Abstract
Background
From the first case of SARS-CoV-2 infection in Wuhan (China), the infection spread all around the world causing a pandemic of coronavirus disease-2019 (COVID-19). Spain has been one of the most severely affected countries, and Madrid has reported a high number of cases and deaths. We discuss our strategies for optimal breast cancer management during COVID-19 pandemic.
Patients and Methods
This was a retrospective observational study at Clínico San Carlos Hospital to analyze the management of patients with breast cancer during the pandemic outbreak and the surgical strategy after the pandemic outbreak. We created a practical and dynamic tool based on a “traffic light” system for prioritizing surgical time. Every patient was contacted by telephone with a preoperative COVID-19 protocol. After surgical procedures, patient satisfaction was assessed using the European Organisation for Research and Treatment of Cancer in-patient satisfaction with cancer care questionnaire (EORTC IN-PATSAT32).
Results
Patients with breast cancer actively treated with surgical procedures were put on a waiting list and received systemic therapy. Telemedicine was used to evaluate any side effects and to avoid unnecessary hospital visits. Surgery was only considered after the pandemic outbreak, and then, only those procedures designed to minimize surgical complications and, therefore, reduce hospital stay. We also measured patients’ satisfaction with medical and nursing scales that resulted in a “very good” evaluation tending to “excellent”.
Conclusion
It is necessary to adapt management of oncology treatment and surgical strategy to optimize resources during the COVID-19 pandemic. Patients’ perception of care quality and the degree of patients’ satisfaction with health services has potential relevance in the absence of outcome data.
Keywords: Breast cancer, Quality care, SARS-CoV-2, Surgical strategy, Systemic therapy
Micro-Abstract
Breast cancer experts share their experience in the management of surgical strategy for patients with breast cancer during the COVID-19 pandemic and subsequent crisis in the health care system. Minimal surgical procedures must be performed when resources are available, and a preoperative protocol is necessary for safe surgery following pandemic outbreak.
Introduction
On December 2019, from the first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Wuhan (China), the infection spread westward all around the world, causing a pandemic. The pandemic escalated into a global lockdown and a health care crisis. As the disease acquired the category of a pandemic, many countries struggled to adapt their health care systems to address this new emergency. Spain has been one of the countries most severely affected by coronavirus disease 2019 (COVID-19), and Madrid has reported a high number of cases and deaths.
The high number of COVID-19 (caused by SARS-CoV-2) cases in Madrid exceeded the existing hospital capacity. This health care crisis seriously threatened the medical attention given to other diseases, including cancer.1 Breast cancer remains one of the major public health problems owing to its high incidence, prevalence, and mortality.2 Our Breast Cancer Unit has been working from the outset of the pandemic to take care of our patients, using our own clinical experience and according to the current recommendations and protocols.3 The results were different clinical points that aimed to balance the prevention of SARS-CoV-2 infection and provide oncologic treatments, while maintaining the highest quality of health care for patients with breast cancer.
Our approach aims to present the treatment strategies adopted by the Breast Cancer Unit at Clínico San Carlos Hospital in Madrid throughout the course of the epidemic. In addition, we investigated patients’ perception of quality of care.
Patients and Methods
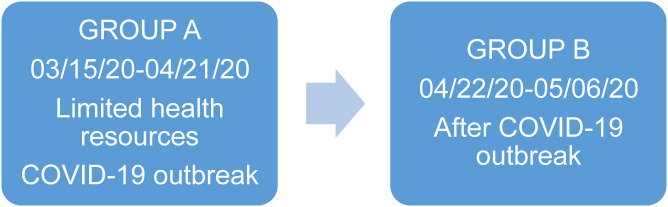
This retrospective observational study included 2 cohorts of patients with breast cancer from different phases of the epidemic (Group A and Group B) (Figure 1 ).
Figure 1.
Groups of Patients With Breast Cancer
Principal Considerations and Conceptual Framework
The 2 principal considerations in approaching breast cancer management during the COVID-19 pandemic at our center are: (1) breast cancer management during the COVID-19 outbreak and (2) surgical strategy after the COVID-19 outbreak.
