and overall survival (OS) rates were only 54% and 58% at 5 years upon treatment with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) [2], remarkably inferior to those of DLBCL patients younger than 60 years [5]. Moreover, most of the elderly patients are ineligible for intensive chemotherapy and/or hematopoietic stem cell transplantation, making effective and safe treatment as an unmet need in this subset of DLBCL.
In addition to clinical parameters, several biological features have been revealed as important prognostic indicators in DLBCL. Based on cell of origin, DLBCL can be classified as germinal center B-cell like (GCB), activated B-cell like (ABC), and unclassified, the latter two referred as non-GCB phenotype [6, 7]. Patients with non-GCB have a worse prognosis than those with GCB phenotype [6]. Double-expressor lymphoma (DEL) is defined as co-expression of BCL2 (> 50%) and MYC (> 40%) proteins by immunohistochemistry without underlying rearrangements [1]. DEL patients respond poorly to standard R-CHOP or intensive chemotherapy followed by stem cell transplantation [8]. As for molecular alterations, epigenetic gene mutations are frequently observed in DLBCL, mainly including histone methyltransferases KMT2C, KMT2D, and EZH2, histone acetyltransferases CREBBP, EP300, and IRF4, chromatin remodeler HIST1H1E and ARID1A, and DNA methylation gene TET2 [9]. GCB DLBCL with CREBBP, EP300, and KMT2D mutations tends to have inferior prognosis [10]. In a SAKK 38/07 prospective cohort, CREBBP mutation is an independent prognostic factor in DLBCL [11]. Elderly DLBCL patients are generally more frequently categorized into those high-risk groups [12, 13], providing clues for searching potential targeted therapeutic strategies.
Novel agents in combination with R-CHOP have been shown to improve clinical outcome of DLBCL. Ibrutinib plus R-CHOP prolongs PFS and OS in patients younger than 60 years [14]. Lenalidomide plus R-CHOP is effective in elderly patients with acceptable tolerability and mitigates the negative impact of non-GCB phenotype on patient prognosis [15]. Histone deacetylase inhibitors (HDACIs) are potent anti-lymphoma agents and synergistic activity between HDACIs and rituximab was observed [16]. Moreover, HDACIs sensitize B-lymphoma cells to chemotherapeutic agents [17] and clinical efficacy of HDACIs has also been investigated in patients with newly diagnosed DLBCL in combination with R-CHOP [18, 19].
Tucidinostat (formerly known as chidamide) is an oral benzamide class of HDACI that selectively inhibits Class I HDAC1, HDAC2, HDAC3, and Class IIb HDAC10, and has been applied to treat relapsed or refractory peripheral T-cell lymphoma as mono- or combinational therapy [20]. In the present study, a phase II study of chidamide plus R-CHOP (CR-CHOP) was conducted to evaluate the efficacy and safety in elderly patients with newly diagnosed DLBCL. Meanwhile, we performed whole genome sequencing (WGS) and targeted sequencing in patients with available tumor samples to explore relevance of molecular biomarkers in this prospective cohort.
Results
Patient characteristics
A total of 49 patients were included in the study between May 10, 2016 and May 2, 2018. Baseline characteristics are summarized in Table 1. Median age was 67 years (range 61–75), and 29 patients (59%) were male. Forty patients (82%) presented advanced Ann Arbor stage, and 41 patients (84%) showed elevated serum lactate dehydrogenase (LDH) level. Twenty-nine patients (59%) had multiple extranodal involvement, mainly as bone (35%), gastrointestinal (24%), and bone marrow (18%). Forty-two patients (86%) were of intermediate-high or high-risk of IPI at diagnosis. Twelve patients had BCL2/MYC double expression with exclusion of MYC and BCL2/BCL6 translocation.
Table 1.
Baseline clinical and pathological characteristics
| Characteristics | Enrolled patients (n = 49) |
|---|---|
| Age (years) | |
| Median | 67 (61–75) |
| Gender | |
| Male | 29 (59%) |
| Female | 20 (41%) |
| ECOG | |
| 0–1 | 38 (78%) |
| 2 | 11 (22%) |
| Ann Arbor stage | |
| II | 9 (18%) |
| III | 15 (31%) |
| IV | 25 (51%) |
| LDH | |
| Normal | 8 (16%) |
| Elevated | 41 (84%) |
| Extranodal sites | |
| 0–1 | 20 (41%) |
| ≥ 2 | 29 (59%) |
| IPI | |
| 2 | 7 (14%) |
| 3 | 17 (35%) |
| 4 | 20 (41%) |
| 5 | 5 (10%) |
| Cell of origin | |
| GCB | 14 (29%) |
| non-GCB | 35 (71%) |
| Double expressor lymphoma | |
| Yes | 12 (25%) |
| No | 37 (75%) |
ECOG Eastern Cooperative Oncology Group, LDH lactate dehydrogenase, IPI International Prognostic Index
Dose intensity
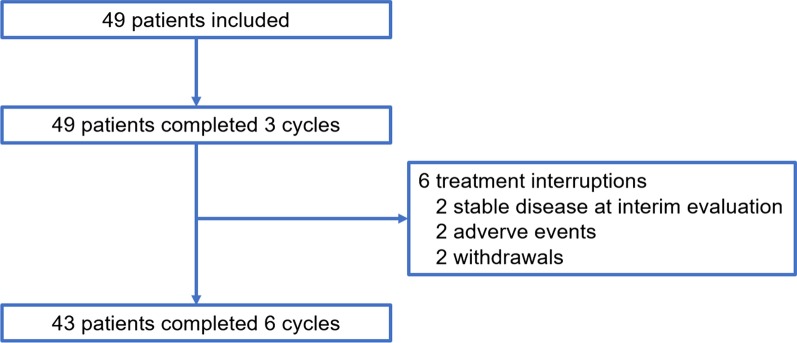
As shown in Fig. 1, 43 patients (88%) completed all six cycles of CR-CHOP. Two patients did not continue after the first three cycles because of stable disease. One patient withdrew consent after four cycles, and another three patients discontinued after five cycles: one withdrew consent, and two stopped due to adverse events (AEs), all of whom were in complete remission.
