Abstract
The coronavirus disease 2019 (COVID 19) pandemic has presented major challenges to ophthalmologists. Reports have shown that ocular manifestations can be the first presenting symptoms of COVID 19 infection and conjunctiva may be a portal of entry for the severe acute respiratory syndrome (SARS) associated coronavirus 2 (SARS CoV 2). The purpose of this article is to provide general guidance for ophthalmologists to understand the prevalence of ocular presentation in COVID 19 patients and to reduce the risk of transmission during practice. Relevant studies published in the period of November 1, 2019, and July 15, 2020, regarding ocular manifestations of COVID 19 and detection of SARS CoV 2 in the eye were included in this systematic review and meta analysis. The pooled prevalence of the ocular manifestations has been estimated at 7% (95% confidence interval [CI]: 0.03–0.10) among COVID 19 patients. The pooled detection rate of SARS CoV 2 from conjunctiva was low (1%, 95% CI: 0.00–0.03). Conjunctival symptoms were the most common ocular manifestations in COVID 19, but the positive detection rate of the SARS CoV 2 virus by reverse transcription–polymerase chain reaction of conjunctival tears or secretions remained low. No study has shown a definite transmission of COVID 19 through ocular mucosa or secretions. In summary, ocular manifestations in COVID 19 patients commonly comprise ocular surface symptoms. Although a low prevalence of ocular symptoms was encountered among patients infected by SARS CoV 2, it is imperative for all ophthalmologists to understand the full spectrum of COVID 19 symptoms or signs including those of the eyes as well as to adopt appropriate protective measures during clinical practice.
Keywords: Coronavirus, coronavirus disease 2019, ocular manifestations, ophthalmology, polymerase chain reaction, severe acute respiratory syndrome-coronavirus 2
Introduction
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), has created a critically distinct medical reality – a persistent and rapidly evolving pandemic which is projected to infect tens of millions around the globe – that all health-care professionals must now live in. As of August 16, 2020, there were over 21 million diagnosed cases and more than 770,000 confirmed deaths worldwide due to COVID-19.[1]
The COVID-19 pandemic has presented major challenges to health-care professionals of different disciplines. Due to the need of frequent close contact between the ophthalmologists and their patients during clinical practice, effective and safe measures must be implemented for mutual protection. This is particularly important because COVID-19 is a highly contagious disease which could be transmitted through discharge, droplets, and fomites from the eyes, nose, or mouth.[2,3] In certain instances, conjunctivitis can be the first presenting symptoms of COVID-19 infection, thus increasing the exposure risk of ophthalmologists to the disease.[4]
It is thus imperative for a comprehensive review of ocular-related information of COVID-19 with the best current evidence for practicing ophthalmologists. Besides, the full spectrum of clinical manifestations of COVID-19 infection should be well understood. Through a systematic review, we aim to answer critical questions regarding COVID-19 in ophthalmology, which comprise:
What is the essential basic science knowledge required to understand the COVID-19?
What are the key ocular manifestations or relevant ocular findings in patients infected by SARS-CoV-2? What is the positive rate of conjunctival swab for the virus?
What are the ocular comorbidities, if any, of COVID-19?
Can SARS-CoV-2 infect or spread through the mucous membranes or secretions of the eye?
How can we prevent or reduce the transmission risk of COVID-19, especially for ophthalmologists?
By answering these questions in this review, we hope that this article can provide essential information and general guidance for ophthalmologists and other medical specialties to better understand the implications and the full clinical spectrum of COVID-19.
Essential basic sciences review regarding coronavirus disease 2019
Pathogenesis review
The pathogenesis of respiratory diseases caused by human coronavirus (HCoV) is well known, but ophthalmic implications are not well elucidated yet. Since the 1960s, there are several known HCoVs, namely the HCoV-229E, HCoV-OC43, and the HCoV SARS-CoV.[5,6] Three of the coronaviruses, namely SARS-CoV, Middle East respiratory syndrome coronavirus, and SARS-CoV-2, can replicate in the lower respiratory tract, thus causing pneumonia.[7]
As the pathogen responsible for COVID-19, SARS-CoV-2 belongs to the betacoronavirus genus and has 79% genetic similarity to SARS-CoV.[8] All viruses from the Coronaviridae family comprise enveloped viruses with plus-strand RNA genome (27–32 kb), with each serology characterized by a specific genome sequence and host range. SARS-CoV-2 is transmitted primarily by respiratory droplets. However, there is a risk of transmission via other routes such as oral-fecal, conjunctival or ocular secretions, and the latter will be discussed in this article. Based on recent reports, the median incubation period is about 4–5 days and >95% of symptomatic patients would have developed symptoms within 11 days.[9,10] This forms the scientific basis on which quarantine period is determined to be 14 days in most nations.
The aforementioned different infection sites of SARS-CoV-2 are dominated by viral surface spikes, which is composed of dipeptidyl peptidase-4 glycoprotein. The spike protein on the virus reacts with a human cell surface receptor known as angiotensin-converting enzyme 2 (ACE2) that has been detected on different cell lines.[11,12] Because ACE2 has been reported to be expressed in corneal cell lines, this raises the concern of the ocular surface serving as a portal of entry for SARS-CoV-2.[13,14] An important study by Hui et al. in investigating tissue tropism of SARS-CoV-2 revealed that SARS-CoV-2 has the ability to infect bronchial epithelium, type 1 pneumocytes in the lung, and the conjunctival mucosa.[15] Interestingly, as compared to SARS-CoV, SARS-CoV-2 demonstrated a greater rate of replication in ex vivo conjunctival cultures.[15]
In addition, transmembrane protease serine 2 (TMPRSS2) is an essential cell surface-associated protease which facilitates entry of virus after interaction of the viral spike protein to ACE2.[16] Multiple reports also revealed the co-expression of ACE2 and TMPRSS2 in the superficial limbal, corneal, and conjunctival epithelium, implicating these as possible target entry cells for SARS-CoV-2 in the ocular surface.[17,18] For instance, all cadaveric and surgical samples of conjunctiva were positive for ACE2 and TMPRSS2 expression demonstrated by immunohistochemistry and Western blot.[17] Pooling all these evidences together, this suggested that ocular surface epithelium might provide an additional portal of entry for SARS-CoV-2, which further exploits the upregulation of ACE2 and TMPRSS2 following inflammation in order to promote infection.[18]
Immune response toward severe acute respiratory syndrome-coronavirus 2
In general, during a healthy immune response, the initial inflammatory signals attract T-cells, which might eliminate those infected cells before spreading of the virus. CD8+ T-cells play an essential role in directly targeting and killing virus-infected cells, whereas CD4+ T-cells are crucial to prime or activate both CD8+ T-cells and B-cells. These roles were described by a study by Zheng et al. which showed that T-cell exhaustion or reduced functional diversity precipitates COVID-19 progression.[19]
In addition, neutralizing antibodies by the host and phagocytosis by macrophages can also avert viral infection, thus promoting recovery. The former occurs simultaneously with T follicular helper response at approximately 1 week after the onset of symptoms.[20] Clinical studies have shown good clinical results in both COVID-19 and SARS patients using convalescent serum therapy, suggesting that antibodies are likely effective against SARS-CoV-2 and SARS-CoV.[21,22,23] However, the specific titer and specificity of antibody repertoire required for protection remain undefined.
