Abstract
Background
Stigma is a significant barrier to healthcare and a factor that drives the global burden of tuberculosis (TB). However, there is a scarcity of information on TB stigma in developing countries. We aimed to characterize, measure, and explore the determinants of TB stigma among people with TB in Cambodia.
Methods
We conducted a mixed-methods study between February and August 2019 using a triangulation convergent design—a cross-sectional survey (n = 730) and nested in-depth interviews (n = 31) among people with TB. Quantitative data were analyzed using descriptive statistics and generalized linear regression models. Qualitative transcripts were thematically analyzed.
Results
A total of 56% and 51% of participants experienced self-stigma and perceived stigma by the community, respectively. We found rural dwellers, knowledge of how TB is transmitted, and knowledge that anybody can get TB were associated with higher levels of self-stigma and perceived stigma by the community. Higher scores on knowledge of TB symptoms were inversely associated with both self-stigma and community stigma. Thematic analyses revealed accounts of experienced stigma, acts of intentional distancing and hiding TB diagnosis from others, and feelings of embarrassment and shame.
Conclusions
Tuberculosis stigma was prevalent, suggesting a need for the incorporation of stigma-reduction strategies in the national TB responses. These strategies should be contextualized and developed through community engagement. Future research should continue to measure the levels and dimensions of TB stigma among people with TB through behavioral surveillance using standardized tools.
Keywords: community, discrimination, self-perceived, stigma, tuberculosis
In Cambodia, TB stigma was prevalent. Rural dwellers, knowledge about TB transmission, and knowledge that anybody can get TB were associated with higher levels of self-stigma and perceived stigma. Accounts of experienced stigma, embarrassment, and shame were reported.
Stigma is a major determinant of health [1] and a barrier to ending tuberculosis (TB) globally [2]. It is a complex matter involving institutional and societal attitudes and personal experience characterized by adverse social judgment either perceived, anticipated, or experienced by people with TB [3]. Perceived stigma in the context of TB refers to one’s perception of how others may act towards or think about individuals who have it [4]. A person may also experience antagonistic attitudes and actions against them, or in other words, discrimination [4], due to others’ undesirable perceptions of TB. There is growing recognition that TB stigma interrupts the cascade of TB care due to its detrimental effect on getting a timely diagnosis, treatment uptake and adherence, and the psychological well-being of people affected by TB [5–7]. Therefore, systematically measuring and addressing stigma affecting people with TB should be a global priority to meet the goal of ending TB by 2030 [2].
Cambodia is one of the 30 high TB-burden countries with a TB incidence rate of 302 per 100 000 population in 2018 [8]. In Cambodia, TB care is largely provided by the public healthcare sector, where persons diagnosed with TB are registered and receive treatment. Tuberculosis treatment is available free of charges within the government health facilities network that spans across different levels (central, provincial and municipal, district, and health center) in the country [9]. Tuberculosis diagnostics services have expanded in the last decade, with 215 microscopy centers and 75 GeneXpert MTB/RIF systems equipped nationwide [9]. Under the National TB Program (NTP) structure, the country has achieved a remarkable treatment success rate of 94% [8]. However, approximately 40% of the cases remain undiagnosed [8]. Missing TB cases or delayed diagnosis is an issue affected by various factors, including stigma. In Cambodia, TB stigma, rural dwellers, seeking private healthcare, and self-medication before TB diagnosis were found to be associated with delayed TB diagnosis [10]. The effects of stigma, by and large, on health are deleterious. Other studies have shown that TB stigma delayed care-seeking, undermined treatment uptake and adherence, and impacted the psychological well-being of people affected by TB [5–7]. Stigma might also lead to social isolation among people with TB, and the effect could be long term [11]. Although biomedical approaches such as TB case detection, treatment, and management have contributed substantially to the country’s fight to end TB, social determinants of health such as stigma are not well understood in developing countries. This study aims to (1) characterize and measure TB stigma and (2) explore the determinants of TB stigma among people with TB in Cambodia.
METHODS
Study Design
We conducted a mixed-method study using a triangulation convergent design [12]—a cross-sectional survey and a series of in-depth interviews (IDIs) with people with TB.
Patient Consent Statement
The National Ethics Committee for Health Research Cambodia (NECHR) (NECHR reference: 024/NECHR) and National University of Singapore (NUS) Institutional Review Board (IRB) (NUS IRB reference: H-19-015) approved the study. We obtained informed consent from study participants.
Setting
This study was conducted in 12 operational districts (OD) in Cambodia between February and August 2019. We purposively selected the 12 ODs, where TB active case finding (ACF) programs were implemented, from the total 194 ODs [13] in Cambodia. We selected 100 health centers from the total 143 health centers in the 12 ODs and recruited people with TB using a probability proportional-to-size sampling method (by the total population each health center serves) without replacement.
Study Population
In the selected health centers, trained data collectors approached all people with TB identified by the ACF programs within 1 month of diagnosis either at the health centers or at the participants’ place of residence (part of the follow-up protocol for the ACF programs). For the IDIs, we purposively selected participants from the same cohort based upon maximum variation on these criteria—care-seeking behavior (short delay/long delay to TB diagnosis), sex (men/women), and residence (urban/rural). The details of the study population and TB diagnostic criteria have been described elsewhere [14].
Key Variables and Definitions
We measured self- (12 items) and perceived (11 items) stigma by the community using validated scales developed by Van Rie et al [15]. The measurements were made on a 4-level Likert scale (0 to 3), with 0 being “strongly disagree” and 3 being “strongly agree.” We standardized the scores for both scales to 50 with higher scores indicating higher stigma. Therefore, the minimum score was 0, and the maximum score was 50. The stigma scale used in this study was internally consistent—Cronbach’s alpha [16] was 0.878 for self-stigma and 0.877 for community stigma.
