Abstract
Currently, the apparition of new SARS-CoV, known as SARS-CoV-2, affected more than 34 million people and causing high death rates worldwide. Recently, several studies reported SARS-CoV-2 ribonucleic acid (RNA) in hospital wastewater. SARS-CoV-2 can be transmitted between humans via respiratory droplets, close contact and fomites. Fecal-oral transmission is considered also as a potential route of transmission since several scientists confirmed the presence of SARS-CoV-2 RNA in feces of infected patients, therefore its transmission via feces in aquatic environment, particularly hospital wastewater. Hospitals are one of the important classes of polluting sectors around the world. It was identified that hospital wastewater contains hazardous elements and a wide variety of microbial pathogens and viruses. Therefore, this may potentially pose a significant risk of public health and environment infection.
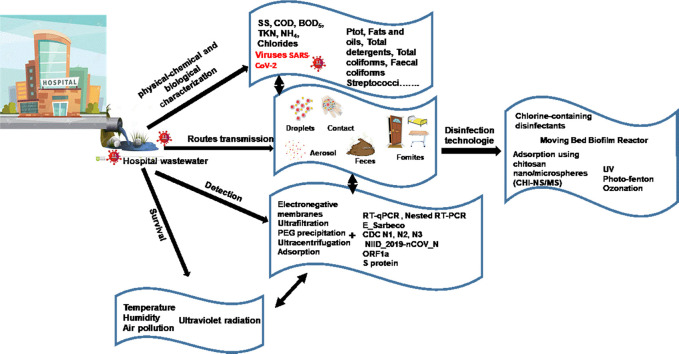
This study reported an introduction about the Physical-chemical and microbiological characterization of hospital wastewater, which can be a route to identify potential technology to reduce the impact of hospital contaminants before evacuation. The presence of SARS-CoV-2 in aqueous environment was reviewed. The knowledge of the detection and survival of SARS-CoV-2 in wastewater and hospital wastewater were described to understand the different routes of SARS-CoV-2 transmission, which is also useful to avoid the outbreak of CoV-19. In addition, disinfection technologies used commonly for deactivation of SARS-CoV-2 were highlighted. It was revealed that, chlorine-containing disinfectants are the most commonly used disinfectants in this field of research. Meanwhile, other efficient technologies must be developed and improved to avoid another wave of the pandemic of COVID-19 infections.
Keywords: SARS-CoV-2, Hospital wastewater, Transmission, Detection, Survival, Disinfection technologies
Graphical abstract
1. Introduction
Coronavirus disease 2019 (COVID-19), also known as SARS-CoV-2, appeared in 31 December 2019 in the city of Wuhan, Central China, and has spread quickly to 213 Countries around the world to date. The virus affected more than 38 million people (38.554.552) and caused more than 1 million deaths in the world (WHO, 2020a). SARS-CoV-2 is a large family of viruses that cause malady. Illness in humans is mostly respiratory, while symptoms can range from common cold to those of more severe lower respiratory infections (Channappanavar and Perlman, 2017). SARS-CoV-2 can also presents other serious manifestations such fatigue, fever, dry cough, myalgia and dyspnoea, with less common symptoms being nasal congestion, headache, runny nose, sore throat, vomiting and diarrhea (Li et al., 2020). For this reason, many scientists were focused to find a rapid and accurate detection of SARS-CoV-2 in environment and aqueous environment, notably hospital wastewater, in order to control his development and to determine the different ways of his transmission.
Hospital wastewater presents a wide range of hazardous pollutants, such as pharmaceutical residues, chemical substances, radioisotopes, and microbial pathogens and viruses as SARS-CoV-2 (Verlicchi et al., 2010a; Basturka et al., 2020; El-Ogri et al., 2016). These substances, which represent a chemical, biological, and physical risk for public and environmental health, in many country, they are discharge into the municipal collector or directly onto surface water without any treatment (Leprat, 1998).
SARS-CoV-2 can be transmitted between humans via respiratory droplets, close contact and fomites. Recently, many studies predicted another route of SARS-CoV-2 transmission; it is fecal-oral transmission (Y. Wu et al., 2020; Yang et al., 2020; Ling et al., 2020; J. Zhang et al., 2020; Y. Zhang et al., 2020; N. Zhang et al., 2020). According to Casanova et al. (2009); Hung (2003) and Leung et al. (2003), the fecal discharge of infected patients by SARS led to viability and persistence of virus in sewage. For this consideration, an increasing attention was reported in order to highlight the presence and persistence of SARS-CoV-2 in aquatic environment. Researches indicated that the presence of SARS-CoV-2 in wastewater was confirmed by several studies in multiple geographical regions (Tai et al., 2020; Green et al., 2020; Sharif et al., 2020). The survival of SARS-CoV-2 and its transfer from wastewater systems, especially infectious disease units such hospitals, require in-deep investigation. Many studies reported that the survival of SARS-CoV-2 could be influenced by different parameters such as temperature, pH, retention time, organic matter, light exposure and aerobic organisms, as well as by pathways used for quantification of RNA viruses (Wang et al., 2005a).
In point of fact, if the transmission of SARS-CoV-2 via the fecal-oral route in the aquatic environment, particularly in hospital wastewater, can survive for long time in sewage from hospitals, efficient disinfection strategies will be recommend, especially in areas with poor sanitation where diagnostic capacity might be limited, such as Africa (Lodder and de Roda Husman, 2020). Nevertheless, very frequently there are no legal requirements for hospital effluent treatment prior to its discharge into the municipal collector or directly onto surface water without treatment. Many treatment technologies of hospital wastewater were investigated by different studies such as ultraviolet irradiation, coagulation-filtration and biocidal agents as gaseous ozone, alcohol, formaldehyde, hydrogen peroxide, peroxyacetic acid, povidone iodine and chlorine-based disinfectants (Kühn et al., 2003; Yu et al., 2013; Chen et al., 2014; Fan et al., 2017; Christensen and Myrmel, 2018). Several other new advances such as supercritical water oxidation, reverse polymerization, plasma, constructed wetlands, and thermal gasification were also developed (Khan et al., 2020; Top et al., 2020). However, there are few numbers of studies investigated the disinfection technologies for deactivation of SARS-CoV-2 from hospital wastewater (D. Zhang et al., 2020a; D. Zhang et al., 2020b; Xing et al., 2020).
In this review, an introduction about the Physical-chemical and microbiological characterization of hospital wastewater was reported. The information on presence of SARS-CoV-2 in aqueous environment was addressed. The knowledge of the detection and survival of SARS-CoV-2 in wastewater and hospital wastewater were described to understand the different routes of SARS-CoV-2 transmission, which is also useful to avoid the outbreak of CoV-19. Furthermore, the inactivation of coronavirus in hospital wastewater using disinfectants and disinfection technologies are discussed. The efficiency, advantages and limitations of these disinfectants were highlighted.
2. Characterization of hospital wastewater
The wastewater amount produced by hospitals reached 200 to 1200 L/bed of hospitalized people/day (Verlicchi et al., 2010a). The increase of wastewater charge depends on various parameters as medical specialty, bed capacity, climate, geographical factors, cultural and nature of services offered by the healthcare institution (e.g., kitchen, bathroom and laundry).
The pollutant substances in hospital wastewater are divided into two main categories as macro-pollutants and micro-pollutants. As shown in Table 1 , micro-pollutants included absorbable organic halogens (AOX), analgesics, cytostatics, hormones, detergents, antiseptics, contrast substances, phenols, antibiotics such as paracetamol, ciprofloxacin and sulfamethoxazole, and heavy metals such as cadmium, chrome, iron, copper, lead, nickel, and zinc. While macro-pollutants substances are physic-chemical parameters as pH, chemical demand of oxygen (COD), biological demand of oxygen (BOD), total demand of oxygen (TOC), suspended solids (SS), ammonium ions and chloride, microbiological taminants as coliforms, bacteria (enterococcus, lococcus, shigella, salmonella) and viruses.
Table 1.
Macro and micro-pollutants of hospital wastewater and their origin.
| Pollutant substances | Parameter | Origin | Reference |
|---|---|---|---|
| Micro-pollutants | AOX | Sterilization of surgical tools, cleaning activities | Verlicchi et al., 2010a |
| Antibiotics | Humans excretion, disposal of unused compounds Chemotherapy drugs |
Diwan et al., 2009; Lien et al., 2016 | |
| Cytostatics | Humans excretion, some materials that humans deal with them on a daily basis (detergents, shampoos, lotions, cosmetics) | Pieczyńska et al., 2017 | |
| Hormones (Progesterone, Cortisol, Aldosterone, Testosterone, Estrogen) | Medical applications (radiology, oncology) | Guedes-Alonso et al., 2014 | |
| Contrast substances | Sterilization of surgical tools, drugs application, humans excretion | Roudbari and Rezakazemi, 2018 | |
| Phenols | Cleaning and building maintenance | Verlicchi et al., 2010a; Basturka et al., 2020 | |
| Detergents | Excreted by oncology patients | Basturka et al., 2020 | |
| Heavy metals Platinum |
Diagnostic agents and active ingredients of disinfectants and diuretics |
El-Ogri et al., 2016 | |
| Mercury | Iodinated contrast media (ICM), magnetic resonance imaging (MRI) | Kummerer et al., 1999; Lenz et al., 2007 | |
| Gadolinium, Silver, nickel, zinc, copper, lead, arsenic | Disinfectants used at diagnosis/examination units and at sterilization units |
Kummerer et al., 1998 Kummerer and Helmers, 2000; Basturka et al., 2020 |
|
| Macro-pollutants | Physic-chemical parameters (COD, BOD, TOC, SS, ammonium ions and chloride) | Diagnosis, equipment disinfection, laboratory activities, anesthetics and sterilization products, nutrient solutions used in microbiology laboratories | Chong and Jin, 2012, Casas et al., 2015, Kumari et al., 2020 |
| Microbiological taminants | Atmosphere, soil, medical devices and water employed in the hospital practice | Nuñez and Moretton, 2007; Kaur et al., 2020 | |
| Viruses | Human fecal matter from infected persons | Xu et al., 2005; Symonds et al., 2009; Prado et al., 2011; Radin, 2014; Gerba et al., 2017; Holshue et al., 2020; F. Wu et al., 2020 |
Based on the above, hospital wastewater is considered as a source of pathogenic microorganisms, toxic chemicals and radioactive elements. It constitutes a source of transmission of infections and epidemic diseases (Sanaa et al., 2019), which may present a potential danger to humans and their environment. Table 2 presented the analysis of the physical-chemical and microbiological characteristics of different hospitals in various countries. Management of this effluent varies from country to country. Generally, it was discharged in municipal sewage network without pre-treatment. Data indicated in Table 2 can be a route to identify potential technology to reduce the impact of hospital contaminants before evacuation.
Table 2.
