Coronavirus disease 2019 (COVID-19) is associated with marked albeit variable hemostasis disturbances and high thrombotic risk [1]. Measurement of plasma D-dimers levels has been put forward as a prognostic marker [2]. However, more comprehensive functional tests, such as thrombin generation and fibrinolysis assay, could also be of clinical relevance to estimate risks of poor outcome and to guide individual anticoagulation. To the best of our knowledge, few studies only describe the longitudinal follow-up of hemostasis parameters - a worrying gap in the close assessment of the course of the hemostasis disturbances during the acute phase of the disease.
The aim of this prospective study was therefore to describe the longitudinal changes in hemostasis parameters assessed daily in 21 COVID-19 patients during their intensive care unit (ICU) stay. Our main findings were that (i) daily standard measurements consistent with a prothrombotic state persisted over the first days and improved thereafter, but did not normalize in all patients; (ii) increased thrombin potential (hypercoagulability) and decreased fibrinolysis were frequent and (iii) a high inter-patient variability was observed.
The study was conducted at the CHU UCL Namur (Godinne site, Belgium) after approval from the local Ethics Committee (NUB: B0392020000031). All adult patients managed at the ICU for an RT-PCR-confirmed SARS-CoV-2 infection from March 27 to April 24, 2020 were considered for inclusion. One patient was not included due to refusal of advanced respiratory support or resuscitation.
Patients were managed according to the internal standard of care. Guidance from the French Interest Group in Perioperative Hemostasis (GIHP) was implemented for anticoagulation management on April 2, 2020 [3].
Blood was collected once a day at approximately 4 a.m. CRP levels were measured on a Vitros 5600 Integrated System (Ortho Clinical Diagnostics, Belgium) with CRP gold latex reagent (DiAgam, Ghislenghien, Belgium) and platelet count by impedance method on a Sysmex XN-20 analyzer with Cellpack reagent (Sysmex Corporation, Kobe, Japan). Hemostasis tests ordered as part of patient's care were performed with plasma prepared from citrated (109 mM; Vacuette, Greiner Bio One, Kremsmünster, Austria) blood centrifuged (1500g 15 min) within 1 h after collection. Remaining plasma underwent a second centrifugation (same conditions) and was stored at −80 °C within 4 h after the first centrifugation. Additional tests were performed using frozen-thawed (37 °C; 5 min) plasma samples. The following laboratory parameters were measured with a STA-R Max analyzer: prothrombin time (PT; STA-NeoPTimal, Diagnostica Stago, Asnières-sur-Seine, France), heparin anti-Xa activity (STA-Liquid anti-Xa, Stago), Clauss fibrinogen (STA-Liquid FIB, Stago), clotting factor VIII (STA-C.K. Prest and STA – Immunodef VIII, Stago), D-dimers (STA–LIATEST D-Di Plus, Stago), antithrombin (STA-Stachrom, Stago), and PAI-1 levels (Stachrom PAI-1, Stago). Thrombin generation was studied with the ST Genesia analyzer using the STG-ThromboScreen reagent (Stago). Heparin was fully neutralized with a hexadimethrine bromide solution (final concentration in the test: 25 μg/mL; polybrene, Sigma Aldrich, Saint-Louis, United States) before thrombin generation measurement. The endogenous thrombin potential (ETP) was normalized against a reference plasma provided with the STG-ThromboScreen kit (Stago). Fibrinolysis was investigated using the global fibrinolytic capacity test (Hyphen Biomed, Neuville-sur-Oise, France), a turbidity-based monitoring of clot dissolution accelerated by exogenous tPA. This test was performed with the Lysis Timer instrument (SD Innovation, Frouard, France): the clot lysis time (in minutes) corresponds to the point where the lysis velocity is the fastest; the longer the lysis time, the lower the fibrinolysis capacity [4].