Alternatively, when host cells are infected with SARS-CoV-2, aggressive host inflammatory responses can be initiated in response to airway cell damage.[24] Active replication and release of the virus upon infecting host cells release damaging-associated molecules such as adenosine triphosphate or nucleic acids, which further trigger the secretion of cytokines and chemokines. These pro-inflammatory substances comprise interleukin-6, interferon-γ, monocyte chemoattractant protein-1, and inducible protein-10.[4] This is known as a dysfunctional immune response, in which overproduction of cytokine eventually damages cell ultrastructures and circulates to other organs, causing multi-organ failure.[25] Thus, disease severity in patients correlates not only with viral infection but also with the host response.
Materials and Methods
Search strategy and study selection
Relevant studies published during the period from November 1, 2019, to July 15, 2020, regarding ocular manifestations of COVID-19 or the presence of SARS-CoV-2 in the cornea, conjunctiva, lacrimal sac, and tears were identified from PubMed, Medline, Cochrane Library, World of Science, as well as ClinicalKey. Search keywords comprised “COVID-19,” “Ocular findings/Signs/Symptoms,” “Ocular/Eye/Ophthalmology,” “2019-nCoV,” and “SARS-CoV-2.”
Criteria of studies included in this meta-analysis were (1) studies or articles which reported ocular symptoms or signs in COVID-19 patients, (2) diagnostic outcomes in both ocular tissue/secretions and nasopharyngeal swab by polymerase chain reaction (PCR) analysis, and (3) ocular comorbidities related to COVID-19.
PRISMA guidelines were used for this systematic review. A reference list of all identified articles were independently hand-searched by two authors (XCL and DHKM). The eligible abstracts were examined, and if eligible, full texts and associated reference lists underwent further evaluation for eligibility. The search was then expanded using a snowballing method applied to the references of retrieved papers. As the number of reports is relatively small, both original articles, editorials, letters, and reviews providing evidence (i.e., prevalence and anecdotal report) about SARS-CoV-2 colonization in ocular and periocular tissues and secretions were all included in the study. All publications were then reassessed by a third senior author (WWC). All the results were then merged using the reference management software EndNote (Version X9.3.3, PDFTron Systems Inc.).
To assess the risk of bias and quality of primary studies or systematic reviews identified from database searches, we utilized the Newcastle–Ottawa Scale, the highest score of which is 9 points.[26]
Meta-analysis was then performed to acquire the pooled prevalence estimates of ocular manifestations in COVID-19 patients. If there was a large heterogeneity among the studies, a random-effects model will be utilized. Heterogeneity was evaluated with the I2 statistic, in which an I2 value over 50% with statistical significance was considered an indicator of substantial heterogeneity.
Results
Search results
Figure 1 illustrates the flowchart of study screening and selection in this systematic review. These studies were published between November 1, 2019, and July 15, 2020. The initial screen yielded 234 citations, of which 208 were ineligible on the basis of the criteria used for screening titles and abstracts, such as nonrelevant reporting and inappropriate population. Following the initial screening of titles, abstracts, and removal of duplicates, we included a total of 14 articles in our review.
Figure 1.
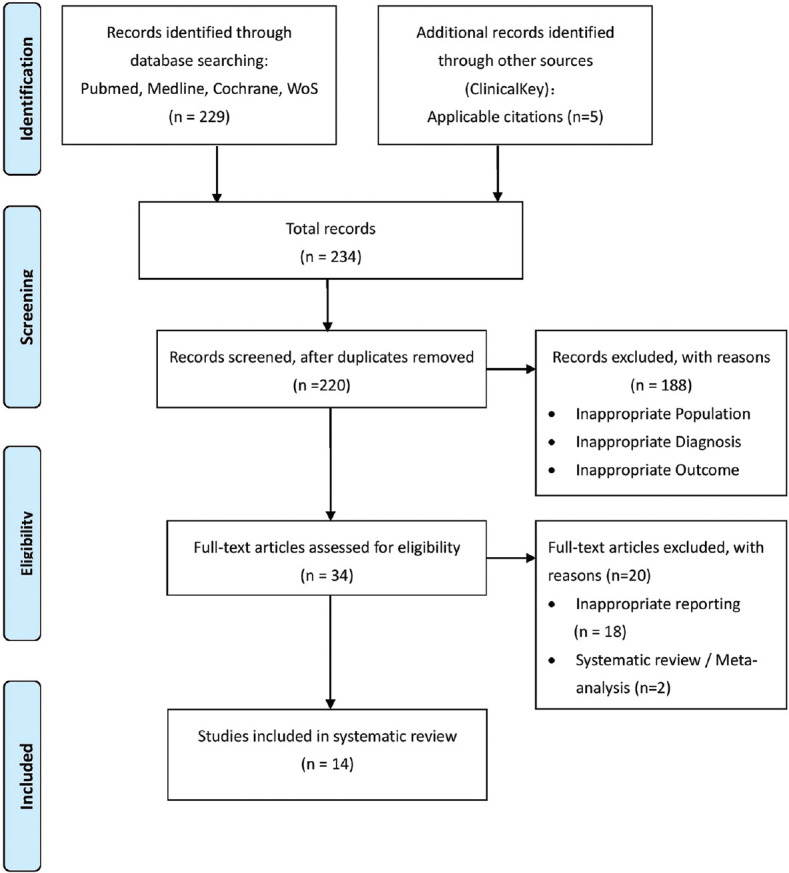
Flowchart of study selection
Study characteristics
All included studies which reported ocular manifestations in patients infected with SARS-CoV-2 were listed in alphabetical order with the details of bias assessment [Table 1]. Of these selected studies, there were nine original articles (including prospective and retrospective studies),[10,16,28,30,32,33,34,35,36] three case reports,[27,29,37] and two letters to the editorials.[17,31]
Table 1.