Physical-chemical and microbiological characterization of hospital wastewater.
| Parameter | [a] | [b] | [c] | [d] | [e] | [f] | [g] | [h] |
|---|---|---|---|---|---|---|---|---|
| pH | 8.6 | 7.28 | 6.94 | 8.2 | 8.28 | 7.5–7.9 | 6.8 | 7.47 |
| Conductivity (mS cm−1) | n.d | n.d | 1468 | n.d | 230 | n.d | n.d | 814 |
| SS (mg L−1) | 138 | 571 | 11 | 236 | n.d | 259–520 | 900 | n.d |
| COD (mg L−1) | 365 | 810 | 710 | 2664 | 1594 | 450–654 | 150 | 441 |
| BOD5 mg L−1 | n.d | 450 | 227 | 1530 | 131 | 220–345 | 80 | 187 |
| TKN (mg L−1) | 94 | 124.1 | 40 | n.d | n.d | 81–120 | 27.6 | n.d |
| NH4+ (mg L−1) | 75 | n.d | n.d | 68 | 2.53 | 18–41 | 16.7 | n.d |
| Total phenols (mg L−1) | 8.4 | 2.26 | n.d | n.d | n.d | n.d | n.d | n.d |
| Ptot (mg L−1) | n.d | 15.1 | n.d | n.d | n.d | 14–19 | n.d | n.d |
| Chlorides (mg L−1) | n.d | n.d | n.d | 359 | n.d | n.d | 110 | 65 |
| AOX (mg L−1) | n.d | 11 | n.d | 1.24 | n.d | n.d | n.d | n.d |
| Fats and oils (mg L−1) | n.d | n.d | 3.5 | n.d | n.d | n.d | n.d | n.d |
| Total coliforms (MPN/100 mL) | 4.16 106 | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Ecotoxicity (TU) (μg/L) | 4.8 | n.d | 163 | n.d | n.d | n.d | n.d | n.d |
| Total copper (Cu) (μg/L) | n.d | n.d | 34 | 110 | n.d | n.d | <1.0 | n.d |
| Total mercury (Hg) (μg/L) | n.d | n.d | 2.8 | n.d | n.d | n.d | n.d | n.d |
| Total zinc (Zn) (μg/L) | n.d | n.d | 95 | 536 | n.d | n.d | 0.077 | n.d |
| Total silver (Ag) (μg/L) | n.d | n.d | 8.1 | n.d | n.d | n.d | n.d | n.d |
| Total chromium (Cr) (μg/L) | n.d | n.d | 121 | 4 | n.d | n.d | 0.042 | n.d |
[a] Munoz et al. (2016); [b] Top et al. (2020); [c] Basturka et al. (2020); [d] Emmanuel et al. (2005); [e] Sanaa et al. (2019); [f] Pirsaheb et al. (2015); [g] Meo et al. (2014); [h] Ouardaa et al. (2019).
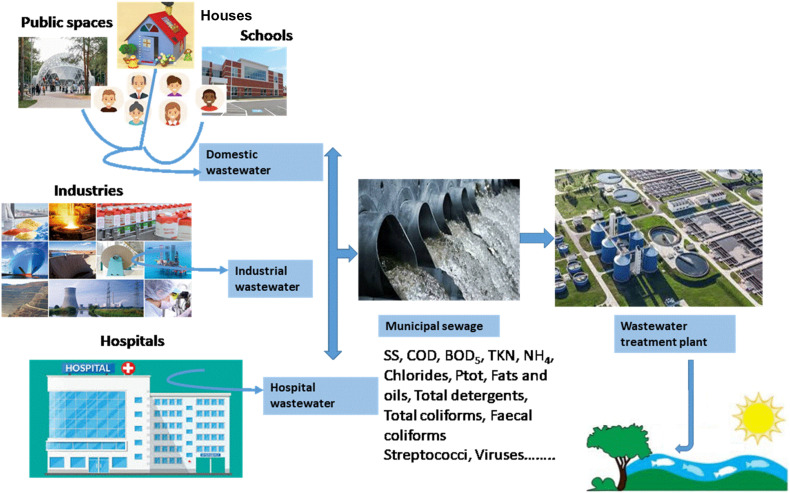
In many countries, the hospital wastewater and urban wastewater are similar in terms of pollutants, concentrations and loads, particularly when they are discharged in municipal sewage and then collected to a wastewater treatment plant (Fig. 1 ) (Leprat, 1998).
Fig. 1.
Collection of domestic, industrial and hospital effluents to wastewater treatment plant.
Moreover, according to several studies, the physical-chemical and microbiological characterization of hospital wastewater and urban wastewater showed that the charge pollutant in hospital effluent is in general higher than in urban wastewater (Tsakona et al., 2007; Mesdaghinia et al., 2009; Verlicchi et al., 2010b; Verlicchi et al., 2012). Referring to comprehensive review of the literature, Table 3 can compiled the main characterization of hospital wastewater and urban wastewater (Verlicchi et al., 2012). As showed, COD, BOD and SS in hospital wastewater are 2–3 times higher than urban wastewater. This observation indicates that the collection of hospital wastewater together with urban wastewater increases more pollutant concentration and a dedicated pre-treatment of hospital wastewater before discharge was recommended (Verlicchi et al., 2010a).
Table 3.
Range of variability of physical-chemical and microbiological characterization of hospital wastewater and urban wastewater.
| Parameter | Hospital wastewater | Urban wastewater |
|---|---|---|
| pH | 6.9–9.18 | 7.5–8.5 |
| Conductivity (mS cm−1) | 750–1000 | 420–1340 |
| SS (mg L−1) | 120–400 | 120–350 |
| COD (mg L−1) | 450–2300 | 500–600 |
| BOD5 mg L−1 | 150–603 | 100–400 |
| TKN (mg L−1) | 30–100 | 20–70 |
| NH4 (mg L−1) | 10–55 | 12–45 |
| Ptot (mg L−1) | 3–8 | 4–10 |
| Chlorides (mg L−1) | 80–400 | 30–100 |
| Fats and oils (mg L−1) | 13–60 | 50–150 |
| Total detergents (mg L−1) | 3–7.2 | 4–8 |
| Total coliforms (MPN/100 mL) | 106–109 | 107–108 |
| Fecal coliforms (MPN/100 mL) | 103–107 | 106–107 |
| E. coli (MPN/100 mL) | 103–106 | 106–107 |
| Streptococci (MPN/100 mL) | 103–105 | 103–105 |
3. Presence of viruses in aqueous environment: SARS-CoV-2
In addition to pathogenic microorganisms, radioactive elements and toxic chemicals, water media contains a wide variety of viruses. International Committee for the Taxonomy of Viruses (ICTV) in 2016 classified 7 orders, 104 families, 505 genera, and 3286 species of viruses including Ebola, Severe acute respiratory syndrome (SARS), Middle East Respiratory Syndrome (MERS), avian influenza (H5N1) as enveloped viruses, and other nonenveloped enteric viruses, such as adenoviruses, hepatitis A (HAV), polioviruses, enteroviruses, noroviruses and rotaviruses (Lago et al., 2003; Lefkowitz et al., 2018). These viruses are some of the principal human pathogens viruses transmissible via water media, notably hospital wastewater. As known, enteric viruses cause waterborne diseases such as diarrhea (Pietruchinski et al., 2006) and are associated with other disease outbreaks (Thongprachum et al., 2018). Enteric viruses may also cause nausea, vomiting, bronchiolitis and fever (Bishop and Kirkwood, 2008; Kocwa-Haluch, 2001).
SARS is a viral respiratory disease caused by a SARS-associated coronavirus (SARS-CoV). It was first identified at the end of February 2003 in China and spread to all world. SARS-CoV 2003 outbreak affected 8098 people and caused 774 deaths, and a case fatality rate of 9.7% (Woolhouse et al., 2015). It is considered as an airborne virus and can spread directly through small droplets of saliva in a similar way to the cold and influenza, and indirectly via surfaces that have been touched by infected individual (WHO, 2020b). SARS-CoV-2 virus is genetically similar to SARS-CoV that was ceased in June 2003 with intense public health mitigation measures. By contrast, the novel SARS-CoV-2 that emerged in December 2019, rapidly caused a global pandemic until now, affected more than 34 million people, and caused more than 1 million deaths in the world (WHO, 2020a).
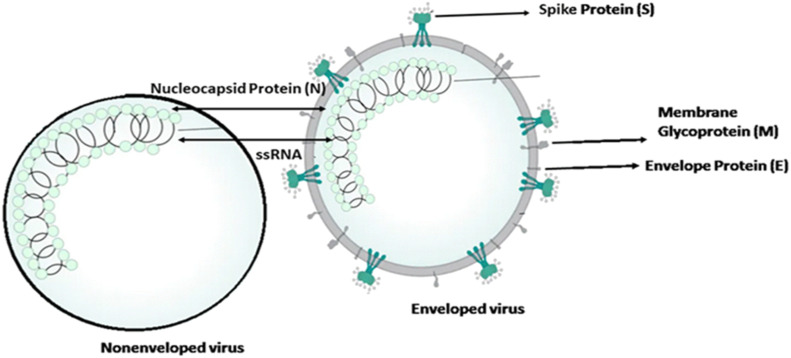
The genetic material of the SARS-CoV-2 is positive single-stranded RNA (ssRNA) and its virion has four main structural proteins including nucleocapsid (N) protein, transmembrane (M) protein, envelope (E) protein, and the spike (S) protein (Fig. 2 ).
Fig. 2.
Structure of enveloped and nonenveloped SARS-CoV-2.
The nucleocapsid named N protein is directly associated with nucleic acid material of the virus (RNA). While the N protein is involved in processes related to the viral genome, it plays a role in the viral replication cycle, and the cellular response of host cells to viral infections (Schoeman and Fielding, 2019, Tai et al., 2020). Another important part of SARS-CoV-2 is M protein, with play a principal role in determining the shape of the virus envelope. M protein may turn cellular membranes into factories where virus and host factors join to make new virus particles. This operation stabilizes N protein-RNA complex, inside the internal virion. Protein S is responsible for the attachment of the virus to host cells via the ACE2 receptor (angiotensin converting enzyme 2). This is mainly expressed in the respiratory and digestive tracts. The smallest protein in the SARS-CoV-2 structure is the envelope protein (E). This protein contributes in the production and maturation of the virus (Schoeman and Fielding, 2019).
SARS-CoV-2 as enveloped virus differ structurally from nonenveloped viruses due to the presence of different protein membranes (M, S, E) that enveloped the viral protein capsid, which contains proteins or glycoproteins. Structure and different functional on the outer surface of SARS-CoV-2 compared to nonenveloped viruses impact its detection, persistence and survival in aqueous environments (Arbely et al., 2006; Gundy et al., 2009; Shigematsu et al., 2014).
4. Modes transmission of SARS-CoV-2 and its survival against environmental factors
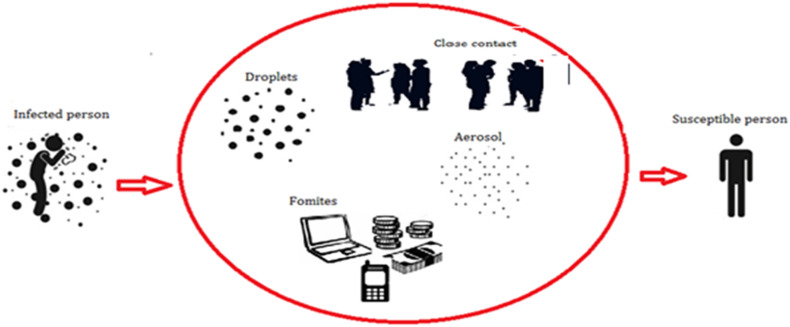
Based on recent and previous published studies, many pathways of SARS-CoV-2 transmission were discussed. As shown in Fig. 3 , SARS-CoV-2 can be transmitted between humans via respiratory droplets, when any individual is in close contact with an infected person, a high risk of contamination is possible mainly when this person coughs, sneezes, talks, or exhales (Morawska and Cao, 2020). Some droplets (0.5–20.0 μm) lingering in the air are more likely to be retained in the respiratory tract and produce the infection (McCluskey et al., 1996). Indeed, some other droplets are too heavy to remain in the air, and fall on floors; consequently, all surfaces become contaminated with SARS-CoV-2, touching by a susceptible person would be infected. The viability of SARS-CoV-2 in droplets form is more stable than aerosols on plastic and stainless steel, copper, cardboard, and glass with durations detected up to 72, 4, 24, and 84 h, respectively. For this reason, maintaining social distance and cleaning hands regularly are the main measure recommended by the World Health Organization (WHO, 2020a). Based on a study carried out by Morawska (2006), it was estimated that some droplets ejected from an infected individual, convert to aerosol particles (small aerodynamic diameters), and then become airborne. For that, the presence of droplets in the air for longer period is limited; evaporation process transforms droplets to bioaerosol residues, which could linger in the air for extended periods.
