Abstract
Characterization of neonates born to mothers with SARS-CoV-2 infection has been partially carried out. There has been no systematic review providing a holistic neonatal presentation including possible vertical transmission. A systematic literature search was performed using PubMed, Google Scholar and Web of Science up to June, 6 2020. Studies on neonates born to mothers with SARS-CoV-2 infection were included. A binary random effect model was used for prevalence and 95% confidence interval. 32 studies involving 261 neonates were included in meta-analysis. Most neonates born to infected mothers did not show any clinical abnormalities (80.4%). Clinical features were dyspnea in 11 (42.3%) and fever in 9 newborns (19.1%). Of 261 neonates, 120 neonates were tested for infection, of whom 12 (10.0%) tested positive. Swabs from placenta, cord blood and vaginal secretion were negative. Neonates are mostly non affected by the mother's SARS-CoV-2 infection. The risk of vertical transmission is low.
Key Words: COVID-19, infant, neonate, newborn, SARS-CoV-2
Abbreviations: COVID-19, Coronavirus disease 2019; SARS-CoV-2, Acute respiratory syndrome-coronavirus-2; rRT-PCR, Real-time reverse transcriptase polymerase chain reaction; MINORS, Methodological Index for Non-Randomized Studies; SGA, Small gestational age; CDC, Centers for Disease Control
1. Introduction
In December 2019, an outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) occurred in Wuhan, China.1 Causing a worldwide pandemic, SARS-CoV-2 was declared a public health emergency by the World Health Organization (WHO).2 On June 28, 2020 Johns Hopkins University reported more than 10 million infections worldwide.3 The clinical spectrum of infection ranges from asymptomatic cases to critically ill patients.4 However, symptoms in children are mostly mild and they seem to be less affected than adults.5 , 6 In the meantime a new syndrome in children was described, Pediatric Multisystem Inflammatory Syndrome (PMIS) or Multisystem Inflammatory Syndrome in Children (MIS-C). It is a systemic disease resembling Kawasaki disease including toxic shock and macrophage activation syndromes.7 , 8 Neonatal SARS-CoV-2 infections are rare9 and there is evidence of intrauterine infection caused by vertical transmission.10 Vivanti et al. were able to demonstrate a transplacental transmission of SARS-CoV-2 in a neonate born to a mother infected in the last trimester.10 However, infected newborns are mostly asymptomatic or present with mild clinical symptoms.11, 12, 13 In a report of 10 neonates with SARS-CoV-2 infection, clinical features were shortness of breath, fever or gastrointestinal symptoms.13 In addition, neonates born to mothers with SARS-CoV-2 infection may also show no clinical abnormalities,14 emphasizing the clinical variety of infection.15 Interestingly, in most case reports swabs from amniotic fluid, vaginal secretion, cord blood, breast milk and neonatal throat swabs were all SARS-CoV-2 negative by real-time reverse transcriptase polymerase chain reaction (rRT-PCR).11 , 14 , 16 To date, large data on clinical characteristics of neonates born to mothers with SARS-CoV-2 infection and possible vertical transmission are limited. Due to low sample sizes, most reports are incomplete and not all pregnant women underwent rRT-PCR testing for SARS-CoV-2. Therefore, we conducted a meta-analysis to characterize a large number of neonates delivered by rRT-PCR confirmed SARS-CoV-2 positive mothers. We provide comprehensive information on birth related data, clinical features, treatment options and rRT-PCR results from SARS-CoV-2 tests in different samples from neonates and their mothers.
2. Material and methods
2.1. Protocol and registration
The present study has been registered at PROSPERO register (www.crd.york.ac.uk/PROSPERO, registration number: CRD42020182444). The meta-analysis was performed in line with recommendations from the Preferred Reporting Items for Systemic reviews and Meta-Analyses (PRISMA statement).17
2.2. Information sources and search strategy
A systematic literature search was performed to identify published reports, which investigated newborns born to SARS-CoV-2 positive mothers. Medline electronic database (PubMed), Google Scholar, and Web of Science were searched using the following search terms ((COVID-19 OR “Novel coronavirus” OR “N. coronavirus 2019” OR “2019 nCoV” OR “Wuhan coronavirus” OR “Wuhan pneumonia” OR “SARS-CoV-2”) AND (demographics OR clinical OR epidemiological OR characteristics OR APGAR) AND (pregnancy OR fetus OR neonate OR neonatal OR infant OR newborn OR delivery OR caesarean section OR vaginal birth)). For initial search, there was no limitation on publication date, language or study design to include as much data as possible. Data base search was conducted until June 6, 2020.
2.3. Eligibility criteria
Potentially eligible studies had to be peer-reviewed articles with neonates born to rRT-PCR confirmed SARS-CoV-2 positive mothers of any age or mode of delivery. According to the WHO definition of newborns, only children ≤28 days of age were included. To be eligible for inclusion, studies had to provide data on demographics, clinical symptoms, laboratory or treatment information of the newborns. Eligible study designs were multi-, and single-center studies, observational-, case-control-, and cohort studies, case series, case reports and short communication reports. For final inclusion, only English language articles were eligible. There was no restriction on publication date. Opinion articles, letters and correspondence articles and studies with missing data were excluded.
2.4. Study selection
Two independent investigators (V.N., F.R.) screened title and abstract of identified studies for eligibility. Any discrepancies between the two reviewers were resolved by consensus through discussion with a third reviewer (H.B.). Full text of eligible studies was analyzed for inclusion in systematic review and meta-analysis. Identified duplicates were removed. Reference lists of identified studies were hand-searched for further relevant studies.
2.5. Data collection
The two investigators (V.N., F.R.) independently extracted data from the included studies to a standardized table in Microsoft Excel (Excel 365; Microsoft Corp., Redmond, Washington, USA). The following information was used for data extraction: first author, date and country of publication, study design (multi-, or single-center study, case series, case report) sample size of mothers and newborns, age of mothers, demographic data of neonates (e.g., gestational age, sex, birthweight, Apgar score, rRT-PCR test for SARS-CoV-2 infection), clinical features (e.g., fever, cough), fetal complications (preterm delivery, intrauterine growth restriction, still birth, low birth weight (<2,500 g)), radiological and laboratory results, and treatment information (e.g., antiviral medication, antibiotics) in neonates. Laboratory data were presented as abnormally high or low according to the reference value reported by the paper or when laboratory-specific ranges were given. Similarly, radiological findings were categorized according to the available description. Authors were contacted via E-mail if full text was not accessible or additional information was required. Extracted data were double checked by the two reviewers (V.N., F.R.). Any discrepancies were solved by consensus through discussion with a third reviewer (H.B.).
