Abstract
Introduction
Parkinson’s disease (PD) is more frequent in the elderly and increases the risk of respiratory infections. Previous data on PD and SARS-CoV-2 are scarce, suggesting a poor prognosis in advanced disease and second-line therapies.
Methods
A retrospective case–control study comparing patients with PD and COVID-19 and patients with PD without COVID-19 was conducted during the pandemic period in Spain (March 1st–July 31st 2020) in a tertiary university hospital.
Results
Thirty-nine (COVID-19 +) and 172 (COVID-19-) PD patients were included. Fifty-nine percent were males in both groups, with similar age (75.9 ± 9.0 COVID-19 + , 73.9 ± 10.0 COVID-19-), disease duration (8.9 ± 6.2 COVID-19 + , 8.5 ± 5.6 COVID-19-) and PD treatments. COVID-19 was mild in 10 (26%), required admission in 21 (54%) and caused death in 8 (21%) patients. Dementia was the only comorbidity more frequent in COVID-19 + patients (36% vs. 14%, p = 0.0013). However, in a multivariate analysis, institutionalization was the only variable associated with COVID-19 + (OR 17.0, 95% CI 5.0–60.0, p < 0.001). When considering severe COVID-19 (admission or death) vs. mild or absent COVID-19, institutionalization, neoplasm, dementia and a lower frequency of dopamine agonists were associated with severe COVID-19. In multivariate analysis, only institutionalization [OR 5.17, 95% CI 1.57–17, p = 0.004] and neoplasm [OR 8.0, 95%CI 1.27–49.8, p = 0.027] remained significantly associated.
Conclusion
In our experience, institutionalization and oncologic comorbidity, rather than PD-related variables, increased the risk of developing COVID-19, and impacted on its severity. These findings suggest that epidemiologic factors and frailty are key factors for COVID-19 morbidity/mortality in PD. Appropriate preventive strategies should be implemented in institutionalized patients to prevent infection and improve prognosis.
Keywords: SARS-CoV-2, COVID-19, Parkinson, Institutionalization, Comorbidity, Prognosis
Introduction
The coronavirus SARS-CoV-2 is the causal agent of the acute respiratory syndrome known as coronavirus disease 2019 (COVID-19). It is estimated that it has already infected more than 23 million people, caused more than 830,000 deaths worldwide [1], and over 29,000 deaths in Spain to date, being one of the most affected countries by the pandemic worldwide [1]. Some factors have been linked to a poor prognosis, including male sex, older age, overweight and diabetes mellitus, although their individual impact is not clearly established yet [2].
Parkinson’s disease (PD) is more frequent in the elderly, contributes to frailty, and increases the risk of respiratory infections, both due to dysphagia (aspirations) and respiratory restriction [3]. This suggests that people affected by PD might be especially vulnerable to SARS-CoV-2 infection and its complications [4, 5]. Along with the infection itself, limited access to healthcare resources, restrictions of mobility, and social interaction due to preventive measures, play a negative role in patients with PD. As a result, the risk of cognitive and motor worsening, as well as other PD-related complications, may be increased [6].
Some drugs commonly used in PD, such as amantadine, have been suggested to play a potential role as antiviral agents against SARS-CoV-2 [7]. Also, regulation of dopa-decarboxylase activity seems to modify angiotensin-converting enzyme 2 (ACE2) functioning, a relevant receptor implicated in SARS-CoV-2 pathogenesis. Therefore, dopa-decarboxylase inhibitors have been suggested to have an impact on COVID-19 [8].
Data on patients with PD and COVID-19 are scarce. An initial study based on ten PD patients, many of them with an advanced disease and living in a nursing home, indicated a very concerning mortality of 40% [9]. In contrast, a case–control survey on 1486 participants found no differences in the rates of hospitalization or death between patients affected by COVID-19 with and without PD. Chronic lung disease was more frequent in people with PD who developed COVID-19, while vitamin D supplementation was more frequent in those who did not [10]. Vitamin D has immune-modulating and potential antiviral properties, which could explain this association [11]. Another study assessing 141 patients with PD in Lombardy found no differences in demographic and clinical variables between PD patients with and without COVID-19. This study had a small sample size and might be underpowered to detect any difference [12]. Finally, a recent multi-center study of 117 community-dwelling PD patients with COVID-19 in Italy, Iran, Spain and the UK found an overall mortality of 19.7%, predictors of poor outcome being dementia and PD duration of PD. There was a trend towards increased mortality with hypertension (p = 0.054) [13].
Here, we aim to assess demographic and clinical variables that may be associated with COVID-19 in patients with PD, as well as those that may have an impact on morbidity and mortality.
