Abstract
Background
Haemophilus influenzae (Hi) serotype b (Hib) vaccination was introduced in Germany in 1990. This study presents a comprehensive overview on the burden of invasive Hi infections for 2001–2016, including serotype distribution and ampicillin resistance.
Methods
Nationwide data from statutory disease surveillance (2001–2016) were linked with laboratory surveillance data (2009–2016). Besides descriptive epidemiology, statistical analyses included multiple imputation to estimate secular trends.
Results
In 2001–2016, 4044 invasive Hi infections were reported. The mean incidence was 3.0 per million inhabitants, higher in males (3.2 vs 2.9 in females) and in the age groups <1 year (15.2) and ≥80 years (15.5). Nontypeable Hi (NTHi) caused 81% (n = 1545) of cases in 2009–2016. Of capsulated cases, 69% were serotype f and 17% serotype b. Of Hib cases eligible for vaccination, 10% (3/29) were fully vaccinated. For 2009–2016, significant increasing trends were observed for NTHi and Hif infections in the age groups <5 years and ≥60 years and for ampicillin resistance in NTHi.
Conclusions
This is one of the most comprehensive Hi data analyses since the introduction of Hib vaccines. NTHi and Hif cause an increasing disease burden among elderly patients and infants. Ampicillin resistance in NTHi must be considered in the treatment of invasive Hi infections.
Keywords: ampicillin resistance, Hib, invasive bacterial infections, NTHi, vaccination
BACKGROUND
Haemophilus influenzae (Hi) is a gram-negative bacillus [1] that colonizes the human nasopharynx and may cause infections of the eyes, ears, and upper and lower respiratory tract. Occasionally, serious systemic infections including pneumonia, meningitis, and septicemia can occur. Six capsular serotypes (a–f) can be distinguished from unencapsulated strains [2], so-called nontypeable H. influenzae (NTHi). Hi serotype b (Hib) has been particularly associated with invasive infections [3–5]. Globally, the burden of invasive Hib disease is still significant especially in children [6]. However, the incidence of invasive Hib cases has been drastically reduced in countries that introduced Hib vaccination in the 1980s–1990s [7]. There is evidence for an increase of NTHi infections, especially in the elderly [8–12] and in newborn children [13–15]. However, robust data covering a large population over a long period of time are scarce. Because aminopenicillins play a crucial role in the treatment of Hi infections, resistance against these antibiotics is of high relevance. Recent studies report a continuous rise of aminopenicillin resistance, especially in NTHi [16].
Routine vaccination against Hib in infants has been recommended by the Standing Committee on Vaccination (STIKO) in Germany since 1990. The current vaccination schedule includes 3 (monovalent) or 4 (hexavalent) vaccinations at 2 [3], 4, and 11–15 months of age with individual catch-up vaccination before the age of 5 years. In 2017, vaccination coverage for a completed series was 91.6% at school entry [17]. In addition, vaccination against Hib is recommended for individuals with anatomic or functional asplenia.
The aim of our study was to provide a comprehensive overview of the burden of invasive Hi infections in a large population (>80 000 000) covering all age groups with an extended surveillance period of 15 years. Special focus was laid on secular trends, serotype distribution, age-specific incidences, and ampicillin susceptibility.
METHODS
Surveillance Data
In Germany, Hi isolated from blood and cerebrospinal fluid (CSF) is notifiable according to the German Infection Protection Act since 2001. Laboratories notify the local health authorities that transmit the pseudonymized information electronically via the respective state health authority to the national public health institute, the Robert Koch Institute (RKI). Since 2009, laboratories have been encouraged to submit clinical isolates to the German National Reference Laboratory for Meningococci and H. influenzae (NRZMHi) for species confirmation, serotyping, and antimicrobial resistance surveillance. Local health authorities are then informed by the NRZMHi of the serotyping results on a case-to-case basis. This allows immediate public health measures, if necessary, and the transmitted results corroborate the notification data.
Anonymized national surveillance data for the years 2001–2016 were extracted using the SurvNet@RKI software package [18]. Case information included month/year of birth, sex, notification week and year, county and state of residence, date of disease onset, clinical presentation, vaccination status, and outcome (survival/death). Using an automated algorithm, notified cases were matched to the cases identified by the NRZMHi, where species diagnosis was confirmed for the clinical isolates during 2009–2016. Matching was based on identical sex, month/year of birth, county and state of residence, ≤7 days between date of illness onset and date of sampling, and the fact that it was a unique match. For potential further matches, a manual search was performed where cases needed to fulfill fewer criteria. Serotype information was available from the NRZMHi. Yearly data exchange between RKI and NRZMHi comprised additional case information including ampicillin resistance. If case information diverged between the 2 data sources, surveillance data were favored over NRZMHi case data, except for the parameter capsule type. Due to German data protection laws, information on date of birth is only available to the NRZMHi. Life months for separate analysis of infants aged <12 months was therefore provided by the NRZMHi.