2.6. Risk of bias assessment
To determine the quality of the included studies the Methodological Index for Non-Randomized Studies (MINORS) was used for multi- and single-center studies.18 Each of the eight items were scored as: 0 (not reported), 1 (reported but inadequate) and 2 (reported and adequate). The maximum ideal score was 16. Scoring more than 70% of a maximum score of 16 (score ≥ 11) conferred a risk of bias that was seen as low with a high quality in included studies. For scores below 11, risk of bias was seen as high. For case series and case reports the Methodological Quality and Synthesis of Case Series and Case Reports Protocol by Murad et al. was used for risk of bias assessment.19 All eight items were scored as: NA (not applicable), 1 (No) and 2 (Yes). Questions 5 and 6 were not applicable for the included study reports. The maximum ideal score was 12. Scoring more than 70% of a maximum score of 12 (score ≥ 8) conferred a risk of bias that was seen as low with a high quality in included studies. For scores below 8, risk of bias was seen as high. Risk of bias was assessed by the two investigators (V.N., F.R.) independently. Discrepancies were solved by consensus through discussion with a third reviewer (H.B.).
2.7. Ethical review
We solely used already published statistical data and therefore ethical approval was not applicable in the current meta-analysis.
2.8. Statistical analysis
Distribution of categorical and continuous variable are described as count (percentage), mean ± standard deviation (SD) or median (Range or IQR: 25%, 75%), respectively. The description of data was in accordance with the original articles. Data in Tables are presented as following: number of studies reporting the characteristic (n) in relation to all (n = 32) included studies (%), number of children with reported characteristic (n) in relation to all children in reporting studies (%), number of children with no report on characteristic (n) in relation to all children in all studies reporting this item (%), and number of children with no information on reported item (n) in relation to all children (n = 261; %). All analyses and graphic illustrations were conducted with Microsoft Excel or GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). For comparison of abnormally distributed data the Mann-Whitney-U-test was used. The meta-analysis was performed using the software OpenMeta [Analyst] (B. Wallace, Boston, MA, USA) for single arm studies. A binary random effect model was used to calculate the prevalence of all variables and their 95% confidence interval (95% CI). For measurement of statistical heterogeneity, I2 statistic was used. In addition, Cochran's Q statistic and the tau-squared test were reported. For all analyses a p-value <0.05 was considered to be statistically significant.
3. Results
3.1. Study selection
A total of 688 studies were identified through database search of PubMed, Google Scholar and Web of Science. With hand search of reference lists an additional 72 studies were identified. After exclusion of duplicates (n = 24), in total 736 studies were assessed for screening of title and abstract. Records were primarily excluded due to missing inclusion criteria (n = 578) and dealing with a different virus than SARS-CoV-2 (n = 12). Overall, 146 records were eligible for full-text review. Therefore, 78 studies were excluded during in-text search due to missing inclusion criteria (n = 75) and other reasons (n = 3). A total of 68 studies were included in qualitative synthesis. Due to ineligible endpoints (n = 36) 32 studies were included in quantitative synthesis. For additional information 3 authors were contacted via E-Mail. However, we received no answers prior to publication (Supplemental Fig. aa).
3.2. Study characteristics
In meta-analysis 32 peer-reviewed studies were included. Studies predominantly originated from China (n = 19); other countries of publication were USA (n = 4), Korea (n = 2), Italy (n = 2), Iran (n = 1), France (n = 1), Canada (n = 1), Iraq (n = 1) and Turkey (n = 1). Study designs of the included publications were multicenter studies (n = 4), single-center studies (n = 4), case series (n = 8), and case reports (n = 16) (Supplemental Table 3).
3.3. Birth-related characteristics
Overall, 261 neonates born to 258 mothers were included in meta-analysis. In all mothers SARS-CoV-2 infection was confirmed by rRT-PCR. Age of mothers, gestational age and neonatal birthweight were presented inhomogenously in included studies and are therefore displayed in supplemental Table (supplemental material). In 18 out of 32 (56.3%) studies reporting, there were 41 out of 60 (68.3%) males (95% CI 0.584 to 0.783). Female gender was reported in 19 out of 32 (59.4%) studies; therefore, 19 out of 60 (31.7%) were females (95% CI 0.221 to 0.418). In 201 (77.0%) children gender was not reported. In 30 out of 32 studies (93.8%) mode of delivery was reported including 217 neonates. 187 out of 217 (86.2%) neonates were delivered by caesarean section (95% CI 0.679 to 0.866), and 30 out of 217 (13.8%) neonates were born by spontaneous vaginal delivery (95% CI 0.065 to 0.160). In 44 out of 261 (16.9%) mode of delivery was not reported. In 11 out of 32 (34.3%) studies reporting, in 33 out of 38 (86.8%) neonates formula feeding was conducted (95% CI 0.862 to 1.008). In 5 out of 32 (15.6%) of the studies reporting, 5 out of 38 (13.2%) neonates were breast fed (95% CI 0.059 to 0.304). In 223 out of 261 (85.4%) neonates feeding was not reported. In all studies reporting Apgar scores, 1-min Apgar score in children discharged from hospital was 7–10 and 5-min Apgar score was 8–10 (Table 1, Table 2 , Supplemental Table 3, Supplemental Figs. a–d, f–g).
Table 1.