Methods
We performed a case–control study comparing the demographic and clinical characteristics of patients with PD and COVID-19 (cases) and patients with PD without COVID-19 (controls) during the pandemic period from March 1st to July 31st of 2020. All patients attend the Movement Disorders Unit at Hospital Ramón y Cajal, that is located in Madrid, the city with the highest incidence of COVID-19 in Spain [14]. Both email and specialist nurse telephone were available for patients and General Practitioners (GP) so they were able to communicate relevant information to our team. When a PD patient was admitted at hospital because of COVID-19, MD specialists were also contacted. Data were retrospectively collected from the electronic medical records.
Patients diagnosed with PD based on MDS diagnostic criteria [15] were included. Atypical and secondary Parkinsonisms were excluded. Cases (COVID-19 +) had a COVID-19 diagnosis meeting clinical and laboratory criteria for probable and confirmed COVID-19 [16]. We classified the severity of COVID-19 as mild (outpatient management), moderate-severe (hospitalization requirement) or death. Controls (COVID-19-) were assessed in our outpatient clinic during the month of July (1–31st July), and were included in a consecutive way if they had remained free of COVID-19 symptoms throughout the pandemic period.
Demographic features such as age, gender and disease duration as well as clinical features such as PD medications (dose of levodopa, treatment with dopamine agonists, amantadine, entacapone, opicapone, and MAO-B inhibitors), advanced therapies (deep brain stimulation, DBS, continuous duodenal infusion of levodopa/carbidopa, continuous perfusion of apomorphine, and MRI-guided focused ultrasound ablations), comorbidities, and institutionalization were collected.
Statistical analyses were performed using G-Stat (version 2.0). Descriptive statistics and univariate (Student’s T, Chi square or Fisher’s test when appropriate) and multivariate analysis (logistic regression including variables with significant differences in univariate analysis) were performed. First, cases (COVID-19 +) and controls (COVID-19 −) were compared. Second, severely affected patients (admission or death due to COVID-19) were compared with those who were mildly affected or had not been suffered COVID-19. Statistical significance was set at < 0.05. Descriptive results are given as mean and standard deviation and percentages. Results of logistic regression are given as odds ratios (OR) with 95% confidence intervals (95% CI). The study was approved by the local Ethics Committee.
Results
A total of 211 patients with PD were included. Of those, 33 were COVID-19 + and 172 COVID-19-, (ratio of 1:4). COVID-19 was mild in 10 (26%), required hospital admission in 21 (54%) and led to death in 8 (21%) cases. Demographic and clinical variables are shown in Table 1.
Table 1.
Demographic and clinical variables in COVID-19 + and COVID-19- groups
| Variable | COVID-19 + (n: 39) | COVID-19- (n: 172) | p |
|---|---|---|---|
| Age (y) mean ± SD | 75.9 ± 9.0 | 73.9 ± 10.0 | NS |
| Gender | NS | ||
| Male | 23(59%) | 101(59%) | |
| Female | 16 (41%) | 71 (41%) | |
|
Duration of disease (y) Mean ± SD |
8.9 ± 6.2 | 8.5 ± 5.6 | NS |
| Institutionalized | 13 (33%) | 4 (2%) | <0.0006 |
| Dementia | 14 (36%) | 24 (14%) | 0.0013 |
| Levodopa daily dose (mg) Mean ± SD | 708.2 ± 514.8 | 645.3 ± 420.7 | NS |
| Dopamine agonists |
9 (23%) Pramipexole 3 Ropinirole 3 Rotigotine 3 Apomorphine 1 |
70 (41%) Pramipexole 31 Ropinirole 15 Rotigotine 23 Apomorphine 1 |
0.0401 |
| MAO-I |
17 (44%) Rasagiline 7 Safinamide 10 Selegiline 0 |
84 (49%) Rasagiline 40 Safinamide 39 Selegiline 5 |
NS |
| Amantadine | 2 (5%) | 21 (12%) | NS |
| Entacapone | 3 (8%) | 17 (10%) | NS |
| Opicapone | 1 (3%) | 18 (10%) | NS |
| Advanced therapies |
7 (18%) DBS 4 L/C duodenal infusion 3 Apomorphine CP 1 HIFU 0 |
15 (9%) DBS 7 L/C duodenal infusion 7 Apomorphine CP 0 HIFU 2 |
NS |
| Vitamin D supplementation | 6 (15%) | 42 (24%) | NS |
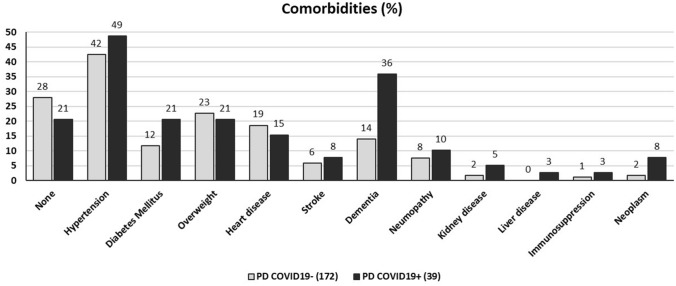
The frequency of common comorbidities was similar between COVID-19 + and COVID-19- groups (Fig. 1), with the exception of dementia, that was significantly more frequent in the group of cases (36% vs. 14%, (p = 0.0013). In a multivariate analysis, only institutionalization remained significantly associated with COVID-19 + (OR 17, 95% CI 5–60, p < 0.001).
