Abstract
Perioperative posterior ischemic optic neuropathy (PION) is a rare but devastating condition. Visual impairment is commonly bilateral, profound, and irreversible. The most frequently associated triggering events are spine surgeries, other orthopedic surgeries, cardiac bypass surgeries, and radical neck dissection. The etiology is multifactorial. The most commonly reported risk factors are severe and prolonged hypotension, anemia, hemodilution, orbital and periorbital edema, direct orbital compression by prone position, and abnormal autoregulation. This review discusses the current literature on perioperative PION and includes a study conducted by our group to investigate the perioperative risk factors of PION in order to better understand the pathogenesis and help identify high-risk patients. Our results provide further corroborating evidence that PION is associated with spinal, cardiovascular, and abdominal surgeries, longer duration of procedure, and facial edema. Anemia and chronic hypertension are frequent risk factors. Treatment for perioperative PION is uncertain and depends largely on the immediate reversal of hemodynamic alterations. Hence, it is important to identify patients at risk and accordingly take prophylactic measures to prevent its occurrence. Optimizing hemoglobin levels, hemodynamic status, and tissue oxygenation is crucial.
Keywords: Anemia, hypoxia, optic nerve, perioperative, posterior ischemic optic neuropathy, tissue perfusion pressure
Introduction
Posterior ischemic optic neuropathy (PION) was described by Hayreh as a distinct clinical entity in 1981.[1] To date, PION has been classified into three types: (1) arteritic PION usually due to giant cell arteritis; (2) nonarteritic PION usually due to hypotensive ischemia; and specifically (3) perioperative PION, a form of nonarteritic PION that occurs during or shortly after certain surgical procedures.[2]
Perioperative PION is characterized by sudden painless profound unilateral or bilateral visual loss, usually noted on waking from anesthesia, though it may be noted a few days later. It is an uncommon but devastating complication of certain surgeries. Stevens et al. reviewed a series of 3,450 spine surgeries over a 9-year period and found three (0.087%) perioperative PION cases.[3] Visual loss has been reported following various nonocular surgeries such as spinal procedures,[4,5,6,7,8,9,10,11,12] radical neck dissection,[13,14,15,16,17,18,19,20,21] venous graft in extremities,[22,23] cardiopulmonary bypass,[24,25,26] hip surgery,[24] other orthopedic surgery,[27] nasal surgery,[28] thoracotomy for hemothorax,[19] laparoscopic nephrectomy,[29] prostatectomy,[30] breast augmentation and abdominal liposuction,[31] and penetrating thoracoabdominal injury.[32] It has also been reported as a rare occurrence following ocular or periocular sinus surgeries[33,34,35,36] though these, not being remote, probably reflect a different pathophysiology. Spine surgery is the most frequently associated procedure, comprising 72% of patients in the American Society of Anesthesiologists (ASA) postoperative visual loss (POVL) registry,[37] and 60% of these cases were PION. Buono and Foroozan reviewed 83 previously published cases of perioperative PION and noted that spinal surgery accounted for 54% of cases, followed by radical neck dissection for 13%.[38] Cardiac surgery is another common procedure associated with ischemic optic neuropathy. The National Inpatient Sample (NIS) studied 20% of inpatient discharges in the U. S. from 1998 to 2012 and found the prevalence of ischemic optic neuropathy (ION) to be 1.02/10,000 after spinal procedures and 1.43/10,000 after cardiac procedures.[39,40] Notably, anterior ischemic optic neuropathy (AION) and PION were not differentiated in the NIS studies. While PION is more commonly seen after spinal surgery, AION is more common after cardiac surgery. Our group conducted a retrospective case series study to investigate the perioperative risk factors of PION to provide a better understanding of the pathogenesis of this condition. While there are many publications studying perioperative ION with both anterior and posterior cases combined, very few studied specifically perioperative PION. Analysis of this large case series may allow for the identification of patients with high-risk profiles and intraoperative factors who undergo high-risk surgeries.
Methods
This is a retrospective chart review consisting of 55 subjects and 103 eyes diagnosed as having PION following a major surgical procedure. The institutional review board (IRB) of the University of California, Los Angeles, approved the study (IRB name: Retrospective Chart Review of Patients with Posterior Ischemic Optic Neuropathy; IRB#20-001518). This study adhered to the tenets of the Declaration of Helsinki. There were 25 men and 30 women meeting the enrollment criteria. The evaluations were performed at Doheny Eye Institute by the same neuro-ophthalmologist (AAS). Diagnostic criteria include an acute decrease in visual acuity in one or both eyes, corresponding visual field defect, presence of a relative afferent pupillary defect in unilateral or asymmetric cases, absence of optic disc swelling or peripapillary flame hemorrhage, and absence of other optic nerve disorders. Subjects were excluded if there were other ocular conditions that could account for the visual loss. The medical history was reviewed and the subject's age, unilateral or bilateral involvement, visual acuity, color vision, type and length of surgery, amount of blood loss, lowest blood pressure, lowest hematocrit, known cardiovascular risk factors, and presence of facial edema were included. Due to the retrospective nature of the study, some patients did not have parts of the intraoperative particulars. Two-sample t-test assuming equal variance and Fisher's exact test were used for the analyses. P values of 0.05 or less were considered statistically significant.