We adapted the World Health Organization TB knowledge, attitude, and practices survey [17] to measure participants’ TB knowledge level based on 8 questions regarding characteristics, symptoms of TB, route of transmission, prevention, and treatment of TB. For each symptom correctly identified—a cough that lasts >3 weeks, hemoptysis, weight loss, fever without a clear cause that lasts >1 week, chest pain, and fatigue—a score of 1 was given, and 0 otherwise. The minimum score was 0, and the maximum possible score was 6, and the total score on the knowledge of TB symptoms was computed for analyses.
For the IDIs, we developed a semistructured interview guide that included broader questions related to (1) participants’ experiences with seeking diagnosis and treatment for TB and (2) the barriers and facilitators in TB care-seeking. The analyses for this study focused on the segment of the interview in which participants were asked about perceived and experienced TB stigma.
Data Collection
Data collectors administered the cross-sectional survey using a paper-based questionnaire that was pretested with other people with TB and TB survivors before the implementation. Participants were reimbursed with US $5 after completing the questionnaire. We checked the questionnaires for completeness before entering the data into KoBoToolbox [18]. Any discrepancies that arose during data entry were resolved by revisiting the questionnaires.
The IDIs were conducted 1:1 by trained interviewers (C.O., S.E., and N.S.) at either the health centers or participants’ homes (on some rare occasions, family members were present during the interview) in 4 ODs using a semistructured interview guide. The interviewers were matched with the interviewees of the same sex. The IDIs were conducted in Khmer, and each IDI lasted between 30 and 60 minutes. Interviews were audio-recorded, and field notes were taken. Participants received US $10 for their participation in the IDIs.
Analyses
We described and presented categorical and continuous data using frequencies and percentages and mean and standard deviation (SD), respectively. In univariate analyses, we compared variables using paired t test, Student’s t test, and one-way analysis of variance.
We investigated the determinants of self-stigma and perceived-community stigma using generalized linear regression models. We considered variables with P ≤ .1 in univariate analyses for inclusion in the models. Beta-coefficients (β coef) were presented with a 95% confidence interval (CI), and we considered 2-tailed P < .05 to be significant. Akaike’s information criterion, Bayesian information criterion, and the plots of residuals were examined to evaluate the best-fitting model. Statistical analyses were made using STATA 14 (StataCorp LP, College Station, TX).
We transcribed all IDIs verbatim and translated the transcripts to English for analyses. We applied thematic analyses [19] and developed a codebook of themes based on the semistructured interview guide using NVIVO 10 (QSR International). The research team independently coded the transcripts, and textual references to the structural themes were retrieved. Themes that emerged were included in the codebook accordingly. No new themes were derived after 31 interviews, and data saturation was achieved. Quantitative and qualitative data were analyzed separately, and the findings were merged for interpretations [12].
RESULTS
This study population comprised 730 people with TB with a mean age of 60.1 years (SD 13.9). Most participants (62.3%) were residing in a rural setting. The cohort comprised men (53.2%), those with primary education and lower (84.1%), and individuals who were married (77.5%) (Table 1).
Table 1.
Characteristics of Participants and Reported Self-Stigma and Perceived Stigma by the Community
| Self-Stigmaa | Perceived Stigma by the Communityb | ||||||
|---|---|---|---|---|---|---|---|
| Variable | n | % | Mean (SD) | P Value | Mean (SD) | P Value | |
| Total study participants | 730 | 100.0 | 25.1 (7.0) | 26.0 (7.2) | |||