The primary endpoints were to describe 2 different strategies throughout the course of the epidemic: (1) systemic oncology therapy according to the breast cancer subtype during the COVID-19 outbreak and (2) surgical procedures (surgical procedures in breast and/or axilla) after the COVID-19 outbreak.
The secondary endpoints included the residual cancer burden present after neoadjuvant treatment and every event related to the surgical procedure and/or adverse outcomes of COVID-19. Additionally, age, waiting time for treatment, and the inpatient stay were also included.
Ethics Approval
This study has been approved by the Clinical Research Ethics Committee of Hospital Clínico San Carlos.
Definitions
Group A
The group A study included all newly diagnosed patients (stage I-III), patients undergoing neoadjuvant therapy, and patients whose surgical treatments were postponed between March 15 and April 21, 2020 (N = 29 patients). These postponed surgical procedures were considered priority B and C (Consortium Priorities)3 during the pandemic outbreak. Every treatment was rescheduled based on immunohistochemical analysis and HER2/neu gene amplification by fluorescence in situ hybridization. Immunohistochemical breast cancer prognostic and therapeutic markers included estrogen receptor (ER), human epidermal growth factor receptor-2 (HER2), Ki-67, and progesterone receptor (PR).
Patients with ER-negative (ER−) and HER2-negative (HER2−) tumors received neoadjuvant chemotherapy and patients with HER2− tumors received neoadjuvant chemotherapy and targeted treatment. For ER-positive (ER+) and HER2-invasive breast cancer, we administered neoadjuvant endocrine therapy prior to surgical intervention. Endocrine responsiveness4, 5, 6 was evaluated with the 21-gene RS assay testing in early breast cancer: cT1c-T3 cN0 G2-3 and cT1b cN0 G3 ER+/HER2-invasive breast cancer.
Telemedicine was used to evaluate any side effects and to avoid unnecessary hospital visits, thus minimizing any exposure to the risk of SARS-CoV-2 infection.
Group B
The B group study included patients with surgical procedures between April 22 and May 6, 2020 (N = 28 patients). Surgical procedures were partially restored in line with the availability of resources, according to the epidemic trend.
Prior to surgery, prognostic clinical points were evaluated weekly by a multidisciplinary breast group, and factors related to risk of delaying surgery were identified: age, tumor biology, active anticancer therapy (cytotoxic chemotherapy, targeted drugs, endocrine therapy, and/or immunotherapy), and cancer status (responding to treatment vs. progressing).
Scale for Prioritizing Surgical Time
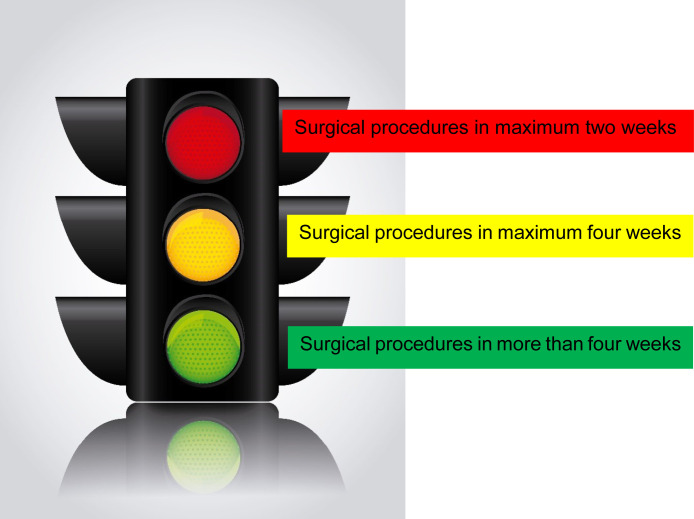
We also created a practical and dynamic tool to prioritize surgical time: a “traffic light” (Figure 2 ).
Figure 2.
“Traffic Light” System to Evaluate Surgical Time
High Priority (Red - Surgical Procedures in Maximum 2 Weeks)
Patient completing neoadjuvant chemotherapy and/or with additional mono-chemotherapy treatment in ER− tumors and progressing cancer.