Fig. 1.
Frequency of comorbidities in COVID-19- and COVID-19 + groups
When comparing severe COVID-19 vs. mild or absent COVID-19, institutionalization, neoplasm, dementia and no dopamine agonists intake were covariates that were significantly associated with severe COVID-19 in the univariate analysis (Table 2). However, when introduced in the multivariate analysis, only institutionalization and neoplasm remained significantly associated with severe COVID-19 + [Institutionalization: OR 5.17 95% CI (1.57–17) p = 0.004; neoplasm: OR 8.0, 95% CI (1.27–49.8) p = 0.027].
Table 2.
Demographic and clinical variables in severe COVID-19 (admission or death) vs. mild or absent COVID-19
| Variable | Severe COVID-19 + (admitted or death) (n: 29) |
Mild COVID-19 + or COVID-19- (n: 182) |
p |
|---|---|---|---|
| Age (y) Mean ± SD | 78 ± 8.7 | 74 ± 9.9 | NS |
| Gender | NS | ||
| Male | 17 (59%) | 107 (59%) | |
| Female | 13 (41%) | 74 (41%) | |
| Duration of disease(y) Mean ± SD | 8.2 ± 6.0 | 8.6 ± 5.6 | NS |
| Institutionalized | 8 (28%) | 9 (5%) | <0.0001 |
| Dementia | 11 (38%) | 27 (15%) | 0.0026 |
| Neoplasm | 3 (10%) | 3 (2%) | 0.0353 |
| Levodopa daily dose (mg) Mean ± SD | 747 ± 5652 | 642.6 ± 415 | NS |
| Dopamine agonists | 5 (17%) | 74 (41%) | 0.0155 |
| MAO-I | 9 (31%) | 92 (51%) | NS |
| Amantadine | 1 (4%) | 22 (12%) | NS |
| Entacapone | 1 (4%) | 19 (11%) | NS |
| Opicapone | 0 | 19 (11%) | NS |
| Advanced therapies | 4 (14%) | 18 (10%) | NS |
| Vitamin D supplementation | 5 (17%) | 43 (23%) | NS |
Discussion
In this single-centre case–control study, we described the demographic and clinical characteristics of PD patients with and without COVID-19. We have found that the only factors that remained associated with COVID-19 infection in PD subjects were institutionalization, and for severe COVID-19, also the presence of neoplasm. There are several explanations that may account for these associations. First, there has been a large community transmission in these institutions in Spain [17]. Also, institutionalized patients tend to be frailer [18]. However, we found no significant differences regarding other comorbidities among patients and controls, suggesting that frailty is a state of increased vulnerability that cannot be inferred only from adding comorbidities. Importantly, a significantly higher presence of neoplasm and previous institutionalization in patients with severe COVID-19 has been also reported in previous studies [17, 19] indicating that patients with PD share similar baseline characteristics than patients with severe COVID-19 without PD. One can, therefore, suggest that some epidemiologic and systemic factors are indeed the most relevant contributing factors for the infection and morbidity of SARS-CoV-2, rather than PD itself. According to Spanish Health Ministry, COVID-19 mortality in the general population of this age during the peak pandemic period was around 20-25%, similar to our study [14].
We found no significant differences between PD patients with and without COVID-19 in terms of age, duration of disease and treatments employed (including advanced therapies such as deep brain stimulation, levodopa/carbidopa intestinal gel, and continuous subcutaneous apomorphine infusion). Our findings are in contrast with previous studies with smaller sample sizes, where a relationship between the uses of advanced therapies with the severity of COVID-19 had been suggested [9]. This is important to reassure patients with PD, their caregivers and healthcare providers, as it does not seem that either PD itself or its treatment may lead to a higher risk of complications of COVID-19. However, further prospective studies are needed to confirm this.
In our cohort, no differences between groups were identified in terms of medications that have been suggested to have a potential benefit in COVID-19 [7, 10, 13]. Similar to recent studies [10, 13], we found that patients with PD and COVID-19 were less frequently treated with dopamine agonists and those without COVID-19 were more frequently on vitamin D. However, these differences disappeared in the multivariate analysis. Nevertheless, further studies are needed to clarify this interesting and potentially useful associations. We acknowledge our study may have been underpowered to detect differences among drug prescriptions between COVID-19-positive and -negative subjects. Nevertheless, alternative confounding factors and/or regional differences in prescription habits may account for the differences observed in previous studies.