Laboratory Testing
Species diagnosis of isolates from blood, CSF, or both submitted to the NRZMHi was confirmed phenotypically by gram staining, positive oxidase reaction, and factor-dependent growth on BBL Hemo ID QUAD plates (BD, Heidelberg, Germany) and genotypically by polymerase chain reaction (PCR) for the presence of the Hi-specific genes fucK or ompP2 [19, 20]. If both genes were missing, sequencing of ompP6 was performed to exclude H. haemolyticus [21]. Hi strains were serotyped by slide agglutination (Remel, ThermoFisher Scientific, Braunschweig, Germany), and capsulation was confirmed by PCR targeting bexA as described previously [22, 23]. Antimicrobial susceptibility to ampicillin was tested as published previously [22]. Minimal inhibitory concentrations (MICs) were determined by gradient agar diffusion tests (Etest, bioMérieux, Nürtingen, Germany) and interpreted using breakpoints of the European Committee for Antibiotic Susceptibility Testing (EUCAST). Additionally, nitrocefin tests (Fluka/Sigma Aldrich, St. Louis, MO, USA) were performed on all isolates.
Statistical Analysis
Cases were stratified into the following age groups (in years): <1, 1–4, 5–9, 10–14, 15–19, 20–39, 40–59, 60–79, and ≥80. Signs of meningitis were defined as 1 of the following: meningism, altered mental status, bulging fontanella, or other signs of elevated intracranial pressure. Incidence data were calculated based on 2001–2016 notification data and population estimates from the Federal Statistics Office. Because reliable serotype data were only available at the NRZMHi since 2009, analyses regarding capsule types were restricted to the years 2009–2016. Proportions and incidences were compared using the chi-square test. For incidence rate ratios (IRRs), Poisson regression was used with 95% CIs.
As reliable serotype data were missing for cases where no Hi isolates had been submitted to the NRZMHi, we used multiple imputation to create different versions of complete data for 2009–2016. The different versions reflect uncertainty about the missing information. Each version was created by sampling from the results of an imputation model that quantified the association of the serotype with the time of diagnosis (given by reporting year and week) and the age group of the patient. In the second step, the secular trend was estimated by a Poisson model in each version of the completed data, and then the results were pooled using Rubin’s rules [24, 25].
For statistical analysis, STATA, version 14.1, was used, and multiple imputation was performed using the mi commands.
RESULTS
Matching
Between 2001 and 2016, a total of 4044 Hi cases were reported to the national disease surveillance system. Of 3253 cases that were reported between 2009 and 2016, 1910 (59%) were able to be matched with invasive isolates that had been sent to the NRZMHi. Of these, 8 were tested by the NRZMHi to be Haemophilus spp. other than H. influenzae, resulting in a total of 4036 reported cases and 1902 matched cases for further analyses. For 6 (0.03%) matched cases, the submitted isolates were not culturable; therefore serotyping was not possible. The proportion of matched cases increased from 45% in 2009 to 63% in 2016 and was higher (74%–86%) among 0–14-year-olds compared with ≥15-year-olds (52–58%). The proportions of matched cases were somewhat higher when cases presented with septicemia (66%) or meningeal signs (63%) than with pneumonia (58%). The proportions of matched cases by federal state ranged from 26% in Bremen (34 cases; population 0.54 million) to 83% in Baden-Wuerttemberg (481 cases; population 11 million). Of the 1902 submitted case isolates, 1726 (91%) were derived from blood samples and 164 (8.6%) from CSF, and in 12 cases (0.6%) blood and CSF were submitted.
Disease Burden According to Age, Sex, and Serotype
The mean incidence for 2001–2016 for all federal states was 3.1/1 million, ranging from 1.9/1 million in Thuringia to 4.1/1 million in Berlin. Incidences were highest in <1-year-olds and ≥80-year-olds (Table 1). Incidence was significantly higher in males than in females overall and in the following age groups: <1 year, 1–4 years, 15–19 years, 60–79 years, and ≥80 years (all P < .05). The case fatality rate (CFR) was highest in the age group ≥80 years, at 13.8%. The proportion of NTHi ranged from 54.4% to 56.0% in the age group <10 years and from 75.0% to 91.7% in the age group ≥10 years. Of the 351 cases that were caused by capsulated strains, 241 (69%) were serotype f (Hif), 58 (17%) Hib, 45 (13%) serotype e (Hie), and 7 (2%) serotype a (Hia). No reported and serotyped Hi cases occurred due to serotypes c or d.