Summary of included studies reporting characteristics of neonates.
| Characteristic | No. of studies reporting this item compared to all studies (n = 32) n (%) | No. of neonates/mothers with positive reported item compared to all neonates in reporting studies n (%) | No. of neonates/mothers with negative reported item compared to all neonates in reporting studies n (%) | No. of neonates/mothers with no reported item compared to all neonates (n = 261) n (%) |
|---|---|---|---|---|
| Sex | ||||
| Female | 19 (59.4) | 19 (31.7) | NA | 201 (77.0) |
| Male | 18 (56.3) | 41 (68.3) | NA | |
| Birth related characteristics | ||||
| Caesarean section | 30 (93.8) | 187 (86.2) | NA | 44 (16.9) |
| Vaginal delivery | 30 (93.8) | 30 (13.8) | NA | |
| Preterm birtha | 15 (46.9) | 46 (28.2) | 117 (71.8) | 100 (38.3) |
| Formula feeding | 11 (34.4) | 33 (86.8) | NA | 223 (85.4) |
| Breast feeding | 5 (15.6) | 5 (13.2) | NA | |
| Clinical symptoms | ||||
| No clinical abnormalitiesa | 9 (28.1) | 37 (80.4) | 9 (19.6) | 215 (82.4) |
| Intrauterine distress | 5 (15.6) | 12 (13.2) | 79 (86.8) | 171 (65.5) |
| Dyspnea | 6 (18.8) | 11 (42.3) | 15 (57.7) | 235 (90.0) |
| Fever | 9 (28.1) | 9 (19.1) | 38 (80.9) | 216 (82.8) |
| SGAa | 4 (12.5) | 7 (8.1) | 79 (91.9) | 177 (67.8) |
| Vomiting | 5 (15.6) | 6 (26.1) | 17 (73.9) | 239 (91.6) |
| Moaning | 2 (6.3) | 3 (37.5) | 5 (62.5) | 253 (96.9) |
| Cough | 4 (12.5) | 2 (15.4) | 11 (84.6) | 248 (95.0) |
| Initial radiological abnormalities | 12 (37.5) | 19 (32.2) | 40 (67.8) | 203 (77.8) |
| Therapy | ||||
| NICU admission | 16 (50.0) | 60 (46.2) | 70 (53.8) | 132 (50.6) |
| Antibiotics | 6 (18.8) | 9 (69.2) | 4 (30.8) | 248 (95.0) |
| Oxygen therapy | 4 (12.5) | 7 (53.8) | 6 (46.2) | 248 (95.0) |
| NIV | 3 (9.4) | 4 (33.3) | 8 (66.7) | 249 (95.4) |
| Invasive ventilation | 2 (6.3) | 1 (2.2) | 45 (97.8) | 215 (82.4) |
| Hospital discharge | 19 (59.4) | 132 (81.5) | 30 (18.5) | 101 (38.7) |
| Death | 22 (68.8) | 3 (1.7) | 177 (98.3) | 83 (31.8) |
| SARS-CoV-2 rRT-PCR test in neonates | ||||
| Tested for SARS-CoV-2 | 27 (84.4) | 120 (46.0) | NA | 141 (54.0) |
| SARS-CoV-2 status | 27 (84.4) | 12 (10.0) | 108 (90.0) | 141 (54.0) |
NIV = non-invasive ventilation; NICU = neonatal intensive care unit; SGA = small for gestational age; SARS-CoV-2 = Severe acute respiratory syndrome-Coronavirus-2; NA = not applicable.
As stated in each individual publication.
Table 2.
SARS-CoV-2 testing in different samples.
| Sample | No. of studies reporting this item compared to all studies (n = 32)n (%) | No. of neonates with positive result compared to all neonates in reporting studies n (%) | No. of neonates with negative result compared to all neonates in reporting studies n (%) | No. of children evaluated for this item n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Nasopharyngeal/Throat swab | 25 | (59.5) | 6 | (5.9) | 95 | (94.1) | 101 | (38.7) |
| Rectal/Anal swab | 4 | (9.5) | 3 | (37.5) | 5 | (62.5) | 8 | (3.1) |
| Blood test newborn | 2 | (4.8) | 1 | (50.0) | 1 | (50.0) | 2 | (0.1) |
| Placenta | 6 | (14.3) | 0 | (0.0) | 7 | (100.0) | 7 | (2.7) |
| Cord blood | 10 | (23.8) | 0 | (0.0) | 28 | (100.0) | 28 | (10.7) |
| Amniotic fluid | 10 | (23.8) | 1 | (2.5) | 33 | (97.5) | 34 | (13.0) |
| Breast milk mother | 8 | (19.0) | 1 | (4.0) | 24 | (96.0) | 25 | (9.6) |
| Vaginal swab mother | 4 | (9.5) | 0 | (0.0) | 9 | (100.0) | 9 | (3.4) |
SARS-CoV-2= Severe acute respiratory syndrome-Coronavirus-2.
3.4. Neonatal outcomes
In 19 out of 32 studies (59.4%) including 160 neonates hospital discharge was reported. Overall 132 out of 160 (82.5%) neonates were discharged from hospital (95% CI 0.729 to 0.508). In 101 out of 261 (38.7%) hospital discharge was not reported. In 22 out of 32 (68.8%) studies including 180 neonates, death was mentioned as an outcome parameter. Only 3 out of 180 (1.7%) newborns died after birth (95% CI 0.012 to 0.062). In 83 out of 261 (31.8%) death was not mentioned as an outcome parameter. Reasons for neonatal death in the 3 newborns were stillbirth at 38 + 7 weeks,20 severe neonatal asphyxia21 and multiple organ failure with refractory shock after delivery.13 In the first neonate reason for stillbirth was unknown. Postmortem examination of the placenta revealed severe chronic villitis but no viral inclusions. PCR testing of placenta and fetal tissues was negative for SARS-CoV-2.20 The second male neonate was delivered at 35 + 2 weeks' gestation by cesarean delivery. The mother experienced severe pneumonia with admission to the intensive care unit and invasive ventilation. The neonate had 1-min and 5-min Apgar scores of 1 and 1, respectively. He was also treated with invasive ventilation due to severe asphyxia and died within 2 h. According to publication, no tests for SARS-CoV-2 were performed.21 The third male neonate was delivered at 34 + 5 weeks' gestation by cesarean section and died on day 9 due to multiple organ failure, disseminated intravascular coagulation and refractory shock. His 1-min and 5-min Apgar scores were 8 and 8, respectively. According to publication, the neonate's SARS-CoV-2 throat swab on day 9 showed negative results. He first presented with an increased heart rate. According to the reported case, the reason for organ failure remains unclear. The mother presented with fever 3 days after delivery, vaginal bleeding occurred in the third trimester (Supplemental Figs. v–w).13
3.5. Clinical features
In 9 out of 32 (28.1%) studies, 37 out of 46 (80.4%) neonates did not show any clinical abnormalities (95% CI 0.671 to 0.937). In 215 out of 261 (82.4%) this characteristic was not reported. The most commonly described clinical features were dyspnea in 11 out of 26 newborns (42.3%, 95% CI 0.183 to 0.619), fever in 9 out of 47 neonates (19.1%, 95% CI 0.106 to 0.431), moaning in 3 out of 8 neonates (37.5%, 95% CI 0.175 to 0.845) and cough in 2 out of 13 neonates (15.4%, 95% CI 0.041 to 0.624). Other clinical signs were mainly gastrointestinal symptoms, namely vomiting in 6 out of 23 newborns (26.1%, 95% CI 0.138 to 0.612). In total, 7 out of 86 (8.1%) neonates presented with small gestational age (SGA) (95% CI 0.005 to 0.327), and 12 out of 90 (13.2%) newborns suffered from intrauterine distress (95% CI 0.052 to 0.181). Regarding fetal complications, 15 out of 32 studies (46.9%) reported preterm birth including 163 neonates. Prematurity was documented in 46 of 163 (28.2%) of the neonates (95% CI 0.163 to 0.378). In 9 out of 15 studies (60.0%), reason for preterm birth was not mentioned.13 , 22, 23, 24, 25, 26, 27, 28, 29 In 3 out of 15 studies (20.0%), the authors stated that prematurity was not associated with SARS-CoV-2. Prematurity occurred to due pre-eclampsia, history of still births, irregular contractions, premature rupture of the membrane11 , 21 and pulmonary state of the mother with obesity.20 Possible association with SARS-CoV-2 was stated in 3 out of 15 studies (20.0%).30, 31, 32 Overall, in 100 out of 261 (38.3%) neonates, preterm birth was not reported. In 5 out of 32 studies (15.6%) low birth weight was reported involving 12 neonates (10 singletons and 1 pair of twins). All of the newborns were delivered by caesarean section,11 , 13 , 29 , 31 , 32 1 newborn died due to multiple organ failure after delivery.13 Birth weight ranged from a minimum of 1527 g13 to a maximum of 2460 g.11 No intrauterine growth restriction was mentioned in the included studies (Table 1, Table 3, Supplemental Fig. e, h–o, Supplemental Table 2).