Our study has several limitations related to its retrospective nature and a relatively small sample size. In addition, no scale of frailty was used, which would have been critical to detect the increased risk of severe disease or death. However, it is one of the largest studies in patients with PD and COVID-19, with a consecutively selected control group, which reduces possible biases.
In conclusion, our study contributes to the scientific literature with a large sample of PD subjects in which institutionalization and to a lesser extent oncologic comorbidity, increased the risk of developing COVID-19, and impacted on its severity. These findings are in line with those observed in the general population, and suggest that some epidemiologic factors and frailty are key factors for COVID-19 morbidity and mortality in PD patients. Other clinical variables such as disease duration and PD treatments do not seem to be critical. Our study highlights the need for nursing homes to be provided with enough healthcare resources to implement focused SARS-CoV-2 preventive strategies in institutionalized patients with PD, to face this challenging situation and prevent infection. Telemedicine with MD neurologists would be useful to maintain patient continuity and treatment of these complex and frail patients.
Funding
This research received no specific Grant from any funding agency in public, commercial, or not-for-profit sectors. The authors received no financial support for the research, authorship, and/or publication of this article.
Compliance with ethical standards
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. Data are available upon request to the authors.
Ethical standard
The study was approved by the local Ethics Committee.
References
- 1.World Health Organization Coronavirus Disease (COVID-19) (2020) https://covid19.who.int. Accessed Aug 31, 2020
- 2.Chaomin Wu, Chen Xiaoyan, Cai Yanping, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiro L, Souza-Machado A, Valderramas S, et al. The effect of levodopa on pulmonary function in Parkinson’s disease: a systematic review and meta-analysis. Clin Ther. 2012;2:1049–1055. doi: 10.1016/j.clinthera.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Prasad S, Holla VV, Neeraja K, et al. Parkinson disease and COVID-19: perceptions and implications in patients and caregivers. Mov Disord. 2020;35:912–914. doi: 10.1002/mds.28088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helmich RC, Bloem BRC The Impact of the COVID-19 pandemic on parkinson disease: hidden sorrows and emerging opportunities. J Parkinsons Dis. 2020;10:351–354. doi: 10.3233/JPD-202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zipprich HM, Teschner U, Witte OW, et al. Knowledge, attitudes, practices and burden during the COVID-19 pandemic in people with Parkinson’s disease in Germany. J Clin Med. 2020;9:1643. doi: 10.3390/jcm9061643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rejdak K, Grieb P. Adamantanes might be protective from COVID-19 in patients with neurological diseases: multiple sclerosis, parkinsonism and cognitive impairment. Mult Scler Relat Disord. 2020;42:102163. doi: 10.1016/j.msard.2020.102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nataf S. An alteration of the dopamine synthetic pathway is possibly involved in the pathophysiology of COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonini A, Leta V, Teo J, et al. Outcome of Parkinson’s disease patients affected by COVID-19. Mov Disord. 2020;35:905–908. doi: 10.1002/mds.28104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano A, Cereda E, Barichella M, et al. COVID-19 in Parkinson’s disease patients living in Lombardy, Italy. Mov Disord. 2020;35:1089–1093. doi: 10.1002/mds.28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hribar CA, Cobbold PH, Church FC. Potential role of vitamin D in the elderly to resist COVID-19 and to slow progression of Parkinson’s disease. Brain Sci. 2020;10:284. doi: 10.3390/brainsci10050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cilia R, Bonvegna S, Straccia G, et al. Effects of COVID-19 on Parkinson’s disease clinical features: a community-based case-control study. Mov Disord. 2020;35:1287–1292. doi: 10.1002/mds.28170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fasano A, Elia AE, Dallocchio C, et al. Predictors of COVID-19 outcome in Parkinson’s disease. Parkinsonism Relat Disord. 2020;78:134–137. doi: 10.1016/j.parkreldis.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministerio de Sanidad (2020) Situación de COVID-19 en España. https://cnecovid.isciii.es/covid19. Accessed July 31, 2020
- 15.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (2020) Coronavirus disease 2019 (COVID-19). Interim Case Definition, Approved April 5, 2020. https://www.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/casedefinition/2020. Accessed on August 31, 2020
- 17.Rada AG. Covid-19: the precarious position of Spain’s nursing homes. BMJ. 2020;369:m1554. doi: 10.1136/bmj.m1554. [DOI] [PubMed] [Google Scholar]
- 18.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosain R, Abdou Y, Singh A, et al. COVID-19 and cancer: a comprehensive review. Curr Oncol Rep. 2020;22:53. doi: 10.1007/s11912-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]