Table 1.
Incidence and Case Fatality Rate for 2001–2016 (n = 4036) and Serotyping Results (Serotypes a–f and Nontypeable) for 2009–2016 (n = 1896) of Reported Invasive Haemophilus influenzae Cases by Age Group in Germany
| Incidence (per 1 million inhabitants) | Case Fatality, % | Serotypes a–f | NTHi | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age, y | All | Male | Female | No. | % | No. | % | No. | % |
| <1 | 15.0 | 18.6 | 11.3 | 3/164 | 1.8 | 29 | 42.6 | 37 | 54.4 |
| 1–4 | 4.9 | 5.5 | 4.2 | 12/215 | 5.6 | 46 | 45.1 | 56 | 54.9 |
| 5–9 | 0.9 | 1.0 | 0.9 | 2/55 | 3.6 | 11 | 44.0 | 14 | 56.0 |
| 10–14 | 0.6 | 0.6 | 0.6 | 1/39 | 2.6 | 4 | 21.1 | 15 | 78.9 |
| 15–19 | 0.6 | 0.8 | 0.4 | 0/41 | 0 | 3 | 18.8 | 13 | 81.3 |
| 20–39 | 0.7 | 0.7 | 0.6 | 2/208 | 1.0 | 13 | 15.5 | 71 | 84.5 |
| 40–59 | 1.5 | 1.5 | 1.5 | 28/578 | 4.8 | 67 | 25.0 | 201 | 75.0 |
| 60–79 | 5.8 | 7.0 | 4.7 | 108/1572 | 6.9 | 135 | 17.6 | 629 | 82.2 |
| ≥80 | 15.5 | 18.0 | 14.1 | 148/1069 | 13.8 | 43 | 7.7 | 509 | 91.7 |
| Total | 3.1 | 3.2 | 2.9 | 304/3941 | 7.7 | 351 | 18.5 | 1545 | 81.2 |
Denominator can differ depending on completeness of information.
Abbreviation: NTHi, nontypeable.
Imputation Model for Serotype
We chose a multinomial logistic model with Hib, Hie, Hif, or NTHi as the categorical outcome. The probability distribution of serotypes changed over time and can be described by a second-order polynomial. In particular, the proportion of NTHi increased from around 70% to >80% and plateaued after 2013. We found that using age group as an additional explanatory variable improved the fit of the model, but there was no significant interaction between age and calendar time. Reported symptoms and mortality status of the patients were not significantly associated with serotype distribution and, hence, were not included in the imputation model.
Serotype Time Trends Per Age Group
Trends for Hib, Hie, Hif, and NTHi by age group were analyzed based on imputed data for 2009–2016 (Figures 1 and 2). The imputation did not include Hia due to the small number of cases (n = 7). Hib incidences fluctuated in the age groups <5 years, with the highest incidence of ≤5 cases/1 million population, but displayed no real overall trend. For the age groups ≥5 years, incidences were continuously <1/1 million population (Figure 1A). Trend analysis of Hie cases showed a slight increase and decrease over the observed time period, with incidences of ≤1/1 million population (Figure 1B). For Hif cases, analysis displayed an overall yearly increase of 12% (IRR, 1.12; 95% CI, 1.06–1.20) that was caused by a continuous increase in the age groups <5 years and ≥60 years (Figure 2A). Among ≥80-year-olds, NTHi incidence increased from ~10 cases/1 million population in 2009 to 40/1 million population in 2015 (IRR, 1.42; 95% CI, 1.31–1.56). An increasing trend was also observed in the age groups <1 year, 1–4 years, and 60–79 years, rising from incidences ≤5 cases/1 million population to ~10 cases/1 million, plateauing in 2015 (Figure 2B).
Figure 1.
Trend analysis for Haemophilus influenzae type b and type e by age group in Germany based on imputed data, 2009–2016.
Figure 2.
Trend analysis for Haemophilus influenzae type f and nontypeable Haemophilus influenzae (NTHi) by age group in Germany based on imputed data, 2009–2016.