| Age, in years, mean (SD) | 60.1 (13.9) | ||||||
| Age group, in yearsc | .331 | .625 | |||||
| 18–24 | 10 | 1.4 | 26.9 (7.3) | 28.0 (6.9) | |||
| 25–34 | 33 | 4.5 | 23.2 (6.4) | 25.0 (6.2) | |||
| 35–44 | 54 | 7.4 | 24.6 (5.8) | 25.6 (6.6) | |||
| 45–54 | 125 | 17.2 | 25.6 (6.5) | 26.9 (7.0) | |||
| 55–64 | 207 | 28.4 | 24.4 (7.0) | 25.4 (7.1) | |||
| ≥65 | 300 | 41.2 | 25.8 (7.3) | 26.2 (7.5) | |||
| Residence | <.001 | .041 | |||||
| Urban | 275 | 37.7 | 23.9 (6.2) | 25.3 (6.0) | |||
| Rural | 455 | 62.3 | 25.9 (7.3) | 26.5 (7.8) | |||
| Sex | .081 | .082 | |||||
| Male | 388 | 53.2 | 24.7 (7.2) | 25.6 (7.3) | |||
| Female | 342 | 46.8 | 25.6 (6.6) | 26.5 (7.0) | |||
| Education levelc | .030 | .300 | |||||
| Primary and lower | 610 | 84.1 | 25.4 (6.9) | 26.2 (7.1) | |||
| Above primary | 115 | 15.9 | 23.9 (7.1) | 25.4 (7.6) | |||
| Marital status | .500 | .544 | |||||
| Never married | 34 | 4.7 | 25.2 (6.8) | 28.2 (6.9) | |||
| Currently married | 566 | 77.5 | 25.3 (7.1) | 25.8 (7.3) | |||
| Divorced/widowed | 130 | 17.8 | 24.5 (6.5) | 26.3 (6.8) | |||
| Ever smokedd | <.001 | .002 | |||||
| Ever smoked | 218 | 29.9 | 23.4 (7.1) | 24.8 (7.6) | |||
| Never smoked | 512 | 70.1 | 25.9 (6.8) | 26.6 (7.0) | |||
| Alcohol usec,e | .002 | .006 | |||||
| Nondrinker | 514 | 70.9 | 25.7 (6.8) | 26.5 (7.1) | |||
| Drinker | 211 | 29.1 | 23.9 (7.2) | 24.9 (7.3) | |||
| Knowledge on TB Symptomsc | |||||||
| Cough that lasts longer than 3 weeks | 418 | 57.4 | 24.3 (6.9) | <.001 | 26.4 (6.8) | .122 | |
| Hemoptysis | 249 | 34.2 | 25.1 (7.7) | .837 | 25.5 (8.1) | .147 | |
| Weight loss | 574 | 78.9 | 24.8 (7.1) | .005 | 26.0 (7.3) | .596 | |
| Fever without clear cause that lasts more than 1 week | 247 | 33.9 | 23.9 (7.5) | <.001 | 25.0 (7.3) | .005 | |
| Chest pain | 360 | 49.5 | 24.9 (7.1) | .328 | 25.3 (7.3) | .006 | |
| Ongoing fatigue | 279 | 38.3 | 24.8 (6.7) | .379 | 26.1 (7.1) | .916 | |
| Ever heard of TB | .436 | .384 | |||||
| Yes | 642 | 88.0 | 25.2 (7.0) | 26.1 (7.3) | |||
| No | 88 | 12.0 | 24.6 (6.5) | 25.4 (6.7) | |||
| TB is transmitted through the air when a person with TB coughs or sneezesc | <.001 | .009 | |||||
| Yes | 692 | 95.2 | 25.3 (6.7) | 26.2 (7.2) | |||
| No | 35 | 4.8 | 20.5 (9.0) | 23.0 (6.8) | |||
| TB transmission can be prevented by covering mouth and nose when coughing/wear a mask | .028 | .002 | |||||
| Yes | 687 | 94.4 | 25.2 (6.8) | 26.3 (7.2) | |||
| No | 41 | 5.6 | 22.8 (8.1) | 22.7 (5.0) | |||
| Knowledge of who can be infected with TBc | .030 | .003 | |||||
| Anybody | 656 | 90.4 | 25.3 (7.0) | 26.3 (7.2) | |||
| Not anybody | 70 | 9.6 | 23.4 (6.0) | 23.7 (6.8) | |||
| Knowledge of the curability of TB | .068 | .738 | |||||
| Can be cured | 701 | 96.0 | 25.0 (7.0) | 26.1 (7.3) | |||
| Not sure if TB can be cured | 29 | 4.0 | 27.4 (6.1) | 25.6 (5.6) | |||
| Perception of seriousness of TB as a disease | .322 | .005 | |||||
| Very serious | 281 | 38.6 | 25.5 (7.1) | 27.0 (7.2) | |||
| Not very serious | 447 | 61.4 | 25.0 (6.8) | 25.5 (7.1) | |||
| Type of TB | .858 | .566 | |||||
| Smear negative/clinician diagnosed TB/extrapulmonary TB | 446 | 61.1 | 25.2 (6.7) | 26.2 (6.9) | |||
| Bacteriologically confirmed TB | 284 | 38.9 | 25.1 (7.4) | 25.8 (7.6) | |||
Abbreviations: SD, standard deviation; TB, tuberculosis.
aEvaluated based on the answers from 12 questions, measured on a Likert scale (0 to 3), with 0 being strongly disagree and 3 being strongly agree. Summary score was standardized to 50 with higher scores indicating higher self-stigma. Minimum score is 0 and the maximum score is 50.
bEvaluated based on the answers from 11 questions, measured on a Likert scale (0 to 3), with 0 being strongly disagree and 3 being strongly agree. Summary score was standardized to 50 with higher scores indicating higher perceived stigma by the community. Minimum score is 0 and the maximum score is 50.
cExclude missing values.
dEver smoked included current and ex-smokers.
eDrinkers reported frequency of alcohol use that ranges from once a month or less to 4 times or more per week. Nondrinkers refer to teetotalers.