Results
The age range of the subjects was 10–94 years. Of the 55 subjects, 49 (89.1%) had bilateral involvement and 6 (10.9%) had monocular involvement. Forty-two eyes (40.8%) had no light perception (NLP), 6 (5.8%) had light perception (LP), 11 (10.7%) had hand motion (HM), 9 (8.7%) had count finger (CF), 4 (3.9%) had 20/400, 6 (5.8%) had 20/200, and 25 (24.3%) had 20/100 or better vision [Figure 1]. Subjects were further subgrouped based on the type of surgery performed prior to visual loss: spinal (44 eyes, 23 subjects), cardiovascular-abdominal (CV-ABD) (32 eyes, 16 subjects), and others (27 eyes, 16 patients). In comparison to the “others” group, spinal (P = 0.04) and CV-ABD (P = 0.007) groups had poorer visual acuity. The median visual acuities for both spinal and CV-ABD groups were LP. The median visual acuity for the other procedures was 20/300. Visual acuity was associated with the type of surgery (P = 0.01) and color vision was associated with age (P = 0.04). The surgery length ranged from 3 to 12 h, with an average of 7.9 h. There were 38 eyes with documented surgery length. There were 24 eyes with NLP vision, three eyes with HM vision, three eyes with CF vision, one eye with 20/200 vision, and seven eyes with vision better than 20/100. The average surgery durations for the NLP, HM, CF, and >20/200 eyes were 8.48, 8.17, 6.33, and 6.63 h, respectively. The NLP group had the longest average surgery length. The length of surgery appeared to trend with visual outcome [Figure 2]. Postoperative facial edema was recorded in seven (30.4%) spinal, one (6.3%) CV-ABD, and zero others groups (P = 0.01) [Figure 3].
Figure 1.
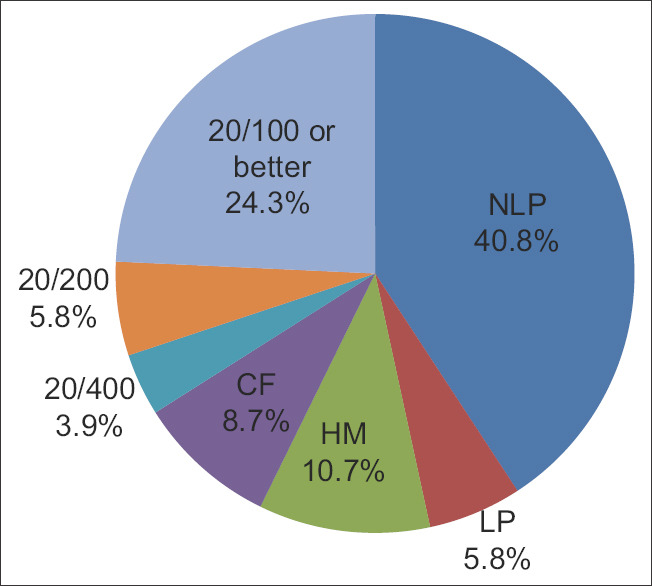
Presenting visual acuity of subjects with posterior ischemic optic neuropathy. Forty-two eyes (41%) had no light perception, 6 (6%) had light perception, 11 (11%) had hand motion, 9 (9%) had count finger, and only 4 (4%) had at least 20/400 vision. The majority of patients had no light perception vision
Figure 2.
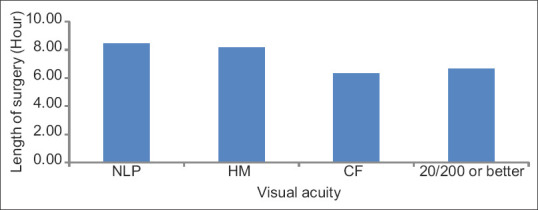
Surgery length ranged from 3 to 12 h with an average of 7.9 h. The average surgery durations for the no light perception, hand motion, count finger, and > 20/200 eyes were 8.5, 8.2, 6.3, and 6.6 h, respectively. Visual acuity appears to trend with surgery duration
Figure 3.
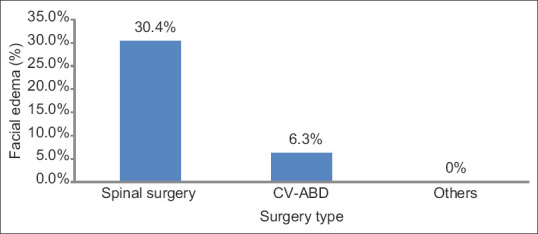
Postoperative facial edema was recorded most often in the spinal group, (P = 0.01)
Discussion
Pathogenesis
The underlying pathogenesis of PION is usually believed to be infarction of the optic nerve posterior to the lamina cribrosa where it may be vulnerable to ischemic insult, as this watershed area is supplied only by the surrounding pial plexus of fine, small-caliber vessels.[41,42] Histopathological studies of three perioperative PION cases all showed intraorbital optic nerve infarction.[38] Ross-Cisneros et al. conducted an animal study using a pressure-controlled hemorrhagic shock model of PION in rats to investigate the underlying mechanism. The result showed a prominent infarct in the central intracanalicular segment of the optic nerve, supporting the watershed ischemia hypothesis.[43]
On the other hand, especially with “disc-at-risk,” the infarct may be at the optic nerve head, mimicking the position and appearance of non-arteritic anterior ischemic optic neuropathy (NAION). We explicitly excluded cases with optic disc edema and peripapillary hemorrhages to not confound these data with spontaneous cases of NAION. There are, however, many postsurgical cases of NAION that probably reflect a version of PION that occurs more anteriorly. In particular, the ASA POVL Registry found no statistical significance between the presentation of AION versus PION cases after spine surgery, with respect to demographics, coexisting diseases, surgical characteristics, or anesthetic management.[37] The distinction between postoperative NAION and PION is, therefore, one of the anatomies, not mechanism.
