Abstract
Objectives
The aim was to investigate the long-term prophylactic efficacy, drug retention and safety of low-dose sulfamethoxazole–trimethoprim (SMX/TMP) prophylaxis against Pneumocystis pneumonia (PCP).
Methods
Adult patients with rheumatic diseases receiving prednisolone ≥0.6 mg/kg/day were randomized into the single-strength group (SS; SMX/TMP 400/80 mg daily), the half-strength group (HS; 200/40 mg daily) or the escalation group (ES; starting at 40/8 mg and increasing incrementally to 200/40 mg daily) and treated for 24 weeks, then observed for 52 weeks. The primary endpoint, the PCP non-incidence rate (non-IR) at week 24, has been reported previously. The secondary endpoints were the PCP non-IR at week 52, treatment discontinuation rate and adverse events.
Results
Fifty-eight, 59 and 55 patients in the SS, HS and ES, respectively, received SMX/TMP. PCP did not develop in any of the patients by week 52. The estimated PCP non-IR in patients receiving SMX/TMP 200/40 mg daily (HS and ES) was 96.8–100%. Throughout the 52-week observation period, the overall discontinuation rate was significantly lower in HS than in SS (22.7 vs 47.2%, P = 0.004). The discontinuation rates attributable to adverse events were significantly lower in HS (19.1%, P = 0.007) and ES (20.3%, P = 0.007) than in SS (41.8%). The IRs of adverse events requiring SMX/TMP dose reduction before week 52 differed among the three groups, with a significantly higher IR in SS than in HS or ES (P = 0.007).
Conclusion
SMX/TMP 200/40 mg had a high PCP prevention rate and was superior to SMX/TMP 400/80 mg in terms of drug retention and safety.
Trial registration
University Hospital Medical Information Network Clinical Trials Registry, UMIN000007727.
Keywords: Pneumocystis pneumonia, sulfamethoxazole–trimethoprim, prophylaxis, efficacy, safety, drug discontinuation rate, rheumatic disease, randomized controlled trial
Key messages
Sulfamethoxazole–trimethoprim dosages for Pneumocystis pneumonia prophylaxis were compared in a randomized controlled trial.
Sulfamethoxazole–trimethoprim 200/40 mg daily has good efficacy and tolerability in patients with rheumatic diseases.
Sulfamethoxazole–trimethoprim 200/40 mg daily is recommended for Pneumocystis pneumonia prophylaxis in Japanese patients with rheumatic diseases.
Introduction
Pneumocystis pneumonia (PCP) can have significant impacts on the clinical course of immunocompromized patients [1]. In particular, in non-HIV patients PCP can result in rapid deterioration and is more likely to have a poor prognosis [2] and should be prevented appropriately. Currently, sulfamethoxazole–trimethoprim (SMX/TMP) is widely used as the first-line drug for PCP prophylaxis [1, 3]. When used properly, the prevention rate is reportedly 89–100%. In a retrospective study of 1522 treatments of rheumatic diseases with high-dose CSs, the preventive efficacy of SMX/TMP was 93% [4]. In a study of RA patients using biologics, the PCP non-incidence rate (non-IR) was 100% in the prophylactic group and 98.4% in the non-prophylactic group [5]. However, many adverse events (AEs) related to this drug have been reported [3], which can necessitate switching to other drugs, discontinuation of prophylaxis and, eventually, development of PCP. Alternative drugs, such as pentamidine isethionate, atovaquone and dapsone, are considered to be less effective [6] and also produce various adverse drug reactions.