Cross-Sectional Study
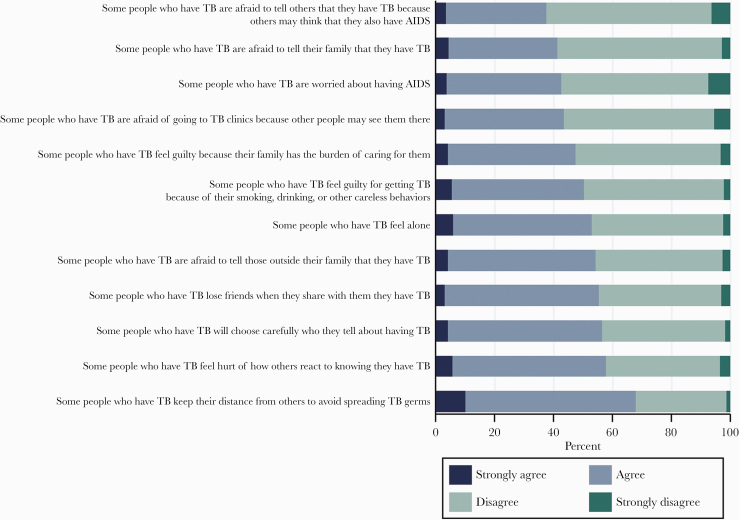
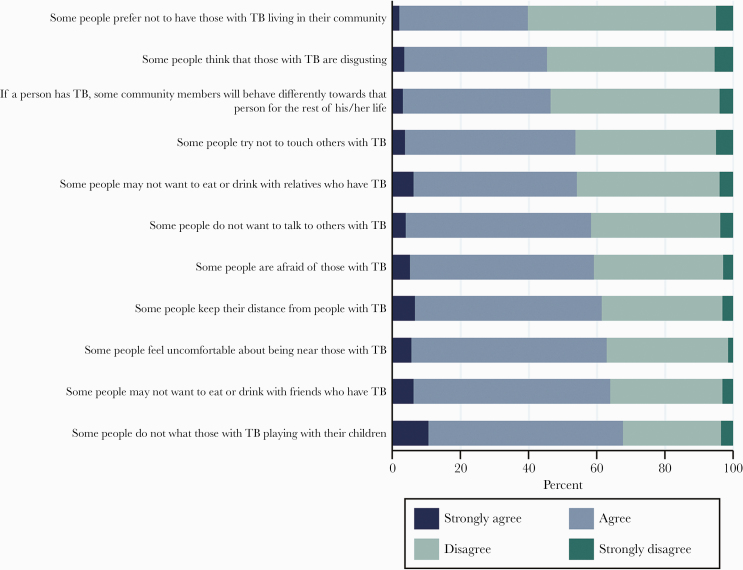
The proportions of respondents who reported that they agreed or strongly agreed with the 11 statements on perceived stigma by the community and 12 statements on self-stigma were 56% and 51%, respectively. The total scores for both scales were normally distributed, and study participants reported higher perceived stigma by the community—mean score: 26.0 (SD 7.2)—compared with self-stigma—mean score: 25.1 (SD 7.0) (P = .001). We illustrated participants’ responses to the individual questions in the scales in Figures 1 and 2. We also illustrated participants’ responses to the questions in the scales by residence (urban/rural) in the Supplementary Materials.
Figure 1.
Participants’ responses to self-stigma. The horizontal axis of the bar showed the percentages of respondents who strongly agreed, agreed, disagreed, and strongly disagreed with the statements used in the scale to measure self-stigma. AIDS, acquired immune deficiency syndrome; TB, tuberculosis.
Figure 2.
Participants’ responses to perceived stigma by the community. The horizontal axis of the bar showed the percentages of respondents who strongly agreed, agreed, disagreed, and strongly disagreed with the statements used in the scale to measure perceived stigma by the community. TB, tuberculosis.
We included age, sex, residence (urban/rural), education levels, ever smoked, and TB knowledge in the multivariate models (Table 2). Rural residence (β coef 1.58; 95% CI, 0.54–2.63), having never smoked (β coef 1.63; 95% CI, 0.43–2.84), and knowledge of how TB is transmitted (β coef 4.33; 95% CI, 2.02–6.63) and that anybody can get TB (β coef 2.42; 95% CI, 0.72–4.12) were associated with higher self-stigma score.
Table 2.
Factors Associated With Stigma
| Self-Stigma | Perceived Stigma by the Community | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Crude β-coef (95% CI) | P Value | Adjusted β-coef (95% CI) | P Value | Crude β-coef (95% CI) | P Value | Adjusted β-coef (95% CI) | P Value |
| Age, years | 0.03 (−0.01 to 0.06) | .133 | 0.01 (−0.03 to 0.04) | .679 | 0.01 (−0.03 to 0.04) | .792 | −0.01 (−0.05 to 0.03) | .614 |
| Sex | .081 | .965 | .082 | .527 | ||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 0.90 (−0.11 to 1.91) | −0.02 (−1.13 to 1.08) | 0.93 (−0.12 to 1.97) | 0.38 (−0.80 to 1.55) | ||||
| Residence | <.001 | .003 | .041 | .018 | ||||
| Urban | Reference | Reference | Reference | Reference | ||||
| Rural | 1.94 (0.90–2.97) | 1.58 (0.54–2.63) | 1.12 (0.05–2.20) | 1.36 (0.24–2.48) | ||||
| Education | .030 | .186 | .300 | .745 | ||||
| Primary and lower | Reference | Reference | Reference | Reference | ||||
| Above primary | −1.53 (−2.92 to −0.15) | −0.94 (−2.34 to 0.46) | −0.76 (−2.20 to 0.68) | −0.25 (−1.73 to 1.24) | ||||
| Ever smokeda | <.001 | .008 | .002 | .114 | ||||
| Ever | Reference | Reference | Reference | Reference | ||||
| Never | 2.51 (1.42–3.60) | 1.63 (0.43–2.84) | 1.79 (0.65–2.93) | 1.03 (−0.25 to 2.31) | ||||
| TB knowledge | ||||||||
| Knowledge of TB symptoms (total score)b | −0.75 (−1.11 to −0.39) | <.001 | −0.88 (−1.24 to −0.52) | <.001 | −0.40 (−0.78 to −0.03) | .034 | −0.72 (−1.11 to −0.34) | <.001 |
| TB is transmitted through the air when a person with TB coughs or sneezes | 4.82 (2.48–7.15) | <.001 | 4.33 (2.02–6.63) | <.001 | 3.23 (0.80–5.66) | .009 | 2.71 (0.27–5.15) | .030 |
| Anybody can get TB | 1.89 (0.18–3.59) | .030 | 2.42 (0.72–4.12) | .005 | 2.66 (0.90–4.42) | .003 | 2.80 (0.99–4.61) | .002 |
| Perception of seriousness of TB as a disease | .322 | .005 | .002 | |||||
| Very serious | Reference | Reference | Reference | |||||
| Not very serious | −0.52 (−1.56 to 0.51) | −1.53 (−2.60 to −0.46) | −1.75 (−2.84 to −0.66) | |||||
Abbreviations: β-coef, beta-coefficient; CI, confidence interval; TB, tuberculosis.
aEver smoked included current and ex-smokers.
bCorrectly identified TB symptoms—cough that lasts more than 3 weeks, hemoptysis, weight loss, fever without a clear cause that lasts more than 1 week, chest pain, fatigue. Each correct answer is given a score of 1. Minimum score is 0 and the maximum score is 6 with higher scores indicating good knowledge of TB symptoms.