Risk factors
The etiology of PION is probably multifactorial and may be influenced by patient-specific susceptibilities. In general, accepted risk factors include anemia and hypotension due to massive blood loss and prolonged surgery,[44,45] hemodilution from large amounts of crystalline intravenous fluids,[46,47] orbital or periorbital edema,[48] and in rare circumstances, direct orbital compression in the prone position.[49,50] Systemic diseases such as diabetes, hypertension, and atherosclerosis may also play a role in the pathogenesis by disrupting normal vasculature autoregulation, thereby increasing resistance and decreasing compensatory blood flow.[21,51,52] Abnormal autoregulation can also be induced by vasospasm and endogenous or pharmacologic vasoconstriction. Risk factors extrapolated from NAION such as disc at risk, obstructive sleep apnea, and use of amiodarone and phosphodiesterase-5 inhibitors have been proposed as potential risk factors for perioperative PION.[53] The POVL Study Group conducted a case-controlled study of 80 ION subjects with 215 control subjects and determined that male sex, obesity, Wilson frame use, anesthesia duration, estimated blood loss, and colloid as a percent of nonblood replacement are major risk factors for ischemic optic neuropathy after spinal fusion surgery.[54] Obstructive sleep apnea, in addition to age and male gender, was included in a predictive model for perioperative ION in spine fusion surgeries.[55] Another study evaluated more than five million patients after cardiac procedures from 1998 to 2013 and found 794 cases (0.014%) of ION. The risk factors were carotid artery stenosis, stroke, diabetic retinopathy, hypertensive retinopathy, macular degeneration, glaucoma, and cataract.[40] Interestingly, these results suggest that concurrent degenerative eye diseases may be associated with perioperative ION, perhaps as a surrogate marker of biological aging.
Clinical presentation
It is a catastrophe for patients, surgeons, and anesthesiologists when the patient awakens blind. Although rare, this condition has become an important medicolegal issue, warranting attention in the general medical community. Symptoms of onset usually occur within 24 h.[56] Examination reveals severe reductions in visual acuity, color vision, and visual field along with a relative afferent pupillary defect in unilateral or asymmetrical bilateral disease. However, at the onset of PION, the optic disc often appears normal, pallor is only seen after 6–8 weeks. The absence of optic disc edema is a key feature distinguishing PION from AION. Despite poor visual acuity, fluorescein angiography, electroretinogram, and routine neuroimaging will likely be normal. There may be other postoperative issues, resulting in a delay in diagnosis. In the setting of profound vision loss, the lack of relative afferent pupillary defect in the presence of a normal fundus examination and normal neuroimaging, functional vision loss may be mistakenly diagnosed.[57] Unlike AION, which can be distinguished by funduscopic examination, the diagnosis of PION remains challenging. More advanced magnetic resonance imaging with diffusion-weighted and fluid-attenuated inversion recovery sequences has been shown in small case reports to demonstrate abnormal hyperintensity within the infarcted intraorbital optic nerves and sparing of the immediately retrobulbar portion of the optic nerve.[58,59] The extent of infarction may be visualized as a high signal on diffusion-weighted imaging within minutes.[60,61] However, larger studies are still required to substantiate these findings.
Our data
The present study data provide further corroborating evidence that PION is associated with spinal, cardiovascular, and abdominal surgeries, longer duration of procedure, and facial edema and strongly implies a mechanism.
The majority of PION subjects in our study had profound vision loss; NLP was the most common visual outcome. This is similar to previous reports. Hayreh described a retrospective series of four surgical PION eyes.[62] Of the four eyes, three were NLP and one was CFs vision. Sadda et al. studied 43 perioperative PION eyes from 28 patients and found that 70% of eyes had visual acuities of CFs or worse.[63] However, unlike our results, they saw bilateral involvement in only 54% of the cases. Limiting their cases to those that followed spinal surgery, 70% had bilateral involvement, a number much closer to our figure of 89.1% [Figure 4]. In Buono's review, CFs vision or worse was seen in 75.8% of eyes and 53.8% of eyes had an initial visual acuity of NLP and bilaterality was seen in 60.9% of the patients.[38]
Figure 4.