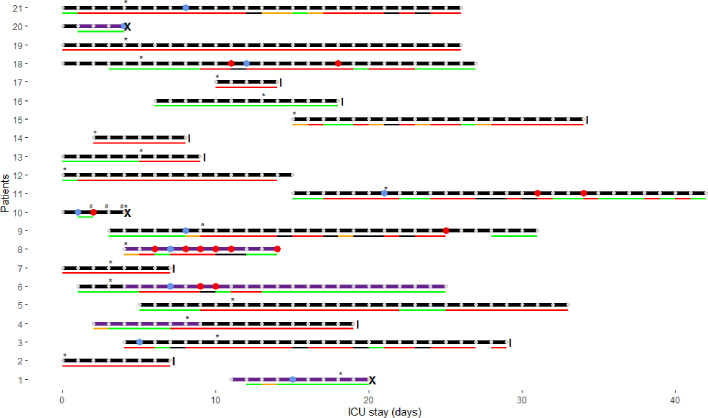
Clinical characteristics of the 21 study patients are presented in Fig. 1 . Median monitoring duration was 15 days (IQR: 7–26, min–max range: 4–28). Median age was 60 (IQR: 57–64, min–max range: 48–74) years. There were 18 males. Half of patients had a BMI ≥ 30 kg/m2 (IQR: 27–32, min–max range: 21–42). Median SOFA score at admission was 6 (IQR: 4–8, min–max range: 2–12). The patient who fulfilled ISTH criteria for overt DIC died, as two others did after confirmed thrombotic complications, which were considered as main contributors of death. Twelve patients presented at least one bleeding event during the study period (major according to the ISTH in six; no deaths).
Fig. 1.
Clinical characteristics of study patients. Horizontal lines with dots represent the follow-up period for each patient; those lines end with a vertical line when the patient was discharged from the ICU and with a cross when he died (from a suspected massive pulmonary embolism (PE), from an ischemic stroke and from bleeding and thrombotic complications in the setting of overt disseminated intravascular coagulopathy (DIC)). Blue dots indicate thrombosis diagnosis (five deep vein thrombosis (DVT), two PE – or thrombosis within pulmonary arteries, one DVT + PE, two ischemic strokes) and red dots represent major bleeding events (i.e. gluteal hematoma requiring two embolizations, intracranial bleeding secondary to thrombolysis, subacute cerebral hematoma, diffuse multiple bleeding requiring blood transfusions and two iatrogenic bleeds – due to an ECMO cannula and to a rectal cannula). Bottom lines represent the degree of anticoagulation actually achieved according to anti-Xa plasma levels measured daily (IU/mL - heparin anti-Xa activity; STA-Liquid anti-Xa; does not contain dextran): excessive in black (i.e. >0.7 for UFH or >1.4 for enoxaparin); increased doses in red (i.e. 0.3–0.7 for UFH or 0.5–1.4 for enoxaparin); prophylactic doses in green (i.e. 0.1–0.3 for UFH or 0.1–0.5 for enoxaparin); orange if unknown (LMWH without peak measurement). The implementation of GIHP guidance [3] is marked with an asterisk: patients deemed at high thrombotic risk were anticoagulated using intermediate doses of heparins (i.e. enoxaparin 1 mg/kg/day; or unfractionated heparin (UFH) for an anti-Xa target between 0.3 and 0.5 IU/mL), and patients at very high risk were anticoagulated with therapeutic doses (i.e. enoxaparin 1 mg/kg twice daily; or UFH for an anti-Xa target between 0.5 and 0.7 IU/mL). The LMWH enoxaparin was preferentially used; UFH was administered if renal failure, extracorporeal oxygen membrane oxygenation (ECMO), or high bleeding risk (e.g. after recent invasive procedure). Overt DIC was present in one patient, represented on the figure by a hash. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
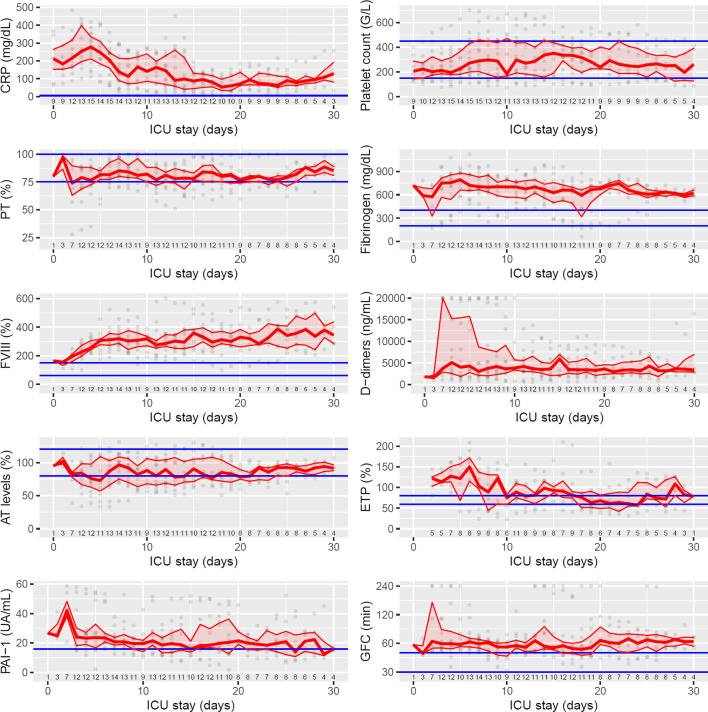
Changes in hemostasis parameters and C-reactive protein (CRP) monitored daily during ICU stay are represented in Fig. 2 ; in total there were 354 patients-days. Patients initially were in a high inflammatory state (median CRP levels of 204 mg/dL during the first ten days after ICU admission); CRP levels progressively decreased over time thereafter. Daily platelet counts were often normal and never below 70 × 109/L. Prothrombin time was only moderately (+3 to +6 s) and transiently increased in four patients, and markedly increased (+6 s) in the patient who fulfilled overt DIC criteria. Fibrinogen levels were markedly and persistently high (median value over the ICU stay: 665 mg/dL). The same held true for factor VIII levels (median value over the ICU stay: 304%).