List of selected articles with ocular manifestations of coronavirus disease 2019 analyzed and reviewed
| Study (year) | Article type | Number of Patients, n | Number of patients with ocular findings, n (%) | Types of ocular findings | Number of patients with both positive tear PCR and ocular findings, n (%) | Risk of bias |
|---|---|---|---|---|---|---|
| Cheema et al. (2020)[27] | Original article (case report) | 1 | N/A | Keratoconjunctivitis | N/A | Selection bias (only 1 case included) |
| Chen et al. (2020)[28] | Original article (cohort study) | 535 | 27 (5.0)* | Conjunctival congestion, increased secretion, ocular pain, FBS, photophobia | N/A | Reporting bias (conjunctival congestion as the only primary outcome) |
| Chen et al. (2020)[37] | Original article (case report) | 1 | N/A | Follicular conjunctivitis | N/A | Selection bias (only 1 case included) |
| Guan et al. (2020)[10] | Original article (retrospective review) | 1099 | 9 (0.8) | Conjunctival congestion | N/A | Low |
| Hong et al. (2020)[30] | Original article (cohort study) | 56 | 15 (26.8) | Conjunctivitis, dry eye, and FBS | 1 (1.8) | Recall bias (ocular symptoms recalled by patients) |
| Khavandi et al. (2020)[31] | Letter to the editor | 1 | N/A | Follicular conjunctivitis | N/A | Selection bias (only 1 case included) |
| Navel et al. (2020)[29] | Original article (case report) | 1 | N/A | Hemorrhagic conjunctivitis with pseudomembrane | N/A | Selection bias (only 1 case included) |
| Seah et al. (2020)[32] | Original article (cohort study) | 17 | 1 (5.9) | Conjunctival injection and chemosis | 0 | Detection bias (two different assays used in detection) |
| Wu et al. (2020)[33] | Original article (case series) | 38 | 12 (31.6) | Conjunctival hyperemia, chemosis, and epiphora | 2 (5.3) | Low |
| Wu et al. (2020)[17] | Letter to the editor | 1 | N/A | Eyelid dermatitis and conjunctivitis | N/A | Selection bias (only 1 case included) |
| Valente et al. (2020)[34] | Original article (prospective study) | 27 | 4 (14.8) | Mild conjunctival hyperemia and secretion | 1 (3.7) | Low |
| Xia et al. (2020)[16] | Original article (prospective case series) | 30 | 1 (3.3) | Conjunctivitis | 0 | Low |
| Zhang et al. (2020)[35] | Original article (retrospective study) | 102 | 2 (2.0) | Conjunctivitis | 1 (1.0) | High (only RCT PCR assay used for laboratory confirmation of COVID-19) |
| Zhou et al. (2020)[36] | Original article (retrospective study) | 121 | 8 (6.6) | Conjunctivitis and FBS | 1 (0.8) | Low |
*Proportion of patients with only conjunctival congestion in this cohort. N/A=Not applicable, FBS=Foreign body sensation, COVID-19=Coronavirus Disease 2019, RCT=Randomized controlled trial, PCR=Polymerase chain reaction
A rough systematic aggregation of the studies revealed that the prevalence of ocular manifestations among COVID-19 patients ranged from 6% to 32%. All the ocular manifestations reported were from patients diagnosed with COVID-19 by laboratory confirmation (nasopharyngeal or tear PCR). Risks of bias for all included studies were allocated in line with PRISMA guidelines.
Ocular manifestations among patients with coronavirus disease 2019
In addition to reporting the total prevalence of ocular manifestations in COVID-19 patients, we have summarized the reported ocular symptoms or signs as according to anatomic sites in Table 2. A general overview revealed that even though ocular symptoms have a relatively low prevalence in COVID-19 patients, they still represent a critical issue, as almost all parts of the eye can have COVID-19-related manifestations. In addition, positive PCR results in tears from conjunctival swab in the reviewed studies also suggested the possibility of ocular transmission.
Table 2.
Major ocular manifestations/symptoms of coronavirus disease 2019 as classified by anatomy
| Anatomy/Signs or symptoms | Number of COVID-19-confirmed patients with ocular symptoms, n (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cheema et al. (2020)[27] | Chen et al. (2020)[28] | Chen et al. (2020)[37] | Khavandi et al. (2020)[31] | Guan et al. (2020)[10] | Hong et al. (2020)[30] | Navel et al. (2020)[29] | Seah et al. (2020)[32] | Wu et al. (2020)[33] | Wu et al. (2020)[17] | Valente et al. (2020)[34] | Xia et al. (2020)[16] | Zhang et al. (2020)[35] | Zhou et al. (2020)[36] | |
| Eyelid | ||||||||||||||
| Dermatitis | NR | NR | NR | NR | NR | NR | NR | NR | NR | Case report (+) | NR | NR | NR | NR |
| Conjunctiva | ||||||||||||||
| Conjunctival hyperemia, n=53 | Case report (+) | 27 (5.0) | Case report (+) | Case report (+) | 9 (0.8) | 2 (3.6) | Case report (+) | 1 (5.9) | 3 (7.9) | Case report (+) | 4; Reported only as conjunctivitis | 1; Reported only as conjunctivitis | 2 (2.78); Reported only as conjunctivitis | 3 (37.5) |
| Increased discharge/secretion, n=66 | Case report (+) | 52 (9.7) | Case report (+) | Case report (+) | NR | 2 (3.6) | Case report (+) | NR | 6 (15.8) | Case report (+) | NR | |||
| Tearing, n=64 | NR | 54 (10.1) | Case report (+) | Case report (+) | NR | NR | NR | NR | 6 (15.8) | NR | NR | |||
| Pain, n=29 | Case report (+) | 23 (4.3) | Case report (+) | NR | NR | 4 (7.1) | NR | NR | NR | NR | NR | N/A | N/A | NR |
| Foreign body sensation, n=70 | NR | 63 (11.8) | Case report (+) | NR | NR | 4 (7.1) | NR | NR | NR | NR | NR | 2 (25.0) | ||
| Dry eyes, n=117 | NR | 112 (20.9) | NR | NR | NR | 5 (8.9) | NR | NR | NR | NR | NR | NR | ||
| Itching/irritation, n=62 | Case report (+) | 53 (9.9) | NR | NR | NR | 3 (5.4) | NR | NR | NR | NR | NR | 5 (62.5) | ||
| Photophobia, n=17 | Case report (+) | 16 (3.0) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Chemosis (+), n=9 | NR | NR | NR | NR | NR | NR | NR | 1 (5.9) | 7 (18.4) | Case report (+) | NR | NR | ||
| Pseudomembrane (+), n=1 | NR | NR | NR | NR | NR | NR | Case report (+) | NR | NR | NR | NR | NR | ||
| Follicles (+), n=4 | Case report (+) | NR | Case report (+) | Case report (+) | NR | NR | Case report (+) | NR | NR | NR | NR | NR | ||
| Cornea | ||||||||||||||
| Subepithelial infiltrates (+), n=1 | Case report (+) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Epithelial defect (+), n=1 | Case report (+) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Retina | ||||||||||||||
| Floaters (+), n=1 | NR | NR | NR | NR | NR | 1 (1.8) | NR | NR | NR | NR | NR | NR | NR | NR |
| Positive conjunctival swab, n (%) | N/A | NR | N/A | N/A | NR | 1 (1.8) | N/A | 0 | 2 (5.3) | N/A | 3 (11.1) | 0 | 1 (1.0) | 1 (0.8) |
| Total patients with ocular symptoms, n (%) | N/A | N/A† | N/A | N/A | 9 (0.8) | 15 (26.8) | N/A | 1 (5.9) | 12 (31.6) | N/A | 4 (14.8) | 1 (3.3) | 2 (2.78) | 8 (6.6) |
Percentage reported as ratio of patients with ocular symptoms/signs to all COVID-19 patients in the respective study. †These could not be acquired because the authors classified patients as conjunctival congestion versus no conjunctival congestion, but patients in the latter group can still have other ocular symptoms, resulting in possible overlapped frequencies. (The highlighted columns represent these particular symptoms or signs were positively reported in patients of this study). NR=Not Reported, COVID-19=Coronavirus disease 2019
Eyelids
The only reported case of eyelid dermatitis related to COVID-19 was by Wu et al.,[17] who described a 2-year-old male with contact history with infected family members. The child first presented with a mild eyelid dermatitis as well as conjunctivitis.[17] SARS-CoV-2 infection was confirmed by nasopharyngeal swab reverse transcription (RT)-PCR in the patient, thereby suggesting that ocular presentation should also be monitored during the pandemic. Correspondingly, the significance of COVID-19 testing in children presenting with ocular symptoms remains to be investigated.