Likewise, we found that rural residence (β coef 1.36; 95% CI, 0.24–2.48) and knowledge of how TB is transmitted (β coef 2.71; 95% CI, 0.27–5.15) and that anybody can get TB (β coef 2.80; 95% CI, 0.99–4.61) were associated with higher perceived stigma by the community. The perception that TB is not very serious (β coef −1.75; 95% CI, −2.84 to −0.66) was associated with lower community stigma score. In addition, in both models, higher scores on knowledge of TB symptoms were inversely associated with self-stigma (β coef −0.88; 95% CI, −1.24 to −0.52) and perceived stigma by the community (β coef −0.72; 95% CI, −1.11 to −0.34), respectively.
In-Depth Interviews
We conducted 31 IDIs with people with TB. Six individuals who were approached refused to participate due to illness and other competing priorities. The characteristics of the participants are provided in Table 3.
Table 3.
Characteristics of In-Depth Interviews Participants
| Characteristic | Frequency | % |
|---|---|---|
| Age, in years (median, IQR) | 56 (45–68) | |
| Sex | ||
| Male | 18 | 58.1 |
| Female | 13 | 41.9 |
| Residence | ||
| Urban | 16 | 51.6 |
| Rural | 15 | 48.4 |
Abbreviations: IQR, interquartile range.
Many participants identified both perceived and experienced stigma at the individual and community levels. There were several reports of participants deliberately distanced by people around them after their diagnosis, primarily due to the fear of acquiring the disease.
They discriminate us. When they speak, they faced away, and they don’t respond. They use a towel or hand to cover their mouth and nose. Some don’t let us speak near them.” (IDI19, male, 66yo)
They do not really want to sit near us, and they are afraid that we will transmit the disease to them.” (IDI12, male, 33yo)
Several participants also reported hiding their diagnosis, fearing being identified as having TB. The fear stemmed from perceived disgust, ill-feelings that other people might harbor against them, and other consequences such as losing jobs.
We are afraid that they will know our illness, and they will find us disgusting. So, we are afraid.” (IDI19, female, 34yo)
It is hard for me to work. If they are scared of me, they do not dare to hire me to work.” (IDI21, male, 45yo)
Notably, respondents alluded that in addition to hiding their diagnosis, they were also blamed for accusing others of having TB when they tried to encourage presumptive TB to seek care.
We do not dare to tell [them about their symptoms]. We say it out of goodwill, but they say we accuse them of TB.” (IDI19, female, 34yo)
Nonetheless, many participants highlighted that encouragement received from family members and other TB survivors in the community were pivotal in facilitating care-seeking.
They [TB survivors] got successful treatment, so they told me [to seek care].” (IDI10, male, 66yo)
My wife said please go to health center, if you don’t go you will die. My wife explained that I had TB, then she forced me to [go] health center.” (IDI03, male, 37yo)
Some respondents also mentioned that they intentionally distanced from others because they were afraid of transmitting TB to them.
When I have the disease, I do not dare to go anywhere to avoid people feeling bad about us, thinking that we have the disease and spreading it to everybody.” (IDI13, female, 51yo)
Shame and embarrassment because of TB were also highlighted by the interviewees. The respondents also felt embarrassed because people around them sidestepped them.
When they [other acquaintances] sit together, I walk in, and then they walk out immediately. So, we feel embarrassed.” (IDI12, male, 33yo)
DISCUSSION
In this study, we found more than 50% of people with TB reported self-stigma and perceived stigma by the community. The prevalence of perceived TB stigma was reported to range between 30% to 60% in several high TB-burden countries [20, 21]. In a study conducted in Vietnam, more than 50% of the study participants reported fear of being known as people with TB in the community [22]. Similar findings (both quantitative and qualitative) on the presence of TB stigma were also reported in studies conducted in neighboring countries in the region [23–25]. Some of the variations in prevalence could be attributed to the different study designs used, data collection tools used, populations sampled, and the cultural life that differed within and across communities [21, 26]. Nevertheless, the findings indicated that stigma is a ubiquitous issue among people with TB.
Our results showed that rural dwellers were associated with higher stigma. Previous studies have yielded heterogeneous findings regarding the relationship between residence setting and TB stigma [3, 27, 28], thereby reinforcing the influence of cultural and geographic variations on TB stigma [20, 26]. Furthermore, we found significant associations between good knowledge of TB—transmission route and that anybody could get TB—and TB stigma. Qualitatively, IDI participants indicated that the fear of transmission and acquisition of infection impelled people with TB to avoid others intentionally and vice versa. In-depth interview participants also further elaborated on shame, embarrassment, and the ill-perception of others against people with TB that could have perpetrated further isolation from other members of the community. Likewise, embarrassment, social isolation to avoid potential discrimination by their communities, and self-stigma as a result of fear of onward transmission have been associated with TB in other settings [26, 29, 30]. These findings are also consistent with the expressions of fear of transmission, perceived risk, and associations of disease with shame and judgment highlighted by other stigmatized illnesses such as human immunodeficiency virus (HIV) and acquired immune deficiency syndrome [4].