Clinical Presentation by Serotype
Clinical presentation differed by serotype and age group (Figure 3). While in ≤4-year-old children Hib and Hif most often caused signs of meningitis, septicemia was most often seen in NTHi cases in this age group. In adults aged ≥40 years, pneumonia and septicemia were the most common clinical presentations in Hib, Hif, and NTHi cases. Other serotypes and age groups are not displayed in Figure 3 due to small case numbers. Pneumonia was reported in 51% of Hie cases, septicemia in 27%. For 9%, death was reported. Hia cases (n = 7) mainly presented with septicemia (43%) or with pneumonia (29%); no deaths were reported.
Figure 3.
Proportions of Haemophilus influenzae–related symptoms and Haemophilus influenzae–caused deaths for serotype b (Hib), serotype f (Hif), and nontypeable Haemophilus influenzae (NTHi) by age groups ≤4 years and ≥40 years in Germany, 2009–2016. aCase data available for death status: 89 cases. bCase data available for death status: 180 cases. cCase data available for death status: 1272 cases.
Infant Cases Aged <12 Months
Of 90 reported infant cases in 2009–2016, 68 (76%) could be matched with NRZMHi data. Of these, 36 (53%) were male and 32 (47%) female; 21 (31%) cases occurred within the first month of life, 27 (40%) between 1 and 5 months, and 20 (29%) between 6 and 11 months. All infections occurring at <1 month of age were caused by NTHi; 13/21 (62%) cases showed signs of septicemia, and 2 (9%) died, 1 of them reportedly due to Hi infection. For the age group 1–5 months, 11/27 (41%) cases were caused by NTHi, 7 (26%) by Hib, 5 (19%) by Hif, and 2 (7%) each by Hia and Hie. The symptoms most often reported were signs of meningitis (n = 7; 26%), followed by septicemia (n = 5; 19%) and pneumonia (n = 3; 11%). In the age group 6–11 months, 10/20 (50%) cases were caused by Hif, 6 (30%) by NTHi, 3 (15%) by Hib, and 1 (5%) by Hie. The symptoms most often reported were signs of meningitis (n = 8; 40%), septicemia (n = 5; 25%), and pneumonia (n = 4; 20%). In the latter 2 age groups, no deaths occurred.
Breakthrough Infections by Hib
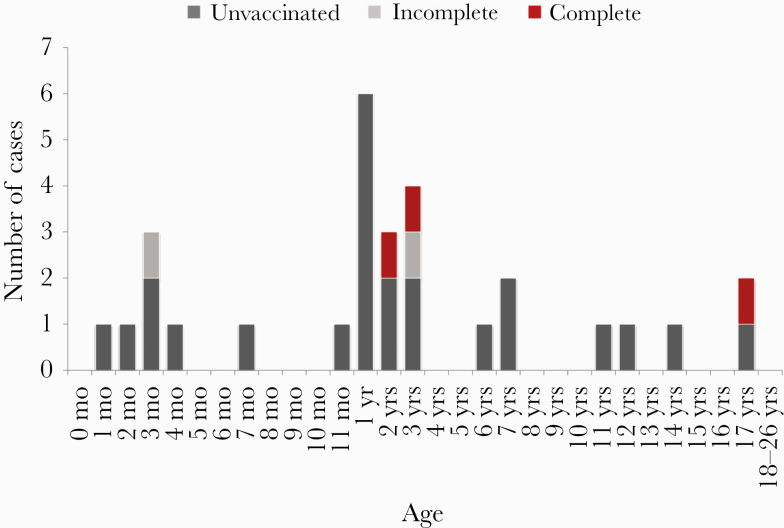
Information on vaccination status was available for 29/35 (83%) Hib cases aged <27 years, who were eligible for Hib vaccination during childhood according to the STIKO recommendation: 24/29 (83%) were unvaccinated, 3 (10%) had received a full course of vaccine (cases 1 and 2: 2 and 3 years of age, both with pneumonia, each had 4 vaccine doses; case 3: 17 years of age, meningitis and septicemia, severe preexisting neurological condition, 3 monovalent vaccinations), and 2 (7%) were incompletely vaccinated (case 4: 3 months of age, arthritis and pneumonia, 1 vaccination; case 5: 3 years of age, epiglottitis, 3 hexavalent vaccinations) (Figure 4).
Figure 4.
Haemophilus influenzae serotype b cases aged <27 years with information on vaccination status (n = 29), Germany, 2009–2016.
Ampicillin Resistance
For 1890/1902 (99%) isolates of matched Hi cases, ampicillin susceptibility was tested. Three hundred six of those (16%) were ampicillin resistant. Among Hib isolates, 9% (5/57) were ampicillin resistant, among Hif isolates 5% (12/237), among Hie isolates 7% (3/45), and among Hia isolates 0% (0/7). For NTHi isolates, ampicillin resistance could be observed in 19% (286/1544). During the observed time period, the proportion of ampicillin-resistant NTHi isolates increased (Figure 5). The trend analysis for the proportion of ampicillin-resistant NTHi isolates showed an odds ratio (OR) of 1.11 (95% CI, 1.04–1.19; P = .001) per year for 2009–2016.