Conjunctiva
As one of the major findings in conjunctivitis, conjunctival hyperemia is the key conjunctival symptom reported in all included studies. A total of 27 patients (5.0%) out of 535 COVID-19 patients presented with conjunctival congestion or hyperemia in a cohort study by Chen et al.[28] Four of these 27 patients presented with conjunctivitis as the initial symptoms. No tear or conjunctival PCR was collected in the study. Overall, there were more accompanying ocular symptoms in patients with conjunctival congestion, including increased conjunctival secretion, ocular pain, photophobia, dry eye, and tearing.
In addition, Zhou et al. reported that 8 (6.6%) out of 121 patients diagnosed with COVID-19 presented with ocular symptoms, among which 5 presented with itching, 3 with redness and tearing, respectively, 2 with discharge as well as 2 with foreign body sensation.[36] Conjunctival swab PCR revealed that 3 out of 121 patients (2.48%) tested positively for SARS-CoV-2. Statistically, a longer duration of COVID-19 infection did not increase the rate of positive detection in this study. However, only one of the aforementioned three patients presented with both conjunctivitis symptoms and positive tear PCR result.
In the largest cohort published to date with patients infected with COVID-19 (n = 1099), Guan et al. reported only nine patients (0.8%) with conjunctival congestion.[10] No conjunctival PCR was collected from the study. Of the nine patients with conjunctival congestion, 5 (0.5%) and 4 (2.3%) patients were graded as nonsevere and severe COVID-19 disease, respectively. All the nine patients did not require mechanical ventilation or intensive care unit (ICU) admission and did not report any mortality at the end point of the study.
Xia et al. enrolled thirty patients diagnosed with COVID-19 in a prospective interventional case series.[16] Tear and conjunctival secretion were collected from all patients for PCR. There was only one patient with conjunctivitis symptoms and whose tear/conjunctival secretion was tested positive for SARS-CoV-2 viral RNA.[16] On the other hand, no virus was detected in the conjunctival secretion and tears in patients without conjunctivitis.
There were several other studies which reported other conjunctival symptoms such as increased conjunctival secretion, tearing, pain, foreign body sensation, dry eyes, and itching.[30,33] The results of these studies signified that conjunctival symptoms were the most commonly occurring ocular symptoms in COVID-19. In relation to that, Wu et al. reported a total of 12 out of 38 (31.6%) patients with conjunctival symptoms including conjunctival hyperemia, chemosis, epiphora, or increased secretion.[33] The study had also shown by univariate analysis that higher white blood cell and neutrophil counts, as well as elevated levels of procalcitonin, C-reactive protein, and lactate dehydrogenase, were correlated with the occurrence of ocular symptoms in the cohort.[33]
Hong et al. recruited 56 patients infected by SARS-CoV-2 who had discharged from the hospital.[30] Evaluation of ocular surface condition before and after the onset of COVID-19 was performed using the Ocular Surface Disease Index (OSDI) and Salisbury Eye Evaluation Questionnaire (SEEQ). The symptoms before COVID-19 were evaluated by asking the patient to recall their ocular symptoms before COVID-19 and to answer the OSDI questionnaire as well as SEEQ accordingly. Fifteen patients (27%) reported ocular symptoms in the course of COVID-19, including sore eyes, itching, foreign body sensation, tearing, redness, dry eyes, eye secretions, and floaters. Worsening ocular surface condition of the patients was shown by a significant increase in mean SEEEQ and OSDI scores after the onset of COVID-19. Among them, six patients (11%) presented with ocular symptoms before the onset of fever or respiratory symptoms.
Zhang et al. performed a retrospective review of 102 patients who were clinically diagnosed with COVID-19 (via clinical symptoms and computed tomography imaging).[35] Seventy-two percent of the 102 patients were further confirmed by SARS-CoV-2 RT-PCR assay, among whom only two patients (2.78%) had conjunctivitis. Only one of the patients (1.39%) with conjunctival symptoms had a positive RT-PCR result in both tears and nasopharyngeal swab. This was the only article included in this systematic review which first recruited a cohort of clinically diagnosed COVID-19 instead of all patients being confirmed by laboratory test.
A prospective study in Singapore by Seah et al. revealed that one of the patients (5.9%) developed conjunctival congestion and chemosis during hospital admission.[32] The study also compared the viral load of tears to nasopharyngeal swab, both of which were sampled at the same time for each patient. All tear samples were negative even when nasopharyngeal swabs persisted to be positive, including the one patient with conjunctival symptoms.[32]
Case reports of patients who were diagnosed with COVID-19 and had presented with ocular symptoms were reviewed as well. Cheema et al. described a case of a young female who presented first with symptoms of conjunctival hyperemia, increased tearing, and photophobia. Follicular change and conjunctival congestion were noticed during physical examination.[27] Corneal signs were also shown, which would later be discussed. In view of progressive conjunctivitis symptoms and the recommendation for COVID-19 test based on travel history, she underwent nasopharyngeal swabbing and was tested positive for SARS-CoV-2. Besides, retrospective testing of eye swab initially sent for gonorrhea or chlamydia PCR revealed weakly positive for SARS-CoV-2 as well. This case emphasizes the importance of considering COVID-19 as one of the differential diagnoses for patients with recent travel who present with acute conjunctivitis.