Fig. 1.
CONSORT diagram of the study
Of the 294 planned treatment cycles, 283 (96%) were given. The full dose was given in 266 (90%) of the 294 planned cycles. Tucidinostat postpones were applied in 8 cycles (3%), due to grade 4 neutropenia on day 11. Cyclophosphamide and doxorubicin dose reductions were applied in 15 cycles (5%), because of treatment delay due to grade 3 infections and grade 3–4 neutropenia. When analyzed by number of patients rather than number of cycles, four and eight patients had postpones of tucidinostat, and reduction of cyclophosphamide and doxorubicin, respectively.
Response and survival
After completion of stage 1, 20 (87%, 95% CI 72–100) of 23 patients achieved complete response, justifying the initiation of stage 2 of Simon’s design. After completion of stage 2, 42 patients (86%, 95% CI 76–96) achieved complete response, and 4 patients (8%) achieved partial response. The four patients with partial response received additional involved-field radiotherapy to sites of residual uptake at final positron emission tomography-computed tomography (PET-CT), and one patient eventually achieved complete response. All three patients who did not respond after three cycles of treatment (including one patient with double hit lymphoma and one patient with triple-hit lymphoma) were salvaged with second-line chemotherapy and died from disease progression.
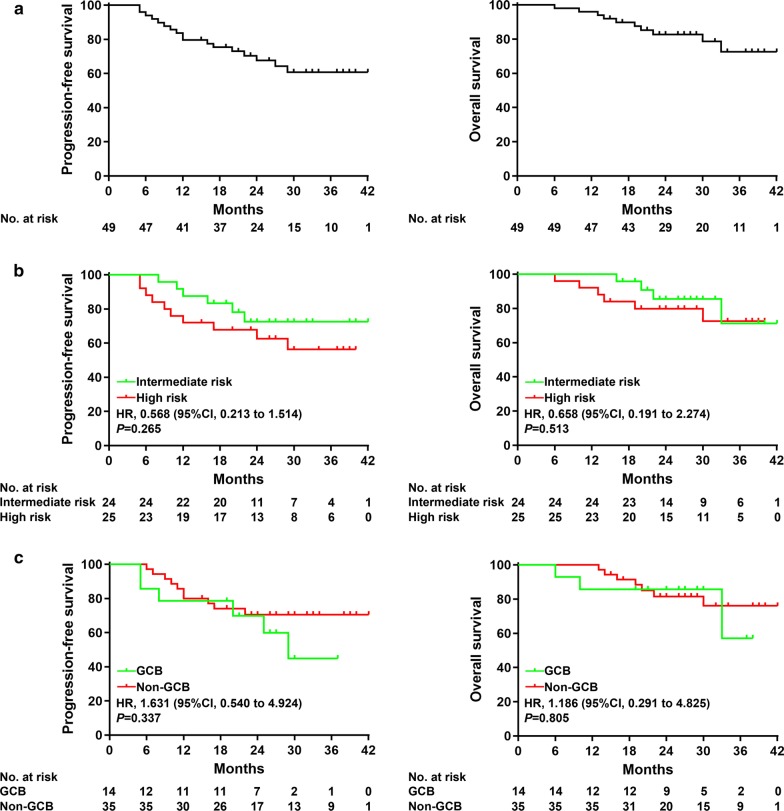
With a median follow-up of 30 months (range 6–42), the 2-year PFS and OS rates were 68% (95% CI 52–79) and 83% (95% CI 68–91), respectively (Fig. 2a). The 2-year PFS was 73% (95% CI 48–87) for patients with intermediate-risk (IPI 2–3) and 63% (95% CI 40–79) for those with high-risk (IPI 4–5) (HR 0.568, 95% CI 0.213–1.514; P = 0.265; Fig. 2b). The 2-year OS was 85% (95% CI 61–95) for patients with intermediate-risk and 80% (95% CI 58–91) for those with high-risk (HR 0.658, 95% CI 0.191–2.274; P = 0.513; Fig. 2b).
Fig. 2.
Outcomes of patients treated with CR-CHOP. a PFS and OS of all patients. b PFS and OS stratified by IPI. c PFS and OS stratified by cell of origin. CR-CHOP = tucidinostat (formerly known as chidamide), rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. PFS = Progression-free survival. OS = Overall survival. IPI = International Prognostic Index
Toxicity
Hematological and non-hematological AEs are summarized in Table 2. For hematological toxicities, grade 3 and 4 neutropenia were present in 31% and 53% of patients, respectively. Neutropenia was of short duration, with a median of 5 days (range, 3–7). Febrile neutropenia was reported in 15 cycles and was of grade 3 in maximum. Grade 3 and 4 thrombocytopenia were reported in 8% and 2% of patients, respectively, without bleeding complications. Grade 3 anemia was observed in 18% of patients. For non-hematological toxicities, grade 3 alanine aminotransferase (ALT) or aspartate aminotransferase (AST) elevation was observed in 8% of patients. No grade 4 non-hematologic toxicities were reported. Epstein–Barr virus DNA (EBV-DNA) was monitored routinely throughout the trial, and no positive event was reported.
Table 2.