Fig. 3.
Transmission of SARS-CoV-2 via respiratory droplets and aerosol.
Airborne transmission route refers to the presence of particles with diameter < 5 μm, who can remain in the air for long periods and can be transmitted to others persons over the distances greater than 1 m (Morawska and Cao, 2020). The World Health Organization denounced, at early hours of manifestation of COVID-19, that airborne transmission did not play any role in disease transmission from 75,465 confirmed COVID-19 cases in China as of March 27, 2020 (WHO, 2020c). However, another recent study reported that SARS-CoV-2 could survive in the air for many hours, causing potential aerosolized transmission (Van Doremalen et al., 2020). Experiences carried out by Ong et al. (2020) corroborated that transmission by airborne is plausible. Their studies indicated that physicians, wearing the personal protective equipment (PPE), exiting the patient rooms were negative for SARS-CoV-2, although, samples from the air outlet exhaust fans in patient-rooms except corridors and anterooms have been reported as positive for SARS-CoV-2. Another study carried out in Singapore reported that COVID-19 was detected in swabs from air exhaust outlets in a hospital room of a symptomatic patient, suggesting the shifting of virus-laden aerosols by airflows and their depositing on equipment (Ong et al., 2020). Another case reported positive of COVID-19 in Inner Mongolia of China, when a person has passed the door of a symptomatic patient several times (Wang and Du, 2020). Read (2020) reported that there was cases of COVID-19 reported positive in cruise ship, when many of the infections occurred after the imposition of isolation that confined passengers for the majority of time to their cabins, and limited direct contact, and with hand hygiene carefully obeyed, giving evidence of the airborne transmission.
Based on the above studies, experiences, and understanding the basic science of viral infection spread, it is strongly plausible that SARS-CoV-2 virus spread through the air. If this is the case, according to Morawska and Cao (2020), it will take at least several months for this to be confirmed by science, especially it is difficult to directly detect the viruses traveling in the air. This is valuable time lost that could be used to properly control the epidemic and prevent more infections and loss of life.
Contact transmission route is caused by close contact, this type of transmission located when viral particles, emitted from infected person, are in contact with object, then another individual touches that object, then touches one of his senses (noise, mouth, eyes). The virus immediately enters to respiratory tract (Chin et al., 2020). Contact transmission also caused when people is in face-to-face contact with a confirmed or probable case for more than 15 min in total over the course of a week, or people shares an enclosed space with a confirmed or probable case for more than 2 h. During the spread of COVID-19, many reports confirmed that the primary route of transmission of SARS-CoV-2 is face-to-face contact transmission, can occur by direct and indirect contact via respiratory droplets such as talking, breathing, sneezing, coughing and close contact with infected people or with surfaces and fomites in the immediate environment or with objects used by the infected person (Morawska, 2006).
Another mode of transmission is fomite transmission (Fig. 3). The World Health Organization reported that droplets expelled by infected individuals could contaminate surfaces and objects, creating fomites (contaminated surfaces) (WHO, 2020c). On depend of ambient environment, SARS-CoV-2 can be found on those surfaces from hours to days. According to World Health Organization, there are not specific reports confirmed the fomite transmission, despite consistent evidence that SARS-CoV-2 survive on surfaces (WHO, 2020c).
Beyond these previous reports concerning the transmission of SARS-CoV-2 from person-to-person by droplets and airborne microorganisms in multiple geographical regions, many recent studies wondered how environmental conditions could influence the persistence of SARS-CoV-2 and remain infectious in the air and on surfaces. The most important environmental factors that could affect the viability of SARS-CoV-2 are temperature, retention time, humidity, PM (particle matter), radiation (light exposure) and open-air (ventilation) (Wang et al., 2005a). Furthermore, Zhu et al. (2020) found that air pollutions could be a risk factor for respiratory infection by carrying microorganisms and affecting body's immunity. Air pollution refers to the presence of gas and contaminating particles in the atmosphere. The gases include (CO), nitrogen oxides (NOx), ozone (O3), sulfur dioxides (SO2), ammonia (NH3), and volatile organic compounds (VOCs). While PM formed of mixture of compounds grouped into five major categories: sulphates, nitrates, elemental carbon, organic carbon, and crustal materials (such as earth and ash). PM, as defined by the Environmental Protection Agency (EPA), is classified as PM10 (diameter < 10 μm) and PM2.5 (diameter < 10 μm). In this trend, the long-term exposure to air pollution may represent a favorable context for the spread of the virus, while the combination of SARS-CoV-2 and particulate matter may produce a loaded airborne (Fattorini and Regoli, 2020; Conticini et al., 2020).
Based on various researchers, Table 4 summarized the correlation between environment factors and SARS-CoV-2 survival.
Table 4.
Influence of environment parameters in survival of SARS-CoV-2.
| Environment factor | Condition | Study area/period | Interpretation | References |
|---|---|---|---|---|
| Temperature | −33.8–26.9 °C | 122 cities (China) 23 January–29 February | Linear relationship with the number of COVID-19 cases in the range of <3 °C and became flat above 3 °C. No evidence supporting that case counts of SARS-CoV-2 could decline when T increase | Zhu et al., 2020 |
| Increasing ambient daily average temperature up to around 13 °C | 31 provinces of Mainland China 23 January–1 March |
Decrease in the rate of progression of COVID-19 with the arrival of spring and summer in the north hemisphere | Oliveiros et al., 2020 | |
| 24.6–28.6 °C | Jakarta Indonesia January–29 March |
Significant correlation with SARS-CoV-2 only at average temperature (°C). Insignificant correlation with minimum and maximum temperature. | Tosepu et al., 2020 | |
| Increase of temperature as spring and summer months | China (North Hemisphere) January 23–10 February |
Negative association of daily number of SARS CoV-2 cases | W. Luo et al., 2020 | |
| −10–10 °C | 31 provinces of mainland (China, Wuhan) January 20–29 February |
Strong association of decrease of SARS-CoV-2 incidence at lower and higher temperatures | Ma et al., 2020 | |
| High temperature | China cities January 19 to 23 U.S cities March 15–16 April |
Reduced Reproductive number (R values) of COVID-19 in China and USA at high temperature | J. Wang et al., 2020a | |
| 13–24 °C | Wuhan (China) October 1–15 December |
SARS-CoV-2 survival between 13 and 24 °C. 19 °C lasting about 60 days is conducive to the spread between the vector and humans | Bu et al., 2020 | |
| −10-30 °C | South Korea, Japan, Iran, Northern Italy, Northwestern United States, Spain, France | Cold temperature include stabilization of the droplet and enhanced propagation in nasal mucosa | Sajadi et al., 2020 | |
| 21 cities/provinces in Italy, 21 cities/provinces in Japan, and 51 other countries around the world January 20–11 March |
Temperature alone could not correlate with the SARS-CoV-2 case counts very well. Combination of several meteorological factors could describe the epidemic trend much better than single-factor models. | B. Chen et al., 2020 | ||
| Wuhan (China) 20 January–29 February |
Negative associations with daily death counts of COVID-19 patients | Ma et al., 2020 | ||
| Humidity | 75%–93% | Jakarta Indonesia January–29 March |
Insignificant correlation with SARS-CoV-2 and humidity | Tosepu et al., 2020 |
| Increase of humidity as spring and summer months | China (North Hemisphere) January 23–10 February |
Negative association of daily number of SARS-CoV-2 cases | W. Luo et al., 2020 | |
| 30–95% | South Korea, Japan, Iran, Northern Italy, Northwestern United States, Spain, France | Low humidity include stabilization of the droplet and enhanced propagation in nasal mucosa | Sajadi et al., 2020 | |
| 50%–80% | Wuhan (China) October 1–15 December |
75% humidity is conducive to the survival of the coronavirus | Bu et al., 2020 | |
| High humidity | China cities January 19 to 23 U.S cities March 15–16 April |
Reduced Reproductive number (R values) of COVID-19 in China and USA | J. Wang et al., 2020a | |
| Wuhan (China) 20 January–29 February |
Negative associations with daily death counts of COVID-19 patients | Ma et al., 2020 | ||
| Air pollution | PM2.5, PM10, CO, NO2, SO2 and O3 | Wuhan (China) 20 January–29 February |
Mortality counts of COVID-19 are negatively associated with PM2.5 and PM10 Mortality counts of COVID-19 are positively associated with SO2, No effect for CO, NO2 and O3 |
Ma et al., 2020 |
| PM2.5, PM10, CO, NO2, SO2 and O3 | 120 (China) January 23–29 February | Short-term exposure to air pollutants (PM2.5, PM10, CO, NO2, O3) is associated with increased risk of COVID-19 Infection Short-term exposure to a higher concentration of SO2 is associated to decreased risk of COVID-19 infection |
Zhu et al., 2020 | |
| PM2.5, PM10, CO, NO2, SO2 and O3 | Prefectures (China), provinces (Italy), Counties (U.S) | Positive significant correlations between COVID-19 infections and air quality variables in each country and concluded that higher mortality was also correlated with poor air quality, namely, with high PM2.5, CO, NO2 values | Pansini and Fornacca, 2020 | |
| PM2.5, PM10, NO2 and O3 | Italian provinces | Long-term exposure to air pollution may represent a favorable context for the spread of the virus |
Fattorini and Regoli, 2020 Conticini et al., 2020 |
|
| PM2.5 | United States Up 22 April |
Small increase in long-term exposure to PM2.5 leads to a large increase in the COVID-19 death rate | X. Wu et al., 2020 | |
| Ultraviolet radiation | UV-B radiation | China cities January 31–29February |
Higher transmission risks for COVID-19 | Liu et al., 2020 |
| UV radiation | 224 cities (China) Early January to early March for |
Spreadability of COVID-19 would not change with increasing UV exposure | Yao et al., 2020 | |
| UV radiation | 173 countries and five continents January 01–10 April |
Ultraviolet (UV) radiation has a statistically significant effect on daily COVID-19 growth rates | Carleton and Meng, 2020 | |
| UV radiation | Jakarta (Indonesia) March 2–10 April |
Sunlight exposure correlated significantly with recovery from Covid-19. However, sunlight exposure did not correlate significantly with the occurrence of and death from Covid-19. | Asyary and Veruswati, 2020 |
5. Fecal-transmission of SARS-CoV-2
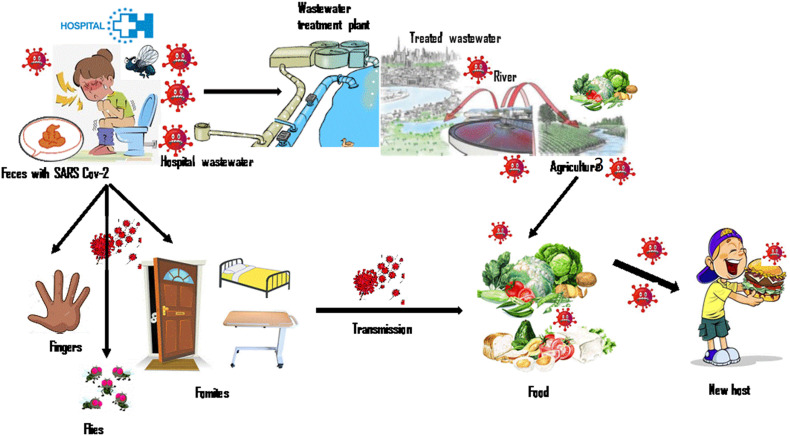
Based on previous studies (Xu et al., 2005; Radin, 2014; Graaf et al., 2017), the transmission of viruses from liquid effluent to humans can be carried out in the case of oral-fecal transmission by direct contact or indirect contact via contaminated fluids, including surface water, food, and carriers such as fomites. Feces are potentially an additional route of transmission. This observation suggests that this may also be a case for SARS-CoV-2 (Fig. 4 ).