In PCP prophylaxis, striking a balance between benefit (i.e. prophylactic effect) and risk (i.e. AEs) is required. Guidelines for patients with HIV infection, haematological malignancies and solid organ transplantation indicate which patients are at risk for PCP and should receive prophylaxis [7–10]. Although PCP prophylaxis is indicated in some patients with rheumatic diseases [1, 5, 11–13], no clear consensus on prophylactic regimens has been developed because of the lack of robust evidence, and no official guidelines for PCP prophylaxis exist for patients with rheumatic diseases. To minimize the risks inherent in PCP prophylaxis, reduction of the AEs of SMX/TMP using an appropriate regimen is desirable. Prasad et al. [14] reported that SMX/TMP 400 mg/80 mg three times a week in renal transplant patients reduced AEs without affecting prophylaxis. Takenaka et al. [15] showed that SMX/TMP 400 mg/80 mg daily with dose escalation was superior to SMX/TMP 400 mg/80 mg daily in terms of the continuation rate in 41 patients with rheumatic diseases who started treatment with CSs. Suyama et al. [16] retrospectively compared SMX/TMP 400 mg/80 mg daily with 400 mg/80 mg in a dose escalation regimen in 59 patients with SLE and found that the latter was safer. In our previous report, using an open-label, randomized controlled trial, we compared the efficacy, drug retention and safety of three regimens (SMX/TMP 400 mg/80 mg daily, 200 mg/40 mg daily or 200 mg/40 mg with dose escalation) in 183 patients with systemic rheumatic diseases who started at a dosage of 0.6 mg/kg/day or more of prednisolone or an equivalent dosage of CS [17]. At week 24, the 200 mg/40 mg daily regimen was superior in efficacy, drug retention and safety. Herein, we report the results of observations at week 52 to verify long-term prophylactic effects and safety of the regimens.
Methods
Patients
The inclusion and exclusion criteria of the present study were described previously [17]. In brief, the inclusion criteria were follows: age ≥20 years; admission to one of the participating institutions for treatment of new-onset or relapsed systemic rheumatic disease in the study period; written informed consent; an oral prednisolone starting dosage at ≥0.6 mg/kg/day or an equivalent dosage of CS regardless of concomitant immunosuppressive drugs; no previous use of SMX/TMP, pentamidine isethionate or dapsone; and serum creatinine values within the upper limit of the normal range according to the institutional standard. Patients were excluded if they received a biologic agent, had a history of PCP or were unable to start SMX/TMP within 10 days of starting prednisolone.
Study design
This study was an open-label, multicentre, randomized controlled trial, and the study design was described previously [17]. In brief, the patients were randomized into one of three arms at a 1:1:1 ratio by using computer-based, central, dynamic allocation with block randomization. Patients in the single-strength dosage group (SS) started SMX/TMP 400 mg/80 mg, the equivalent of a single-strength tablet, and patients in the half-strength dosage group (HS) started SMX/TMP 200 mg/40 mg, and both groups continued the same dosage for 24 weeks. Patients in the escalation group (ES) started SMX/TMP 40 mg/8 mg, the equivalent of 10% of a single-strength tablet, and the dosage was increased by 40 mg/8 mg weekly up to 200 mg/40 mg and continued for 24 weeks. All patients received SMX/TMP in granule form. After week 24 or discontinuation of the study, SMX/TMP use, including the dosage, interval and treatment duration, was left to the discretion of the attending physicians. The observation period was 52 weeks irrespective of continuation or discontinuation of the study regimen. This study was approved by the ethics committee of Tokyo Medical and Dental University Hospital (#2349) and the other participating institutions. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000007727).
Endpoints
PCP non-IRs at week 24 between SS and ES (the primary endpoint of this study) and the other comparisons between groups at week 24 have been reported previously [17]. The PCP non-IRs, treatment discontinuation rates and AEs at 52 weeks are reported here. PCP was diagnosed clinically by symptoms, laboratory tests and imaging by site investigators, with verification by the clinical event review committee, which included experts in pulmonary medicine, infectious diseases and rheumatology.
Statistical analyses
The calculated sample size was 58 patients per group, assuming the PCP non-IR of SS to be 93% and that of ES to be 98%. The non-inferiority limit was set at 5%, one-sided α at 0.05, and β at 0.20 [15, 17]. For statistical analyses, treatment discontinuation rates were analysed using the Kaplan–Meier method and the log-rank test. Fisher’s exact test with adjusted residuals was used to compare the incidences of AEs. As a post hoc analysis, the PCP non-IRs of each group and the combined group of HS and ES were estimated using the Clopper–Pearson exact confidence interval [18] and the rule of three [19], because PCP developed in none of the patients.