Hematological and non-hematological adverse events
| Adverse events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Number of cycles in which hematological adverse events were reported (n = 283) | ||||
| Neutropenia | 11 (4%) | 28 (10%) | 58 (20%) | 113 (40%) |
| Thrombocytopenia | 70 (25%) | 48 (17%) | 17 (6%) | 10 (4%) |
| Anemia | 85 (30%) | 34 (12%) | 11 (4%) | 0 |
| Febrile neutropenia | 0 | 0 | 15 (5%) | 0 |
| Number of patients with hematological adverse events (n = 49) | ||||
| Neutropenia | 2 (4%) | 3 (6%) | 15 (31%) | 26 (53%) |
| Thrombocytopenia | 12 (24%) | 10 (20%) | 4 (8%) | 1 (2%) |
| Anemia | 17 (35%) | 12 (24%) | 9 (18%) | 0 |
| Febrile neutropenia | 0 | 0 | 6 (12%) | 0 |
| Number of patients with non-hematological adverse events (n = 49) | ||||
| Liver function abnormalities | 5 (10%) | 1 (2%) | 4 (8%) | 0 |
| Infection | 2 (4%) | 6 (12%) | 8 (16%) | 0 |
| Fatigue | 2 (4%) | 3 (6%) | 0 | 0 |
| Vomiting | 3 (6%) | 1 (2%) | 0 | 0 |
| Diarrhea | 2 (4%) | 1 (2%) | 0 | 0 |
| Hypoalbuminemia | 5 (10%) | 0 | 0 | 0 |
| Hypokalemia | 4 (8%) | 0 | 0 | 0 |
| Heart failure | 0 | 1 (2%) | 0 | 0 |
| Atrial fibrillation | 0 | 1 (2%) | 0 | 0 |
| Neurological | 2 (4%) | 0 | 0 | 0 |
No death occurred during the study as a result of toxicity related to treatment. Overall, nine patients died: eight for lymphoma progression, and one for severe pulmonary infection and heart failure in complete remission.
Impact of phenotype on response to CR-CHOP
Twelve (86% [95% CI 65–100]) of the 14 patients with GCB achieved complete response, as did 30 (86% [95% CI 74–98]) of the 35 patients with non-GCB phenotype. The 2-year PFS was 70% (95% CI 38–88) in the GCB group and 71% (95% CI 52–83) in the non-GCB group (HR 1.631, 95% CI (0.540–4.924); P = 0.337; Fig. 2c). The 2-year OS was 86% (95% CI 54–96) in the GCB group and 81% (95% CI 63–91) in the non-GCB group (HR 1.186, 95% CI (0.291–4.825); P = 0.805; Fig. 2c). Outcomes for non-GCB patients tended to be improved upon CR-CHOP, as compared to historical control (2-year PFS and OS of 60% [95% CI 49–68] and 72% [95% CI 62–80], respectively).
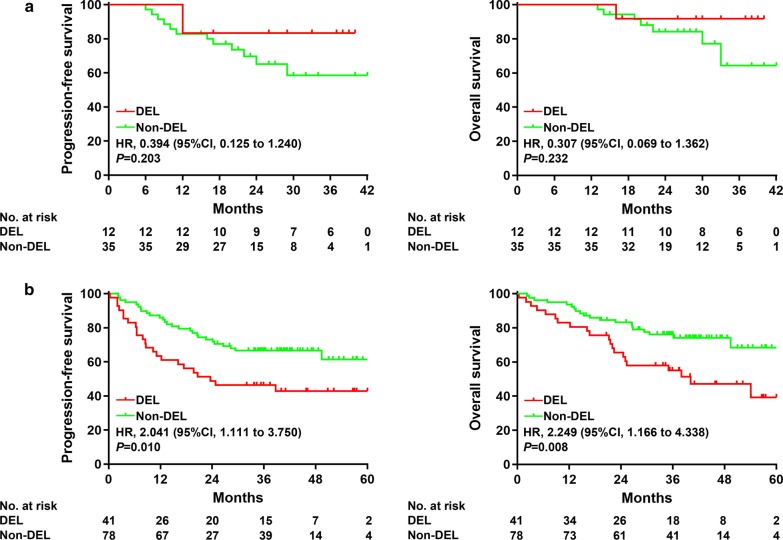
All twelve patients (100%) with DEL phenotype achieved complete response, as did 30 (86% [95% CI 74–98]) of the 35 patients with non-DEL phenotype. The 2-year PFS and OS rates of patients with DEL phenotype were 83% (95% CI 48–96) and 92% (95% CI 54–79), which were comparable to those of non-DEL phenotype (PFS: HR 0.394, 95% CI 0.125–1.240, P = 0.203; OS: HR 0.307, 95% CI 0.069–1.362, P = 0.232; Fig. 3a). Instead, in historical control (NCT01852435) upon R-CHOP treatment, the 2-year PFS and OS rates of patients with DEL phenotype were 46% (95% CI 31–61) and 63% [95% CI 46–76], which were significantly lower than those of non-DEL phenotype (PFS: HR 2.041, 95% CI 1.111–3.750, P=0.010; OS: HR 2.249, 95% CI 1.166–4.338, P = 0.008; Fig. 3b).
Fig. 3.