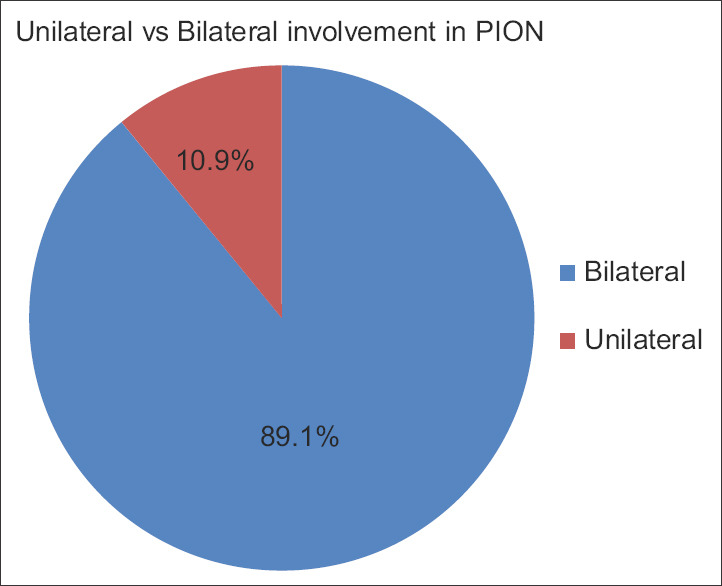
About 90% of our posterior ischemic optic neuropathy cases had both eyes affected
In our study, postoperative facial edema was noted significantly more frequently in the spinal, though also in CV-ABD surgery cases. The prone and head-down position increases gravity-dependent head edema, and the increased venous pressure would logically predispose to PION. In a review of previously published 37 cases, Myers et al. found 36 cases to be in prone positioning.[4] Cheng et al. found that prone positioning increases intraocular pressure during anesthesia.[64] This is very meaningful as intraocular pressure is a surrogate marker for the venous pressure behind the eye. The eye's aqueous humor drains into the episcleral veins that drain into the orbital veins, so a rise in pressure of the orbital venous system elevates the eye pressure. Lam and Douthwaite found an increase in intraocular pressure after only 8 min in the prone position.[65] Since ocular perfusion pressure is a function of mean arterial pressure minus intraocular pressure, ocular perfusion pressure decreases in spite of normal blood pressure during anesthesia.[64] Prone position leads to increased orbital venous pressure from increased abdominal venous pressure as well.[21] In spinal surgery, the Trendelenburg position further increases orbital and facial edema, resulting in increased orbital venous pressures.[7,8,9] In some cases, compression of the delicate capillary plexus supplying the optic nerve might result from directly increased orbital pressure. However, unlike the “headrest syndrome,” PION can occur even when surgery and head positioning were appropriately performed and the eye and orbit are not directly compressed.
Risk stratification formula
PION is a consequence of hypoxia of the optic nerve. The amount of oxygen delivered to the optic nerve is a product of the oxygen-carrying capacity in the blood and the amount of blood flow to the optic nerve. Anemia, intraoperative blood loss, and hemodilution can all contribute to a decrease in the oxygen-carrying capacity of the blood. A reduction in perfusion pressure would result from hypotension or elevated tissue pressure; both would compromise blood flow to the tissues of the optic nerve. Taken in combination, low oxygen-carrying capacity plus less blood flow increases the risk of hypoxia to the optic nerve. In the setting of good oxygen saturation, the hematocrit level is a good proxy for the amount of oxygen in the blood. The perfusion pressure in the optic nerve is the difference between the blood pressure and the tissue perfusion pressure. Therefore, the risk of PION is inversely related to hematocrit × (blood pressure–tissue perfusion pressure). This formula may be useful in risk stratification for certain surgeries [Figure 5]. A decrease in the perfusion pressure may be caused by (1) hypotension as measured by the mean arterial pressure or (2) increases in venous pressure due to prolonged head-down and prone position. In Buono and Foroozan's review, the mean of the drop in postoperative hematocrit was reportedly 14.4%, the mean of the drop in systolic blood pressure was 53 mmHg, and the mean intraoperative blood loss was 3.7 L.[38] In our study, we found that the average blood loss was 2.3 L (ranging 0.8 L-7.6 L). On average, the lowest hematocrit was 26.1% (range: 20%–36.6%) about 2/3 of the 40.2% at baseline. Some orthopedic surgeons chose to deliberately maintain arterial hypotension in the thought that this reduces intraoperative arterial bleeding, though this probably increased the risk of PION. Furthermore, in response to intraoperative hypotension, some anesthesiologists give vasopressor agents to raise the blood pressure. However, while these agents can increase systemic blood pressure, vasoconstriction of terminal arterioles can also reduce blood flow in the capillary bed, in essence interfering with autoregulation by shunting blood away from critical tissues such as the optic nerve, resulting in more optic nerve hypoxia. Chronic atherosclerosis and hypertension also contribute to poor autoregulation.
Figure 5.
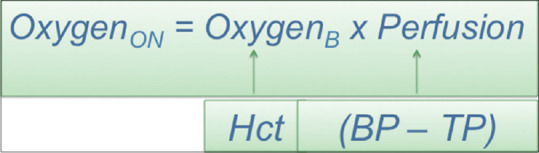
The oxygen level in the optic nerve (oxygenON) is a product of the oxygen-carrying capacity in the blood (oxygenB) and the amount of blood flow to the optic nerve (perfusion). The hematocrit is a proxy for oxygen in the blood (oxygenB). The perfusion pressure is the difference between the blood pressure and tissue pressure. The risk of posterior ischemic optic neuropathy is inversely related to hematocrit × (blood pressure–tissue perfusion pressure)
Lee et al. manipulated these parameters in a study that compared the effects of anemia and hypotension on cerebral blood flow and optic nerve blood flow in pigs.[66] The authors concluded that while compensatory mechanisms for porcine cerebral blood flow were able to maintain stable oxygen delivery in the brain, compensatory mechanisms were not sufficient to preserve oxygen delivery to the optic nerve, highlighting that the optic nerve is particularly susceptible to physiologic hemodynamic changes.