Figure 5.
Ampicillin-resistant nontypeable Haemophilus influenzae (NTHi) cases in Germany, 2009–2016 (n = 1544).
DISCUSSION
Twenty-seven years after the introduction of Hib vaccination into the childhood vaccination schedule, our analysis showed that Hib has become a rare disease in Germany, with ≤5 cases per 1 million inhabitants. In the prevaccine era, incidences in <5-year-olds ranged between 200 and 500 per million inhabitants in Europe [26]. During our study period, birth cohorts in Germany ranged between 663 000 and 792 000 [27], and vaccination coverage for a full vaccination series has been 92%–94% since 2010 [28]. Nevertheless, only 35 cases of Hib infections were reported. Of those, only 3 cases were due to breakthrough infections in fully vaccinated individuals, whereas more than three-quarters of the cases occurred in unvaccinated persons including infants that were too young for vaccination. The results again highlight the effectiveness of Hib vaccination programs with high vaccine coverage in preventing Hib disease.
Invasive Hi infections in Germany have been foremost caused by NTHi, followed by Hif. Individuals aged ≥80 years were the most affected age group with an Hi incidence of >15 per million and a CFR of 14%. The observed shift toward a high burden of disease in the elderly caused by NTHi is in line with findings in other countries [29, 30], although German case numbers are still at a low level [8, 29]. Our data also suggest an increasing temporal trend in invasive NTHi and Hif infections in the age groups ≥60 years. Reasons for the augmented case numbers among the elderly may be found in prolonged life spans and thus larger numbers of elderly individuals with more severe comorbidities and waning immunity [31]. Because our data did not systematically include information on comorbidities or immunosuppression/immunodeficiency, we were not able to examine their potential influence on the observed increase.
Other factors contributing to the increase in NTHi cases might be improvements in the quality of serotyping data of NTHi. Hib may have been taken as a synonym for Haemophilus influenzae in the prevaccine era, as serotyping was not always carried out and Hib was the most prevalent type in that period. Since 2009, the NRZMHi has been sending laboratory reports to local public health offices on a routine basis. This resulted in a rise in specimens submitted to the NRZMHi. Thus, notification morale and disease awareness could have been improved. In addition, changes in the management of notifications supported increasing notification rates. The German Infection Protection Act was established in 2001, and notification, public health action in controlling infectious diseases, and distinction between the notions of Hi, Hib, and NTHi may have improved. Nevertheless, the increasing trend of NTHi cases is following developments in other countries and continued after stabilization of submission rates.
It is open for debate whether disease clusters may contribute to the high numbers of invasive NTHi infections. Disease outbreaks have been reported sporadically, especially for nursing homes [32]. However, public health authorities never reported suspicious clusters of NTHi cases during the observation time. A recent study revealed high diversity of NTHi in France [33]. Future analyses are needed to detect spatio-temporal clusters that include genomic typing of NTHi.
Among the highly affected infants, one-third of these infections occurred in the first month of life. NTHi predominated (95%) and led to a CFR of 9%. Increasing numbers of invasive NTHi infections in newborns [13, 14, 34] and an association with high case fatality [15] have been documented before and have become the focus of interest of recent studies [15, 35]. To further assess the burden of invasive Hi disease in neonates and to identify potential risk factors—such as preterm birth, status of vaginal colonization of the mother, birth mode, or maternal age—prospective multicenter studies are needed. This could help to implement preventive measures. In the second half of the first year of life, most invasive Hi infections in our population were caused by Hif, and predominant symptoms shifted from signs of septicemia to signs of meningitis. Deaths did not occur. However, it needs to be noted that our overall case numbers in infants were relatively low, and these results need to be interpreted carefully.
Besides high incidences of invasive NTHi infections, Hif was the most frequent serotype causing invasive disease during the observation period. This finding, as well as the increasing trend of Hif, particularly in the elderly, is consistent with developments in other countries [11, 12]. The dominance of NTHi and increasing trends of Hif raise the question of whether the introduction of Hib vaccination has led to a gradual serotype replacement. However, invasive infections due to NTHi and Hif seem to have a different disease nature than the ones caused by Hib. Hib used to attack mainly children and could manifest as epiglottitis, meningitis, and septicemia. By contrast, Hi is most prevalent in elderly patients and, to a lesser extent, in neonates by most frequently causing septicemia. In line with these speculations, an analysis of European data from 2000–2004 did not show signs of serotype replacement [36]. Furthermore, our analysis shows that infections with different Hi serotypes may cause different symptoms in different age groups: Meningeal signs were the most common symptom for Hib and Hif, whereas septicemia was the predominant manifestation for NTHi in the <5-year-olds. In individuals aged ≥40 years, Hib, Hif, and NTHi most frequently caused pneumonia.