Fig. 2.
Temporal changes of hemostasis parameters since intensive care unit (ICU) admission. Medians and interquartile ranges (IQR) are represented till day 30 (few patients had longer ICU stay during study period). Grey dots represent individual values and blue lines reference ranges (according to the manufacturer's, determined locally (for prothrombin time and global fibrinolytic capacity (GFC)) or according to Calzavarini et al. (for ETP) [8]). The population with laboratory testing at each day is shown under the individual plots. D0 is the day of admission to an ICU, but not necessarily in Namur (there were 11 transfers from a Belgian ICU to Namur ICU and one patient already admitted to Namur ICU before the start of the study). Of note there were less tests during the first few days (transfers), and less results as well beyond D20 (censoring or discharge). Results of GFC are represented with a logarithmic scale.
CRP, C-reactive protein; FVIII, coagulation factor VIII; ETP, ‘endogenous thrombin potential’ (area under the thrombin generation curve); AT, antithrombin; PAI-1, plasminogen activators inhibitor 1; GFC, ‘global fibrinolytic capacity’. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The median value of D-dimers levels was 3440 ng/mL, reaching very high levels in seven patients, above the upper limit of measurement (20,000 ng/mL), and tended to decrease over time. These findings are in line with previous reports [2]. In addition they show that the increase in D-Dimers levels is a sustained process despite heparin administration (even with intensified prophylactic regimens), clinical improvement and decrease in CRP levels.
Antithrombin deficiency (<80%) was detected in 13 patients, severe (<50%) in three, contributing to hypercoagulability. There was very little evidence for a consumptive process though since platelet counts were preserved, clotting times were only slightly prolonged, and fibrinogen was increased, not decreased.
One of the best approaches to evidence hypercoagulability in vitro is thrombin generation, which is now more accessible in the clinical environment thank to automated analyzers. Neutralization of heparin permitted the use of reagents more sensitive to coagulation abnormalities (i.e. STG-ThromboScreen) [5]. At variance with previous reports [[5], [6], [7]], we observed increased thrombin potential, median ETP values being above the published reference range (8) over the first week after ICU admission. Further work is required to understand the discrepant reports.
PAI-1 plasma levels were increased at some time-points at least since the start of the observation period of all patients, fitting with the reduced global fibrinolytic capacity we observed. Published data so far are consistent with defective fibrinolysis in COVID-19 patients (which is not unique to this infectious condition), using viscoelastometric assays modified with tPA addition [5,6,9]. It is intriguing that D-dimers plasma levels can be so high with defective fibrinolysis. As already hypothesized, this could be due to extravascular (e.g. pulmonary alveoli) fibrin deposits and tissue, not intravascular, fibrinolysis [10].
Importantly, laboratory markers showed complex temporal profiles during the ICU stay, which were quite variable among patients (Fig. 2 – see also companion paper with individual data). For the two functional integrative tests (ETP and GFC), median ranges between daily extreme values among patients were 96% and 94 min respectively; median ranges for intraindividual extreme values over the whole ICU observation period were 49% and 25 min, respectively. This is a hint for a varying thrombotic risk, and anticoagulation intensity could be tailored in a timely manner with frequent reassessments to minimize the bleeding risk.
This is the first study reporting the daily changes of relevant parameters of hemostasis of severe COVID-19 patients, including functional integrative tests for thrombin generation and fibrinolysis. It is limited by the small number of patients, but there was a close monitoring of each patient (354 patients-days). Day of ICU admission as the baseline for the longitudinal follow-up could not represent the same moment in individual disease courses. Moreover, effective degree of anticoagulation achieved was variable among patients and over time for a given individual. This could have influenced the changes in hemostasis parameters and complicates their interpretation, but this is inherent to studies performed in such settings. One challenging task is to determine whether increased intensities of anticoagulation actually improve relevant hemostasis biomarkers. Regarding endogenous anticoagulants, other components than AT should be taken into account as well. Finally, the absence of a control group precludes the identification of specificities by comparison to other severe infections.