Indeed, PION in children and healthy young adults usually occurs without concomitant vascular injury in other organs including the central nervous system. Furthermore, even though anemia, hypotension, and many factors are not uncommon after major surgery, very few patients develop perioperative PION, suggesting the multifactorial etiology and importance of individual susceptibility, which may be greater with age and comorbidities such as disease. Even within the same risk factor category, there is great variability. For instance, the surgery length varies widely within each vision group. However, the overall visual outcome appears to be inversely related to the length of the surgery. Most of our cases of PION would be considered prolonged. In our study, 29/38 (76.3%) of eyes had average surgery length longer than 6.5 h, the duration deemed prolonged by the ASA consultants and specialty society members.[67] The ASA published advisory guidelines state that the risk of perioperative ischemic optic neuropathy may be higher in patients undergoing prolonged procedures or having sustained significant blood loss. The updated advisory in 2019 redefined prolonged procedures as spine procedures > 4 h and substantial blood loss as blood loss > 800 ml.[68] It is probably prudent to consider these parameters before surgery, especially in high-risk patients, on a case-by-case basis. Measures such as continuous systemic blood pressure and central venous pressure monitoring add safety. In cases of substantial blood loss, the addition of colloids to crystalloids will reduce third-spacing and the resultant increase in tissue pressure. Periodic intraoperative monitoring of hemoglobin or hematocrit is sensible. Guidelines for the use of vasopressors and transfusion have not reached consensus. Staged surgery may also be considered for high-risk patients. Postoperatively, high-risk patients should be evaluated for potential vision loss as soon as patients become alert. Prudence demands optimizing hemoglobin, hematocrit, hemodynamic status, and arterial oxygenation.
Treatments and prevention
Our present study is retrospective and hence subject to the usual limitations. Much of the surgical data were not available. However, our work and the recent literature highlight that several factors are shown to be important. Arterial hypotension, low hematocrits, and dependent position of the head should be avoided, especially in long cases. In high-risk cases with extreme blood loss, it is prudent to use colloids in proportion to crystalloids to prevent hemodilution. The duration of surgery may be mitigated by staging long procedures. Significant Trendelenburg is best avoided. The correction of hemodynamic derangements has shown promising results when initiated in a timely manner.[3] A study by Rubin DS et al. showed a nearly threefold decrease in perioperative ION secondary to spinal fusion surgery between 1998 and 2012.[39] Minimally invasive spine surgery, staging of complex procedures, and improved anesthesia practices probably reduced the length of surgery, blood loss, and other factors. Systemic corticosteroid therapy has rarely been successful and is unlikely to be of benefit.[62,69] Corticosteroids cannot reverse the risk factors leading to PION. Similarly, antiplatelet medication[17] and intraocular pressure-lowering measures[53] do not address the pathophysiology of PION and are unlikely to be effective. The use of vasopressors to elevate blood pressure is controversial. Therefore, we recommend maintaining the hematocrit and blood pressure within the patient's particular tolerances. Avoidance of long surgeries or the excessive use of crystalloids without concomitant colloids help to optimize the hemodynamic parameters and should be approached on a case-to-case basis. In the high-risk patient, vision should be assessed in the recovery room as soon as the patient becomes alert and urgent ophthalmologic consultation be obtained if a decrease in vision is noted. Reports of successful treatment after visual loss in PION[70] suggest that the window of reversibility may vary from hours to a day or two and that the risk factors such as bleeding, hemodilution, and tissue edema can continue for a period of time after surgery. Parallel efforts should be undertaken to optimize all three hemodynamic components (hematocrit, blood pressure, and tissue pressure) to mitigate the ischemic insult involving the optic nerve in PION.
Conclusion
Although the accurate preoperative prediction of PION is not possible, it is important to identify vascular risk factors such as anemia and hypotension and carefully monitor high-risk patients. Early correction of hemodynamic abnormalities is likely to be beneficial.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hayreh SS. Posterior ischemic optic neuropathy. Ophthalmologica. 1981;182:29–41. doi: 10.1159/000309085. [DOI] [PubMed] [Google Scholar]
- 2.Hayreh SS. Ischemic optic neuropathies-where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251:1873–84. doi: 10.1007/s00417-013-2399-z. [DOI] [PubMed] [Google Scholar]