Fig. 4.
Fecal transmission of SARS-CoV-2.
Currently, all reported data on COVID-19 mentioned that SARS-Cov-2 was detected in feces from infected patients. Holshue et al. (2020) reported that RT-PCR assay carried out for a patient in United States (Washington) revealed the presence of SARS-Cov-2 RNA in a stool specimen collected on day 7 of the patient's illness. Although, authors observed that the serum specimens from this patient were repeatedly negative for SARS-Cov-2. This observation is in agreement with that indicated by Y. Wu et al. (2020); Yang et al. (2020); Ling et al. (2020) and J. Zhang et al. (2020). Viral RNA can be persistently shed in feces for a maximum of 33 days after the patient has tested negative for respiratory viral RNA. Potential presence of SARS-Cov-2 in stool specimens has been also reported by Y. Zhang et al. (2020); Tang et al. (2020) and Y. Chen et al. (2020). Authors noted that fecal transmission is a new finding in the transmission routes of SARS-Cov-2. In this framework, Yeo et al. (2020) indicated that whether SARS-Cov-2 is confirmed to be fecally transmissible, health care and laboratory settings pose another potential target for preempting further spread of the disease. Regarding the mechanism transmission, the experiences carried out by limited studies try to explain the potential for fecal-oral SARS-CoV-2 transmission. Ong et al. (2020) collected samples from the bathroom of a patient infected by COVID-19. The samples from the surface of the toilet bowl, inside bowl of the sink and the door handle were positive results, while post-cleaning samples were negative. In the same context, the details given by Van Doremalen et al. (2020), reported that viable virus existed for at least 3 h in aerosols after their formation, and for up to 2 or 3 days on plastic and stainless steel surfaces. N. Zhang et al. (2020) observed that the median duration of virus emission was 10 days in swabs from respiratory tracts and up to 22 days in feces. Based on Ong et al. (2020); N. Zhang et al. (2020) and Van Doremalen et al. (2020) studies, the exposure to areas with poor sanitation (e.g. toilet public) may cause oral-fecal transmission where individuals touch their mouth, nose or eyes with contaminated hands. However, Xu et al. (2020) noted that the transmission mechanism of SARS-CoV-2 via fecal-oral route remains unclear, although viral shedding from the digestive system can last longer than shedding from the respiratory tract. In the same trend, Y. Wu et al. (2020) indicated that this route of transmission remains yet unquantified as pathway for increased exposure during the current outbreak. Tian et al. (2020) proclaimed that more clinical and experimental data about virus viability in feces and varying environmental conditions such as temperature and relative humidity are needed. Other studies also indicated that more works are needed to explore the potential for fecal-oral SARS-CoV-2 transmission (Nghiem et al., 2020; Collivignarelli et al., 2020).
Based on all studies cited previously, there is literature agreement about the detection of SARS-Cov-2 in feces of infected individuals, while the occurrence in the urine has not been always confirmed. W. Wang et al. (2020) also indicated that no study has shown the presence of SARS-CoV-2 in the urine. Ling et al. (2020) reported that the transmission by urine or blood might occur less frequently because of the low rate of positive findings in patients. This finding is in agreement with the series of clinical studies involving affected patients carried out by Lescure et al. (2020). They observed that among five patients admitted to French hospitals, the virus was detected in the feces of two patients, while no urines samples appeared positive.
Another approach of coronavirus transmission was determined by Mullis et al. (2013) and Yépiz-Gomez et al. (2013). They concluded that the involvement of fecally-contaminated food in coronavirus transmission cannot be ignored, given the survival of the human or animal coronavirus on vegetables (Fig. 4).
6. Detection and survival of SARS-CoV-2 in wastewater and hospital wastewater
As mentioned in Table 2, hospital wastewater contains a wide range of many macro and micro-pollutants including many bacteria and viruses as SARS-CoV-2, discharges from different units of hospital such as operation room, laboratories, laundry, kitchen, rooms and research lab. Currently, several research studied the presence and survival of SARS-CoV-2 in water, wastewater and river (Latorre et al., 2020; Rimoldi et al., 2020; Ahmed et al., 2020; Carducci et al., 2020; Casanova et al., 2009; Lodder and de Roda Husman, 2020; Naddeo and Liu, 2020). However, in spite of all the health risks associated with viruses, very few research oriented their investigations specifically to detection of SARS-CoV-2 in hospital wastewater, and thus, requires special attention due to the fact that, it is one of the main sources to spread the pathogenic and deadly viral diseases (D. Zhang et al., 2020a; J. Wang et al., 2020b). Indeed, this negligence likely due to direct discharge of hospital wastewater into municipal sewage network without analysis and characterization.
Several types of methods were developed for detecting SARS-CoV-2 in wastewater, notably hospital wastewater. The detection process will be precede by virus concentration step(s) such as ultrafiltration, PEG precipitation, and electronegative membrane adsorption, and follows by direct RNA extraction. According to numerous studies, detection of SARS-CoV-2 RNA primarily relies on RT-qPCR or (nested) RT-PCR (Ahmed et al., 2020; Medema et al., 2020; Nemudryi et al., 2020; F. Wu et al., 2020; Wurtzer et al., 2020; Randazzo et al., 2020; Lodder and de Roda Husman, 2020; La Rosa et al., 2020; Haramoto et al., 2020; J. Wang et al., 2020b; D. Zhang et al., 2020b). Table 5 summarized concentration methods and main RT-qPCR and nested RT-PCR assays available for SARS-CoV-2.
Table 5.
Detection of SARS-CoV-2 in wastewater and hospital wastewater.
| Wastewater sampling | Wastewater type | Study area | Virus concentration method | Detection method | Finding and interpretation | Reference |
|---|---|---|---|---|---|---|
| Untreated wastewater (nine samples) | Sewage, Wastewater treatment plant |
Netherlands (Hague, Utrecht, Apeldoorn, Amersfoort, Schiphol, Tilburg) | Ultrafiltration | RT-qPCR: E_Sarbeco CDC N1, N2, N3 |
Sensitivity of the primer/probe sets of N1 = N3 > N2 on SARS-CoV-2 RNA. Positive rate (58%). | Medema et al., 2020 |
| Untreated wastewater | Swage, Wastewater treatment plant |
Australia (Southeast Queensland) | Electronegative membranes Ultrafiltration |
RT-qPCR: N_Sarbeco NIID_2019-nCOV_N |
N_Sarbeco assay produced positive signals for two wastewater samples (12 copies/100 mL) with 22% positive rate, while the same samples were negative when tested using NIID_2019-nCOV_N. N_Sarbeco assay is more sensitive than the NIID_2019-nCOV_N assay. | Ahmed et al., 2020 |
| Untreated wastewater | Wastewater treatment plant | U.S.A (Bozeman, Montana) | Ultrafiltration | RT-qPCR: CDC N1, N2 |
Number of COVID-19 cases should have a linear relationship with viral RNA copies in wastewater. 98.5% SARS-CoV-2 genome sequence from the wastewater | Nemudryi et al., 2020 |
| Untreated wastewater | Raw and filtered swage | U.S.A (Massachusetts) | PEG precipitation | RT-qPCR: CDC N1, N2, N3 |
Presence of SARS-CoV-2 at high titers (>2 105 copies/L) in the period from March 18–25. Viral titers observed were significantly higher than expected based on clinically confirmed cases as of March 25. | F. Wu et al., 2020 |
| Wastewater | Untreated wastewater | France (Paris) | Ultracentrifugation | RT-qPCR: E_Sarbeco |
Increase of genome units in raw wastewater accurately followed the increase of human COVID-19 cases. SARS-CoV-2 concentration exceeds 3.16 106 and less 105 with 100% and 75% of positive rate for untreated and tread wastewater respectively. | Wurtzer et al., 2020 |
| Treated wastewater | France (Paris) | Ultracentrifugation | RT-qPCR: E_Sarbeco |
|||
| Wastewater | Influent, secondary and tertiary treated effluent | Spain (Murcia, Cartagena, Molina de Segura, Lorca, Cieza, Totana) | Aluminum hydroxide adsorption-precipitation | RT-qPCR: CDC N1, N2, N3 |
From 42 influent 35 are positive for SARS-CoV-2 RNA. From 18 effluent (secondary treated) 2 are positive for SARS-CoV-2 RNA. From 12 effluent (tertiary treated) 0 is positive for SARS-CoV-2 RNA. SARS-CoV-2 RNA positive wastewater samples were detected in three municipalities 12–16 days ahead of confirmed COVID-19 cases. |
Randazzo et al., 2020 |
| Wastewater | Untreated wastewater (Amsterdam Airport Schiphol) | Holland (Haarlemmermeer, Netherlands) | Not available | RT-qPCR: Not available |
Samples tested positive for virus RNA 4 days after the first cases of coronavirus disease 2019 (COVID-19) were identified in the Netherlands on Feb 27, 2020 | Lodder and de Roda Husman, 2020 |
| Wastewater | Swage Wastewater |
Italy (Milan, Rome) | PEG-dextran | Nested RT-PCR | SARS-CoV-2 RNA detected in 6/12 (50%) of wastewater samples. Design of a novel nested RT-PCR assay for SARS-CoV-2. |
La Rosa et al., 2020 |
| Wastewater River water |
Influent, secondary treated effluent | Japan (Yamanashi) | Electronegative membranes Adsorption |
RT-qPCR: E_Sarbeco, CDC N1, N2, NIID_2019-nCOV_N |
SARS-CoV-2 RNA was detected in one of five secondary-treated wastewater samples (2.4 × 103 copies/L) by N_Sarbeco qPCR assay following the Electronegative membranes method. | Haramoto et al., 2020 |
| Nested RT-PCR: ORF1a, S protein |
ARS-CoV-2 RNA was not detected in any of the five influent and three river water samples tested with N_Sarbeco, NIID_2019-nCOV_N, CDC-N1, and CDC-N2assays and two nested PCR (ORF1a and S protein) assays. | |||||
| Hospital wastewater (Hospital of Zhejiang University) | Sewage pools | China (Shanghai) | Not available | Not available | Three sewage samples from the inlets of sewage disinfection pool were positive for SARS-CoV-2 RNA (Cycle threshold value 29.37, 30.58, and 32.42). | J. Wang et al., 2020b |
| Hospital wastewater (Jinyintan Hospital, Huoshenshan Hospital, Wuchang Fangcang Hospital) | Wastewater (adjusting tank) | China (Wuhan) | PEG precipitation | RT-qPCR: CCDC-ORF1, CCDCN |
SARS-CoV-2 in Jinyintan Hospital was only detected in water from the adjusting tank (255 copies/L), but undetected in other tanks and effluents. Hospital wastewater in the adjusting tank of Huoshenshan Hospital contained 633 copies/L of SARS-CoV-2, |
D. Zhang et al., 2020b |
As reported in Table 5, most of the recent studies indicated that the detection of this virus in wastewater and hospital wastewater needs to be investigated in order to avoid the outbreak of CoV-19. In fact, various studies in France, Netherlands, Spain, Italy, USA and recently in Australia reported that SARS-CoV-2 RNA was successfully detected in wastewater and hospital wastewater using different types of virus concentration methods.