Khavandi et al. reported that a 65-year-old Caucasian male who did not have a remarkable travel history or typical COVID-19 symptoms first presented with mucoid discharge and follicular conjunctivitis.[31] Two days later, he developed a sudden-onset fever and bilateral ground-glass opacity of the lungs, after which he was diagnosed with COVID-19. RT-PCR testing of the conjunctival secretion was positive for SARS-CoV-2 on two occasions during admission.
Chen et al. reported a 30-year-old male with bilateral follicular conjunctivitis on days 13 after the onset of COVID-19.[37] Conjunctival swabs collected immediately after the onset of conjunctivitis (days 13) were positive for SARS-CoV-2. The conjunctival swabs remained positive subsequently on days 14, 17, and 19, albeit with decreasing titer values. The detection of SARS-CoV-2 in a conjunctival specimen in this patient for several days represents a possible source of transmission, especially in view of the high viral titer during the acute stage of ocular presentation.
In addition to the common conjunctivitis symptoms, the multiplicity and severity of COVID-19-associated ocular presentation have raised concern. For instance, Navel et al. reported a case of hemorrhagic conjunctivitis and pseudomembrane in a patient treated for COVID-19 in the ICU.[29] His ocular symptoms started at around days 17 after admission, after which exacerbation occurred with follicles, petechiae, chemosis, pseudomembranes, and tarsal hemorrhages despite treatment with physiologic serum as well as artificial tears. No abnormal posterior inflammation or retinal abnormality was observed. Conjunctival scrapings and swabs were negative for any other bacterial or viral etiology, including PCR for SARS-CoV-2. This case implicated that all physicians should be aware of possible late (>14 days) ocular complications in severe COVID-19 patients.
Cornea and anterior segment
In comparison with conjunctivitis, corneal involvement in COVID-19 was comparatively rarer. The patient reported by Cheema et al. had developed corneal pathologies in addition to conjunctivitis.[27] For instance, small pseudodendrites first appeared in tandem with conjunctivitis, before morphing into small subepithelial infiltrates with overlying defect. The central implication from this case is that clinical presentation can vary or fluctuate and even progress if left unattended. To date, there is no published evidence which shows anterior segment involvement of COVID-19. However, care has to be practiced in order to grasp the full clinical spectrum of COVID-19 in the eyes.
Retina and vitreous
With respect to the presentation in the posterior segment of the eye, Hong et al. reported one patient with floaters in the right eye after hospitalization for COVID-19.[30] However, a direct relationship could not be ascertained, and no patient infected with COVID-19 had reported a blurred vision in the cohort.
In a study by Marinho et al. using optical coherence tomography (OCT) to evaluate retinal changes, 12 adults were examined 11–33 days after the onset of COVID-19 symptom. All patients exhibited hyperreflective lesions at the ganglion cell level and prominent inner plexiform layers at the papillomacular bundle.[18] Even so, the results of OCT-angiography and ganglion cell complex analysis appeared normal. These aforementioned findings might be related to neurologic events in COVID-19 patients.
Pooled prevalence of ocular manifestations in coronavirus disease 2019
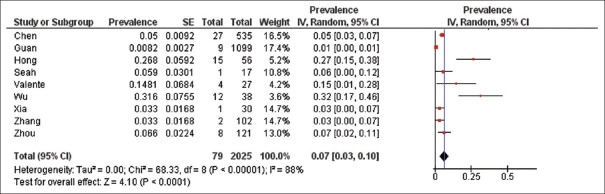
With regard to the prevalence of ocular manifestations among COVID-19 patients, a rough estimate of the prevalence range was between 1% and 32%. A meta-analysis of 9 studies, with an accumulated number of 2025 patients, was performed. Overall, a pooled prevalence of ocular manifestations among COVID-19 patients was 7% (95% confidence interval [CI]: 0.03–0.10). A random-effects model was utilized due to the heterogeneity (I2 = 88%) between studies [Figure 2].
Figure 2.
Forest plot of the nine studies estimating the pooled prevalence of ocular manifestations among coronavirus disease 2019 patients
The pooled detection rate of conjunctival swab was calculated from 7 studies with collection of tear/conjunctival secretion samples from a total of 391 patients. The pooled positive rate of ocular tissue or secretions in detecting SARS-CoV-2 was very low (1%, 95% CI: 0.00–0.03). This confirmed that the detection rate of tear or conjunctival swab PCR remained low for SARS-CoV-2, thus making it impractical to make the confirmative diagnosis of COVID-19 using samples from the eye.
Ocular comorbidities related to coronavirus disease 2019
A review of articles regarding ocular comorbidities associated with COVID-19 was performed. A case series from Turbin et al. reported two adolescent cases who presented with unilateral painful orbital swelling and rhinorrhea to the emergency department.[39] There was no description of fever, chills, anosmia, dysgeusia, and lower respiratory symptoms. CT revealed orbital cellulitis and sinusitis in both cases. For precaution, nasopharyngeal swab collected within 6 h of first visit turned out to be positive for SARS-CoV-2 in both patients.
While it was unclear if the detection of SARS-CoV-2 in these two cases was a contributing factor or merely a coincidence, the study had shown that there was a possibility that secondary upper respiratory symptoms of COVID-19 could compromise mucociliary clearance, resulting in sinus obstruction and thus bacterial orbital infection. It further highlighted that the full spectrum of COVID-19 presentation had not been well known and that even serious ocular comorbidities can be associated with the disease.
Furthermore, during the early outbreak of COVID-19, empirical treatment was proposed with chloroquine and hydroxychloroquine at a dosage of 1000 mg/day for 10 days of chloroquine, followed by 800 mg 1st day and then 400 mg/day for 5 days of hydroxychloroquine.[40] However, the aforementioned doses were both considerably higher than the maximum safe dosages for retinal toxicity. In cases of COVID-19 treated with chloroquine and hydroxychloroquine, the major risk comprises the use of doses higher than those recommended, though usually only for a week, it might still cause a certain risk for retinal toxicity.
In a report by Ruamviboonsuk et al., macula abnormalities on retinal imaging and multifocal electroretinogram were observed in two out of seven patients (28.6%) who received a high dose of hydroxychloroquine for treating COVID-19.[41] History review revealed that these patients did not have any known risk factors such as renal insufficiency, concomitant retinotoxic drug, or predisposing retinal condition. In view of the data, routine baseline ocular examination might not be absolutely necessary for patients receiving hydroxychloroquine or chloroquine treatment for COVID-19, but the risks of irreversible retinal toxicity and visual loss may far outweigh the unproven benefits of both agents in certain patients, especially patients with previous history of retinal or macular disease.