Results
Randomization and follow-up
One hundred and eighty-three patients were randomized into one of three arms, with 58, 59 and 55 patients in SS, HS and ES, respectively, starting treatment with SMX/TMP. Twenty-nine, 43 and 34 patients in SS, HS and ES, respectively, continued the same regimen until week 52. Details are given in Supplementary Fig. S1, available at Rheumatology Advances in Practice online. Reasons for discontinuation of the regimens were AEs, prescription errors or the investigators’ discretion. All the patients were followed for 52 weeks, except those who died or were transferred to another hospital.
Baseline characteristics of the patients
Supplementary Table S1, available at Rheumatology Advances in Practice online, shows the baseline characteristics of the patients. The mean age, percentage of female patients and mean body weight were similar across the groups. There were no significant differences in terms of a background of rheumatic diseases, co-morbidities or treatments before enrolment. The range of prednisolone-equivalent CS dosages was 0.94–0.97 mg/kg/day at baseline, 10–12.5 mg/day at week 24, and 7–8 mg/day at week 52. The proportion of the patients receiving i.v. pulsed methylprednisolone between weeks 0 and 12 was 20–32.2%. The proportion of those receiving an immunosuppressant was 67.8–81.8, 65.5–78.2, 63.8–71.2 and 65.5–74.6%, at weeks 0–12, 12–24, 24–36 and 36–52, respectively.
Efficacy and drug discontinuation rate
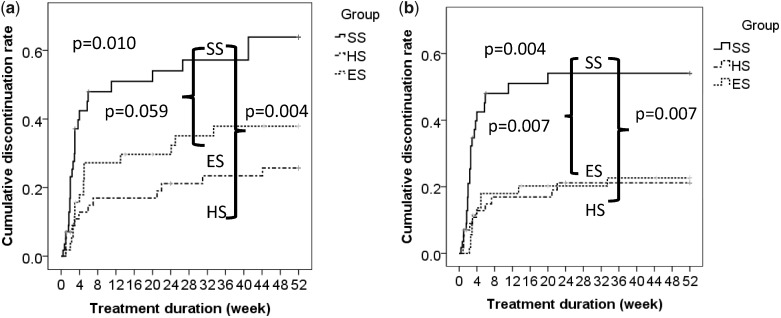
No PCP cases were reported up to week 52, and the PCP non-IR was estimated by using the Clopper–Pearson exact confidence interval in post hoc analysis [18]. The estimated non-IR at week 52 in SS, HS and ES was 93.8–100, 93.9–100 and 93.5–100%, respectively. Given that the patients in HS and ES received 200 mg/40 mg SMX/TMP daily for 52 and 47 weeks, respectively, we combined these two groups and estimated the PCP non-IR of the combined group to be 96.8–100% (n = 114). Estimation using the rule of three showed almost the same results [19]. The cumulative discontinuation rates due to any reason are shown in Fig. 1a using the Kaplan–Meier curves. HS showed a significantly lower cumulative discontinuation rate than SS (47.2 vs 22.7%, P = 0.004), which also tended to be lower in ES (31.6%) than in SS (P = 0.059). Fig. 1b shows the cumulative discontinuation rates attributable to AEs. A significant difference was observed between SS (41.8%) and ES (20.3%) (P = 0.007) and between SS and HS (19.1%) (P = 0.007).
Fig. 1.
Discontinuation rates of the allocated treatments by Kaplain–Meier analysis
The discontinuation rates of the allocated treatments attributable to any reasons (a) and attributable to adverse events (b) are shown. Cumulative treatment discontinuation rates were compared using the log-rank test among groups. ES: escalation group; HS: half-strength dosage group; SS: single-strength dosage group.