Despite the effect of stigma on delayed TB diagnosis [7], IDI participants also reported that encouragements from family members, friends, and notably TB survivors were instrumental in facilitating TB care-seeking. One apparent link to the current TB policy in Cambodia was the implementation of the community directly observed treatment, short-course (C-DOTS). The C-DOTS was established in the early 2000s, by involving the communities in providing health education, case-finding, supporting TB treatment (DOTS services) in the community, and the members of the community from accessing TB services [9]. Although our study did not evaluate the effect of C-DOTS on TB stigma, it reinforced the notion of community mobilization and support in TB care. A separate study on the same population has empirically shown that a TB case-finding strategy that mobilizes the community to find and refer people presumptive of TB living in the same community has led to early identification of TB and treatment initiation [14]. Therefore, it is vital to continue recognizing the roles of the people affected by TB and rally community-driven approaches in monitoring and combatting stigma and ending TB.
Notwithstanding the paucity of information on the effectiveness of interventions to reduce TB stigma, approaches to improve knowledge, change people’s attitude towards TB, and support people affected by TB have been found to be effective in reducing TB stigma [31]. Although our study is unable to elucidate the causal pathway of stigma among people with TB in Cambodia, the findings could inform interventions that are salient and contextualized to the local settings. For instance, good knowledge of TB that included the cause of the disease, route of transmission, curability, and symptoms as a whole was inversely associated with stigma [32]. In this study, we investigated the elements of TB knowledge separately and found that stigma was associated with good knowledge of how TB is transmitted and the risk of acquiring the infection. However, we found that knowledge of TB symptoms specifically had a negative relationship with TB stigma. Our findings thereby highlighted that interventions to reduce stigma should include an interplay of educating the public in identifying TB-related symptoms, the importance of care-seeking when a person exhibits the symptoms, and how the transmission could be terminated should an infectious individual be promptly treated. It is also essential to recognize that a ground-up approach, akin to HIV activism, is needed to effect changes in behaviors and practices that perpetuated TB stigma and discrimination [33]. Person-centered interventions through the empowerment of people affected by TB focusing at the intrapersonal and community level have been found to be effective [34]. However, to achieve and sustain a more significant impact, grassroots initiatives that emerged should be used to influence and drive organizational and policy change.
Besides targeting people and communities affected by TB, the practices of the general population towards people with TB needs to be improved, especially in the rural areas. As the internet is gaining traction as the primary source of information in Cambodia [35], public health education messages that are designed in collaboration with the affected communities could be disseminated via social media for a quicker and wider reach. The propagation of public health messages to the general public could lead to normalization and a broader acceptance of the affected communities. In the context of HIV, the concept of normalization and the use of media and social networks had positively impacted stigma and effected behavior change [36, 37]. However, the use of mass media could be a double-edged sword whereby inappropriately curated messages disseminated by the media has been associated with TB stigma [38]. Because the manifestations of stigma are influenced by cultural contexts, messages disseminated via the mass media should be appropriately verified and tailored to the local audiences.
To our knowledge, this is the first study to characterize TB stigma in Cambodia. We adopted the triangulation convergent design where quantitative and qualitative findings were corroborated within a single study for comprehensiveness. We also took a community-driven approach to the study by including community health workers, people with TB, and TB survivors in designing the study, refining the data collection tools, and data collection. In the qualitative component, we ensured sex and urban/rural dwellings-balanced representations for diverse perspectives. The interviewers were matched with the interviewees by sex to reduce potential bias.
We acknowledged several limitations in this study. The generalizability of our findings was limited, because not all ODs in Cambodia were included in the sampling frame. However, the sex ratio and the trends in the proportion of bacteriologically confirmed TB by age groups [10] were comparable to the most recent national TB prevalence survey [39]. In comparison with the cases notified to the NTP in 2018 [40], the age and sex distribution of our sample also reflected a similar trend. We were unable to verify the representativeness of our sample in comparison with the profile and demographics of other people with TB at the selected sites. Participants were not systematically selected based on the level of stigma that they have faced. Hence, we believe that the impact of selection bias on the outcome of interest is thereby nominal. Nevertheless, the findings from this study only represented the perspectives of people with TB and not the general population. The stigma scale that we used adopted an indirect framing approach and may lead to an overestimation of the prevalence of stigma [41]. We also did not measure experienced stigma quantitatively, and incongruity between the perceived and actual experience of stigma could be present [41]. However, we attempted to elicit accounts of experienced stigma through qualitative IDIs.