Medium Priority (Yellow - Surgical Procedures in Maximum 4 Weeks)
Patients with endocrine therapy without genomic testing. We gave higher priority to younger women with neoadjuvant endocrine therapy.
Low Priority (Green - Surgical Procedures More Than 4 Weeks)
Endocrine therapy in elderly patients, patients with anti-HER2 monotherapy, re-excision procedures, and in situ ductal carcinoma (ER+/ER−).
It was assumed that all benign, cosmetic, and risk-reducing surgery could be deferred.
Furthermore, we completed the triage by taking into account other comorbidities requiring active treatment, such as hypertension, diabetes, and heart disease, and also taking into account the patient’s preferences. The most common surgery option was a minimal surgical procedure, to avoid surgical complications and reduce hospital stay, thus minimizing the risk of exposure to the virus.
Preoperative COVID-19 Protocol
Every patient was telephoned and checked according to our hospital preoperative COVID-19 protocol (see Supplemental Appendix 1 in the online version).
Patients’ Perception of Care Quality
In-patient satisfaction with cancer care was measured in group B with the European Organisation for Research and Treatment of Cancer in-patient satisfaction with cancer care questionnaire (EORTC IN-PATSAT32).7 Patients were contacted by telephone after their discharge from hospital, were informed of the objectives and procedures of the survey, and were invited to take part. None of the patients refused to participate.
The EORTC IN-PATSAT32 questionnaire is composed of 32 questions designed to assess cancer patients’ perception of the quality of hospital-based care, relevant across country settings. This questionnaire was developed according to the guidelines and procedures recommended by the EORTC Quality of Life Group.
The survey was carried out via phone call as a structured interview with closed questions.
The patient can choose from 5 alternative responses on the Likert scale (“poor,” “fair,” “good,” “very good,” and “excellent”). This type of response scale has been shown to have methodological advantages over other types of response scales. All scores are linearly transformed to a 0 to 100 scale. The selected time frame was each individual hospital stay. This test can discriminate between patients with different care expectations and varying preexisting intentions as to recommending their hospital to other potential health care users. A higher score reflects a higher level of satisfaction.
The EORTC IN-PATSAT327 test shows a great psychometric solidity, a Cronbach alpha of 0.80 to 0.90, and is adapted to the Spanish language.
Statistical Analysis
Descriptive statistics were used to describe patient and treatment characteristics. Continuous data were reported as means plus standard deviation, or as medians and interquartile range (IQR), depending on the distribution. The Mann-Whitney test was used to compare the median of waiting list times between both periods. Statistical analysis was performed using the IBM SPSS Statistics for Windows, Version 22.0. A P value below .05 was considered statistically significant.
Results
Breast Cancer Management During the COVID-19 Outbreak
From March 15, 2020, to April 21, 2020, 36 patients pending surgery at the time of the COVID-19 outbreak pandemic were included on a waiting list. Of these, 29 patients had invasive breast cancer and 7 patients had ductal in situ carcinoma (Consortium Priority B and C). The oncologic team rescheduled their treatments during the worse weeks of the pandemic. Invasive breast cancer subtype and patients’ oncology treatments are shown in Table 1 .
Table 1.
Management of Oncology Treatment During COVID-19 Outbreak
| Oncology Primary Treatment In Group A (N = 29) | n |
|---|---|
| ER− HER2− breast cancer: docetaxel + carboplatin | 6 |
| HER2+ either ER+ or ER− breast cancer: anti-HER2-targeted therapy (taxanes plus trastuzumab and pertuzumab) | 10 |
| ER+ breast cancer: | 13 |
| Taxane-based neoadjuvant chemotherapy | 2 |
| Endocrine therapy (tamoxifen w/o LH-RH analogs or NSAI) | 11 |
| 21-gene assay | 8 |
| Low RS (≤ 25) | 6 |
| High RS (> 25) | 2 |
Abbreviations: ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; LH-RH = luteinizing hormone releasing hormone; NSAI = non-steroidal aromatase inhibitor; RS = recurrence score.