Table 3.
Outcome of meta-analysis (random effect model).
| Characteristic | Neonates |
|||||
|---|---|---|---|---|---|---|
| 95% CI | n | Q | I2 | τ2 | p-value | |
| Sex | ||||||
| Female | 0.320 (0.221–0.418) | 19 | 12.936 | 0.000 | 0.000 | <0.001 |
| Male | 0.684 (0.584–0.783) | 41 | 12.671 | 0.000 | 0.000 | <0.001 |
| Birth related characteristics | ||||||
| Caesarean section | 0.773 (0.679–0.866) | 187 | 127.535 | 77.261 | 0.038 | <0.001 |
| Vaginal delivery | 0.113 (0.065–0.160) | 30 | 47.494 | 38.940 | 0.004 | <0.001 |
| Preterm birtha | 0.271 (0.163–0.378) | 46 | 44.112 | 68.262 | 0.025 | 0.011 |
| Formula feeding | 0.657 (0.415–0.899) | 33 | 65.555 | 84.746 | 0.121 | <0.001 |
| Breast feeding | 0.181 (0.059–0.304) | 13 | 23.299 | 48.496 | 0.016 | 0.004 |
| Clinical symptoms | ||||||
| No clinical abnormalitiesa | 0.804 (0.671–0.937) | 37 | 15.843 | 49.504 | 0.017 | <0.001 |
| Intrauterine distress | 0.117 (0.052–0.181) | 12 | 2.309 | 0.000 | 0.000 | 0.002 |
| Dyspnea | 0.401 (0.183–0.619) | 11 | 8.847 | 43.487 | 0.031 | <0.001 |
| Fever | 0.268 (0.106–0.431) | 9 | 23.367 | 65.763 | 0.032 | 0.001 |
| SGAa | 0.166 (0.005–0.327) | 7 | 13.243 | 69.795 | 0.020 | 0.043 |
| Vomiting | 0.381 (0.138–0.612) | 6 | 10.542 | 52.571 | 0.044 | 0.002 |
| Moaning | 0.510 (0.175–0.845) | 3 | 2.835 | 29.459 | 0.027 | 0.003 |
| Cough | 0.332 (0.041–0.624) | 2 | 8.959 | 55.351 | 0.057 | 0.025 |
| Initial radiological abnormalities | 0.370 (0.202–0.538) | 19 | 38.732 | 69.018 | 0.053 | <0.001 |
| Therapy | ||||||
| NICU admission | 0.577 (0.291–0.863) | 60 | 723.241 | 97.926 | 0.295 | <0.001 |
| Antibiotics | 0.657 (0.454–0.860) | 9 | 2.925 | 0.000 | 0.000 | <0.001 |
| Oxygen therapy | 0.454 (0.222–0.686) | 7 | 2.660 | 0.000 | 0.000 | <0.001 |
| NIV | 0.419 (0.171–0.668) | 4 | 3.540 | 15.249 | 0.010 | <0.001 |
| Invasive ventilation | 0.145 (−0.105 – 0.395) | 1 | 5.871 | 0.030 | 65.934 | 0.256 |
| Hospital discharge | 0.818 (0.729–0.908) | 132 | 67.296 | 71.766 | 0.019 | <0.001 |
| Death | 0.037 (0.012–0.062) | 3 | 12.229 | 0.903 | 0.000 | 0.004 |
| SARS-CoV-2 rRT-PCR test in neonates | ||||||
| Tested for SARS-CoV-2 | 0.762 (0.643–0.881) | 120 | 173.711 | 85.003 | 0.063 | <0.001 |
| SARS-CoV-2 negative | 0.670 (0.527–0.813) | 108 | 265.268 | 90.199 | 0.103 | <0.001 |
| SARS-CoV-2 positive | 0.128 (0.066–0.190) | 12 | 61.247 | 57.549 | 0.010 | <0.001 |
95% CI = 95% confidence interval; Q = Cochrane's Q statistic for heterogeneity; I2 = Index for the degree of heterogeneity.
t2 = Tau-squared measure of heterogeneity; NIV = non-invasive ventilation; NICU = neonatal intensive care unit; SGA = small for gestational age; SARS-CoV-2 = Severe acute respiratory syndrome-Coronavirus-2.
As stated in each individual publication.