- 3.Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976) 1997;22:1319–24. doi: 10.1097/00007632-199706150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Myers MA, Hamilton SR, Bogosian AJ, Smith CH, Wagner TA. Visual loss as a complication of spine surgery.A review of 37 cases. Spine (Phila Pa 1976) 1997;22:1325–9. doi: 10.1097/00007632-199706150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Lee AG. Ischemic optic neuropathy following lumbar spine surgery.Case report. J Neurosurg. 1995;83:348–9. doi: 10.3171/jns.1995.83.2.0348. [DOI] [PubMed] [Google Scholar]
- 6.Katz DM, Trobe JD, Cornblath WT, Kline LB. Ischemic optic neuropathy after lumbar spine surgery. Arch Ophthalmol. 1994;112:925–31. doi: 10.1001/archopht.1994.01090190073024. [DOI] [PubMed] [Google Scholar]
- 7.Roth S, Nunez R, Schreider BD. Unexplained visual loss after lumbar spinal fusion. J Neurosurg Anesthesiol. 1997;9:346–8. doi: 10.1097/00008506-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Alexandrakis G, Lam BL. Bilateral posterior ischemic optic neuropathy after spinal surgery. Am J Ophthalmol. 1999;127:354–5. doi: 10.1016/s0002-9394(98)00343-2. [DOI] [PubMed] [Google Scholar]
- 9.Lee LA, Roth S, Todd MM, Posner KL, Polissar NL, Neradilek MB, et al. The postoperative visual loss study group.Risk factors associated with ischemic optic neuropathy after spinal fusion surgery. Anesthesiology. 2012;116:15–24. doi: 10.1097/ALN.0b013e31823d012a. [DOI] [PubMed] [Google Scholar]
- 10.Chalam KV, Shah VA. Severe bilateral posterior ischemic optic neuropathy as a complication of spinal surgery. Eye (Lond) 2005;19:367–8. doi: 10.1038/sj.eye.6701482. [DOI] [PubMed] [Google Scholar]
- 11.Chang SH, Miller NR. The incidence of vision loss due to perioperative ischemic optic neuropathy associated with spine surgery: The Johns Hopkins Hospital Experience. Spine (Phila Pa 1976) 2005;30:1299–302. doi: 10.1097/01.brs.0000163884.11476.25. [DOI] [PubMed] [Google Scholar]
- 12.Samdani AF, Rutter L, Betz RR, Mulcahey MJ. Vision loss after spinal fusion for scoliosis in a child with spinal cord injury. J Spinal Cord Med. 2009;32:591–4. doi: 10.1080/10790268.2009.11754567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks SC, Jaques DA, Hirata RM, Saunders JR., Jr Blindness following bilateral radical neck dissection. Head Neck. 1990;12:342–5. doi: 10.1002/hed.2880120412. [DOI] [PubMed] [Google Scholar]
- 14.Nawa Y, Jaques JD, Miller NR, Palermo RA, Green WR. Bilateral posterior optic neuropathy after bilateral radical neck dissection and hypotension. Graefes Arch Clin Exp Ophthalmol. 1992;230:301–8. doi: 10.1007/BF00165935. [DOI] [PubMed] [Google Scholar]
- 15.Schobel GA, Schmidbauer M, Millesi W, Undt G. Posterior ischemic optic neuropathy following bilateral radical neck dissection. Int J Oral Maxillofac Surg. 1995;24:283–7. doi: 10.1016/s0901-5027(95)80030-1. [DOI] [PubMed] [Google Scholar]
- 16.Milner GA. A case of blindness after bilateral neck dissection. J Laryngol Otol. 1960;74:880–5. doi: 10.1017/s0022215100057339. [DOI] [PubMed] [Google Scholar]
- 17.Kirkali P, Kansu T. A case of unilateral posterior ischemic optic neuropathy after radical neck dissection. Ann Ophthalmol. 1990;22:297–8. [PubMed] [Google Scholar]
- 18.Pazos GA, Leonard DW, Blice J, Thompson DH. Blindness after bilateral neck dissection: Case report and review. Am J Otolaryngol. 1999;20:340–5. doi: 10.1016/s0196-0709(99)90039-x. [DOI] [PubMed] [Google Scholar]
- 19.Warner ME, Warner MA, Garrity JA, MacKenzie RA, Warner DO. The frequency of perioperative vision loss. Anesth Analg. 2001;93:1417–21. doi: 10.1097/00000539-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Worrell L, Rowe M, Petti G. Amaurosis: A complication of bilateral radical neck dissection. Am J Otolaryngol. 2002;23:56–9. doi: 10.1053/ajot.2002.28782. [DOI] [PubMed] [Google Scholar]
- 21.Dunker S, Hsu HY, Sebag J, Sadun AA. Perioperative risk factors for posterior ischemic optic neuropathy. J Am Coll Surg. 2002;194:705–10. doi: 10.1016/s1072-7515(02)01210-3. [DOI] [PubMed] [Google Scholar]
- 22.Wessels IF. Posterior ischemic optic neuropathy during general surgery. Am J Ophthalmol. 1987;104:555–6. doi: 10.1016/s0002-9394(14)74131-5. [DOI] [PubMed] [Google Scholar]
- 23.Remigio D, Wertenbaker C. Post-operative bilateral vision loss. Surv Ophthalmol. 2000;44:426–32. doi: 10.1016/s0039-6257(00)00107-7. [DOI] [PubMed] [Google Scholar]
- 24.Rizzo JF, Lessell S. Posterior ischemic optic neuropathy during general surgery. Am J Ophthalmol. 1987;103:808–11. doi: 10.1016/s0002-9394(14)74398-3. [DOI] [PubMed] [Google Scholar]