CONCLUSIONS
In conclusion, we found rural residence, a good knowledge of how TB is transmitted, and an understanding that anybody can get TB were associated with TB stigma. The perception that TB is not very serious and a good knowledge of TB symptoms had inverse relationships with TB stigma. Historically, attention and investments in TB have prioritized biomedical models of care [42]. Today, countries, including Cambodia, have committed to reaching 90% of the key and vulnerable populations [43] with essential TB services and ending TB stigma and all forms of discrimination [2]. Therefore, measuring and addressing TB stigma that prevents people from seeking and accessing care should be prioritized. Based on this study’s findings, we opined that the knowledge and awareness of the affected communities and the public on TB stigma should be constantly shaped using tools relevant to the current times. It is also important to recognize the role of community involvement in TB care and support and the potential benefits of community mobilization in stigma reduction. Nevertheless, interventions should be contextualized to the local setting, and diverse stakeholder groups—people with TB, healthcare workers, and the general population—should be engaged. Our study also calls for future research and action to explore the levels and dimensions of TB stigma among key populations in different settings through behavioral surveillance using validated and standardized tools, such as the Stop TB Partnership TB Stigma Assessment [44]. The assessment’s findings will be instrumental in commencing a national and multisectoral dialog in Cambodia on TB stigma, establishing a national baseline of TB stigma, measuring its trends, monitoring the progress of stigma reduction interventions, and in realizing Cambodia’s commitment to end TB stigma by 2022 [43].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the study participants for their contribution to this project. We also thank Dr. Chansophal Ly, Seyha Ong, and the field staff in coordinating the data collection processes.
Author contributions. A. K. J. T., L. Y. H., and S. Y. conceptualized and designed the study. A. K. J. T., C. O., S. T., S. E., and N. S. supported project implementation and data collection. A. K. J. T., R. K. J. T., and S. Y. supported the design and statistical analysis of the study. A. K. J. T., S. T., L. Y. H., and S. Y. obtained ethical approval for the project. A. K. J. T., R. K. J. T., C. S., V. S., and S. Y. contributed to the drafting of the manuscript. All authors reviewed and approved the final manuscript.
Disclaimer. The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Financial support. The study was funded by the Saw Swee Hock School of Public Health Infectious Diseases Program (Grant SSHSPH ID-PRG/PILOTGRANT/2018/01) and the National University of Singapore President’s Graduate Fellowship, and Stop TB Partnership TB REACH (Grant STBP/TBREACH/GSA/W5SU-01).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Hatzenbuehler ML, Phelan JC, Link BG. Stigma as a fundamental cause of population health inequalities. Am J Public Health 2013; 103:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daftary A, Mitchell EMH, Reid MJA, et al. To end TB, first-ever high-level meeting on tuberculosis must address stigma. Am J Trop Med Hyg 2018; 99:1114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Courtwright A, Turner AN. Tuberculosis and stigmatization: pathways and interventions. Public Health Rep 2010; 125 Suppl 4:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zelaya CE, Sivaram S, Johnson SC, et al. Measurement of self, experienced, and perceived HIV/AIDS stigma using parallel scales in Chennai, India. AIDS Care 2012; 24:846–55. [DOI] [PubMed] [Google Scholar]
- 5. Xu M, Markström U, Lyu J, Xu L. Survey on tuberculosis patients in rural areas in China: tracing the role of stigma in psychological distress. Int J Environ Res Public Health 2017; 14:1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cremers AL, de Laat MM, Kapata N, et al. Assessing the consequences of stigma for tuberculosis patients in urban Zambia. PLoS One 2015; 10:e0119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teo AKJ, Singh SR, Prem K, et al. Determinants of delayed diagnosis and treatment of tuberculosis in high-Burden Countries: a mixed-methods systematic review and meta-analysis. In Review; 2020. Available at: https://www.researchsquare.com/article/6dbe6955-78e6-49ec-9249-a0ae9baffe8c/v1. Accessed 14 March 2020.
- 8. World Health Organization. Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019. [Google Scholar]
- 9. National Center for Tuberculosis and Leprosy Control (CENAT). Technical Guidelines on Tuberculosis Control. 2nd ed. Phnom Penh: Ministry of Health; 2016. [Google Scholar]
- 10. Teo AKJ, Ork C, Eng S, et al. Determinants of delayed diagnosis and treatment of tuberculosis in Cambodia: a mixed-methods study. Infect Dis Poverty 2020; 9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo N, Marra F, Marra CA. Measuring health-related quality of life in tuberculosis: a systematic review. Health Qual Life Outcomes 2009; 7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Creswell JW, Piano Clark VL. Designing and Conducting Mixed Methods Research. 2nd ed. Thousand Oaks, California: SAGE Publications, Inc; 2011. [Google Scholar]
- 13. National Institute of Statistics. Economic Census of Cambodia 2011. Ministry of Planning 2012. Available at: http://nis.gov.kh/nis/EC2011/EC2011_Final_Results_Revised.pdf. Accessed 2 May 2020.
- 14. Teo AKJ, Prem K, Tuot S, et al. Mobilising community networks for early identification of tuberculosis and treatment initiation in Cambodia: an evaluation of a seed-and-recruit model. ERJ Open Res 2020; 6:00368-02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Rie A, Sengupta S, Pungrassami P, et al. Measuring stigma associated with tuberculosis and HIV/AIDS in southern Thailand: exploratory and confirmatory factor analyses of two new scales. Trop Med Int Health 2008; 13:21–30. [DOI] [PubMed] [Google Scholar]
- 16. Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ 2011; 2:53–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. Advocacy, communication and social mobilization for TB control: a guide to developing knowledge, attitude, and practice surveys. Geneva: World Health Organization; 2008. [Google Scholar]
- 18. KoBoToolbox. KoBoToolbox: data collection tools for challenging environments. KoBoToolbox. Available at: https://kobotoolbox.org/. Accessed 4 October 2019.