Safety
Table 1 summarizes the AEs and their breakdown during the study period. Although no significant difference in the overall IRs of AEs and serious AEs was observed, there was a significant difference in the proportion of patients with AEs who required SMX/TMP dosage reduction (P = 0.007) and of patients with AEs of special interest (P = 0.001) across all three groups, with SS showing the highest proportion. Among the AEs of special interest, thrombocytopenia and hyponatraemia were observed more frequently in SS, but the difference was not tested statistically owing to the relatively small number of cases.
Table 1.
Adverse events reported by week 52
| Adverse event | SS (n = 58) | HS (n = 59) | ES (n = 55) | P-value a |
|---|---|---|---|---|
| AE, n (%) | 34 (58.6) | 25 (42.4) | 26 (47.3) | 0.201 |
| Serious AEb, n (%) | 9 (15.5) | 12(20.3) | 6 (10.9) | 0.408 |
| AE requiring SMX/TMP dose reduction, n (%) | 12 (20.7) | 2 (3.4)c | 4 (7.3)c | 0.007 |
| AE requiring SMX/TMP discontinuation, n (%) | 12 (20.7) | 8 (13.6) | 6 (10.9) | 0.330 |
| AE leading to death, n (%) | 1 (1.7) | 3 (5.1) | 1 (1.8) | 0.622 |
| AE of special interest, n (%) | 25 (43.1) | 10 (16.9)c | 9 (16.4)c | 0.001 |
| Fever, n (%) | 2 (3.4) | 0 (0) | 0 (0) | ND |
| Rash, n (%) | 5 (8.6) | 2 (3.4) | 1 (1.8) | ND |
| Appetite loss, n (%) | 1 (1.7) | 0 (0) | 1 (1.8) | ND |
| Anaemia, n (%) | 1 (1.7) | 1 (1.7) | 0 (0) | ND |
| Leucocytopenia, n (%) | 1 (1.7) | 1 (1.7) | 0 (0) | ND |
| Thrombocytopenia, n (%) | 9 (15.5) | 3 (5.1) | 4 (7.3) | ND |
| Elevated LFT, n (%) | 6 (10.3) | 4 (6.8) | 4 (7.3) | ND |
| Elevated serum creatinine, n (%) | 3 (5.2) | 0 (0) | 1 (1.8) | ND |
| Hyponatraemia, n (%) | 6 (10.3) | 1 (1.7) | 0 (0) | ND |
| Hyperpotassaemia, n (%) | 3 (5.2) | 1 (1.7) | 1 (1.8) | ND |
Adverse events (AEs) reported in each group by week 52 were shown. Neither the incidence rates of overall AEs and serious AEs nor the rate of AEs requiring SMX/TMP dose reduction and AEs of special interest differed significantly among the three groups.
By Fisher’s exact test.
Serious AE: sepsis, organizing pneumonia, severe liver failure, flare of rheumatic disease, rash requiring hospitalization, thrombocytopenia requiring hospitalization, mental disorder requiring hospitalization or AE resulting in death.
P < 0.05 by adjusted residues vs SS.
AE: adverse event; ES: escalation group; HS: half-strength dosage group; LFT: liver function test; ND: not detected; SMX/TMP: sulfamethoxazole–trimethoprim; SS: single-strength dosage group.
Discussion
In the present study, PCP did not develop in any of the patients in the 52-week observation period. The accumulated overall discontinuation rate for 52 weeks was significantly lower in HS than in SS, suggesting that the half-strength regimen had the better benefit–risk balance. A number of reasons might account for the lack of development of PCP in the present study. The physicians in charge at the participating institutions were proficient in treating rheumatic diseases and were knowledgeable about PCP prophylaxis. Many patients continued PCP prophylaxis even after stopping the allocated treatment with SMX/TMP; the numbers of patients without prophylaxis were 6 in weeks 0–12, 10 in weeks 12–24, and 17 in weeks 24–52. The lack of development of PCP in this study is understandable in view of the fact that the incidence of PCP without prophylaxis is reportedly 2.3–8.97% [5] and that the median prednisolone dosage administered at week 24 in this study was <15 mg/day, at which discontinuation of prophylaxis may be considered safely [4].