- 25.Kalyani SD, Miller NR, Dong LM, Baumgartner WA, Alejo DE, Gilbert TB. Incidence of and risk factors for perioperative optic neuropathy after cardiac surgery. Ann Thorac Surg. 2004;78:34–7. doi: 10.1016/j.athoracsur.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Nuttall GA, Garrity JA, Dearani JA, Abel MD, Schroeder DR, Mullany CJ. Risk factors for ischemic optic neuropathy after cardiopulmonary bypass: a matched case/control study. Anesth Analg. 2001;93:1410–6. doi: 10.1097/00000539-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Kudo D, Yamamura H, Nishiuchi T, Ishikawa K, Mizushima Y, Matsuoka T, et al. Anterior and posterior ischemic optic neuropathy related to massive fluid resuscitation after blunt trauma. J Trauma. 2010;68:E67–70. doi: 10.1097/TA.0b013e31816275de. [DOI] [PubMed] [Google Scholar]
- 28.Savino PJ, Burde RM, Mills RP. Visual loss following intranasal anesthetic injection. J Clin Neuroophthalmol. 1990;10:140–144. [PubMed] [Google Scholar]
- 29.Metwalli AR, Davis RG, Donovan JF. Visual impairment after laparoscopic donor nephrectomy. J Endourol. 2004;18:888–90. doi: 10.1089/end.2004.18.888. [DOI] [PubMed] [Google Scholar]
- 30.Weber ED, Colyer MH, Lesser RL, Subramanian PS. Posterior ischemic optic neuropathy after minimally invasive prostatectomy. J Neuroophthalmol. 2007;27:285–7. doi: 10.1097/WNO.0b013e31815b9f67. [DOI] [PubMed] [Google Scholar]
- 31.Rath EZ, Falick Y, Rumelt S. Posterior ischemic optic neuropathy following breast augmentation and abdominal liposuction. Can J Ophthalmol. 2009;44:346–7. doi: 10.3129/i09-060. [DOI] [PubMed] [Google Scholar]
- 32.Asensio JA, Forno W, Castillo GA, Gambaro E, Petrone P. Posterior ischemic optic neuropathy related to profound shock after penetrating thoracoabdominal trauma. South Med J. 2002;95:1053–7. [PubMed] [Google Scholar]
- 33.Huang TW, Liu CM, Cheng PW, Yang CH. Posterior ischemic optic neuropathy following endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2003;129:448–50. doi: 10.1016/s0194-5998(03)00624-7. [DOI] [PubMed] [Google Scholar]
- 34.Luscavage LE, Volpe NJ, Liss R. Posterior ischemic optic neuropathy after uncomplicated cataract extraction. Am J Ophthalmol. 2001;132:408–9. doi: 10.1016/s0002-9394(01)00955-2. [DOI] [PubMed] [Google Scholar]
- 35.Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531–46. doi: 10.2147/opth.s9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarrett WH, Brockhurst RJ. Unexplained blindness and optic atrophy following retinal detachment surgery. Arch Ophthalmol. 1965;73:782–91. doi: 10.1001/archopht.1965.00970030784006. [DOI] [PubMed] [Google Scholar]
- 37.Lee LA, Roth S, Posner KL, Cheney FW, Caplan RA, Newman NJ, et al. The American society of Anesthesiologists postoperative visual loss registry: Analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105:652–9. doi: 10.1097/00000542-200610000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: Review of the literature. Surv Ophthalmol. 2005;50:15–26. doi: 10.1016/j.survophthal.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Rubin DS, Parakati I, Lee LA, Moss HE, Joslin CE, Roth S. Perioperative visual loss in spine fusion surgery: Ischemic optic neuropathy in the United States from 1998 to 2012 in the nationwide inpatient sample. Anesthesiology. 2016;125:457–64. doi: 10.1097/ALN.0000000000001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubin DS, Matsumoto MM, Moss HE, Joslin CE, Tung A, Roth S. Ischemic optic neuropathy in cardiac surgery: Incidence and risk factors in the United States from the national inpatient sample 1998 to 2013. Anesthesiology. 2017;126:810–21. doi: 10.1097/ALN.0000000000001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams EL, Hart WM, Jr, Tempelhoff R. Postoperative ischemic optic neuropathy. Anesth Analg. 1995;80:1018–29. doi: 10.1097/00000539-199505000-00029. [DOI] [PubMed] [Google Scholar]
- 42.Rucker JC, Biousse V, Newman NJ. Ischemic optic neuropathies. Curr Opin Neurol. 2004;17:27–35. doi: 10.1097/00019052-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Ross-Cisneros FN, Sultan WC, Asanad S, Sadun AA. Rat model of posterior ischemic optic neuropathy. Invest Ophthalmol Visual Sci. 2019;60:2265. [Google Scholar]
- 44.Brown RH, Schauble JF, Miller NR. Anemia and hypotension as contributors to perioperative loss of vision. Anesthesiology. 1994;80:222–6. doi: 10.1097/00000542-199401000-00033. [DOI] [PubMed] [Google Scholar]
- 45.Johnson MW, Kincaid MC, Trobe JD. Bilateral retrobulbar optic nerve infarctions after blood loss and hypotension.A clinicopathologic case study. Ophthalmology. 1987;94:1577–84. doi: 10.1016/s0161-6420(87)33236-1. [DOI] [PubMed] [Google Scholar]
- 46.Archer DP. The role of bloodletting in the prevention and treatment of asthenic apoplexy. J Neurosurg Anesth. 1994;6:51–3. doi: 10.1097/00008506-199401000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Roth S. The effects of isovolumic hemodilution on ocular blood flow. Exp Eye Res. 1992;55:59–63. doi: 10.1016/0014-4835(92)90092-7. [DOI] [PubMed] [Google Scholar]
- 48.Hayreh SS. Management of ischemic optic neuropathies. Indian J Ophthalmol. 2011;59:123–36. doi: 10.4103/0301-4738.77024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delattre O, Catonne Y, Saillant G. Ocular complications of surgery of the spine.Proposal for a new head-rest. Chirurgie. 1994;120:31–4. [PubMed] [Google Scholar]