One of the first detections of SARS-CoV-2 in wastewater was reported by Medema et al. (2020) in Netherlands. This study indicated the presence of SARS-CoV-2 in wastewater samples of six cities and the airport using four qRT-PCR assays. In the same trend, Ahmed et al. (2020) confirmed the presence of the viral RNA of SARS-CoV-2 in the untreated wastewater in Australia using the same clinical specimen testing (qRT-PCR). Other study in Chennai (India) reported that the sewage samples collected from five sewage-pumping stations across the city were analyzed by qRT-PCR technique. The results indicated that COVID-19 viral RNA was detected in two samples and a correlation of confirmed cases was also established in the locations from where samples were lifted (Lakshmi, 2020). Study carried out by Wurtzer et al. (2020) in Paris (France) demonstrated the detection of viral genome before the exponential phase of the epidemic. A similar study conducted in Murcia (Spain) by Randazzo et al. (2020) indicated that SARS-CoV-2 could be detected weeks before the first confirmed case. La Rosa et al. (2020) revealed that 6 out of 12 influent sewage samples, collected between February and April 2020 from wastewater treatment plants in Milan and Rome (Italy) were positive. A published study by F. Wu et al. (2020) announced the presence of SARS-CoV-2 at high titers in the period from March 18–25 using RT-qPCR in tested wastewater collected at a major urban treatment facility in Massachusetts (United States).
On the other hand, Gormley et al. (2020) and Carducci et al. (2020) reported that aerosols generated from wastewater system are considered as a potential transmission route of COVID-19. According to Casanova et al. (2009), the aerolization of the virus can be formed particularly during the pumping of wastewater through sewerage systems and at the wastewater treatment works, and during its discharge and subsequent transport through the catchment drainage network. Gormley et al. (2020) noted that hospitals and health-care buildings could be as a high-risk transmission setting. In addition, Carducci et al. (2020) indicated that sewage generated from hospital, especially infectious disease units, might contain epidemic virus.
Beyond these previous reports concerning the detection of SARS-CoV-2 in wastewater and hospital wastewater in multiple geographical regions, more recent studies wondered how long this virus can survive and remain infectious one discharged into wastewater. A scientific literature search was thus carried out to highlight the current state of the art and knowledge gaps regarding survival of coronavirus in wastewater. The survival of coronaviruses in wastewater depends on a number of parameters such as temperature, pH, retention time, organic matter, light exposure and aerobic organisms (Wang et al., 2005a). In general, at higher temperature the survivability of enveloped RNA viruses decreases (Hasija et al., 2013). Otherwise, studies carried out by Wang et al. (2005a) revealed that at low temperatures (4 °C) the virus maintained its vitality up to 14 d in domestic and hospital sewage, while it was inactivated only after 2d at higher temperatures (20 °C). In the same trend, Gundy et al. (2009) observed an inactivation of virus (23 °C) after 2.36 days and 1.85 days in urban wastewater after primary and secondary treatment, respectively.
According to previous studies, the exposure to UV light can also decrease the activity of coronavirus, especially SARS-CoV, in aquatic environment (Darnell et al., 2004). It was demonstrated that UV-B (315–280 nm) and UV-C (190–290 nm) cause a significant and rapid decrease in infectious SARS-CoV (Pratelli, 2008). The effectiveness of UV light in the inactivation of SARS-CoV-2 is not yet explored to date. However, if this behavior occurs, it is evident to take into account the variation of season and geography in UV light availability (Grigalavicius et al., 2016).
On the other hand, the presence of suspended organic matter in wastewater can also affect coronavirus survive. As reported by Murray and Jackson (1992), suspended solids can provide shielding from light and affect settling behavior. Although, it may also influence the viral diffusion coefficient and potentially result in clusters of viruses. Evaluation of the presence of SARS-CoV-2 viral RNA in septic tanks of Wuchang Cabin Hospital by D. Zhang et al. (2020a) indicated a high level of (0.5–18.7) × 103 copies/L after disinfection with sodium hypochlorite. This result set evidence to release of virus embedded in patient's stool, protected by organic matters, in septic tanks.
The detection of coronavirus and then its survival in wastewater can be influenced by pathway used for quantification of RNA viruses. Literature reviews showed that several qRT-PCR assays have been designed for the detection of SARS-CoV-2, which are efficient for wastewater surveillance (Vogels et al., 2020; Corman et al., 2020; Chan et al., 2020). However, despite the fact that qPCR method is rapid, sensitive and accurate, it remains limited due to the presence of high load of organic contaminants in wastewater, especially in hospital wastewater, which often interfere with downstream virus detection.
Nonetheless, many other emerging technologies were tested for SARS-CoV-2 detection in wastewater samples, including digital PCR, isothermal amplification and biosensors (Farkas et al., 2020). However, these methods are more expensive and less sensitive (Ishii et al., 2014).
Knowledge of the presence of the coronavirus (and more specifically of SARS-CoV-2) in wastewater, along with its survival generates a great debate since the outbreak of COVID-19. Xiao et al. (2020a) and Sun et al. (2020) reported that the infectivity of SARS-CoV-2 in wastewater has not been assessed, even though culturable viral particles have been detected in the feces of infected individuals. Venugopal et al. (2020) indicated that the survival of the viruses decreased drastically when the parameters such temperature, UV-light and organic matter were unfavorable. In addition, the presence of solvents and detergents in wastewater can compromise the viral envelope (Gundy et al., 2009). However, according to World Health Organization (WHO, 2020c), there is no evidence on the persistence of SARS-CoV-2 virus in wastewater. Along these same lines, Naddeo and Liu (2020) showed that the persistence of SARS-CoV-2 in the environment could be short, although little is known concerning survival of this virus in wastewater.
Although the presence, survival of SARS-CoV-2 and its transfer from wastewater systems, especially infectious disease units such hospitals, accommodate many sources of uncertainty, it is recommended to be treated and disinfected in order to limit its outbreak in the population, and its effect on public health.
7. Disinfection technology for inactivation of coronavirus from Hospital wastewater: SARS-CoV-2
In many countries such as Australia, Iran, Egypt, India, Japan, South Africa, and Thailand, hospital wastewater is usually discharged in the municipal sewage, where they combined with other charged effluent before being collected to a wastewater treatment plant. However, in many other countries such as Algeria, Bangladesh, Congo, Ethiopia, India, Nepal, Pakistan, Taiwan, and Vietnam, hospital wastewater is discharged into drainage systems, rivers without any pre-treatment (Al Aukidy et al., 2018). This practice have an adverse impact on environment and human health through spread of pathogenic microorganisms, viruses, antibiotic resistant bacteria, pharmaceuticals, and chemical pollutants. Several technologies were adopted acting as primary, secondary, and tertiary steps, the most widely applied technologies for hospital wastewater disinfection are activated sludge, membrane bioreactor, ozonation, coagulation-flocculation and Fenton-oxidation studies (Kühn et al., 2003; Yu et al., 2013; Chen et al., 2014; Fan et al., 2017; Christensen and Myrmel, 2018). Recently, other promising technologies were developed such as constructed wetland (Khan et al., 2020) and supercritical water oxidation (Top et al., 2020).
Methodical researches regarding the disinfection of SARS-CoV in wastewater and hospital wastewaters, particularly distinct disinfection propositions throughout the coronavirus pandemic, were targeted by various studies (Kühn et al., 2003; Yu et al., 2013; Chen et al., 2014; Fan et al., 2017; Christensen and Myrmel, 2018). The complete deactivation of SARS-CoV can be achieved by combination of three steps of water processing.
The first stage, known as primary treatment, concerning the remove of material that will either float or readily settle out by gravity by sedimentation or flotation processes. The second stage, known as secondary treatment, concerning remove of the soluble organic matter that escapes primary treatment and the suspended solids, which protect virus from disinfection, by free chlorine. Removal is usually accomplished by biological processes (trickling filter, activated sludge process, and oxidation pond) in which microbes consume the organic impurities as food, converting them into carbon dioxide, water, and energy for their own growth and reproduction. This stage is generally considered a practical and available hospital wastewater treatment technology that is particularly well suited to destroying pathogens, as relatively long retention times, combined with sunlight, elevated pH levels, biological activity and other factors serve to accelerate pathogen destruction (WHO, 2020d). The third stage, as known tertiary treatment, aimed to improve the quality of water. Many technologies can be included in this stage for treatment of hospital wastewater such as activated carbon (granular and powdered activated carbon), membrane technique (microfiltration, ultrafiltration, and reverse osmosis), coagulation-floculation (zirconium, chitosan and polyaluminium chloride) and oxidation (UV, UV/H2O2, photo-fenton, ozonation).
On the other hand, Chen et al. (2014) reported that the efficient technologies of deactivation of viruses as SARS-CoV from hospital wastewater are Chlorine (Cl2), Sodium hypochlorite (NaOCl), Chlorine dioxide (ClO2), Ozone (O3) and UV irradiation. Every disinfection technology presents advantages and disadvantages; selection of efficient methods depends on different parameters such as operation cost, disinfectants toxicity, safety conditions, energy consumption, nature and amount of wastewater, operating parameters, and reliability (Chen et al., 2014) (Table 6 ).
Table 6.
Comparison of disinfection technologies in hospital wastewater (Chen et al., 2014).
| Parameter | Cl2 | NaOCl | ClO2 | O3 | UV |
|---|---|---|---|---|---|
| Concentration (g/L) | 10 | 10–15 | 2–5 | 10 | 300 g/m2 |
| Time (min) | 10–30 | 10–30 | 10–20 | 5–10 | 10s |
| Reliability | Good | Better | Good | Good | Better |
| Effects to endospore | Bad | Bad | Good | Better | Better |
| Secondary pollution | Yes | Yes | Yes | No | No |
| Toxicity of disinfection | More | More | More | Less | No |
| Energy consumption | Lower | Low | Lower | High | Lower |
| Influence of pH to disinfection | More | More | More | Little | No |
| Application | Water supply plant and sewage treatment Traditional method |
Seldom used | Medium scale projects, especially in hospital wastewater treatment | Advanced waste treatment in water supply plant and industrial water consumption | Large-scale municipal water supply and wastewater treatment in food industry |
Inactivation of SARS-CoV in hospital wastewater can be completely after 30 min of disinfection with more than 10 mg L−1 chlorine (0.4 mg L−1 of free residual chlorine). However, chlorine dioxide was less effective for the inactivation of SARS-CoV than chlorine. 40 mg L−1 of chlorine dioxide (2.19 mg L−1 of free residual chlorine) can inactive completely SARS-CoV about 30 min (Wang et al., 2005a).
Removal of another kind of Human coronavirus (HCoV) NL63 and (HCoV) OC43 by adsorption using modified chitosan nano/microspheres N-(2-hydroxypropyl)-3-trimethyl chitosan (HTCC-NS/MS) was attempted. HTCC-NS/MS adsorb strongly (HCoV) NL63, but ineffective in removing (HCoV) OC43 coronavirus. The result of this study also showed an increase of HCoV-NL63 adsorption effect with the increase of degree of cationization of modified chitosan (Ciejka et al., 2017).
In case of COVID-19, few numbers of studies were developed the disinfection of wastewater and hospital wastewater spiked with SARS-CoV-2 (Table 7 ). This scarcity of information can be caused by the lack of environmental research on COVID-19, insufficient of laboratory equipment and/or the risk to workers handling wastewater. The review reported by J. Wang et al. (2020c) represented the disinfection technologies commonly used for hospital wastewater such as ultraviolet light (UV), ozone, Chlorine-containing disinfectants, and provided just suggestions for hospital wastewater disinfection during COVID-19 pandemic in China. To determine the sustainable technology to inactive SARS-Cov-2, J. Wang et al. (2020c) indicated that the disinfection technologies adopted during SARS epidemic could be used as good reference to inactivation of SARS-Cov-2 in hospital wastewater, due to the similarities between SARS-CoV-1 and SARS-CoV-2. The disinfection with free chlorine for effective centralized disinfection was also suggested by World Health Organization (WHO, 2020d) under optimal conditions (pH < 8.0, contact time least 30 min and concentration dosage ≥0.5 mg L−1).