With respect to ocular comorbidities related to COVID-19, Stevens et al. described a 74-year-old male with severe COVID-19 who developed orbital emphysema due to extension of subcutaneous emphysema from orotracheal intubation.[42] The emphysema extended to unilateral conjunctiva and bilateral eyelids. However, there was no evidence of orbital compartment syndrome or vascular occlusion.
Discussion
This systematic review and meta-analysis provides the most comprehensive review on the ocular manifestations of COVID-19 in different parts of the eye and to demonstrate the detection rate of conjunctival swab/tear PCR. A total of 2025 patients and more than 14 studies were accounted for in the meta-analysis. The pooled prevalence of the ocular manifestations in COVID-19 is estimated to be 7% (95% CI: 0.03–0.10) in our meta-analysis. The conjunctival symptoms, which included conjunctival hyperemia, increased secretion, pain, and foreign body sensation, were the most common ocular manifestations in COVID-19. All studies included in the systematic review had reported patients with a presentation of conjunctivitis. Rare instances of corneal signs such as pseudodendrites, epithelial defect, and surface complications such as pseudomembranes were also reported in separate case reports. There was a patient who reported floaters after admission for COVID-19, but the causation relationship could not be ascertained. Currently, no clinically significant involvement of the aqueous, iris, vitreous, or retina has been reported. Potential comorbidities including orbital cellulitis and retinal toxicity due to the use of chloroquine/hydroxychloroquine beyond the daily recommended dosage were among the possible and serious ocular complications following COVID-19.
Detection of severe acute respiratory syndrome-coronavirus 2 from tears or conjunctival secretions
Although the most common ocular symptom presented by COVID-19 patients is conjunctivitis, the positive detection rate of the SARS-CoV-2 virus from RT-PCR of conjunctival tears or secretions remained low. The range of positive conjunctival tear PCR results in patients with ophthalmic symptoms was about 1%–5% according to our systematic review. The pooled positive rate of the conjunctival swab/tear PCR in our meta-analysis was low (1%, 95% CI: 0.00–0.03), in spite of high specificity of the test. The causes of low but positive detection rate of SARS-CoV-2 in tears or conjunctival secretion could be as following:
-
(a) The window period of viral shedding in ocular tissues might be too short and is not definitively known as in what phase of COVID-19 does it occur. However, there might be certain clues based on our systematic review. Wu et al. had shown that ocular manifestations were more likely in severe COVID-19, and 91.7% of these patients with ocular presentation were tested positive for SARS-CoV-2 from conjunctival swab.[33] Xia et al. had also shown that no virus was detected in the tears or conjunctival secretions from patients without conjunctivitis, in contrast to positive detection in the tears of one patient with conjunctivitis.[16] Altogether, this suggested that patients with conjunctival symptoms were more likely to have positive detection from tears or conjunctival swab than those without.
Prospectively, Seah et al. investigated the viral shedding in tears as compared to nasopharyngeal swab throughout a 2-week course of active COVID-19 infection in 17 patients.[32] Although there was no positive result of tear viral shedding during the course, it is important to note that only one among the 17 patients had presented with conjunctivitis, and that most patients sought treatment a few days after symptoms onset, thus leading to difficulty in early sampling during infection.
In conclusion, the optimal window period has not been determined yet, and the sampling condition was also not yet ascertained. This is one of the major reasons that conjunctival swab is not feasible for definite diagnosis purpose
-
(b) ACE2/TMPRSS2 expression in the eye facilitates viral colonization or transmission via ocular route, however, ocular antiviral measures might explain the low frequency of eye involvement. The presence of SARS-CoV-2 in conjunctival secretion or tears has raised the concern of COVID-19 transmission through the conjunctiva. It was also shown that the entry of SARS-CoV-2 depended on SARS-CoV-2 spike protein/ACE2 receptor interaction.[43] It has also been reported that there is a high possibility that SARS-CoV-2 and SARS-COV can both interact with ACE2, which is located prominently on lung alveolar epithelial cells and small intestinal enterocytes.[44,45]
Ocular structures contain its own renin–angiotensin–aldosterone system and ACE receptors, most of them intraocular, such as aqueous humor,[46] retinal pigmented epithelia,[47] and retina.[48] However, reports of such protein expression in the conjunctiva and cornea were limited in the literature before COVID-19.
A few reports have shown the expression of such proteins in both conjunctiva and cornea. An in vitro model by Sun et al. revealed the positive expression of ACE2 in corneal and conjunctiva cells. Subsequently, these cells were shown to bind to the S240 proteins of SARS-CoV.[13] The expression of ACE2 was also found on the surface of corneal endothelial cells. As mentioned in the pathogenesis section, the co-expression of ACE2 and TMPRSS2 in ocular surface (limbal, corneal, and conjunctival) epithelia from recent studies raised the possibility of ocular surface serving as a portal of entry for SARS-CoV-2.[14,43,49] All these evidence, despite rare, might explain the positive detection of viral genome from conjunctival swab and raise the possibility of transmission through ocular tissue.
Even so, given the scarce nature of currently available evidence regarding SARS-CoV-2 transmission through ocular tissues, no strong conclusion can be made to ascertain whether infection from ocular structures is possible or if the virus can reach the respiratory tract via nasolacrimal duct.[50] One of the possible explanations for the low frequency of viral transmission via ocular route is the adoption of antiviral capacity by ocular surface epithelia. For example, while microarray or transcriptome sequencing confirmed ACE2 expression in the conjunctiva, RNA-editing enzymes such as APOBEC3A and ADAR-1 were also highly expressed in conjunctival epithelial and ex vivo cornea samples by Leonardi et al.[51] This implicates that countermeasures can develop in ocular surface by local expression of antiviral factors, which explains the low prevalence of ocular manifestations as well as the low yield of viral tests from the conjunctiva
(c) Low viral load in conjunctival secretion/tissues and inconsistencies in sample handling. As shown in our systematic review, the SARS-CoV-2 RT-PCR assay for tears or conjunctival swab is inconsistent in its detection rate. Overall, the detection rate remained low in all studies, which might be due to inherent low viral load in tears or conjunctival secretion, the absence of viral culture, and the lack of standardized handling or testing protocol for collected samples.[32,38] This issue is further exacerbated as the sensitivity of nucleic acid amplification test in detecting SARS-CoV-2 varied at different sites.[52]
As previously mentioned, although ACE2 has been located on the ocular surface, it is not currently clear if SARS-CoV-2 can thrive in the microenvironment of ocular surface. This would explain the low detection rate of SARS-CoV-2 in tears or conjunctival tissue because it might not be a suitable niche environment for colonization of the virus.