Outcomes of patients treated with CR-CHOP and historical control based on BCL2/MYC double expression. a PFS and OS of patients treated with CR-CHOP. b PFS and OS of patients from historical control (NCT01852435). CR-CHOP = tucidinostat (formerly known as chidamide), rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. PFS = Progression-free survival. OS = Overall survival
Impact of molecular alterations on response to CR-CHOP
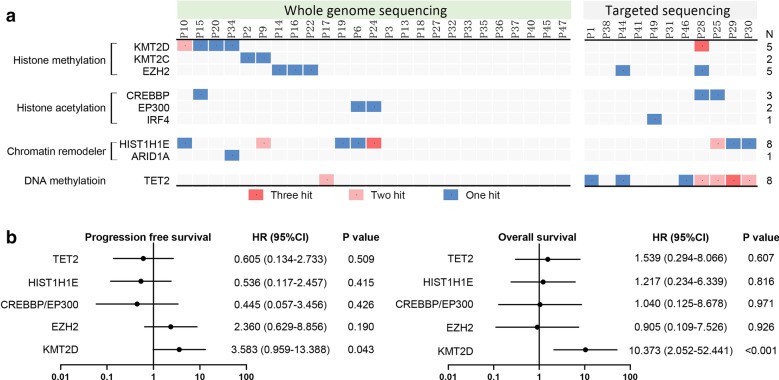
As shown in Fig. 4a, epigenetic gene mutations were identified in 21 of 36 (58%) patients, including genes related to histone methylation (KMT2D [5/36, 14%], KMT2C [2/36, 6%], and EZH2 [5/36, 14%]), histone acetylation (CREBBP [3/36, 8%], EP300 [2/36, 6%], and IRF4 [1/36, 3%]), chromatin remodeler (HIST1H1E [8/36, 22%] and ARID1A [1/36, 3%]), and DNA methylation (TET2 [8/36, 22%]). Overall, a total of 47 non-silent somatic mutations were identified, including 34 missense, 9 insertion or deletion, 4 nonsense, and a preference for C > T/A > G alterations analogous to the somatic single nucleotide variation (SNV) spectrum in other cancers.
Fig. 4.
Correlation of genomic alterations with response to CR-CHOP. a Epigenetic gene mutations by whole genome sequencing and targeted sequencing in 36 patients. The number of patients with mutations was listed on the right. b Forest plot of univariate analysis on PFS and OS in patients with or without epigenetic gene mutations. PFS = Progression-free survival. OS = Overall survival
In terms of the influence of epigenetic gene mutations on clinical outcome, mutations in KMT2D, but not in CREBBP/EP300, were associated with inferior PFS and OS (P = 0.043 and < 0.001, respectively, Fig. 4b). The 2-year PFS and OS rates were 40% (95% CI 5–75) and 40% (95% CI 5–75) in patients with KMT2D mutation, significantly shorter than those without mutation (68% [95% CI 47–82] and 89% [95% CI 70–96]). Although mutations in CREBBP/EP300 were associated with inferior PFS and OS in patients of historical control (mutation rate 15%, PFS: HR 3.292, 95% CI 1.140–9.502, P = 0.028; OS: HR 4.628, 95% CI 1.379–15.530, P = 0.013), there was no significant difference of PFS and OS between patients with or without CREBBP/EP300 mutation upon treatment with CR-CHOP (P = 0.426 and 0.971, respectively; Fig. 4b). The 2-year PFS and OS rates were 80% (95% CI 20–97) and 80% (95% CI 20–97) in patients with CREBBP/EP300 mutation, 61% (95% CI 40–87) and 83% (95% CI 63–92) in patients without CREBBP/EP300 mutation, respectively.
Discussion
DLBCL in the elderly is associated with adverse clinical outcome and limited treatment options. In this phase II study, we showed that CR-CHOP was effective in first-line therapy of elderly patients with newly diagnosed DLBCL. After six cycles of CR-CHOP, the complete response rate was higher than those of previous reports in Western countries (71–76%) [2, 3] and in China (72%) [13], all of which contained low-risk IPI patients. In a recent phase I study of valproate plus R-CHOP in newly diagnosed DLBCL (26% of the patients had intermediate-high or high-risk IPI), improvement of clinical outcome has been achieved, with PFS of 84.7% and OS of 96.8% at 2 years [18]. The 2-year survival time was also encouraging with our study on CR-CHOP, considering that more than 80% of patients had intermediate-high or high-risk IPI. Of note, CR-CHOP was equally effective in high-risk IPI patients as intermediate-high IPI patients, and compared favorably to standard R-CHOP reported by LNH985 (2-year PFS and OS as approximately 50% and 60%) [2] and by our previous study (2-year PFS and OS as 41% and 63%, respectively) [13].
Another encouraging finding of our study was the clinical efficacy of CR-CHOP in the DEL phenotype of DLBCL. Though in historical control (NCT01852435), the outcome of DEL patients was significantly inferior to that of non-DEL patients upon R-CHOP treatment, no apparent difference was observed in patients treated with CR-CHOP. Improved outcomes were also reported for DEL in a phase I/II trial of vorinostat plus R-CHOP (SWOG S0806), as compared to those of R-CHOP (SWOG S0433) (2-year PFS 73% vs. 58%, 2-year OS 91% vs. 75%) [19]. These clinical findings are supported by experimental data showing that MYC acts as a biomarker of the anti-cancer action of HDACI romidepsin and entinostat in DLBCL [21, 22]. In other hematological malignancies, tucidinostat inhibits MYC and BCL2 in acute myeloid leukemia [23, 24], and tucidinostat also downregulates BCL2 in combination with doxorubicin in peripheral T-cell lymphoma [25]. Together, tucidinostat may improve the outcome of DLBCL patients through targeting MYC and BCL2. To further confirm the role of tucidinostat plus R-CHOP in DEL phenotype of DLBCL patients, a phase III, randomized, double-blind, placebo-controlled, multicenter study is currently ongoing in China (NCT04231448).