Table 7.
Disinfection of SARS-CoV by different technologies.
| Virus removed | Wastewater sampling | Disinfection technology | Detection method | Inactivation rate (%) | Result and interpretation | Reference |
|---|---|---|---|---|---|---|
| SARS-CoV | Hospital wastewater (Xiao Tang Shan Hospital) | Chlorine Chlorine dioxide |
RT-qPCR | 100 | 10 mg L−1 of chlorine resulted in 100% inactivation of SARS-CoV. 40 mg L−1 of chlorine resulted in 100% inactivation of SARS-CoV. Chlorine dioxide was less effective for the inactivation of SARS-CoV than chlorine. |
Wang et al., 2005b |
| Human coronavirus NL63 (HCoV-NL63), human coronavirus OC43 (HCoV-OC43) | Aqueous virus suspensions | Adsorption using chitosan nano/microspheres (CHI-NS/MS) | RT-qPCR | 92 | 5 mg of modified CHI-NS/MS was mixed with the virus suspension and incubated at 23 °C for 30 min with mixing. RT-qPCR analysis revealed the decrease of virions (92%). No significant adsorption was observed in the case of human coronavirus OC43 (HCoV-OC43). |
Ciejka et al., 2017 |
| SARS-CoV-2 | Hospital wastewater (Wuchang Cabin Hospital) | Sodium hypochlorite | RT-qPCR | Not available | Absence of SARS-CoV-2 viral RNA in the effluent of septic tanks after disinfection with 800 g/m3of sodium hypochlorite. Presence of SARS-CoV-2 viral RNA in the influent of septic tanks with the increase of sodium hypochlorite (6700 g/m3), suggested that SARS-CoV-2 might be embedded in patient's stools, protected by organic matters from disinfection, and slowly release when free chlorine declines. |
D. Zhang et al., 2020a |
| SARS-CoV-2 | Hospital wastewater (Jinyintan Hospital, Huoshenshan Hospital, Wuchang Fangcang Hospital) | Adjusting tank, Moving Bed Biofilm Reactor (MBBR)-sedimentation-disinfection (sodium hypochlorite) | RT-qPCR | Not available | 255 copies/L of SARS-CoV-2 viral RNA were detected only in adjusting tank of Jinyintan Hospital. SARS-CoV-2 viral RNA was found in first adjusting tank (633 copies/L), MBBR (not detected-505 copies/L), and sedimentation tank (not detected-2208 copies/L). SARS-CoV-2 RNA was detected in wastewater from the septic tanks disinfected by 800 mg L−1 of sodium hypochlorite, ranging from 557 to 18,744 copies/L, and it declined to non-detected after the dosage of sodium hypochlorite increased to 6700 mg L−1. |
D. Zhang et al., 2020a |
According to previous study (J. Wang et al., 2020c; How et al., 2017), chlorine-based disinfectants are widely used for their broad sterilization spectrum, high inactivation efficiency and easy decomposition with little residue, as well as represents the best economic solution. However, excess use of chlorine-based disinfectants can generate more than 600 kinds of disinfection by-products, which are harmful to ecosystems and human health (Table 6) (Richardson, 2011; Wang et al., 2014; Y. Luo et al., 2020). On the other hand, chlorine reacts with ammonia contains in wastewater and forms a new product (chloramine), which behaves differently to free chlorine during disinfection (Wang et al., 2005b). As part of an emergency treatment of hospital wastewater containing SARS-CoV-2, China has launched a guideline requiring free chlorine ≥6.5 mg L−1 at 1.5 h of contact time and without condition of pH.
D. Zhang et al. (2020a) studied the presence of SARS-CoV-2 viral RNA in septic tanks of Wuchang Fangcang Hospital (Wuhan, China) in order to evaluate the disinfection performance and optimize disinfection strategies to prevent SARS-CoV-2 from spreading through drainage pipelines. They concluded that despite of addition of 800 g/m3 of sodium hypochlorite as indicated by the guideline for emergency treatment of medical sewage containing SARS-CoV-2 suggested by China CDC, SARS-CoV-2 viral RNA was present in the effluents at 12-h after sodium hypochlorite addition when free chlorine declined to no detectable. Xing et al. (2020) explained this behavior by the fact that SARS-CoV-2 embedded in feces particles, which are rich in organic compounds, could escape from disinfection and slowly release into aqueous phase, as well as in drainage pipelines and then in septic tanks. Therefore, disinfection technology recommended by WHO and China CDC cannot ensure a complete deactivation of SARS-CoV-2 in hospital wastewater.
The study of detection and disinfection of SARS-CoV-2 from three hospitals (Jinyintan Hospital, Huoshenshan Hospital, Wuchang Fangcang Hospital) located in China (Wuhan) was investigated by D. Zhang et al. (2020a). The treatment technologies applied in Jinyintan Hospital are an adjusting tank, an aeration tank for biodegradation, a secondary sedimentation tank and a disinfection tank. Only in adjusting tank, 255 copies/L of SARS-CoV-2 viral RNA were detected, while the virus was undetected in other tanks and effluents. The wastewater of Huoshenshan Hospital comprised a series of treatment steps including adjusting tank-septic tank adjusting tank-moving bed biofilm reactor (MBBR)-sedimentation-disinfection, in which SARS-CoV-2 viral RNA was found in first adjusting tank (633 copies/L), MBBR (not detected-505 copies/L), and sedimentation tank (not detected-2208 copies/L). In the third hospital treatment unit (Wuchang Fangcang Hospital), there was two disinfection units (preliminary disinfection tank followed by septic tank). SARS-CoV-2 RNA was detected in wastewater from the septic tanks disinfected by 800 mg L−1 of sodium hypochlorite, ranging from 557 to 18,744 copies/L, and it declined to non-detected after the dosage of sodium hypochlorite increased to 6700 mg L−1.
Recently other disinfection technologies such as radiation, reverse polymerization, plasma, constructed wetlands, and thermal gasification were also tested for hospital wastewater. They approve certain promotion value, but due to the high investment costs and the efficient equipment, these technologies have not been applicable at a large scale.
8. Conclusion
This review highlighted the detection, survival and route transmission of SARS-CoV-2 in hospital wastewater during outbreak of CoV- 19. The disinfection technologies of this virus were also discussed, with special emphasis on efficiency of disinfection technologies used commonly for deactivation of SARS-CoV-2 from hospital wastewater.
Physical-chemical and biological characterization of hospital wastewater presents a wide range of hazardous pollutants, such as pharmaceutical residues, chemical substances, radioisotopes, and microbial pathogens and viruses as SARS-CoV-2. The discharge of this effluent into the municipal collector or directly onto surface water without any treatment represents a chemical, biological, and physical risk for public and environmental health, and allows a rapid outbreak of CoV-19.
There are many pathways of SARS-CoV-2 transmission; it can be transmitted between humans via respiratory droplets, aerosol, close contact and fomites. Fecal-oral transmission is considered also as a potential route of transmission since several scientists confirmed the presence of SARS-CoV-2 RNA in feces of infected patients. The survival of SARS-CoV-2 could be influenced by different parameters such as temperature, pH, retention time, organic matter, light exposure and aerobic organisms, as well as by pathways used for quantification of RNA viruses.
Many techniques were developed to removal of organic pollutants and microbial pathogens and viruses from hospital wastewater. According to few studies investigating the deactivation of SARS-Co V-2 showed that chlorine-based disinfectants are widely used for their broad sterilization spectrum, high inactivation efficiency and easy decomposition with little residue, as well as represents the best economic solution. The complete deactivation of SARS-CoV-2 can be achieved by combination of other technologies (biological and/or physical-chemical processes).
In the meantime, we should to develop more secure, efficient, economical disinfection technologies in order to limit the transmission of COVID-19 and to avoid other waves of the pandemic of COVID-19 infections.
Funding
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
CRediT authorship contribution statement
Mounia ACHAK, Younes CHHITI, Fatima Ezzahrae M'hamdi Alaoui and Noureddine Barka analyzed, interpreted the research data and contributed to writing the manuscript.
Soufiane Alaoui Bakri and Wafaa Boumya analyzed literature data and prepared manuscript.
All authors reads and approved final paper.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
None.