- 50.Wolfe SW, Lospinuso MF, Burke SW. Unilateral blindness as a complication of patient positioning for spinal surgery.A case report. Spine (Phila Pa 1976) 1992;17:600–5. doi: 10.1097/00007632-199205000-00023. [DOI] [PubMed] [Google Scholar]
- 51.Hayreh SS, Bill A, Sperber GO. Effects of high intraocular pressure on the glucose metabolism in the retina and optic nerve in old atherosclerotic monkeys. Graefes Arch Clin Exp Ophthalmol. 1994;232:745–52. doi: 10.1007/BF00184278. [DOI] [PubMed] [Google Scholar]
- 52.Williams I. Risk factors for ischemic optic neuropathy after cardiopulmonary bypass: A matched case/control study. Surv Ophthalmol. 2003;48:237–8. doi: 10.1016/s0039-6257(02)00452-6. [DOI] [PubMed] [Google Scholar]
- 53.Roth S, Moss HE. Update on perioperative ischemic optic neuropathy associated with non-ophthalmic surgery. Front Neurol. 2018;9:557. doi: 10.3389/fneur.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Postoperative Visual Loss Study Group. Risk factors associated with ischemic optic neuropathy after spinal fusion surgery. Anesthesiology. 2012;116:15–24. doi: 10.1097/ALN.0b013e31823d012a. [DOI] [PubMed] [Google Scholar]
- 55.Shah SH, Chen YF, Moss HE, Rubin DS, Joslin CE, Roth S. Predicting risk of perioperative ischemic optic neuropathy in spine fusion surgery: A cohort study using the national inpatient sample. Anesth Analg. 2020;130:967–74. doi: 10.1213/ANE.0000000000004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho VT, Newman NJ, Song S, Ksiazek S, Roth S. Ischemic optic neuropathy following spine surgery. J Neurosurg Anesthesiol. 2005;17:38–44. [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy MA. Bilateral posterior ischemic optic neuropathy after lumbar spine surgery. Ophthalmology. 2003;110:1454–7. doi: 10.1016/S0161-6420(03)00480-9. [DOI] [PubMed] [Google Scholar]
- 58.Purvin V, Kuzma B. Intraorbital optic nerve signal hyperintensity on magnetic resonance imaging sequences in perioperative hypotensive ischemic optic neuropathy. J Neuroophthalmol. 2005;25:202–4. doi: 10.1097/01.wno.0000177295.52468.5b. [DOI] [PubMed] [Google Scholar]
- 59.Vaphiades MS. Optic nerve enhancement in hypotensive ischemic optic neuropathy. J Neuroophthalmol. 2004;24:235–6. doi: 10.1097/00041327-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Al-Zubidi N, Stevens S, Fung SH, Lee AG. Diffusion-weighted imaging in posterior ischemic optic neuropathy. Can J Ophthalmol. 2014;49:e21–5. doi: 10.1016/j.jcjo.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Park JY, Lee IH, Song CJ, Hwang HY. Diffusion MR imaging of postoperative bilateral acute ischemic optic neuropathy. Korean J Radiol. 2012;13:237–9. doi: 10.3348/kjr.2012.13.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayreh SS. Posterior ischaemic optic neuropathy: Clinical features, pathogenesis, and management. Eye (Lond) 2004;18:1188–206. doi: 10.1038/sj.eye.6701562. [DOI] [PubMed] [Google Scholar]
- 63.Sadda SR, Nee M, Miller NR, Biousse V, Newman NJ, Kouzis A. Clinical spectrum of posterior ischemic optic neuropathy. Am J Ophthalmol. 2001;132:743–50. doi: 10.1016/s0002-9394(01)01199-0. [DOI] [PubMed] [Google Scholar]
- 64.Cheng MA, Todorov A, Tempelhoff R, McHugh T, Crowder CM, Lauryssen C. The effect of prone positioning on intraocular pressure in anesthetized patients. Anesthesiology. 2001;95:1351–5. doi: 10.1097/00000542-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 65.Lam AK, Douthwaite WA. Does the change of anterior chamber depth or/and episcleral venous pressure cause intraocular pressure change in postural variation? Optom Vis Sci. 1997;74:664–7. doi: 10.1097/00006324-199708000-00028. [DOI] [PubMed] [Google Scholar]
- 66.Lee LA, Deem S, Glenny RW, Townsend I, Moulding J, An D, et al. Effects of anemia and hypotension on porcine optic nerve blood flow and oxygen delivery. Anesthesiology. 2008;108:864–72. doi: 10.1097/ALN.0b013e31816c8a30. [DOI] [PubMed] [Google Scholar]
- 67.American Society of Anesthesiologists Task Force on Perioperative Visual Loss. Practice advisory for perioperative visual loss associated with spine surgery: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Visual Loss. Anesthesiology. 2012;116:274–85. doi: 10.1097/ALN.0b013e31823c104d. [DOI] [PubMed] [Google Scholar]
- 68.Practice Advisory for Perioperative Visual Loss Associated with Spine Surgery 2019: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Visual Loss the North American Neuro-Ophthalmology Society and the Society for Neuroscience in Anesthesiology and Critical Care. Anesthesiology. 2019;130:12–30. doi: 10.1097/ALN.0000000000002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee AG. Reversible loss of vision due to posterior ischemic optic neuropathy. Can J Ophthalmol. 1995;30:327–9. [PubMed] [Google Scholar]
- 70.Connolly SE, Gordon KB, Horton JC. Salvage of vision after hypotension-induced ischemic optic neuropathy. Am J Ophthalmol. 1994;117:235–42. doi: 10.1016/s0002-9394(14)73082-x. [DOI] [PubMed] [Google Scholar]