Histone modifying genes are critically involved in tumor progression of DLBCL [26]. Inactivation of CREBBP and EP300 abrogates germinal center B cell formation and contributes to lymphomagenesis, as revealed by conditional germinal center-directed deletion mouse models targeting Crebbp or Ep300 [27]. Clinically, CREBBP mutation is an independent prognostic factor in DLBCL in a prospective SAKK 38/07 trial [11]. Disruption of KMT2D also perturbs germinal center B cell development [28, 29] and KMT2D mutations are the most frequent relapse-specific events in DLBCL [30]. In consistence with experimental data showing that HDACIs can rescue deficits in histone acetylation induced by CREBBP/EP300 mutations in B-cell lymphoma [26], our results provided clinical evidence that tucidinostat may mitigate the negative prognostic impact of CREBBP/EP300 mutations on DLBCL. However, mutations in KMT2D remained to be an adverse prognostic factor in DLBCL treated with CR-CHOP, which could be alternatively targeted by hypomethylating agents [31].
Safety profile is a major issue for the addition of novel agent to an existing combination, including HDACIs. Vorinostat plus R-CHOP is associated with high rates of AEs in patients with newly diagnosed advanced stage DLBCL (SWOG S0806) [19]. Also, unexpected auditory AEs occur in valproate plus R-CHOP [18]. Here, we recorded no grade 4 non-hematological AEs and no deaths due to toxicity. Grade 3–4 neutropenia was recorded in almost 20%–40% of cycles despite the use of granulocyte-colony stimulating factor (G-CSF), but it was of short duration and did not translate into an excess of infections. Most AEs in our trial were of mild-to-moderate intensity, and similar to those recorded with standard R-CHOP in elderly patients with DLBCL. No new clinically significant toxicity was noted with the addition of tucidinostat to R-CHOP. EBV reactivation is of concern in the administration of HDACI [32] and was not observed in this study.
With regard to dose intensity and the feasibility of the CR-CHOP regimen, the analysis of drug delivery showed that patients received full dose of tucidinostat and at least 90% of the doses of R-CHOP drugs in more than 90% of cycles. This frequency is acceptable when comparing with other studies of standard R-CHOP, in which the percentage of the patients who received more than 90% of the dose intensity of the drugs varied from 64 to 90% [14, 15]. Therefore, the addition of tucidinostat does not impair full delivery of R-CHOP, provided that G-CSF is used.
Conclusion
CR-CHOP was well tolerated and showed promising clinical activity in DLBCL. Our encouraging data warrant validation in a future phase III randomized trial to elucidate the relevance of DEL phenotype and molecular biomarkers on response to R-CHOP in combination with tucidinostat in DLBCL.
Methods
Eligibility criteria
Patients were eligible if they were 61–75 years; had newly diagnosed, histologically confirmed CD20-positive DLBCL, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, IPI risk of intermediate or high (IPI ≥ 2), and a life expectancy of more than 6 months. Patients were excluded if they had previous chemotherapy or stem cell transplantation; history of malignancy (other than skin cancers or carcinomas in situ of the cervix); uncontrollable cardio-cerebral vascular, coagulation, autoimmune, infectious disease; primary central nervous system (CNS) lymphoma; left ventricular ejection fraction ≤ 50% [4, 33]. They were also eliminated from the study if, at enrollment, their neutrophil count < 1.5 × 109/L, platelets < 75 × 109/L, ALT or AST > 2 × upper limit of normal (ULN), AKP or bilirubin > 1.5 × ULN, or creatinine > 1.5 × ULN (unless they were caused by the lymphoma). They were not enrolled if they were not able to comply with the protocol for mental or other unknown reasons; pregnancy or lactation; or positive for hepatitis B virus (HBV-DNA) and human immunodeficiency virus.
Pathological diagnosis was performed according to the 2016 World Health Organization classification [1]. Cell of origin profile was determined by Hans algorithm, with 30% cutoff value of CD10, BCL6, and MUM-1 [6]. As for DEL phenotype, cutoff value of BCL2 and MYC was 50% and 40%, respectively [1]. Fluorescence in situ hybridization of BCL2, BCL6, and MYC rearrangements was performed for each patient.
Study design and procedures
This was an investigator-initiated, open-label, single-arm, phase II study. The dose and administration schedule of CR-CHOP were as follows: rituximab 375 mg/m2 given intravenously on day 0, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, and vincristine 1.4 mg/m2 (maximum 2.0 mg) intravenously on day 1, prednisone 60 mg/m2 (maximum 100 mg) orally on days 1–5, and tucidinostat 20 mg orally on days 1, 4, 8, and 11, according to the maximum tolerated dose in combination with CHOP and etoposide in peripheral T-cell lymphoma (ClinicalTrials.gov Identifier: NCT02987244) [34]. The regimen was repeated every 21 days with a total of six cycles.
Tucidinostat should be postponed on the occurrence of grade ≥ 3 hematological or non-hematological toxicities. There was no plan for dose reduction of tucidinostat. The administration was resumed when the AEs were to grade 1 or pre-treatment levels. G-CSF prophylaxis (recombinant human pegylated G-CSF of 100 ug/kg) was given from the second cycle of chemotherapy if grade ≥ 3 neutropenia was present in the first cycle. Lamivudine was administered in occult carriers of HBV to prevent HBV reactivation. Prophylaxis for CNS relapse was given to patients with involvement of bone marrow, nasal or paranasal sinuses, orbit, breast, or testis. Tumor lysis prophylaxis and radiation therapy were performed for patients with bulky disease, or with residual disease at the end of treatment, at the discretion of physicians.