Inconsistencies in sample testing were also observed. Although Seah et al. tested both nasopharyngeal swab and tears for SARS-CoV-2 in the same patients, both samples were differently handled by the respective clinical and research laboratories.[32] The limitation of detection by the research laboratory was not reported in the study. Besides, a limited quantity of tears and conjunctival secretions was collected, thus resulting in insufficient sample concentration for RT-PCR.
Pediatric ocular presentation in coronavirus disease 2019
In the current literature, ocular presentation in COVID-19 patients was mostly reported only in adults. Valente et al. reported conjunctivitis in 3 out of 27 children with mild clinical course. It is hypothesized that children might have more benign systemic manifestations of COVID-19 as compared to adults.[34] Similarly, the positive rate of conjunctival swabs was low in pediatric patients with conjunctivitis.
A COVID-19-infected child with conjunctivitis and eyelid dermatitis as reported by Wu et al. had self-resolving symptoms 5 days after onset.[17] However, muscle enzyme elevation was noted initially, thus suggesting that although symptoms might be milder in children, the blood indices of children with ocular manifestations should be closely monitored.
Finally, a case series by Chiotos et al., which primarily highlighted a multisystem inflammatory syndrome observed in critically ill children with COVID-19, had also reported conjunctivitis as one of the presenting symptoms.[53]
Transmission risk of severe acute respiratory syndrome-coronavirus 2 via conjunctiva or tears
In spite of the low prevalence of conjunctival symptoms in COVID-19 patients, the positive detection of viral genome in tears still demonstrates that SARS-CoV-2 has the capacity to cause ocular disease or even viral transmission using the eye as a portal of entry. It is known that HCoV commonly spreads through respiratory droplets or direct contact with virus-contaminated fomites.[54] As an exposed mucosal structure, the conjunctiva is susceptible to infectious droplets and fomites during close contact with infected patients. This is consistent with the fact that respiratory viruses, such as human adenovirus (species D) and avian influenza virus (H7), can cause highly contagious keratoconjunctivitis or conjunctivitis. Tears and conjunctival secretions can also contain and spread the virus.[54,55] Currently, there is no clinical cohort or populational study to statistically evaluate the transmission risk of COVID-19 from conjunctiva tissues or tears.
Prevention strategies for ophthalmologists in managing coronavirus disease 2019 patients
Based on our previous report on the proposed measures for ophthalmologists during the COVID-19 pandemic, the essential strategies for ophthalmologists in surmounting the challenges of COVID-19 comprise proper personal protective equipment (PPE); patient volume control; detailed travel, occupation, contact, and cluster screening; and effective infection control measures in clinics, inpatient wards, and operation theaters.[56] In addition, any possibility of cross infection can be effectively reduced with close health monitoring of all healthcare professionals and strict measures by policy makers as well to enforce mask-wearing and social distancing in the hospital.
In view of the transmission risk of COVID-19 by close contact of ophthalmologists with their patients, telemedicine is also utilized and promoted during the pandemic. For instance, a strategy involving telemedicine technology in the emergency department was implemented before seeing the patients personally.[56] Doctors could perform history taking and a brief inspection of the external eyes before deciding if the patients require further examination. Through this measure, patients could receive a detailed evaluation, and long-time close contact can be avoided.[56]
Finally, our systematic review suggested that patients infected with COVID-19 might present with ocular manifestations first. Therefore, all ophthalmologists must adhere to proper infection control protocols and personal protection including face masks, PPE, and gloves when examining all patients, regardless of travel or contact history. Essential and nonessential ophthalmic services can also be written in the interim guidelines to guide ophthalmologists to balance the risks and necessity of providing crucial ophthalmic services.
Limitations
Although this is a systematic review and meta-analysis of the largest number of patients to date, it has several limitations. First, most of the included studies were of retrospective nature and case series/reports. However, with respect to the scant evidence available and the urgency of the matter, these studies to investigate the prevalence of ocular manifestations or viral shedding in conjunctiva/tears were evaluated in detail by multiple reviews, as only studies with good quality and low risk of bias were included. Second, studies were conducted in the midst of a worldwide outbreak. There is a possibility that certain studies have not yet been published, which might affect the results in the future. In spite of this, most included papers have been assessed critically for its risk of bias and value of evidence, thus providing an objective evaluation of the issue at hand.
Conclusion
Ocular manifestations in COVID-19 patients commonly comprise ocular surface symptoms, such as conjunctival hyperemia, foreign body sensation, and rarely corneal subepithelial infiltrates. The prevalence of ocular presentation among COVID-19 patients is low, as is shown in our meta-analysis, and there has been no definite report of transmission from the conjunctiva, but it is imperative for ophthalmologists and all health professionals to be aware of the full spectrum of COVID-19 symptoms or signs. The multifaceted mechanisms of ocular colonization or viral shedding from tears of SARS-CoV-2 are still not clear yet. Further experimental and clinical research should be conducted to investigate the importance of ocular manifestations, as well as its underlying mechanisms, in the progression or transmission of COVID-19.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that there are no conflicts of interests of this paper.
Acknowledgment
This work did not receive funding from any research grant or any sponsoring organization. Study design, data analysis or interpretation, and writing were all completed independently by all the authors.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization. 2020. [[Last accessed on 2020 Jul 15]]. Available from: https://covid19whoint .
- 2.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–7. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–94. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyrrell DA, Bynoe ML. Cultivation of a novel type of common-cold virus in organ cultures. Br Med J. 1965;1:1467–70. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendley JO, Fishburne HB, Gwaltney JM., Jr Coronavirus infections in working adults.Eight-year study with 229 E and OC 43. Am Rev Respir Dis. 1972;105:805–11. doi: 10.1164/arrd.1972.105.5.805. [DOI] [PubMed] [Google Scholar]
- 7.Tay MZ, Poh CM, Renia L, MacAry PA, Ng LF. The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–44. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann Intern Med. 2020;172:577–82. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj VS, Mou H, Smits SL, Dekkers DH, Müller MA, Dijkman R, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–4. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–9. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Liu L, Pan X, Jing M. Mechanism of the action between the SARS-CoV S240 protein and the ACE2 receptor in eyes. Int J Ophthalmol. 2006;6:783–6. [Google Scholar]
- 14.Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf. 2020;18:537–44. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui KP, Cheung MC, Perera RA, Ng KC, Bui CH, Ho JC, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: An analysis in ex vivo and in vitro cultures. Lancet Respir Med. 2020;8:687–95. doi: 10.1016/S2213-2600(20)30193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–94. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu P, Liang L, Chen C, Nie S. A child confirmed COVID-19 with only symptoms of conjunctivitis and eyelid dermatitis. Graefes Arch Clin Exp Ophthalmol. 2020;258:1565–6. doi: 10.1007/s00417-020-04708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinho PM, Marcos AA, Romano AC, Nascimento H, Belfort R., Jr Retinal findings in patients with COVID-19. Lancet. 2020;395:1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17:541–3. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thevarajan I, Nguyen TH, Koutsakos M, Druce J, Caly L, van de Sandt CE, et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat Med. 2020;26:453–5. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XY, Du B, Wang YS, Kang HY, Wang F, Sun B, et al. The keypoints in treatment of the critical coronavirus disease 2019 patient (2) Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:277–81. doi: 10.3760/cma.j.cn112147-20200224-00159. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–6. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh KM, Chiueh TS, Siu LK, Lin JC, Chan PK, Peng MY, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–22. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–8. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2020. [[Last accessed on 2020 Jul 15]]. Available from: http://wwwohrica/programs/clinicalepidemiology/oxfordasp .