3.6. Treatment
Of the studies reporting, 60 out of 130 (46.2%) neonates were admitted to the neonatal intensive care unit (NICU) for immediate isolation or intensive care treatment (95% CI 0.291 to 0.863). Antibiotics were given to 9 out of 13 (69.2%) neonates (95% CI 0.454 to 0.860), and 7 out of 13 (53.8%) newborns required oxygen therapy (95% CI 0.222 to 0.686). Non-invasive ventilation (NIV) was necessary in 4 out of 12 (33.3%) neonates (95% CI 0.171 to 0.668), while invasive ventilation was reported in only 1 out of 46 (2.2%) newborn (95% CI -0.105 to 0.395) (Table 2, Table 3, Supplemental Fig. p–u).
3.7. SARS-CoV-2 testing in different samples
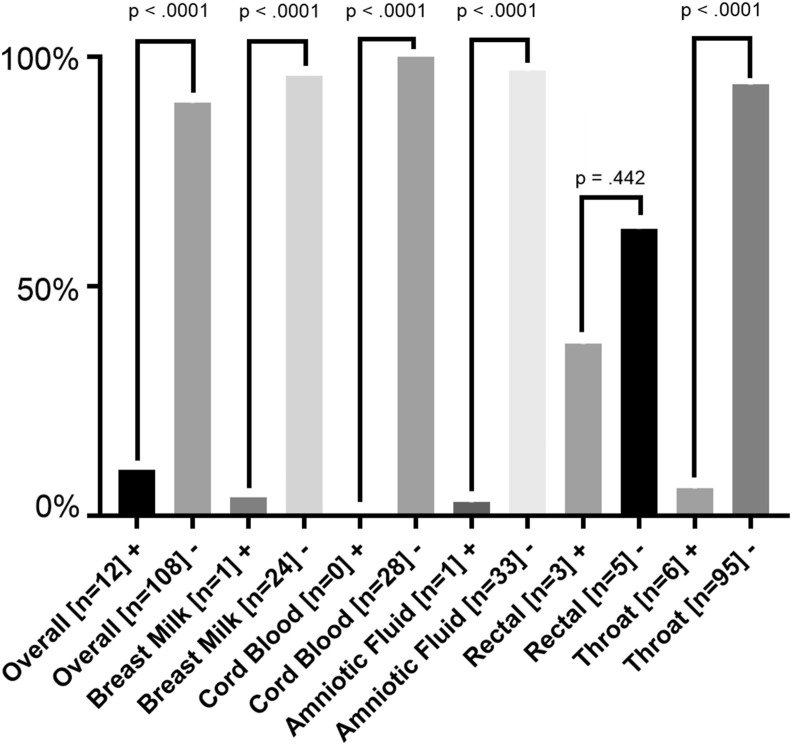
Overall, 120 (46.0%) neonates were tested for SARS-CoV-2 (95% CI 0.643 to 0.881). Different samples for neonates were taken from different locations (nasopharyngeal/throat swabs, rectal/anal swabs, blood tests, placenta, cord blood and amniotic fluid). In mothers 34 tests were performed from breast milk and vaginal secretion. Of all neonates tested, 12 out of 120 (10%) neonates showed positive test results (95% CI 0.066 to 0.190) and 108 out of 120 (90%) neonates tested negative (95% CI 0.527 to 0.813). Nasopharyngeal or throat swabs were positive in 6 (5.9%) neonates and negative in 95 (94.1%). Rectal or anal swabs were positive in 3 (37.5%) and negative in 5 (62.5%), blood test was positive in 1 (50.0%) and negative in 1 (50.0%), placenta positive in 0 (0.0%) and negative in 7 (100.0%), cord blood positive in 0 (0.0%) and negative in 28 (100.0%) and amniotic fluid was positive in 1 (2.5%) and negative in 33 (97.5%) of the cases. Test of breast milk was positive in 1 (4.0%) case and negative in 24 (96.0%). Vaginal swabs were positive in 0 (0.0%) and negative in 9 (100.0%) mothers (Table 1, Table 2, Table 3, Fig. 1 , Supplemental Figs. x–z).
Figure 1.
Overview of rRT-PCR tests for SARS-CoV-2 from different samples. Fig. 2 summarizes the performed SARS-CoV-2 rRT-PCR tests in different samples. X axis represents the different sample swabs, Y axis represents ratio (%) of positive or negative tested neonates compared to all neonates with performed tests. P-values were determined by Mann-Whitney-U-test. + = positive test result by rRT-PCR; - = negative test result by rRT-PCR; SARS-CoV-2 = Severe acute respiratory syndrome-Coronavirus-2.
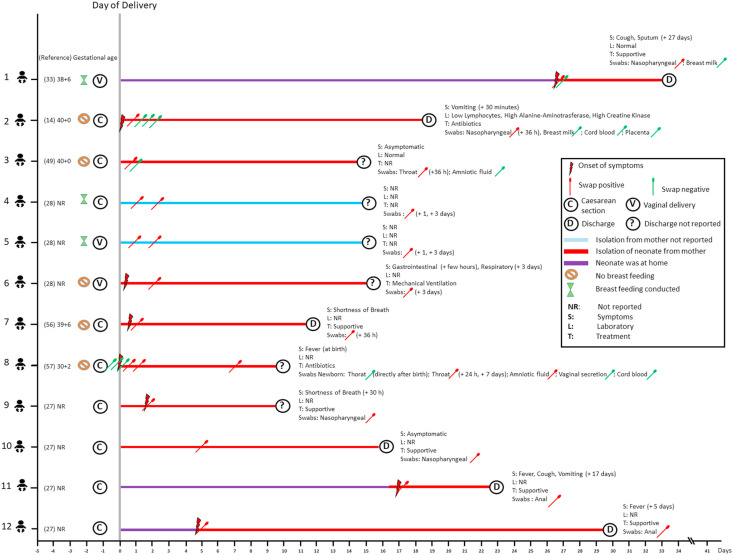
3.8. Characterization of 12 SARS-CoV-2 positive neonates
Overall, 12 newborns from infected mothers tested positive for SARS-CoV-2. In total, 3 out of 12 (25.0%) neonates were born by vaginal delivery (No. 1, 5–6), and all other newborns (9 out of 12 (75.0%)) were delivered by caesarean section (No. 2–4, 7–12). Of these, 2 out of 12 (16.7%) cases were reported to be asymptomatic (No. 3, 10), 5 out of 12 (41.7%) cases presented with respiratory symptoms (No. 1, 6–7, 9, 11), 3 out of 12 (25.0%) cases with gastrointestinal symptoms (No. 2, 6, 11) and 3 out of 12 (25.0%) cases with fever (No. 8, 11–12). In 2 out of 12 (16.7%) neonates clinical features were not reported (No. 4–5). In 9 out of 12 (75.0%) cases laboratory results were not reported (No. 4–12), in 2 out of 12 neonates (16.7%) laboratory results were stated as normal (No. 1, 3), and 1 other neonate (8.3%) presented with low lymphocytes, alanine-aminotransferase and creatine kinase (No. 2). Seven out of 12 (58.3%) neonates were immediately isolated after birth (No. 2, 3, 6–10). Nasopharyngeal or throat swabs were positive the earliest 24 h after birth (No. 8), 1 day after delivery (No. 4–5, 8) and 4 days after delivery (No. 6). In the 12 children who tested positive, swabs from vaginal secretion, breast milk, cord blood and placenta were negative. Amniotic fluid was positive in 1 out of 12 (8.3%) (No.8). Supportive treatment was conducted in 6 out of 12 (50.0%) neonates (No. 1, 7, 9–12), 2 out of 12 (16.7%) cases received antibiotics (No. 2, 8) and 1 out of 12 (8.3%) neonates required invasive ventilation (No. 6) (Fig. 2 ).