In light of these results and of the current knowledge on hemostasis disturbances of COVID-19 patients, we suggest that a close monitoring of sensible set hemostatic parameters would be useful to assess individual thrombotic risk. We identified an increased thrombin potential and a decreased fibrinolytic capacity using newly available tests, which are suitable for clinical use and decision making in real time. Further prospective and preferably multicenter studies using standard operating protocols for the management of COVID-19 patients are required to validate the clinical usefulness of such a monitoring approach.
Acknowledgments
Acknowledgements
The authors would like to thank Professor Bernard Chatelain (Université catholique de Louvain) for providing very sound and helpful advice on the content of the manuscript. The authors would like also to thank Mrs Justine Baudar, Mrs Maité Guldenpfennig and Mr Philippe Devel for performing the experiments. Finally, we would like to thank Ms Norma Ceesay for carefully editing the manuscript.
Sources of funding
This study was supported by the Fonds National de la Recherche Scientifique (Belgium): ‘Anticoagulation fibrinolysis COVID19’ (reference: 40002796). The instrument and reagents for Global Fibrinolytic Capacity assay (Lysis Timer) were provided by Hyphen Biomed.
Declaration of competing interest
The authors have no conflict of interest related to this work.
References
- 1.Warkentin T.E., Kaatz S. COVID-19 versus HIT hypercoagulability. Thromb. Res. 2020;196:38–51. doi: 10.1016/j.thromres.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Sole F, Farcomeni A, Loffredo L, Carnevale R, Menichelli D, Vicario T, et al. Features of severe COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020: e13378. [DOI] [PMC free article] [PubMed]
- 3.Susen S., Tacquard C.A., Godon A., Mansour A., Garrigue D., Nguyen P. Prevention of thrombotic risk in hospitalized patients with COVID19 and hemostasis monitoring: proposals from the French Working Group on Perioperative Haemostasis (GIHP) the French Sdy Group on Thrombosis and Haemostasis (GFHT), in collaboration with the French Society for Anaesthesia and Intensive Care (SFAR) Crit. Care. 2020;24:364. [Google Scholar]
- 4.Amiral J., Laroche M., Seghatchian J. A new assay for global fibrinolysis capacity (GFC): investigating a critical system regulating hemostasis and thrombosis and other extravascular functions. Transfus. Apher. Sci. 2018;57(1):118–126. doi: 10.1016/j.transci.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Hardy M, Douxfils J, Bareille M, Lessire S, Gouin-Thibault I, Fontana P, et al. Studies on hemostasis in COVID-19 deserve careful reporting of the laboratory methods, their significance and their limitations. J Thromb Haemost. 2020. Accepted manuscript. Doi: 10.1111/jth.15061. [DOI] [PMC free article] [PubMed]
- 6.Nougier C., Benoit R., Simon M., Desmurs-Clavel H., Marcotte G., Argaud L. Hypofibrinolytic state and high thrombin generation may play a major role in sars-cov2 associated thrombosis. J. Thromb. Haemost. 2020;18:2215–2219. doi: 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White D., MacDonald S., Edwards T., Bridgeman C., Hayman M., Sharp M. Evaluation of COVID-19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays. Int. J. Lab. Hematol. 2020;00:1–8. doi: 10.1111/ijlh.13329. [DOI] [PubMed] [Google Scholar]
- 8.Calzavarini S., Brodard J., Quarroz C., Maire L., Nutzi R., Jankovic J. Thrombin generation measurement using the ST Genesia Thrombin Generation System in a cohort of healthy adults: normal values and variability. Res. Pract. Thromb. Haemost. 2019;3(4):758–768. doi: 10.1002/rth2.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss E., Roux O., Moyer J.D., Paugam-Burtz C., Boudaoud L., Ajzenberg N. Fibrinolysis resistance: a potential mechanism underlying COVID-19 coagulopathy. Thromb. Haemost. 2020;120(9):1343–1345. doi: 10.1055/s-0040-1713637. [DOI] [PubMed] [Google Scholar]
- 10.Hardy M., Lecompte T., Douxfils J., Lessire S., Dogne J.M., Chatelain B. Management of the thrombotic risk associated with COVID-19: guidance for the hemostasis laboratory. Thrombosis J. 2020;18:17. doi: 10.1186/s12959-020-00230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]