Some differences were observed between HS and ES in the drug discontinuation rates. The cumulative discontinuation rates by AEs did not differ significantly between HS and ES; the observed AEs, such as thrombocytopenia, elevated liver function test and hyponatraemia, were mostly dose dependent, with the exception of rash. This breakdown of AEs is consistent with previous reports [15, 16]. In contrast, HS had a lower drug discontinuation rate for all reasons than ES, mainly resulting from prescription errors in ES. In patients with allergy to multiple drugs, SMX/TMP may be administered under the escalation regimen. Recently, Suyama et al. [16] reported that patients with SLE, especially those with positive anti-Ro/SSA antibodies, had a higher incidence of adverse drug reactions to SMX/TMP. If true, the escalation regimen might be a better treatment option for these patients.
This study has some limitations. First, there was a detection bias attributable to nonblinding. The investigators might have expected more AEs in SS. Second, the efficacy and safety of a follow-up period >52 weeks are unknown. However, the median time from the start of treatment with CS to the onset of PCP was reportedly 12 weeks [20], and a 52-week observation period appeared to be sufficient in this context. Third, patients with reduced renal function and patients with low body weight were excluded. In addition, >60% of the patients were female; the mean body weight of the patients was <60 kg in all three groups, and only five patients weighed >80 kg. Fourth, not a large number of patients were enrolled in this study. Fifth, we did not test an alternate-day regimen in this study. This could be a challenge for the future.
Conclusion
In patients with the characteristics included in the present study, SMX/TMP dosage reduction decreased the cumulative drug discontinuation rate and AEs requiring SMX/TMP dosage reduction. In patients with normal serum creatinine concentrations and similar body weights to those enrolled in the present study, SMX/TMP 200 mg/40 mg might provide a favourable benefit–risk balance in PCP prophylaxis.
Supplementary Material
Acknowledgements
We thank the following researchers for their contribution to recruiting the patients and collecting the clinical data: Michi Tsutsumino (Department of Pharmacovigilance, Tokyo Medical and Dental University), Tsuyoshi Takeda (Third Department of Internal Medicine, Obihiro-Kosei General Hospital), Hiroto Nakano and Kenichiro Tokunaga (Kameda Medical Center), Yohko Murakawa (Department of Rheumatology, Faculty of Medicine, Shimane University), Kayoko Kaneko and Funiaki Kondo (Department of Rheumatology, Soka Municipal Hospital). We also thank Ms Marie Kokido for her contribution as the secretary of this study. We sincerely thank all the rheumatologists and medical staff who care for the patients enrolled in this study. The detailed protocols and aggregate data of this study are available upon request.
Funding: This work was supported by unrestricted research grants to the Department of Pharmacovigilance, Tokyo Medical and Dental University and to the Division of Epidemiology and Pharmacoepidemiology, Institute of Rheumatology, Tokyo Women’s Medical University. This work was also supported by a research grant from the Ministry of Health, Labour, and Welfare of Japan (H30-menneki-shitei-002). Funders of the research grants had no roles in this clinical trial.
Disclosure statement: Tokyo Medical and Dental University (TMDU) received unrestricted research grants for the Department of Pharmacovigilance from Abbvie Japan Co., Ltd, Astellas Pharma Inc., Bristol-Myers Squibb K.K., Chugai Pharmaceutical Co., Ltd, Eisai Co., Ltd, Mitsubishi Tanabe Pharma Co., Ono Pharmaceuticals, Pfizer Japan Inc., Sanofi-Aventis K.K., Santen Pharmaceutical Co., Ltd, Takeda Pharmaceutical Co., Ltd, and UCB Japan, with which TMDU paid the salaries of K.N., R.S., H.Y. and M.H. K.S. received a manuscript fee from Bristol-Myers Squibb K.K. M.K. received research funding from Chugai Pharmaceutical Co., Eisai Co., Ltd, and Mitsubishi Tanabe Pharma Co. H.Y. is currently an employee of AbbVie GK. T.N. received research funding from Chugai Pharmaceutical Co. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Advances in Practice online.