Figure 2.
Characteristics of SARS-CoV-2 positive neonates.
3.9. Risk of bias
From the included multi- and single-center studies only 2 studies had a risk of bias score below 70%. In total, 6 studies scored 11 or higher, making these reports less susceptible to bias. For case series and case reports, 1 report had a risk of bias score below 70%. Overall, 23 studies scored eight or higher (Supplemental Table 1).
4. Discussion
Since SARS-CoV-2 has become a worldwide pandemic, questions concerning possible vertical transmission and outcome of neonates born to infected mothers are raised. To assess this topic, this meta-analysis was conducted. The present meta-analysis includes 32 studies with 261 neonates born to mothers with infection.
Our analysis revealed that most of the neonates born to infected mothers did not show any clinical abnormalities (80.4%). Symptoms in infected newborns were mostly mild. In total, 13.3% of the neonates suffered from intrauterine distress. Neonatal death was reported in 3 cases.13 , 20 , 21 One neonate was still born at 38 + 7 weeks due to severe chronic villitis. PCR testing showed no viral inclusions. However, the authors state that there was a delay between fetal demise and RNA preservation for PCR analysis, so that results may be inaccurate.20 The second neonate died due to severe asphyxia. Before delivery the mother was put under mechanical ventilation. However, the authors did not state whether the neonate`s death was associated with SARS-CoV-2 infection.21 The third neonate died due to multiple organ failure. PCR test for SARS-CoV-2 was performed in this neonate with negative results.13 Therefore, with limited data on neonatal infection, it remains unclear whether neonatal death was associated with SARS-CoV-2 infection or not. Case reports have demonstrated a wide range of symptoms in neonates; for example, Zhu et al. analyzed 10 neonates, of whom 9 presented with symptoms after birth. Respiratory symptoms were present in 6, fever in 2 and tachycardia in 1 case.13 In our meta-analysis out of 120 neonates tested, 12 tested positive for infection. Eight out of 12 (66.7%) neonates presented with symptoms. When children were still in hospital, the onset of clinical symptoms ranged from 30 min to 3 days after delivery. Our results suggest that symptoms start early after delivery in case of infection. The onset of symptoms at home may be due to secondary infection from other family members. For example in the report by Han et al. mother and grandparents were confirmed with infection.33 Our analysis revealed that most infected neonates were discharged from hospital (82.5%), supporting the mild course of infection.34 Pregnant women with SARS-CoV-2 are prone to adverse neonatal complications including still birth, preterm birth and intrauterine growth restriction. In our study, premature birth occurred in 28.6% of the neonates. In the majority of included studies, reason for preterm birth was not mentioned13 , 22, 23, 24, 25, 26, 27, 28, 29 or authors did not detect an association with SARS-CoV-2.11 , 20 , 21 Possible association of prematurity and SARS-CoV-2 infection was only stated in 3 studies, namely pre-eclampsia, history of still births, irregular contractions, premature rupture of the membrane and pulmonary state of the mother.30, 31, 32 In another study, up to half of the infected mothers delivered preterm infants. However, they stated that fetal growth during third trimester did not seem to be affected35 and therefore reasons for prematurity were still unclear.11, 12, 13 Some authors hypothesized that morbidity in newborns could be related to possible hypoxemia in the infected mother, which increases the risk of perinatal adverse events.34
Literature reveals that vertical transmission of SARS-CoV-2 infection is possible10 and therefore it is still an object of current research for clinicians worldwide with respect to frequency and clinical relevance.36 Vivantis and colleagues were able to demonstrate a transplacental transmission of SARS-CoV-2 in a neonate born to a mother infected in the third trimester by comprehensive virological and pathological investigations.10 Regarding the risk of possible intrapartum transmission, data reveal that caesarean section is the preferred mode of delivery.36 The same results were demonstrated in our study, in which the majority of patients underwent caesarean section. In addition, another study revealed possible viral transmission through maternal breast milk.37 Here, RNA from SARS-CoV-2 was found in the breast milk from two mothers. Their neonates also showed SARS-CoV-2 rRT-PCR positive results in swabs; however, it remains unclear whether the neonates were infected by breastfeeding or other modes of transmission.37 Breast milk has many benefits including the passive transmission of antibodies.38 Our analysis shows that in studies reporting breast feeding was only conducted in 5 out of 38 (13.1%) neonates. In total, 24 out of 25 tests of breast milk for PCR of SARS-CoV-2 were negative. Most international associations recommend breastfeeding from mothers with confirmed SAR-CoV-2 under strict measures of infection control.39 , 40 The American Academy of Paediatrics (AAP) recommends separating the newborns from mothers with confirmed or suspected SARS-CoV-2 infection into separate isolation rooms.41 On April 4, 2020 the Centers for Disease Control (CDC) stated that the decision to separate newborns should be made on an individual basis.42 In our study, the separation of the mother and her newborn directly after birth did not prevent the SARS-CoV-2 infection in the majority of the infected newborns. It rather had a negative impact on the mother-child-bond.43 Therefore, this drastic sanction is questionable. In our analysis, swabs from vaginal secretion, cord blood and placenta were all negative. Amniotic fluid was positive in 1 case. In this case, the first neonatal throat swab immediately after birth was negative. Further throat swabs in the same neonate 24 h and 7 days after delivery showed positive results. The authors stated that the neonate was isolated directly after birth. Since the SRAS-CoV-2 rRT-PCR test was positive for amniotic fluid and neonatal throat swab, the route of infection may have been intrauterine. However, the first throat swab was negative and then a subsequent swab was positive 24 h after birth. Thus, secondary infection cannot be excluded.32 In other studies, tests performed from vaginal secretion, cord blood and placenta were also reported to be negative.11 , 16 The first proven transplacental transmission of SARS-CoV-2 and our findings reveal that vertical transmission of SARS-CoV-2 is a possible rare event. Further studies on perinatal SARS-CoV-2-infections with detailed analysis on both maternal and neonatal parameters are necessary to create a solid basis on potential effects of this infection on mother and newborn.