Baseline evaluations were performed within 28 days prior to therapy, including physical examination, complete blood cell count, serum biochemistry with LDH, coagulation function, HBV markers and DNA, EBV-DNA, electrocardiogram, echocardiography, bone marrow aspiration and trephine biopsy, and PET-CT. The patients were staged according to Ann Arbor staging system, and IPI [35] was calculated. Treatment response was assessed according to the standardized response criteria established by Cheson et al. [36]. Interim efficacy was evaluated by PET-CT after three cycles. Patients who had achieved a complete or partial response received another three cycles. Patients who did not achieve a complete or partial response stopped receiving CR-CHOP at this point. Final evaluation was performed by PET-CT one month after the end of the last cycle of treatment. CT of the neck, thorax, abdomen, and pelvis was repeated every 3 months thereafter to monitor disease progression until 1 year, then every 6 months until 2 years, and every year thereafter.
Study end points and assessments
The primary endpoint was complete response rate assessed by PET-CT. Secondary endpoints were PFS, OS, overall response rate, and AEs. PFS was measured from diagnosis to date of progression, relapse, or death from any cause. Patients with a partial response who were given an additional treatment (i.e., radiation therapy) without apparent disease progression were not considered as an event for PFS analysis. OS was measured from diagnosis to death of any cause or date of last follow-up. AEs were categorized and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 4.0).
Historical control cohort
Elderly patients from 20 centers of the Multicenter Hematology/Oncology Programs Evaluation System (M-HOPES) in China with newly diagnosed DLBCL, treated with regular R-CHOP50 (doxorubicin 50 mg/m2) or regular R-CEOP70 (rituximab, cyclophosphamide, epirubicin 70 mg/m2, vincristine, and prednisone) between May 15, 2013 and March 16, 2016 in our previous multicenter, phase III, randomized, controlled trial (NCT01852435) [13], and who met the same inclusion/exclusion criteria as those treated with CR-CHOP were referred as the historical control cohort and analyzed for outcome on the basis of DLBCL subtypes.
DNA sequencing
WGS was performed on frozen tumor samples of 25 patients with genomic DNA extracted using Wizard® Genomic DNA Purification Kit (Promega, Wisconsin-Madison, USA). The depth of samples measured with WGS was 50–200× , with 83–99% of the target sequence being covered sufficiently deep for variant calling (≥ 10 × coverage). A total of 9 genes reported to be hypermutation (> 5%) or related to lymphoma pathogenesis in DLBCL were selected. SNVs and indels were called by Genome Analysis Toolkit (GATK, v3.7.0) Haplotype Caller and GATK Unified Genotyper, and mapped to the genome location using the UCSC Genome Browser (https://genome.ucsc.edu/). The reference genome was the Refseq database (Human Reference Genome version hg19). All the somatic functional mutations, including non-synonymous SNVs, frameshift or in-frame indels, stopgain or stoploss, were obtained. Visual inspection was used to exclude potential false positive results. Homemade pipeline was used to filter SNVs and indels detected by the above software, excluding: (1) mutations reported with low confidence; (2) germline mutations detected from control samples; (3) population-related variants reported in 1000 Genomes (dbSNP 138) as common SNPs and not included in COSMIC (the Catalogue of Somatic Mutations in Cancer) version v77.
Targeted sequencing was performed on formalin-fixed paraffin-embedded tumor samples of 11 patients with genomic DNA extracted using GeneRead DNA formalin-fixed paraffin-embedded Tissue Kit (Qiagen, Hilden, Germany). The depth of samples measured with targeted sequencing was 1000–2000× , with 85–98% of the target sequence being covered sufficiently deep for variant calling (≥ 10× coverage).
Statistical analysis
This phase II study was designed according to Simon’s two-stage minimax design [37]. Our objective was to show a 15% improvement in frequency of complete response with the new regimen relative to an expected frequency of 70% with R-CHOP alone [38], with an α of 0.05, 80% power. A total of 49 patients were required to obtain the hypothesis, 23 of whom were to be enrolled during stage 1 and 26 during stage 2. The study would be stopped early if fewer than 16 (70%) of the patients achieved complete response in stage 1. Similarly, if 39 (80%) or fewer patients achieved complete response by trial completion, the hypothesis would also be rejected.
Efficacy and safety analyses were by intention to treat. Statistical analyses were performed by Statistical Package for the Social Science (SPSS) 23.0 software (SPSS Inc., Chicago, IL, USA). Survival estimates were calculated by Kaplan–Meier method, and survival curves were compared by log-rank test. A two-sided P value of < 0.05 was considered statistically significant.
Acknowledgements
We appreciate the effort the physicians for enrolling patients and thank all the patients involved for allowing us to analyze their clinical data.
Abbreviations
- DLBCL
Diffuse large B-cell lymphoma
- IPI
International Prognostic Index
- PFS
Progression-free survival
- OS
Overall survival
- R-CHOP
Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
- GCB
Germinal center B-cell
- ABC
Activated B-cell
- DEL
Double-expressor lymphoma
- HDACI
Histone deacetylase inhibitor
- CR-CHOP
Tucidinostat (formerly known as Chidamide), rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
- WGS
Whole genome sequencing
- LDH
Lactate dehydrogenase
- AE
Adverse event
- PET-CT
Positron emission tomography-computed tomography
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- EBV-DNA
Epstein–Barr virus DNA
- SNV
Single nucleotide variation
- G-CSF
Granulocyte-colony stimulating factor
- ECOG
Eastern Cooperative Oncology Group
- CNS
Central nervous system
- ULN
Upper limit of normal
- HBV-DNA
Hepatitis B virus DNA
- CTCAE
Common Terminology Criteria for Adverse Events
Authors' contributions
L.W., P.-P.X, W.-L.Z. conceived and designed the study. M.-C.Z., L.W., S.C., Y.H., C.-F.W., X.-F.J., Q.S., P.-P.X., and W.-L.Z. provided executive support, active data surveillance, and performed patient selection process. M.-C.Z., Y.F., L.W., S.C., D.F., Y.Z, and P.-P.X. collected data and provided data management. M.-C.Z., D.F. P.-P.X., and W.-L.Z. performed data analysis. M.-C.Z., P.-P.X., and W.-L.Z. interpreted the results and wrote the manuscript. All authors reviewed the manuscript critically and approved the content. All authors read and approved the final manuscript.