References
- 1. Thomas CFJ, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004;350:2487–98. [DOI] [PubMed] [Google Scholar]
- 2. Kovacs JA, Hiemenz JW, Macher AM. et al. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med 1984;100:663–71. [DOI] [PubMed] [Google Scholar]
- 3. Stern A, Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev 2014;(10):CD005590. [DOI] [PMC free article] [PubMed]
- 4. Park JW, Curtis JR, Moon J. et al. Prophylactic effect of trimethoprim-sulfamethoxazole for Pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2018;77:644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 2014;16:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ioannidis JP, Cappelleri JC, Skolnik PR, Lau J, Sacks HS. A meta-analysis of the relative efficacy and toxicity of Pneumocystis carinii prophylactic regimens. Arch Intern Med 1996;156:177–88. [PubMed] [Google Scholar]
- 7. Baden LR, Swaminathan S, Angarone M. et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016;14:882–913. [DOI] [PubMed] [Google Scholar]
- 8. Maertens J, Cesaro S, Maschmeyer G. et al. ECIL guidelines for preventing Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 2016;71:2397–404. [DOI] [PubMed] [Google Scholar]
- 9. Cooley L, Dendle C, Wolf J. et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014;44:1350–63. [DOI] [PubMed] [Google Scholar]
- 10. Martin SI, Fishman JA; AST Infectious Diseases Community of Practice. Pneumocystis pneumonia in solid organ transplantation. Am J Transplant 2013;13(Suppl 4):272–9. [DOI] [PubMed] [Google Scholar]
- 11. Sepkowitz KA, Brown AE, Armstrong D. Pneumocystis carinii pneumonia without acquired immunodeficiency syndrome. More patients, same risk. Arch Intern Med 1995;155:1125–8. [PubMed] [Google Scholar]
- 12. Roux A, Gonzalez F, Roux M. et al. Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Med Mal Infect 2014;44:185–98. [DOI] [PubMed] [Google Scholar]
- 13. Harigai M, Koike R, Miyasaka N; Pneumocystis Pneumonia under Anti-Tumor Necrosis Factor Therapy (PAT) Study Group. Pneumocystis pneumonia associated with infliximab in Japan. N Engl J Med 2007;357:1874–6. [DOI] [PubMed] [Google Scholar]
- 14. Prasad GVR, Beckley J, Mathur M. et al. Safety and efficacy of prophylaxis for Pneumocystis jirovecii pneumonia involving trimethoprim-sulfamethoxazole dose reduction in kidney transplantation. BMC Infect Dis 2019;19:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takenaka K, Komiya Y, Ota M, Yamazaki H, Nagasaka K. A dose-escalation regimen of trimethoprim-sulfamethoxazole is tolerable for prophylaxis against Pneumocystis jiroveci pneumonia in rheumatic diseases. Mod Rheumatol 2013;23:752–8. [DOI] [PubMed] [Google Scholar]
- 16. Suyama Y, Okada M, Rokutanda R. et al. Safety and efficacy of upfront graded administration of trimethoprim-sulfamethoxazole in systemic lupus erythematosus: a retrospective cohort study. Mod Rheumatol 2016;26:557–61. [DOI] [PubMed] [Google Scholar]
- 17. Utsunomiya M, Dobashi H, Odani T. et al. Optimal regimens of sulfamethoxazole-trimethoprim for chemoprophylaxis of Pneumocystis pneumonia in patients with systemic rheumatic diseases: results from a non-blinded, randomized controlled trial. Arthritis Res Ther 2017;19:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clopper C, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–13. [Google Scholar]
- 19. Hanley JA, Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA 1983;249:1743–5. [PubMed] [Google Scholar]
- 20. Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc 1996;71:5–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.