Editor: Paola Verlicchi
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., Brien J.W.O.…Mueller J.F. First confirmed detection of SARSCoV- 2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Science of the Total Environment. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Aukidy M., Al Chalabi S., Verlicchi P. Hospital wastewater treatments adopted in Asia, Africa, and Australia. Hospital Wastewaters. 2018;60:171–188. [Google Scholar]
- Arbely E., Granot Z., Kass I., Orly J., Arkin I.T.A. Trimerizing GxxxG motif is uniquely inserted in the severe acute respiratory syndrome (SARS) coronavirus spike protein transmembrane domain. Biochemistry. 2006;45:11349–11356. doi: 10.1021/bi060953v. [DOI] [PubMed] [Google Scholar]
- Asyary A., Veruswati M. Sunlight exposure increased Covid-19 recovery rate: a study in the central pandemic area of Indonesia. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.139016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basturka I., Varankb G., Murat-Hocaoglua S., Yazici-Guvenc S., Oktem-Olguna E.E., Canli O. Characterization and treatment of medical laboratory wastewater by ozonation: optimization of toxicity removal by central composite design. The Journal of the International Ozone Association: Ozone: Science & Engineering. 2020 doi: 10.1080/01919512.2020.1794794. [DOI] [Google Scholar]
- Bishop R.F., Kirkwood C.D. Enteric viruses, encyclopedia of virology. Elsevier Ltd. 2008:116–123. [Google Scholar]
- Bu J., Peng D.D., Xiao H., Yue Q., Han Y., Lin Y., Hu G., Chen J. 2020. Analysis of Meteorological Conditions and Prediction of Epidemic Trend of 2019-nCoV Infection in 2020. medRxiv. [Google Scholar]
- Carducci A., Federigi I., Liu D., Thompson J.R., Verani M. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton T., Meng K.C. 2020. Causal Empirical Estimates Suggest COVID-19 Transmission Rates Are Highly Seasonal. medRxiv. [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M.E., Chhetri R.K., Ooi G., Hansen K.M.S., Litty K., Christensson M.…Bester K. Biodegradation of pharmaceuticals in hospital wastewater by staged Moving Bed Biofilm Reactors (MBBR) Water Res. 2015;83:293–302. doi: 10.1016/j.watres.2015.06.042. [DOI] [PubMed] [Google Scholar]
- Chan J.F.W., Yip C.C.Y., To K.K.W., Tang T.H.C., Wong S.C.Y., Leung K.H., Tsoi Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020 doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Zhou H., Yu B., Huang Z.W. Comparison study on hospital wastewater disinfection technology. Adv. Mater. Res. 2014;884-885:41–45. [Google Scholar]
- Chen B., Liang H., Yuan X., Hu Y., Xu M., Zhao Y., et al. 2020. Roles of Meteorological Conditions in COVID-19 Transmission on a Worldwide Scale. medRxiv. [Google Scholar]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L.…Cheng Z. The presence of SARS-CoV-2 RNA in the feces of COVID-19patients. J Med Virol. 2020;92:833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Chin A., Chu J., Perera M., Hui K., Yen H.L., Chan M., Peiris M., Poon L. 2020. Stability of SARS-CoV-2 in Different Environmental Conditions. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong M.N., Jin B. Photocatalytic treatment of high concentration carbamazepine in synthetic hospital wastewater. J. Hazard. Mater. 2012;199– 200:135–142. doi: 10.1016/j.jhazmat.2011.10.067. [DOI] [PubMed] [Google Scholar]
- Christensen E., Myrmel M. Coagulant residues influence on virus enumeration as shown in a study on virus removal using aluminium, zirconium and chitosan. J. Water Health. 2018;6:600–613. doi: 10.2166/wh.2018.028. [DOI] [PubMed] [Google Scholar]
- Ciejka J., Wolski K., Nowakowska M., Pyrc K., Szczubiałka K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater. Sci. Eng C. 2017;76:735–742. doi: 10.1016/j.msec.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collivignarelli M.C., Collivignarelli C., Miino M.C., Abbagrave A., Pedrazzani R., Bertanz G. 2020. SARS-CoV-2 in Wastewater Treatment Plants. medRxiv. [Google Scholar]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W.…Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT–PCR. Euro Surveill. 2020;25:1–8. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M.E.R., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan V., Tamhankar A.J., Aggarwal M., Sen Shanta, Khanda R.K., Lundborg C.S. Detection of antibiotics in hospital effluents in India. urrent Science. 2009;97:12–25. [Google Scholar]
- El-Ogri F., Ouazzani N., Boraâm F., Mandi L. A survey of wastewaters generated by a hospital in Marrakech city and their characterization. Desalin. Water Treat. 2016:1–14. [Google Scholar]
- Emmanuel E., Perrodina Y., Keckc G., Blanchardb J.M., Vermandeb P. Ecotoxicological risk assessment of hospital wastewater: a proposed framework for raw effluents discharging into urban sewer network. Journal of Hazardous Materials A. 2005;117:1–11. doi: 10.1016/j.jhazmat.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Fan D., Tian Y., Han L., Liu Z., Teng Y., Li B. Catalytic electrolysis of sodium chlorite to prepare highly pure chlorine dioxide. J. Funct. Mater. 2017;48:9150–9156. [Google Scholar]
- Farkas K., Mannion F., Hillary L.S., Malham S.K., Walke D.I. Emerging technologies for the rapid detection of enteric viruses in the aquatic environment. Curr Opin Environ Sci Heal. 2020;16:1–6. [Google Scholar]
- Fattorini D., Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ. Pollut. 2020;264 doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C.P., Betancourt W.Q., Kitajima M. How much reduction of virus is needed for recycled water: a continuous changing need for assessment? Water Res. 2017;108:25–31. doi: 10.1016/j.watres.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A. COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob. Health. 2020;8:e643. doi: 10.1016/S2214-109X(20)30112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graaf M.D., Beck R., Caccio S.M., Duim B., Fraaij P.L., Guyader F.S.L.…Schultsz C. Sustained fecal-oral human-to-human transmission following a zoonotic event. Current Opinion in Virology. 2017;22:1–6. doi: 10.1016/j.coviro.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Wilder M., Collins M., Fenty A., Gentile K., Brittany L.…Larsen D.A. 2020. Quantification of SARS-CoV-2 and cross-assembly phage (crAssphage) From wastewater to monitor coronavirus transmission Within communities. MedRxiv. [Google Scholar]
- Grigalavicius M., Moan J., Dahlback A., Juzeniene A. Daily, seasonal, and latitudinal variations in solar ultraviolet A and B radiation in relation to vitamin D production and risk for skin cancer. Int. J. Dermatol. 2016;55:e23–e28. doi: 10.1111/ijd.13065. [DOI] [PubMed] [Google Scholar]
- Guedes-Alonso R., Montesdeoca-Esponda S., Sosa-Ferrera Z., Santana-Rodríguez J.J. Liquid chromatography methodologies for the determination of steroid hormones in aquatic environmental systems. Trends Environ Anal Chem. 2014;3:14–27. [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1:10–14. [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;37140405 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasija M., Li L., Rahman N., Ausar S.F. Forced degradation studies: an essential tool for the formulation development of vaccines. Vaccin. Dev. Ther. 2013;3:11–33. [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H.…Pillai M.D. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- How Z.T., Kristiana I., Busetti F., Linge K.L., Joll C.A. Organic chloramines in chlorine-based disinfected water systems: a critical review. J Environ Sci-China. 2017;58:2–18. doi: 10.1016/j.jes.2017.05.025. [DOI] [PubMed] [Google Scholar]
- Hung L.S. The SARS epidemic in Hong Kong: what lessons have we learned? J. R. Soc. Med. 2003;96:374–378. doi: 10.1258/jrsm.96.8.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Kitamura G., Segawa T., Kobayashi A., Miura T., Sano D., Okabe S. Microfluidic quantitative PCR for simultaneous quantification of multiple viruses in environmental water samples. Appl. Environ. Microbiol. 2014;80:7505–7511. doi: 10.1128/AEM.02578-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R., Yadav B., Tyagi R.D. Microbiology of hospital wastewater. Current Developments in Biotechnology and Bioengineering. 2020;2020:103–148. [Google Scholar]
- Khan N.A., El Morabet R., Khan R.A., Sirajuddin A., Dhingra A., Alsubih M., Khan A.R. Horizontal sub surface flow constructed wetlands coupled with tubesettler for hospital wastewater treatment. J. Environ. Manag. 2020;267:110627. doi: 10.1016/j.jenvman.2020.110627. [DOI] [PubMed] [Google Scholar]
- Kocwa-Haluch R. Waterborne enteroviruses as a hazard for human health. Pol. J. Environ. Stud. 2001;10:485–487. [Google Scholar]
- Kühn K.P., Chaberny I.F., Massholder K., Stickler M., Erdinger L. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere. 2003;53:71–77. doi: 10.1016/S0045-6535(03)00362-X. [DOI] [PubMed] [Google Scholar]
- Kumari A., Maurya N.S., Tiwari B. In: Current Developments in Biotechnology and Bioengineering Environmental and Health Impact of Hospital Wastewater. Tyagi R.D., Tiwari B., Drogui P., Pandey A., Sellamuthu B., Yan S., et al., editors. Elsevier; 2020. Hospital wastewater treatment scenario around the globe; pp. 549–570. [Google Scholar]
- Kummerer K., Helmers E. Hospital effluents as a source of gadolinium in the aquatic environment. Environ Sci Technol. 2000;34:573–577. [Google Scholar]
- Kummerer K., Erbe T., Gartiser S., Brinker L. AOX-emissions from hospital into municipal wastewater. Chemosphere. 1998;36:2437–2445. doi: 10.1016/s0045-6535(97)10200-4. [DOI] [PubMed] [Google Scholar]
- Kummerer K., Helmers E., Hubner P., Mascart G., Milandri M., Reinthaler F., Zwakenberg M. European hospitals as a source for platinum in the environment in comparison with other sources. Sci. Total Environ. 1999;225:155–165. doi: 10.1016/s0048-9697(98)00341-6. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., et al. First detection of SARS-CoV-2. In untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago P.M., Gary H. E.J., Perez L.S., Caceres V., Olivera J.B., Puentes R.P.…Cruz R.G. Poliovirus detection in wastewater and stools following an immunization campaign in Havana. Cuba. Int. J. Epidemiol. 2003;32:772–777. doi: 10.1093/ije/dyg185. [DOI] [PubMed] [Google Scholar]
- Lakshmi K. 2020. Coronavirus Metrowater Tests Show Prevalence of Virus RNA in Sewage Collected From Chennai. (in-sewage-collected-from-chennai/article31485182.ece) [Google Scholar]
- Latorre L.G., Ballesteros I., Villacrés-Grand I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. 2020;743:140832. doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz E.J., Dempsey D.M., Hendrickson R.C., Orton R.J., Siddell S.G., Smith D.B. Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV) Nucleic Acids Res. 2018;46:D708–D717. doi: 10.1093/nar/gkx932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz K., Mahnik S.N., Weissenbacher N., Mader R.M., Krenn P., Hann S.…Fuerhacker M. Monitoring, removal and risk assessment of cytostatic drugs in hospital wastewater. Water Sci Technol. 2007;56:141–149. doi: 10.2166/wst.2007.828. [DOI] [PubMed] [Google Scholar]
- Leprat P. Les rejets liquides hospitaliers, quels agents et quelles solutions techniques? Revue Techniques Hospitalières. 1998;632:49–52. [Google Scholar]
- Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., Gaymard…Yazdanpanah Y. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.K., To K.F., Chan P.K., Chan H.L., Wu A.K., Lee N., Yuen K.Y., Sung J.J. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liu S.M., Yu X.H., Tang S.L., Tang C.K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien L.T.Q.N., Hoa Q., Chuc N.T.K., Thoa N.T.M., Phuc H.D., Diwan V.…Lundborg C.S. Antibiotics in Wastewater of a Rural and an urban hospital before and after Wastewater Treatment, and the Relationship with Antibiotic Use—A One Year Study from Vietnam. Int. J. Environ. Res: Public Health. 2016;13:588. doi: 10.3390/ijerph13060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Xu S.B., Lin Y.X., Tian D., Zhu Z.Q., Dai F.H.…Lu H.Z. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Wang S., Zhang Y., Zhong R. Relationship between the COVID-19 outbreak and temperature, humidity, and solar radiation across China. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3594115. [DOI] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020;5:533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Majumder M.S., Liu D., Poirier C., Mandl K.D., Lipsitch M., Santillana M. 2020. The Role of Absolute Humidity on Transmission Rates of the COVID-19 Outbreak. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Feng L., Liu Y., Zhang L. Disinfection by-products formation and acute toxicity variation of hospital wastewater under different disinfection processes. Sep. Purif. Technol. 2020;238 [Google Scholar]
- Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S., Luo B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020;2020:1–7. doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey R., Sandin R., Greene J. Detection of airborne cytomegalovirus in hospital rooms of immuno-compromised patients. J. Virol. Methods. 1996;56:115–118. doi: 10.1016/0166-0934(95)01955-3. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R. 2020. Presence of SARS-Coronavirus-2 in Sewage. medRxiv. [DOI] [PubMed] [Google Scholar]
- Meo M.I., Haydar S., Nadeem O., Hussain G., Rashid H. Characterization of hospital wastewater, risk waste generation and management practices in Lahore. Proceedings of the Pakistan Academy of Sciences. 2014;51:317–329. [Google Scholar]