Infections due to Hie were relatively rare and comparable to the case numbers of Hib infections. This is in line with reports from other European countries [13] and overseas [37–39]. Consistent with the epidemiology in other European countries, Hia was a rarity in Germany [12, 13]. This stands in contrast to the epidemiologic situation in Canada and the United States [40, 41], where Hia incidences are particularly high in the indigenous population [42].
Similar to data from Europe [12], we did observe gender differences in Hi incidences. Male individuals among young children (<5 years), teenagers (15–19 years), and the elderly (≥60 years) were significantly more often affected. Findings from the United States, however, show that females are more often affected, although gender differences varied between age groups [43]. The reasons for and importance of this finding remain unclear.
Finally, our study provides information relevant to the antibiotic treatment of invasive Hi infections. Because aminopenicillins are first-choice antibiotics, monitoring of resistance to these antibiotics is important. Our data show an increasing trend in ampicillin resistance overall since 2009 and especially since 2012. This trend is due to an 11% increase in resistance of NTHi strains per year since 2009. These observations support common recommendations of combined antibiotic treatment using both third-generation cephalosporines and aminopenicillins for the empirical treatment of severe diseases such as meningitis potentially caused by Hi [44].
Our study had some limitations. The observation period of 8 years for analysis of cases with serotype information might not have been sufficient to generate reliable statements on strain time trends. Therefore, further trend analyses in future years might have to be awaited to see if the trends described here continue. Furthermore, because our data lacked information on comorbidities and immunosuppression/immune-deficiency, we could not analyze whether there is an association between the increasing number of NTHi infections in the elderly and these factors. Finally, our strain analysis is partly based on imputations because there was no strain information available for all cases. As we saw a difference in the percentage of available strain analysis by region (range of isolates matched per federal state, 26%–83%) and age group, imputations might have introduced a bias. However, there is no evidence that relevant regional differences in Hi strain distribution in Germany exist. As a higher proportion of cases in younger age groups could be matched, and as in these age groups infections were more often caused by encapsulated strains than in older age groups, the increasing trend for NTHi especially might have been underestimated in our findings.
In conclusion, our findings indicate that the Hib vaccination strategy in Germany is highly effective to prevent Hib infections in young children and needs no adaptation at the moment. However, NTHi and Hif are responsible for an increasing burden of disease, especially in the elderly and infants. In connection with rising ampicillin resistance rates in NTHi, it might be warranted to look into the development of an NTHi vaccine that could prevent an increasing burden of disease with a CFR of up to 14%.
Acknowledgments
We thank Wiebke Hellenbrand (RKI) for scientific advice and Eberhardt Pape (RKI) for creating the automated matching algorithm and for technical support in the yearly matching processes. We thank Sabrina Hebling and Alexandra Sikora (both NRZMHi) for excellent technical assistance. We would also like to thank all local health authorities for collecting case data and initiating the submission of isolates as well as all collaborating laboratories for submitting the clinical isolates to the NRZMHi.
Financial support. The German National Reference Laboratory for Meningococci and Haemophilus influenzae is supported by the Robert Koch Institute with funds from the Federal Ministry of Health (funding code 1369-237).
Potential conflicts of interest. The authors do not have a commercial or other association that might pose a conflict of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. The study analyses anonymized surveillance data. According to regulations in Germany, patient consent or approval of an ethical committee was not needed in this case.
Author affiliations. Affiliations of all authors are as stated on the title page (Anja Takla is on parental leave and cannot be contacted via institutional e-mail but via anjatakla@gmail.com).