- 27.Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A, et al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19) Can J Ophthalmol. 2020;55:e125–9. doi: 10.1016/j.jcjo.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Deng C, Chen X, Zhang X, Chen B, Yu H, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan. China: A cross-sectional study Acta Ophthalmol; 2020. Doi: https://doiorg/101111/aos14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navel V, Chiambaretta F, Dutheil F. Haemorrhagic conjunctivitis with pseudomembranous related to SARS-CoV-2. Am J Ophthalmol Case Rep. 2020 doi: 10.1016/j.ajoc.2020.100735. pii: 100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong N, Yu W, Xia J, Shen Y, Yap M, Han W. Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol. 2020 doi: 10.1111/aos.14445. Doi: https://doiorg/101111/aos14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khavandi S, Tabibzadeh E, Naderan M, Shoar S. Corona virus disease-19 (COVID-19) presenting as conjunctivitis: Atypically high-risk during a pandemic. Cont Lens Anterior Eye. 2020;43:211–2. doi: 10.1016/j.clae.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seah IY, Anderson DE, Kang AE, Wang L, Rao P, Young BE, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127:977–9. doi: 10.1016/j.ophtha.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–8. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valente P, Iarossi G, Federici M, Petroni S, Palma P, Cotugno N, et al. Ocular manifestations and viral shedding in tears of pediatric patients with coronavirus disease 2019: A preliminary report. J AAPOS. 2020;S1091-8531:30115–4. doi: 10.1016/j.jaapos.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Chen X, Chen L, Deng C, Zou X, Liu W, et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. 2020;18:360–2. doi: 10.1016/j.jtos.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Duan C, Zeng Y, Tong Y, Nie Y, Yang Y, et al. Ocular findings and proportion with conjunctival SARS-COV-2 in COVID-19 patients. Ophthalmology. 2020;127:982–3. doi: 10.1016/j.ophtha.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol. 2020;104:748–51. doi: 10.1136/bjophthalmol-2020-316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin C, Ye R, Xia YL. A meta-analysis to evaluate the effectiveness of real-time PCR for diagnosing novel coronavirus infections. Genet Mol Res. 2015;14:15634–41. doi: 10.4238/2015.December.1.15. [DOI] [PubMed] [Google Scholar]
- 39.Turbin RE, Wawrzusin PJ, Sakla NM, Traba CM, Wong KG, Mirani N, et al. Orbital cellulitis, sinusitis and intracranial abnormalities in two adolescents with COVID-19. Orbit. 2020;39:305–10. doi: 10.1080/01676830.2020.1768560. [DOI] [PubMed] [Google Scholar]
- 40.Zhou D, Dai SM, Tong Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75:1667–70. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruamviboonsuk P, Lai TY, Chang A, Lai CC, Mieler WF, Lam DS, et al. Chloroquine and hydroxychloroquine retinal toxicity consideration in the treatment of COVID-19. Asia Pac J Ophthalmol (Phila) 2020;9:85–7. doi: 10.1097/APO.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevens DV, Tran AQ, Kim E. Complications of orbital emphysema in a COVID-19 patient. Ophthalmology. 2020;127:990. doi: 10.1016/j.ophtha.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus.A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–92. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holappa M, Vapaatalo H, Vaajanen A. Many faces of renin-angiotensin system-focus on eye. Open Ophthalmol J. 2017;11:122–42. doi: 10.2174/1874364101711010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao L, Qiu Y, Fu X, Lin R, Lei C, Wang J, et al. Angiotensin-converting enzyme 2 activator diminazene aceturate prevents lipopolysaccharide-induced inflammation by inhibiting MAPK and NF-κB pathways in human retinal pigment epithelium. J Neuroinflammation. 2016;13:35. doi: 10.1186/s12974-016-0489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinores SA, Wang Y, Vinores MA, Derevjanik NL, Shi A, Klein DA, et al. Blood-retinal barrier breakdown in experimental coronavirus retinopathy: Association with viral antigen, inflammation, and VEGF in sensitive and resistant strains. J Neuroimmunol. 2001;119:175–82. doi: 10.1016/S0165-5728(01)00374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collin J, Queen R, Zerti D, Dorgau B, Georgiou M, Djidrovski I, et al. Co-expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface. Ocul Surf. 2020;S1542-0124:30097–5. doi: 10.1016/j.jtos.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qing H, Li Z, Yang Z, Shi M, Huang Z, Song J, et al. The possibility of COVID-19 transmission from eye to nose. Acta Ophthalmol. 2020;98:e388. doi: 10.1111/aos.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leonardi A, Rosani U, Brun P. Ocular surface expression of SARS-CoV-2 receptors. Ocular Immunol Inflammat. 2020;28:735–8. doi: 10.1080/09273948.2020.1772314. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–4. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: A case series. J Pediatric Infect Dis Soc. 2020;9:393–8. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belser JA, Rota PA, Tumpey TM. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev. 2013;77:144–56. doi: 10.1128/MMBR.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedrosa PB, Cardoso TA. Viral infections in workers in hospital and research laboratory settings: A comparative review of infection modes and respective biosafety aspects. Int J Infect Dis. 2011;15:e366–76. doi: 10.1016/j.ijid.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin JY, Kang E, Yeh PH, Ling X, Chen HC, Chen KJ, et al. Proposed measures to be taken by ophthalmologists during the coronavirus disease 2019 pandemic: Experience from Chang Gung Memorial Hospital, Linkou, Taiwan. Taiwan J Ophthalmol. 2020;10:80–6. doi: 10.4103/tjo.tjo_21_20. [DOI] [PMC free article] [PubMed] [Google Scholar]