Six (20.7%) patients with invasive triple negative breast cancer underwent docetaxel plus carboplatin neoadjuvant chemotherapy. Two additional single carboplatin cycles were rescheduled in 2 (6.9%) patients from this group before surgical procedure during this phase. Finally, both patients achieved pathologic complete response at surgery.
Ten (34.4%) patients with HER2+ breast cancer received neoadjuvant chemotherapy, trastuzumab, and pertuzumab. Trastuzumab and pertuzumab were maintained until surgery time.
Patients newly diagnosed with hormone receptor-positive breast cancer (HR+) (including 1 patient with N1 nodal involvement) began endocrine therapy. The 21-gene recurrence score (RS) assay at the time of the diagnosis was carried out in 8 of 13 patients in this group. High RS (> 25) was found in 2 patients, so they received taxane-based neoadjuvant chemotherapy. Patients with low RS (≤ 25) continued with neoadjuvant endocrine therapy up to the time of surgery. We did not prescribe endocrine therapy in patients with ER+ ductal carcinoma in situ.
In this cohort of patients with systemic therapy, only 2 patients required hospitalization owing to SARS-CoV-2 infection. Both patients recovered from the infection.
Surgical Strategy After the COVID-19 Outbreak
In total, 28 patients with breast cancer (COVID-19–negative by polymerase chain reaction [PCR]) with a median age of 57 years (IQR, 51-62 years) underwent breast surgery. Twenty of 28 surgical procedures included patients who had received neoadjuvant oncology treatment: 13 patients with neoadjuvant chemotherapy and 7 patients with hormone therapy drugs. Axillary lymph node dissection was more common in the neoadjuvant setting (10 patients).
The different types of breast surgery and the characteristics of the tumors are detailed in Table 2 . The use of oncoplastic resources was kept to a minimum. Four of 18 conservative surgeries required minimal use of oncoplastic procedures, and in 3 of 10 mastectomies, an immediate breast reconstruction with an implant was considered. Because risk-reducing surgeries were deferred, the surgery was less than planned in 3 patients. Surgical procedures postponed in patients with ductal carcinoma in situ would be performed in 6 patients when resources were established.
Table 2.
Breast Cancer Subtype, Surgery, and Histopathologic Characteristics Following COVID-19 Outbreak
| Breast Cancer Subtype, Surgery, and Histopathologic Features in Group B (N = 28) | n |
|---|---|
| Breast cancer subtype (IHQ) | |
| ER+ | 11 |
| HER2+ | 2 |
| ER+ and HER2+ | 3 |
| ER− and HER2− | 5 |
| In situ carcinoma | 6 |
| Breast surgery | |
| Breast conservation | 18 |
| Mastectomy | 10 |
| Axillary surgery | |
| Sentinel lymph node | 14 |
| Axillary lymphadenectomy dissection | 10 |
| Histopathologic features | |
| Invasive ductal carcinoma | 21 |
| Invasive lobular carcinoma | 1 |
| In situ ductal carcinoma | 6 |
| Residual cancer burden (n = 13) | |
| RCB 0 | 5 |
| RCB 1 | 3 |
| RCB 2 | 4 |
| RCB 3 | 1 |
Abbreviations: ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; IHQ = Immunohistochemistry; RCB = residual cancer burden.
During the study period, the average time spent on the waiting list varied according to the trend in the epidemic and the surgical resources available. For patients with surgery scheduled for March, the median waiting list time was 75 days (IQR, 48.5-81.3 days), for April, it was 28 days (IQR, 11-42.5 days), and for May, 13.5 days (IQR, 11.8-27.5 days) (P < .001).
Patients awaiting surgical procedures were screened for COVID-19 symptoms prior to arrival at the hospital, and those patients who reported symptoms were referred for further evaluation 2 weeks later. This triage was done by telephone call using our hospital preoperative COVID-19 protocol (see Supplemental Appendix 1 in the online version).