4.1. Limitations
This meta-analysis has several limitations. First of all, most information on neonates was retrieved from case reports. Additionally, many of the analyzed parameters of our meta-analysis were only reported in a minority of the cited publications. The lack of higher quality studies is mostly due to the novelty of the virus. Given the lack of high quality evidence, this type of meta-analysis may be helpful for a better understanding of clinical characteristics of neonates and its possible vertical transmission and underlines the urgent need of detailed, high-quality studies in this field. Furthermore, the number of studies on neonates from infected mothers are limited. However, we included 261 neonates, which represents the largest number of included cases so far. Another main limitation is the difficulty to retrieve the full text of some Chinese studies. Thus, we had to rely on English articles only. This strategy might lead to possible selection bias. In addition, most studies are from China, representing just one country of the major infection outbreak. This could be a bias, either for genetic or cultural reasons. An equal number of studies from other countries are needed for better understanding. Also, laboratory results of interest were not consistently reported and reference ranges not always clearly defined. In future reports on neonates born to SARS-CoV-2 positive mothers, cases with fetal complications like intrauterine growth restriction, low birth weight and preterm delivery should be reported in more detail, as these represent common fetal complications developed by infected pregnant women.
5. Conclusion
Of 261 neonates born to SARS-CoV-2 infected mothers only 120 neonates have been tested for SARS-CoV-2 infection. Out of these 120 neonates, 12 (10.0%) tested positive. Most neonates did not show any clinical abnormalities (80.4%), and symptoms in infected newborns were mostly mild. Most neonates with SARS-CoV-2 infection were discharged from hospital. The risk for vertical transmission is low. Nucleic acid test from vaginal secretions, cord blood and placenta were all negative. Tests from breast milk and amniotic fluid were positive in 1 case. To separate the mother from her newborn directly after birth did not prevent the SARS-CoV-2 infection in the majority of the infected newborns in our analysis. Larger epidemiological and more detailed studies are urgently needed to create a valid scientific data-basis for risk-adapted clinical decisions in the highly problematic field of perinatal SARS-CoV-2 transmission.
Funding/support
No funding was secured for this study.
PROSPERO registration
Registration number: CRD42020182444.
Contributors' statement
Dr. Vanessa Neef and Dr. Florian J. Raimann conceptualized and designed the study, performed the literature research, drafted the initial manuscript, calculated the statistics, reviewed and revised the manuscript.
Dr. Horst Buxmann collected data, carried out the initial analyses, and reviewed and revised the manuscript.
Prof. Dr. Horst Rabenau and Prof. Dr. Dr. Kai Zacharowski coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declaration of competing interest
All authors claim to have no conflicts of interest in connection with the subject of this publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pedneo.2020.10.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhou T., Liu Q., Yang Z., Liao J., Yang K., Bai W. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019-nCoV. J Evid Base Med. 2020;13:3–7. doi: 10.1111/jebm.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation . World Health Organization; 2020. Coronavirus disease (COVID-19) pandemic 2020.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at. Accessed May 27, 2020. [Google Scholar]
- 3.Johns Hopkins University . Johns Hopkins University; 2020. COVID-19 dashboard by the center for Systems science and engineering (CSSE) at Johns Hopkins university (JHU)https://coronavirus.jhu.edu/map.html Available at. Accessed May 27, 2020. [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi S.H., Kim H.W., Kang J.M., Kim D.H., Cho E.Y. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63:125–132. doi: 10.3345/cep.2020.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Center for Disease Control. Multisystem Inflammatory Syndrome in Chil (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Available at https://emergency.cdc.gov/coca/calls/2020/callinfo_051920.asp?cid=EPR-homepage. Accessed June 15, 2020.
- 8.Royal College of Paediatrics and Child Health. Guidance - Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS)2020. Available at https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims. Accessed June 15, 2020.