- Mesdaghinia A., Naddafi K., Nabizadeh R., Saeedi R., Zamanzadeh M. Wastewater characteristics and appropriate method for wastewater management in the hospitals. Iranian Journal Public Health. 2009;38:34–40. [Google Scholar]
- Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 2006;16:335–347. doi: 10.1111/j.1600-0668.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-: the world should face the reality. Environ. Int. 2020;105730 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis L., Saif L.J., Zhang Y., Zhang X., Azevedo M.S. Stability of bovine coronavirus on lettuce surfaces under household refrigeration conditions. Food Microbiol. 2013;30:180–186. doi: 10.1016/j.fm.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M., Garcia-Muñoz P., Pliego G., de Pedro Z.M., Zazo J.A., Jose A.C., Rodriguez J.J. Application of intensified Fenton oxidation to the treatment of hospital wastewater: kinetics, ecotoxicity and disinfection. Journal of Environmental Chemical Engineering. 2016;(4):4107–4112. [Google Scholar]
- Murray A., Jackson G.A. Viral dynamics: a model of the eects of size, shape, motion and abundance of single-celled planktonic organisms and other particles. Mar. Ecol. Prog. Ser. 1992;89:103–116. [Google Scholar]
- Naddeo V., Liu H. Editorial perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ. Environ. Sci.: Water Res. Technol. 2020;6:1213–1216. [Google Scholar]
- Nemudryi A., Nemudraia A., Surya K., Wiegand T., Buyukyoruk M., Wilkinson R., Wiedenheft B. 2020. Temporal Detection and Phylogenetic Assessment of SARS-CoV-2 in Municipal Wastewater. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short D.M. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Studies in Chemical and Environmental Engineering. 2020;1 doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez L., Moretton J. Disinfectant-resistant bacteria in Buenos Aires city hospital wastewater. Braz. J. Microbiol. 2007;38(4) [Google Scholar]
- Oliveiros B., Caramelo L., Ferreira N.C., Caramelo F. 2020. Role of Temperature and Humidity in the Modulation of the Doubling Time of COVID-19 Cases. medRxiv. [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardaa Y., Bouchardb F., Azaïsa A., Vaudreuilc M.A., Droguia P., Tyagia R.D.…Dubé R. Electrochemical treatment of real hospital wastewaters and monitoring ofpharmaceutical residues by using surrogate models. urnal of Environmental Chemical Engineering. 2019;7:103332. [Google Scholar]
- Pansini R., Fornacca D. 2020. Initial Evidence of Higher Morbidity and Mortality Due to SARS-CoV-2 in Regions With Lower Air Quality. medRxiv. [Google Scholar]
- Pieczyńska A., Borzyszkowska A.F., Ofiarska A., Siedlecka E.M. Removal of cytostatic drugs by AOPs: a review of applied processes in the context of green technology. Critical Reviews in Environmental Science and Technolog. 2017;47:1282–1335. [Google Scholar]
- Pietruchinski E., Benati F., Lauretti F., Kisielius J., Ueda M., Volotão E.M.…Santos N. Rotavirus diarrhea in children and adults in a southern city of Brazil in 2003 : distribution of G/P types and finding of a rare G12 strain. J. Med. Virol. 2006;78:1241–1249. doi: 10.1002/jmv.20686. [DOI] [PubMed] [Google Scholar]
- Pirsaheb M., Mohamadi M., Mansouri A.M., Lorestani Zinatizadeh A.A., Sumathi S., Sharafi K. Process modeling and optimization of biological removal of carbon, nitrogen and phosphorus from hospital wastewater in a continuous feeding & intermittent discharge (CFID) bioreactor. Korean J. Chem. Eng. 2015;32:1340–1353. [Google Scholar]
- Prado T., Silva D.M., Guilayn W.C., Rose T.L., Gaspar A.M.C., Miagostovich M.P. Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Res. 2011;45:1287–1297. doi: 10.1016/j.watres.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Pratelli A. Canine coronavirus inactivation with physical and chemical agents. Vet. J. 2008;177:71–79. doi: 10.1016/j.tvjl.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin D. New trends in food-and waterborne viral outbreaks. Arch Biol Sci. 2014;66:1–9. [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;118:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read R. Now Dozens of Members Have COVID-19 and Two Are Dead Los Angeles Times. 2020. A choir decided to go ahead with rehearsal. [Google Scholar]
- Richardson S.D. Disinfection by-products: formation and occurrence in drinking water. Encyclopedia of Environmental Health. 2011:110–136. [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Gismondo Salerno F. 2020. Presence and Vitality of SARS-CoV-2 Virus in Wastewaters and Rivers medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudbari A., Rezakazemi M. Hormones removal from municipal wastewater using ultrasound. AMB Express. 2018;8:91. doi: 10.1186/s13568-018-0621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi M.M., Habibzadeh P., Vintzileos A., Shokouhi S., Miralles-Wilhelm F., Amoroso A. 2020. Temperature and Latitude Analysis to Predict Potential Spread and Seasonality for COVID-19. (Available at SSRN 3550308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaa D., Bouchaib B., Nadia B., Said E.A. Diagnostic de la gestion des effluens liquides hospitaliers de la region de Casablanca-Settat. Eur. Sci. J. 2019;15:171–190. [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019:16–69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif S., Ikram A., Khurshid A., Salman M., Mehmood N., Arshad Y.…Ali N. 2020. Detection of SARs-CoV-2 in wastewater, using the existing environmental surveillance network :an epidemiological gateway to an early warning for COVID-19 in communities. MedRxiv. [Google Scholar]
- Shigematsu S., Dublineau A., Sawoo O., Batejat C., Matsuyama T., Leclercq I., Manuguerra J. Influenza A virus survivalin water is influenced by the origin species of the host cell. Influenza Other Respir. Viruses. 2014;8:123–130. doi: 10.1111/irv.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., Shi Y., Zhang Z., Chen S., Liu X. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Letter. 2020:991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds E.M., Griffin D.W., Breitbart M. Eukaryotic viruses in wastewater samples from the United States. Appl. Environ. Microbiol. 2009;75:1402–1409. doi: 10.1128/AEM.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020:1–8. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A.N., Tong Z.D., Wang H.L., Dai Y.X., Li K.F., Liu J.N.…Yan J. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerging Infection Diseases. 2020;26:1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongprachum A., Fujimoto T., Takanashi S., Saito H., Okitsu S., Shimizu H., Ushijima H. Detection of nineteen enteric viruses in raw sewage in Japan. Infect. Genet. Evol. 2018;63:17–23. doi: 10.1016/j.meegid.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Top S., Akgün M., Kıpçak E., Bilgili M.S. Treatment of hospital wastewater by supercritical water oxidation process. Water Res. 2020;185 doi: 10.1016/j.watres.2020.116279. [DOI] [PubMed] [Google Scholar]
- Tosepu R., Gunawan J., Effendy D.S., Lestari H., Bahar H., Asfian P. Correlation between weather and Covid-19 pandemic in Jakarta, Indonesia. Sci. Total Environ. 2020:138436. doi: 10.1016/j.scitotenv.2020.138436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakona M., Anagnostopoulou E., Gidarakos E. Hospital waste management and toxicity evaluation: a case study. Waste Manag. 2007;27:912–920. doi: 10.1016/j.wasman.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D., et al. 2020. Aerosol and Surface Stability of HCoV-19 (SARS-CoV-2) Compared to SARS-CoV-1. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal A., Ganesan H., Raja S.S.S., Govindasamy V., Arunachalam M., Narayanasamy A.l.…Vellingiri B. Novel wastewater surveillance strategy for early detection of coronavirus disease 2019 hotspots. Environmental Science & Health. 2020;17:8–13. doi: 10.1016/j.coesh.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlicchi P., Galletti A., Petrovic M., Barcelo D. Hospital effluents as a source of emerging pollutants: an overview of micropollutants and sustainable treatment options. J. Hydrol. 2010;389:416–428. [Google Scholar]
- Verlicchi P., Galletti A., Masotti L. Management of hospital wastewaters: the case of the effluent of a large hospital situated in a small town. Water Sci. Technol. 2010;61:2507–2519. doi: 10.2166/wst.2010.138. [DOI] [PubMed] [Google Scholar]
- Verlicchi P., Galletti A., Petrovic M., Barcelo D. Micro-pollutants in hospital effluent: their fate, risk and treatment options. Emerging Organic Contaminants and Human Health. 2012:139–171. [Google Scholar]
- Vogels C.B.F., Brito A.F., Wyllie A.L., Fauver J.R., Ott I.M., Kalinich…Grubaugh N.D. 2020. Analytical sensitivity and efficiency comparisons of SARS-COV-2 qRT-PCR assays. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Du G. COVID-19 may transmit through aerosol. Ir. J. Med. Sci. 2020:1–2. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., et al. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J., Guo T., Zhen B., Kong Q., Yi B.…Li J.W. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital of the Chinese People’s Liberation Army. Water Science Technology. 2005;52:213–221. [PubMed] [Google Scholar]
- Wang W., Qian Y.C., Li J.H., Moe B., Huang R.F., Zhang H.Q., Hrudey S.E., Li X.F. Analytical and toxicity characterization of halo-hydroxyl-benzoquinones as stable halobenzoquinone disinfection byproducts in treated water. Anal. Chem. 2014;86:4982–4988. doi: 10.1021/ac5007238. [DOI] [PubMed] [Google Scholar]
- Wang J., Tang K., Feng K., Lv W. 2020. High Temperature and High Humidity Reduce the Transmission of COVID-19. arXiv:2003.05003. [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARSCoV- 2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARSCoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W.…Pan L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. World Health Organization,Coronavirus Disease (COVID-19) Situation Report. [Google Scholar]
- WHO . 2020. World Health Organization, Consensus Document on the Epidemiology of Severe Acute Respiratory Syndrome (SARS) p. 2003. [Google Scholar]
- WHO . 2020. World Health Organization, WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) Report. [Google Scholar]
- WHO . 2020. World Health Organization, Water, Sanitation, Hygiene and Waste Management for the COVID-19 Virus. Technical Brief. [Google Scholar]
- Woolhouse M.E., Rambaut A., Kellam P. Lessons from Ebola: improving infectious disease surveillance to inform outbreak management. Sci. Transl. Med. 2015;7:307rv5. doi: 10.1126/scitranslmed.aab0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X.…Kuang L. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W., Kauffman K.…Alm E. 2020. SARS-CoV-2 titers in wastewater are higher Than expected From clinically confirmed cases. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Exposure to air pollution and COVID-19 mortality in the United States. 2020. https://projects.iq.harvard.edu/covid-pm [DOI] [PMC free article] [PubMed]
- Wurtzer S., Marechal V., Mouchel J., Moulin L. 2020. Time Course Quantitative Detection of SARS-CoV-2 in Parisian Wastewaters Correlates With COVID-19 Confirmed Cases. medRxiv. [Google Scholar]
- Xiao F., Sun J., Xu Y., Li F., Huang X., Li H., et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26:1920–1922. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Ni W., Wu Q., Li W., Li G., Tong J., Song X., Xing Q. 2020. Prolonged Presence of SARS-CoV-2 in Feces of Pediatric Patients During the Convalescent Phase. medRxiv. [Google Scholar]
- Xu D., Zhang Z., Jin L., Chu F., Mao Y., Wang H.…Wang F.S. Persistent shedding of viable SARS-CoV in urine and stool of SARS patients during the convalescent phase. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24:165–171. doi: 10.1007/s10096-005-1299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y.…Zhang H. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nature Medicine. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Li G., Dai X., Liu G., Li G., Jie Y. Three cases of novel coronavirus pneumonia with viral nucleic acids still positive in stool after throat swab detection turned negative. Chin J Dig. 2020;40:E002. [Google Scholar]
- Yao Y., Pan J., Liu Z., et al. No association of COVID-19 transmission with temperature or UV radiation in Chinese cities. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00517-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible ? Lancet. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yépiz-Gomez M.S., Gerba C.P., Bright K.R. Survival of respiratory viruses on fresh produce. Food Environ Virol. 2013;5:150–156. doi: 10.1007/s12560-013-9114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.L., Li Q., Yan S.C. Design and running for a hospital wastewater treatment project. Adv. Mater. Res. 2013;777:356–359. [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coro- navirus-infected pneumonia. J. Med. Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen H., Zhu S., Shu C., Wang D., Song J.…Xu W. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2:123–124. [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Gong Y., Meng F., Bi Y., Yang P., Wang F. Virus Shedding Patterns in Nasopharyngeal and Fecal Specimens of COVID-19 Patients. MedRxiv. 2020. [DOI] [PMC free article] [PubMed]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C.…Qu J. 2020. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of fangcang hospital. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Yang Y., Huang X., Jiang J., Li M., Zhang X., Xie J. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724:138201. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.