Funding
This study was supported, in part, by research funding from the National Natural Science Foundation of China (81520108003, 81830007, 81670176, and 82070204), Chang Jiang Scholars Program, Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (20152206 and 20152208), Clinical Research Plan of Shanghai hospital development center (SHDC, 16CR2017A), Multicenter Clinical Research Project by Shanghai Jiao Tong University School of Medicine (DLY201601), Collaborative Innovation Center of Systems Biomedicine, and the Samuel Waxman Cancer Research Foundation. The funding source had no involvement in the study, manuscript preparation, or decision to submit the study for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available in Mendeley Data through the 10.17632/nmxgxsvbk5.1 and National Omics Data Encyclopedia (NODE, https://www.biosino.org/node/) under Accession Number OEP001040.
Ethics approval and consent to participate
The final protocol, amendments, informed consent, and any other appropriate documents were reviewed and approved by the ethics committee and institutional review board of Shanghai Ruijin Hospital (Reference Number 2016-24). Trial registration: ClinicalTrial.gov, NCT02753647. Registered on April 28, 2016, https://clinicaltrials.gov/ct2/show/NCT02753647?term=NCT02753647&draw=2&rank=1. The study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients prior to enrollment.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mu-Chen Zhang, Ying Fang and Li Wang have contributed equally to this manuscript.
Contributor Information
Peng-Peng Xu, Email: pengpeng_xu@126.com.
Wei-Li Zhao, Email: zhao.weili@yahoo.com.
References
- 1.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, Christian B, Lepage E, Tilly H, Morschhauser F, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23(18):4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 3.Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9(2):105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, Pocock C, Ardeshna KM, Radford JA, McMillan A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817–1826. doi: 10.1016/S0140-6736(13)60313-X. [DOI] [PubMed] [Google Scholar]
- 5.Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, Gill DS, Walewski J, Pettengell R, Jaeger U, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 6.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 7.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 8.Riedell PA, Smith SM. Double hit and double expressors in lymphoma: definition and treatment. Cancer. 2018;124(24):4622–4632. doi: 10.1002/cncr.31646. [DOI] [PubMed] [Google Scholar]
- 9.Lunning MA, Green MR. Mutation of chromatin modifiers; an emerging hallmark of germinal center B-cell lymphomas. Blood Cancer J. 2015;5:e361. doi: 10.1038/bcj.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer AL, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juskevicius D, Jucker D, Klingbiel D, Mamot C, Dirnhofer S, Tzankov A. Mutations of CREBBP and SOCS1 are independent prognostic factors in diffuse large B cell lymphoma: mutational analysis of the SAKK 38/07 prospective clinical trial cohort. J Hematol Oncol. 2017;10(1):70. doi: 10.1186/s13045-017-0438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 13.Xu PP, Fu D, Li JY, Hu JD, Wang X, Zhou JF, Yu H, Zhao X, Huang YH, Jiang L, et al. Anthracycline dose optimisation in patients with diffuse large B-cell lymphoma: a multicentre, phase 3, randomised, controlled trial. Lancet Haematol. 2019;6(6):e328–e337. doi: 10.1016/S2352-3026(19)30051-1. [DOI] [PubMed] [Google Scholar]
- 14.Younes A, Sehn LH, Johnson P, Zinzani PL, Hong X, Zhu J, Patti C, Belada D, Samoilova O, Suh C et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in Non-Germinal Center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019:Jco1802403. [DOI] [PMC free article] [PubMed]
- 15.Vitolo U, Chiappella A, Franceschetti S, Carella AM, Baldi I, Inghirami G, Spina M, Pavone V, Ladetto M, Liberati AM, et al. Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol. 2014;15(7):730–737. doi: 10.1016/S1470-2045(14)70191-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhao WL, Wang L, Liu YH, Yan JS, Leboeuf C, Liu YY, Wu WL, Janin A, Chen Z, Chen SJ. Combined effects of histone deacetylase inhibitor and rituximab on non-Hodgkin's B-lymphoma cells apoptosis. Exp Hematol. 2007;35(12):1801–1811. doi: 10.1016/j.exphem.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Xue K, Gu JJ, Zhang Q, Mavis C, Hernandez-Ilizaliturri FJ, Czuczman MS, Guo Y. Vorinostat, a histone deacetylase (HDAC) inhibitor, promotes cell cycle arrest and re-sensitizes rituximab- and chemo-resistant lymphoma cells to chemotherapy agents. J Cancer Res Clin Oncol. 2016;142(2):379–387. doi: 10.1007/s00432-015-2026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drott K, Hagberg H, Papworth K, Relander T, Jerkeman M. Valproate in combination with rituximab and CHOP as first-line therapy in diffuse large B-cell lymphoma (VALFRID) Blood Adv. 2018;2(12):1386–1392. doi: 10.1182/bloodadvances.2018019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persky DO, Li H, Rimsza LM, Barr PM, Popplewell LL, Bane CL, Von Gehr A, LeBlanc M, Fisher RI, Smith SM, et al. A phase I/II trial of vorinostat (SAHA) in combination with rituximab-CHOP in patients with newly diagnosed advanced stage diffuse large B-cell lymphoma (DLBCL): SWOG S0806. Am J Hematol. 