At the time of writing, and in line with the current state of the epidemic, the basal preoperative protocol only includes epidemiology, clinical, and PCR COVID-19 testing with a nasopharyngeal swab. Chest x-ray and blood serologic testing may also be considered depending on the results of the basal protocol. Only 2 surgeries were delayed 2 weeks because of a PCR COVID-19–positive nasopharyngeal swab. Both patients had undergone chemotherapy neoadjuvant treatment.
After surgery, the patients were hospitalized in COVID-19–free areas, and no perioperative complications or SARS-CoV-2 infection were reported.
The median total hospital length of stay was 2.0 days (IQR, 0-2.0 days). For breast-conserving surgery, the median total hospital length of stay was 1.0 day (IQR, 0-2.0 days); for mastectomy, it was 3.0 days (IQR, 2.5-3.5 days), and mastectomy with immediate reconstruction with an implant had a median of 4.0 days (IQR, 2.5-4 days).
Patients’ Perception of Care of Quality After Surgery
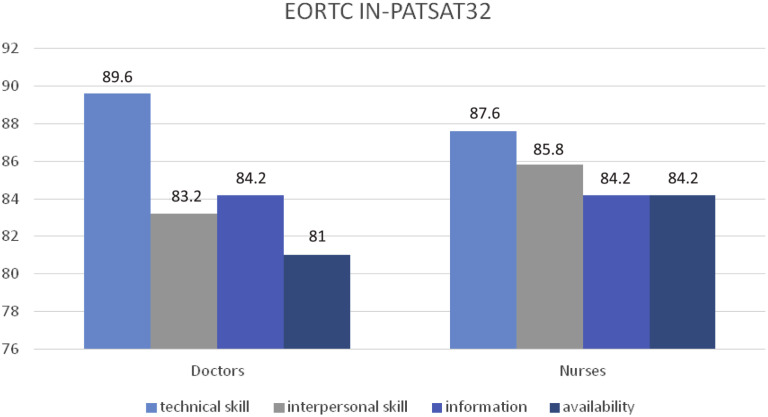
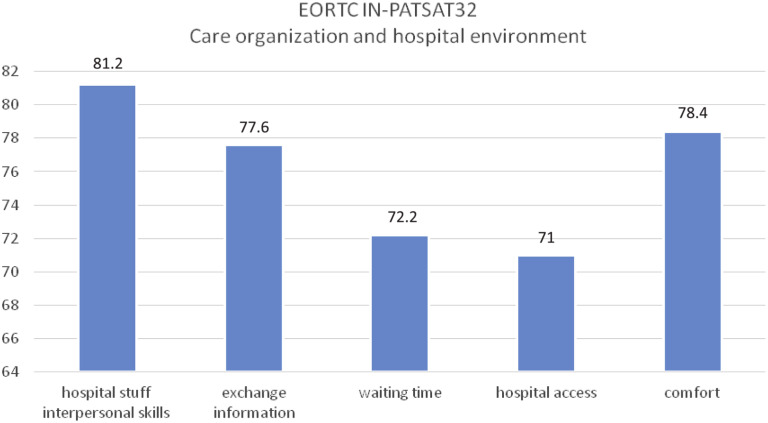
The following aspects of patients’ perception of care quality after surgery procedures were recorded (Figures 3 and 4 ). For satisfaction with medical care, the data obtained in the 4 evaluated scales are shown next: technical skills (89.6), interpersonal skills (83.2), information administration (84.2), and availability (81). For satisfaction with nursing care, the same 4 scales were used, obtaining the following data: technical skills (87.6), interpersonal skills (85.8), information administration (84.2), and availability (84.2). For satisfaction with organization quality and services provided by the hospital, different scales were taken into consideration: hospital staff interpersonal skills (81.2), the exchange of information (77.6), waiting time (72.2), hospital access (71), and comfort (78.4).
Figure 3.
Perception of the Quality of Care With Doctors, Nurses, and Hospital
Abbreviation: EORTC IN-PATSAT32 = European Organisation for Research and Treatment of Cancer in-patient satisfaction with cancer care questionnaire.