References
- 1. Wen S, Feng D, Chen D, et al. Molecular epidemiology and evolution of Haemophilus influenzae. Infect Genet Evol 2020; 80:104205. [DOI] [PubMed] [Google Scholar]
- 2. Pittman M. Variation and type specificity in the bacterial species Hemophilus influenzae. J Exp Med 1931; 53:471–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pittman M. The action of type-specific Hemophilus influenzae antiserum. J Exp Med 1933; 58:683–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fothergill LD, Chandler CA, Spencer M. Observations on the dissociation of meningitic strains of H. influenzae. J Immunol 1936; 31:401–15. [Google Scholar]
- 5. Shapiro ED, Ward JI. The epidemiology and prevention of disease caused by Haemophilus influenzae type b. Epidemiol Rev 1991; 13:113–42. [DOI] [PubMed] [Google Scholar]
- 6. Wahl B, O’Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Global Health 2018; 6:e744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev 2000; 13:302–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giufrè M, Cardines R, Caporali MG, et al. Ten years of Hib vaccination in Italy: prevalence of non-encapsulated Haemophilus influenzae among invasive isolates and the possible impact on antibiotic resistance. Vaccine 2011; 29:3857–62. [DOI] [PubMed] [Google Scholar]
- 9. Campos J, Hernando M, Román F, et al. ; Group of Invasive Haemophilus Infections of the Autonomous Community of Madrid, Spain Analysis of invasive Haemophilus influenzae infections after extensive vaccination against H. influenzae type b. J Clin Microbiol 2004; 42:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hargreaves RM, Slack MP, Howard AJ, et al. Changing patterns of invasive Haemophilus influenzae disease in England and Wales after introduction of the Hib vaccination programme. BMJ 1996; 312:160–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Resman F, Ristovski M, Ahl J, et al. Invasive disease caused by Haemophilus influenzae in Sweden 1997-2009; evidence of increasing incidence and clinical burden of non-type b strains. Clin Microbiol Infect 2011; 17:1638–45. [DOI] [PubMed] [Google Scholar]
- 12. Whittaker R, Economopoulou A, Dias JG, et al. Epidemiology of invasive Haemophilus influenzae disease, Europe, 2007–2014. Emerg Infect Dis 2017; 23:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ladhani S, Slack MP, Heath PT, et al. Invasive Haemophilus influenzae disease, Europe, 1996–2006. Emerg Infect Dis 2010; 16:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soeters HM, Blain A, Pondo T, et al. Current epidemiology and trends in invasive Haemophilus influenzae disease—United States, 2009–2015. Clin Infect Dis 2018; 67:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collins S, Litt DJ, Flynn S, et al. Neonatal invasive Haemophilus influenzae disease in England and Wales: epidemiology, clinical characteristics, and outcome. Clin Infect Dis 2015; 60:1786–92. [DOI] [PubMed] [Google Scholar]
- 16. Heinz E. The return of Pfeiffer’s bacillus: rising incidence of ampicillin resistance in Haemophilus influenzae. Microb Genom 2018; 4:e000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robert Koch-Institut. Impfquoten bei der schuleingangsuntersuchung in Deutschland 2017 [Vaccination coverage at school entry examinations in Germany 2017]. Epidemiol Bull 2019; 18:147–53. [Google Scholar]
- 18. Faensen D, Claus H, Benzler J, et al. SurvNet@RKI - a multistate electronic reporting system for communicable diseases. Euro Surveill 2006; 11:7–8. [DOI] [PubMed] [Google Scholar]
- 19. Meats E, Feil EJ, Stringer S, et al. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol 2003; 41:1623–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hobson RP, Williams A, Rawal K, et al. Incidence and spread of Haemophilus influenzae on an Antarctic base determined using the polymerase chain reaction. Epidemiol Infect 1995; 114:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy TF, Brauer AL, Sethi S, et al. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis 2007; 195:81–9. [DOI] [PubMed] [Google Scholar]
- 22. Lâm TT, Claus H, Elias J, et al. Ampicillin resistance of invasive Haemophilus influenzae isolates in Germany 2009-2012. Int J Med Microbiol 2015; 305:748–55. [DOI] [PubMed] [Google Scholar]
- 23. Lâm TT, Claus H, Frosch M, Vogel U. Analysis of non-typeable Haemophilus influenzae in invasive disease reveals lack of the capsule locus. Clin Microbiol Infect 2016; 22:63.e7–8. [DOI] [PubMed] [Google Scholar]
- 24. Rubin D. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 25. Little RJA, Rubin DB. Statistical Analysis With Missing Data. 2nd ed. New York: John Wiley & Sons; 2002. [Google Scholar]
- 26. Kalies H, Siedler A, Gröndahl B, et al. Invasive Haemophilus influenzae infections in Germany: impact of non-type b serotypes in the post-vaccine era. BMC Infect Dis 2009; 9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Statistisches Bundesamt (Destatis). Lebendgeborene: Deutschland, jahre, geschlecht [Live births: Germany, years, sex]. 2020. Available at: https://www-genesis.destatis.de/genesis/online/link/tabellen/12612*. Accessed 21 August 2020.