2018;93(4):486–493. doi: 10.1002/ajh.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Jia B, Xu W, Li W, Liu T, Liu P, Zhao W, Zhang H, Sun X, Yang H, et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol. 2017;10(1):69. doi: 10.1186/s13045-017-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin DY, Kim A, Kang HJ, Park S, Kim DW, Lee SS. Histone deacetylase inhibitor romidepsin induces efficient tumor cell lysis via selective down-regulation of LMP1 and c-myc expression in EBV-positive diffuse large B-cell lymphoma. Cancer Lett. 2015;364(2):89–97. doi: 10.1016/j.canlet.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Frys S, Simons Z, Hu Q, Barth MJ, Gu JJ, Mavis C, Skitzki J, Song L, Czuczman MS, Hernandez-Ilizaliturri FJ. Entinostat, a novel histone deacetylase inhibitor is active in B-cell lymphoma and enhances the anti-tumour activity of rituximab and chemotherapy agents. Br J Haematol. 2015;169(4):506–519. doi: 10.1111/bjh.13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Chen K, Zhou Y, Xiao Y, Deng M, Jiang Z, Ye W, Wang X, Wei X, Li J, et al. A new strategy to target acute myeloid leukemia stem and progenitor cells using chidamide, a histone deacetylase inhibitor. Curr Cancer Drug Targets. 2015;15(6):493–503. doi: 10.2174/156800961506150805153230. [DOI] [PubMed] [Google Scholar]
- 24.Zhao S, Guo J, Zhao Y, Fei C, Zheng Q, Li X, Chang C. Chidamide, a novel histone deacetylase inhibitor, inhibits the viability of MDS and AML cells by suppressing JAK2/STAT3 signaling. Am J Transl Res. 2016;8(7):3169–3178. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Dong L, Chen Q, Kong L, Meng B, Wang H, Fu K, Wang X, Pan-Hammarstrom Q, Wang P, et al. Synergistic antitumor effect of histone deacetylase inhibitor and Doxorubicin in peripheral T-cell lymphoma. Leuk Res. 2017;56:29–35. doi: 10.1016/j.leukres.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Ortega-Molina A, Geng H, Ying HY, Hatzi K, Parsa S, McNally D, Wang L, Doane AS, Agirre X, et al. CREBBP inactivation promotes the development of HDAC3-dependent lymphomas. Cancer Discov. 2017;7(1):38–53. doi: 10.1158/2159-8290.CD-16-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer SN, Scuoppo C, Vlasevska S, Bal E, Holmes AB, Holloman M, Garcia-Ibanez L, Nataraj S, Duval R, Vantrimpont T, et al. Unique and shared epigenetic programs of the CREBBP and EP300 acetyltransferases in Germinal Center B cells reveal targetable dependencies in lymphoma. Immunity. 2019;51(3):535–47.e9. doi: 10.1016/j.immuni.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Dominguez-Sola D, Hussein S, Lee JE, Holmes AB, Bansal M, Vlasevska S, Mo T, Tang H, Basso K, et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat Med. 2015;21(10):1190–1198. doi: 10.1038/nm.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortega-Molina A, Boss IW, Canela A, Pan H, Jiang Y, Zhao C, Jiang M, Hu D, Agirre X, Niesvizky I, et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat Med. 2015;21(10):1199–1208. doi: 10.1038/nm.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juskevicius D, Lorber T, Gsponer J, Perrina V, Ruiz C, Stenner-Liewen F, Dirnhofer S, Tzankov A. Distinct genetic evolution patterns of relapsing diffuse large B-cell lymphoma revealed by genome-wide copy number aberration and targeted sequencing analysis. Leukemia. 2016;30(12):2385–2395. doi: 10.1038/leu.2016.135. [DOI] [PubMed] [Google Scholar]
- 31.Ji MM, Huang YH, Huang JY, Wang ZF, Fu D, Liu H, Liu F, Leboeuf C, Wang L, Ye J, et al. Histone modifier gene mutations in peripheral T-cell lymphoma not otherwise specified. Haematologica. 2018;103(4):679–687. doi: 10.3324/haematol.2017.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SJ, Kim JH, Ki CS, Ko YH, Kim JS, Kim WS. Epstein-Barr virus reactivation in extranodal natural killer/T-cell lymphoma patients: a previously unrecognized serious adverse event in a pilot study with romidepsin. Ann Oncol. 2016;27(3):508–513. doi: 10.1093/annonc/mdv596. [DOI] [PubMed] [Google Scholar]
- 33.Vitolo U, Trneny M, Belada D, Burke JM, Carella AM, Chua N, Abrisqueta P, Demeter J, Flinn I, Hong X, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017;35(31):3529–3537. doi: 10.1200/JCO.2017.73.3402. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Su L, Liu L, Gao Y, Wang Q, Su H, Song Y, Zhang H, Shen J, Jing H, et al. Combination of chidamide with the CHOEP regimen in previously untreated patients with Peripheral T-Cell Lymphoma (PTCL): a prospective, multicenter, Single-Arm, Phase 1b/2 Trial. Blood 2019, 134(Supplement_1):4036. [DOI] [PMC free article] [PubMed]
- 35.A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993, 329(14):987–94. [DOI] [PubMed]
- 36.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 38.Xu PP, Zhong HJ, Huang YH, Gao XD, Zhao X, Shen Y, Cheng S, Huang JY, Chen SJ, Wang L, et al. B-cell function gene mutations in diffuse large b-cell lymphoma: a retrospective cohort study. EBioMedicine. 2017;16:106–114. doi: 10.1016/j.ebiom.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available in Mendeley Data through the 10.17632/nmxgxsvbk5.1 and National Omics Data Encyclopedia (NODE, https://www.biosino.org/node/) under Accession Number OEP001040.