- 19. Guest G, Macqueen K, Namey E. Applied Thematic Analysis. Thousand Oaks, California: SAGE Publications, Inc; 2012. [Google Scholar]
- 20. Cervantes J. Tuberculosis. Digging deep in the soul of humanity. Respir Med 2016; 119:20–2. [DOI] [PubMed] [Google Scholar]
- 21. Duko B, Bedaso A, Ayano G, Yohannis Z. Perceived stigma and associated factors among patient with tuberculosis, Wolaita Sodo, Ethiopia: cross-sectional study. Tuberc Res Treat 2019; 2019:5917537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoa NP, Diwan VK, Co NV, Thorson AE. Knowledge about tuberculosis and its treatment among new pulmonary TB patients in the north and central regions of Vietnam. Int J Tuberc Lung Dis 2004; 8:603–8. [PubMed] [Google Scholar]
- 23. Thu A, Win H, Nyunt M-T, Lwin T. Knowledge, attitudes and practice concerning tuberculosis in a growing industrialised area in Myanmar. Int J Tuberc Lung Dis 2012; 16:330–5. [DOI] [PubMed] [Google Scholar]
- 24. Ngamvithayapong-Yanai J, Luangjina S, Thawthong S, et al. Stigma against tuberculosis may hinder non-household contact investigation: a qualitative study in Thailand. Public Health Action 2019; 9:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kipp AM, Pungrassami P, Nilmanat K, et al. Socio-demographic and AIDS-related factors associated with tuberculosis stigma in southern Thailand: a quantitative, cross-sectional study of stigma among patients with TB and healthy community members. BMC Public Health 2011; 11:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang SH, Cataldo JK. A systematic review of global cultural variations in knowledge, attitudes and health responses to tuberculosis stigma. Int J Tuberc Lung Dis 2014; 18:168–73, i–iv. [DOI] [PubMed] [Google Scholar]
- 27. Zhang T, Liu X, Bromley H, Tang S. Perceptions of tuberculosis and health seeking behaviour in rural Inner Mongolia, China. Health Policy 2007; 81:155–65. [DOI] [PubMed] [Google Scholar]
- 28. Mushtaq MU, Shahid U, Abdullah HM, et al. Urban-rural inequities in knowledge, attitudes and practices regarding tuberculosis in two districts of Pakistan’s Punjab province. Int J Equity Health 2011; 10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Long NH, Johansson E, Diwan VK, Winkvist A. Fear and social isolation as consequences of tuberculosis in VietNam: a gender analysis. Health Policy 2001; 58:69–81. [DOI] [PubMed] [Google Scholar]
- 30. Khan A, Walley J, Newell J, Imdad N. Tuberculosis in Pakistan: socio-cultural constraints and opportunities in treatment. Soc Sci Med 2000; 50:247–54. [DOI] [PubMed] [Google Scholar]
- 31. Sommerland N, Wouters E, Mitchell EMH, et al. Evidence-based interventions to reduce tuberculosis stigma: a systematic review. Int J Tuberc Lung Dis 2017; 21:S81–6. [DOI] [PubMed] [Google Scholar]
- 32. Yin X, Yan S, Tong Y, et al. Status of tuberculosis-related stigma and associated factors: a cross-sectional study in central China. Trop Med Int Health 2018; 23:199–205. [DOI] [PubMed] [Google Scholar]
- 33. Daftary A, Frick M, Venkatesan N, Pai M. Fighting TB stigma: we need to apply lessons learnt from HIV activism. BMJ Glob Health 2017; 2:e000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heijnders M, Van Der Meij S. The fight against stigma: an overview of stigma-reduction strategies and interventions. Psychol Health Med 2006; 11:353–63. [DOI] [PubMed] [Google Scholar]
- 35. Phong K, Srou L, Solá J. Mobile Phones and Internet Use in Cambodia 2016. The Open Institute. 2016. Available at: https://www.open.org.kh/research/phones_2016.pdf. Accessed 10 May 2020.
- 36. Hutchinson PL, Mahlalela X, Yukich J. Mass media, stigma, and disclosure of HIV test results: multilevel analysis in the Eastern Cape, South Africa. AIDS Educ Prev 2007; 19:489–510. [DOI] [PubMed] [Google Scholar]
- 37. Zuch M, Lurie M. ‘A virus and nothing else’: the effect of ART on HIV-related stigma in rural South Africa. AIDS Behav 2012; 16:564–70. [DOI] [PubMed] [Google Scholar]
- 38. Tadesse S. Stigma against tuberculosis patients in Addis Ababa, Ethiopia. PLoS One 2016; 11:e0152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. National Center for Tuberculosis and Leprosy Control (CENAT). Second National Prevalence Survey, 2011. Phnom Penh: Ministry of Health; 2012. [Google Scholar]
- 40. World Health Organization. TB Country Profile: Cambodia. Geneva: World Health Organization; 2019. [Google Scholar]
- 41. Kipp AM, Audet CM, Earnshaw VA, et al. Re-validation of the Van Rie HIV/AIDS-related stigma scale for use with people living with HIV in the United States. PLoS One 2015; 10:e0118836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rasanathan K, Sivasankara Kurup A, Jaramillo E, Lönnroth K. The social determinants of health: key to global tuberculosis control. Int J Tuberc Lung Dis 2011; 15 Suppl 2:30–6. [DOI] [PubMed] [Google Scholar]
- 43. Stop TB Partnership. Global plan to end TB. The paradigm shift 2018–2022. Stop TB Partnership; 2019. Available at: http://www.stoptb.org/assets/documents/global/plan/GPR_2018-2022_Digital.pdf. Accessed 27 May 2020.
- 44. Stop TB Partnership. TB Stigma Assessment Implementation Handbook. Stop TB Partnership; 2019. Available at: http://www.stoptb.org/assets/documents/communities/STP%20TB%20Stigma%20Assessment%20Implementation%20Handbook.pdf. Accessed 15 May 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.