- 28. Robert Koch Institute. Impfquoten bei den schuleingangsuntersuchungen in Deutschland [Vaccination coverage at school entry examinations in Germany]. 2019. Available at: https://www.rki.de/DE/Content/Infekt/Impfen/Impfstatus/schulanfaenger/schuleingangsuntersuchungen_inhalt.html. Accessed 24 April 2018.
- 29. Van Eldere J, Slack MP, Ladhani S, Cripps AW. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis 2014; 14:1281–92. [DOI] [PubMed] [Google Scholar]
- 30. McVernon J, Howard AJ, Slack MP, Ramsay ME. Long-term impact of vaccination on Haemophilus influenzae type b (Hib) carriage in the United Kingdom. Epidemiol Infect 2004; 132:765–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jalalvand F, Riesbeck K. Update on non-typeable Haemophilus influenzae-mediated disease and vaccine development. Expert Rev Vaccines 2018; 17:503–12. [DOI] [PubMed] [Google Scholar]
- 32. Andersson M, Resman F, Eitrem R, et al. Outbreak of a beta-lactam resistant non-typeable Haemophilus influenzae sequence type 14 associated with severe clinical outcomes. BMC Infect Dis 2015; 15:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deghmane AE, Hong E, Chehboub S, et al. High diversity of invasive Haemophilus influenzae isolates in France and the emergence of resistance to third generation cephalosporins by alteration of ftsI gene. J Infect 2019; 79:7–14. [DOI] [PubMed] [Google Scholar]
- 34. Gkentzi D, Slack MP, Ladhani SN. The burden of nonencapsulated Haemophilus influenzae in children and potential for prevention. Curr Opin Infect Dis 2012; 25:266–72. [DOI] [PubMed] [Google Scholar]
- 35. Collins S, Vickers A, Ladhani SN, et al. Clinical and molecular epidemiology of childhood invasive nontypeable Haemophilus influenzae disease in England and Wales. Pediatr Infect Dis J 2016; 35:e76–84. [DOI] [PubMed] [Google Scholar]
- 36. Ladhani S, Ramsay ME, Chandra M, Slack MP; EU-IBIS No evidence for Haemophilus influenzae serotype replacement in Europe after introduction of the Hib conjugate vaccine. Lancet Infect Dis 2008; 8:275–6. [DOI] [PubMed] [Google Scholar]
- 37. Urwin G, Krohn JA, Deaver-Robinson K, et al. Invasive disease due to Haemophilus influenzae serotype f: clinical and epidemiologic characteristics in the H. influenzae serotype b vaccine era. The Haemophilus influenzae Study Group. Clin Infect Dis 1996; 22:1069–76. [DOI] [PubMed] [Google Scholar]
- 38. Desai S, Jamieson FB, Patel SN, et al. The epidemiology of invasive Haemophilus influenzae non-serotype B disease in Ontario, Canada from 2004 to 2013. PLoS One 2015; 10:e0142179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wan Sai Cheong J, Smith H, Heney C, et al. Trends in the epidemiology of invasive Haemophilus influenzae disease in Queensland, Australia from 2000 to 2013: what is the impact of an increase in invasive non-typable H. influenzae (NTHi)? Epidemiol Infect 2015; 143:2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsang RS, Mubareka S, Sill ML, et al. Invasive Haemophilus influenzae in Manitoba, Canada, in the postvaccination era. J Clin Microbiol 2006; 44:1530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soeters HM, Oliver SE, Plumb ID, et al. Epidemiology of invasive Haemophilus influenzae serotype a disease-United States, 2008–2017 [published online ahead of print June 26, 2020]. Clin Infect Dis. 2020; doi: 10.1093/cid/ciaa875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsang RS, Bruce MG, Lem M, et al. A review of invasive Haemophilus influenzae disease in the indigenous populations of North America. Epidemiol Infect 2014; 142:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dworkin MS, Park L, Borchardt SM. The changing epidemiology of invasive Haemophilus influenzae disease, especially in persons > or = 65 years old. Clin Infect Dis 2007; 44:810–6. [DOI] [PubMed] [Google Scholar]
- 44. Pfister H-W. S2k-leitlinie ambulant erworbene bakterielle (eitrige) meningoenzephalitis im erwachsenenalter [S2k guideline community acquired bacterial meningoencephalitis in adults]: deutsche gesellschaft für neurologie. 2015. Available at: https://www.awmf.org/uploads/tx_szleitlinien/030-089l_S2k_Ambulant_erworbene_Meningoenzephalitis_2016-08-verlaengert_01.pdf.