- 9.Yang H., Wang C., Poon L.C. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. 2020;55:435–437. doi: 10.1002/uog.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J. A case report of neonatal 2019 coronavirus disease in China. Clin Infect Dis. 2020;71:853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng L.K., Tao X.W., Yuan W.H., Wang J., Liu X., Liu Z.S. First case of neonate infected with novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58:E9. doi: 10.3760/cma.j.issn.0578-1310.2020.0009. [Article in Chinese] [DOI] [PubMed] [Google Scholar]
- 16.Peng Z., Wang J., Mo Y., Duan W., Xiang G., Yi M. Unlikely SARS-CoV-2 vertical transmission from mother to child: a case report. J Infect Public Health. 2020;13:818–820. doi: 10.1016/j.jiph.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 18.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 19.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lokken E.M., Walker C.L., Delaney S., Kachikis A., Kretzer N.M., Erickson A. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2020;223 doi: 10.1016/j.ajog.2020.05.031. 911.e1–911.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan J., Guo J., Fan C., Juan J., Yu X., Li J. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:111 e1–14. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breslin N., Baptiste C., Gyamfi-Bannerman C., Miller R., Martinez R., Bernstein K. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2:100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R., Zhang Y., Huang L., Cheng B.H., Xia Z.Y., Meng Q.T. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients. Can J Anaesth. 2020;67:655–663. doi: 10.1007/s12630-020-01630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S., Liao E., Cao D., Gao Y., Sun G., Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020;92:1556–1561. doi: 10.1002/jmv.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y., Peng H., Wang L., Zhao Y., Zeng L., Gao H. Infants born to mothers with a new coronavirus (COVID-19) Front Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020;71:2035–2041. doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W., Wang J., Li W., Zhou Z., Liu S., Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020;14:193–198. doi: 10.1007/s11684-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y., Liu C., Dong L., Zhang C., Chen Y., Liu J. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. BJOG. 2020;127:1109–1115. doi: 10.1111/1471-0528.16276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang P., Wang X., Liu P., Wei C., He B., Zheng J. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. 2020;127:104356. doi: 10.1016/j.jcv.2020.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-kuraishy H.M., Al-Maiahy T.J., Al-Gareeb A.I., Musa R.A., Ali Z.H. COVID-19 pneumonia in an Iraqi pregnant woman with preterm delivery. Asian Pac J Reprod. 2020;9:156–158. [Google Scholar]
- 31.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A Case of 2019 Novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020;71:844–846. doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamaniyan M., Ebadi A., Aghajanpoor S., Rahmani Z., Haghshenas M., Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat Diagn. 2020;40:1759–1761. doi: 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han M.S., Seong M.W., Heo E.Y., Park J.H., Kim N., Shin S. Sequential analysis of viral load in a neonate and her mother infected with SARS-CoV-2. Clin Infect Dis. 2020;71:2236–2239. doi: 10.1093/cid/ciaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mustafa N.M., A Selim L. Characterisation of COVID-19 Pandemic in paediatric age group: a systematic review and meta-analysis. J Clin Virol. 2020;128:104395. doi: 10.1016/j.jcv.2020.104395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullins E., Evans D., Viner R.M., O'Brien P., Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55:586–592. doi: 10.1002/uog.22014. [DOI] [PubMed] [Google Scholar]
- 36.Muhidin S., Behboodi Moghadam Z., Vizheh M. Analysis of maternal coronavirus infections and neonates born to mothers with 2019-nCoV; a systematic review. Arch Acad Emerg Med. 2020;8:e49. [PMC free article] [PubMed] [Google Scholar]
- 37.Groß R., Conzelmann C., Müller J.A., Stenger S., Steinhart K., Kirchhoff F. Detection of SARS-CoV-2 in human breastmilk. Lancet. 2020;395:1757–1758. doi: 10.1016/S0140-6736(20)31181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanson L.A., Korotkova M. The role of breastfeeding in prevention of neonatal infection. Semin Neonatol. 2002;7:275–281. doi: 10.1016/s1084-2756(02)90124-7. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Breastfeeding advice during the COVID-19 outbreak: World Health Organization. Available at https://reliefweb.int/report/world/who-emro-breastfeeding-advice-during-covid-19-outbreak-march-2020. Accessed May 27, 2020.
- 40.Davanzo R., Moro G., Sandri F., Agosti M., Moretti C., Mosca F. Breastfeeding and coronavirus disease-2019: Ad interim indications of the Italian society of neonatology endorsed by the union of European neonatal & perinatal societies. Matern Child Nutr. 2020;16 doi: 10.1111/mcn.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puopolo KM. American Academy of Pediatrics Committee on Fetus and Newborn. Section on Neonata Perinatal Medicine, and Committee on Infectious Diseases. Initial Guidance: Management of Infants Born to Mothers with COVID-19 2020. Available at https://www.aappublications.org/news/2020/04/02/infantcovidguidance040220. Accessed May 27, 2020.
- 42.Center for Disease Control . 2020. Considerations for inpatient obstetric Healthcare settings.https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html Available at. Accessed May 27, 2020. [Google Scholar]
- 43.Linnér A., Westrup B., Lode-Kolz K., Klemming S., Lillieskold S., Markhus Pike H. Immediate parent-infant skin-to-skin study (IPISTOSS): study protocol of a randomised controlled trial on very preterm infants cared for in skin-to-skin contact immediately after birth and potential physiological, epigenetic, psychological and neurodevelopmental consequences. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-038938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- 44.Kalafat E., Yaprak E., Cinar G., Varli B., Ozisik S., Uzun C. Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obstet Gynecol. 2020;55:835–837. doi: 10.1002/uog.22034. [DOI] [PubMed] [Google Scholar]
- 45.Liu D., Li L., Wu X., Zheng D., Wang J., Yang L. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020;215:127–132. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 46.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang H., Sun G., Tang F., Peng M., Gao Y., Peng J. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J Infect. 2020;81:e40–e44. doi: 10.1016/j.jinf.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z.J., Yu X.J., Fu T., Liu Y., Jiang Y., Yang B.X. Novel coronavirus infection in newborn babies aged <28 days in China. Eur Respir J. 2020;55:2000697. doi: 10.1183/13993003.00697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee D.H., Lee J., Kim E., Woo K., Park H.Y., An J. Emergency cesarean section performed in a patient with confirmed severe acute respiratory syndrome Coronavirus-2 -a case report. Korean J Anesthesiol. 2020;73:347–351. doi: 10.4097/kja.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu X., Gao J., Luo X., Feng L., Liu W., Chen J. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID-19) pneumonia. Obstet Gynecol. 2020;136:65–67. doi: 10.1097/AOG.0000000000003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrazzi E., Frigerio L., Savasi V., Vergani P., Prefumo F., Barresi S. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. BJOG. 2020;127:1116–1121. doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyra J., Valente R., Rosário M., Guimarães M. Cesarean section in a pregnant woman with COVID-19: first case in Portugal. Acta Med Port. 2020;33:429–431. doi: 10.20344/amp.13883. [DOI] [PubMed] [Google Scholar]
- 53.Kelly J.C., Dombrowksi M., O’Neil-Callahan M., Kernberg A.S., Frolova A.I., Stout M.J. False-negative testing for severe acute respiratory syndrome coronavirus 2: consideration in obstetrical care. Am J Obstet Gynecol MFM. 2020;2:100130. doi: 10.1016/j.ajogmf.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schnettler W.T., Al Ahwel Y., Suhag A. Severe respiratory distress syndrome in coronavirus disease 2019-infected pregnancy: obstetric and intensive care considerations. Am J Obstet Gynecol MFM. 2020;2:100120. doi: 10.1016/j.ajogmf.2020.100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song L., Xiao W., Ling K., Yao S., Chen X. Anesthetic management for emergent cesarean delivery in a parturient with recent diagnosis of coronavirus disease 2019 (COVID-19): a case report. Transl Perioper & Pain Med. 2020;7:234–237. [Google Scholar]
- 56.Vlachodimitropoulou Koumoutsea E., Vivanti A.J., Shehata N., Benachi A., Le Gouez A., Desconclois C. COVID-19 and acute coagulopathy in pregnancy. J Thromb Haemost. 2020;18:1648–1652. doi: 10.1111/jth.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.