Figure 4.
Aspects of Care Management and Hospital Environment
Abbreviation: EORTC IN-PATSAT32 = European Organisation for Research and Treatment of Cancer in-patient satisfaction with cancer care questionnaire.
Finally, overall satisfaction with the hospital stay obtained a value of 91.4.
Discussion
The COVID-19 pandemic has arisen in a context of high multidisciplinary complexity.8 The adverse impact of the pandemic has caused a change in breast cancer care and, consequently, in breast cancer protocols, a direct result of this health emergency.
The European Society for Medical Oncology (ESMO),9 the American Society of Breast Surgeons (ASBrS), the National Accreditation Program for Breast Centers (NAPBC), the National Comprehensive Care Network (NCCN), the Commission on Cancer (CoC), and the American College of Radiology (ACR) all agree in recommending the postponement of non-surgical emergencies during the COVID-19 pandemic outbreak.10, 11, 12 In line with global scientific opinion, our multidisciplinary committee considered postponing breast cancer surgeries (Consortium Priority B and C) during this period. Every patient (breast cancer I-III stages included) underwent chemotherapy, targeted treatments, and endocrine therapy as a safe alternative approach, in an adaptation of breast oncology practice in our center at a time of minimal burdening of hospital resources. We did not prescribe primary endocrine therapy in ductal carcinoma in situ prior to surgery, in contrast to other teams.3 , 13 Nevertheless, it is a valuable option to be considered.
Salient features about immunocompromised patients with cancer emerge in this pandemic scenario, owing to their cancer and its treatment. Initial reports suggested that patients with a history of an active cancer might be at increased risk of SARS-CoV-2 infection and related complications, as per Liang et al,8 who even suggested postponing adjuvant chemotherapy as well as surgery for stable patients with cancer in endemic areas. In contrast, only 2 patients from our data group required hospital admission, as a result of presenting respiratory symptoms, but with an inconclusive COVID-19 diagnosis (COVID-19 PCR-negative). Zhang et al14 observed fatal outcomes in immunosuppressed patients with cancer, and their rate of infection for SARS-CoV-2 could be up to 30%. These authors propose that chemotherapy treatment must not be ended in these patients, avoiding unnecessary patient hospital visits, especially inside designated COVID-19 hospitals.
The latest research on the effects of COVID-19 on patients with cancer15 suggests that mortality in patients with cancer is exacerbated by advancing age and the presence of other non-cancer–related comorbidities. Gender has been found to be an important risk factor in the infection, and Grasselli et al16 describe a significantly lower risk of developing a critical illness from infection in women compared with men. More specific research related to mortality and SARS-CoV-2 infection in patients with breast cancer carried out by Vuagnat et al17 found that age and hypertension were associated with disease severity rather than the extent of disease or ongoing cancer therapy (including all stages of breast cancer). Recent studies suggest that breast cancer per se is not a major contributor to COVID-19 mortality, even in patients at high risk owing to an immunosuppressive treatment.18
However, limited and inconclusive data on the effect of the pandemic on surgical procedures in patients with SARS-Cov2 infection are known,16 and a triage is still necessary prior to establishing surgical procedures. No postoperative complications were reported in our data, probably resulting from the precautions used to avoid the spread of the pandemic and the SARS-CoV-2 infection at the time of surgery, such as other authors postulated.2 , 3 , 19 More research is needed to provide us with more information on surgery in patients with SARS-CoV-2 infection.
Cancer progression must also be considered in this scenario, and authors such as Liang et al8 and general medical best practice lead us to the view that a delay in breast cancer surgery could lead to cancer progression, increase perioperative morbidity/mortality, and increase disease spread as a result of the delay. In contrast, we have reported 5 patients with residual cancer burden in 0 of 12 surgeries in patients that underwent chemotherapy treatment. However, more time is necessary to predict the biological behavior and influence of the treatments in this group of patients with breast cancer. Further studies are necessary in order better to predict long-term outcomes in patients with breast cancer where cancer treatment has been postponed.20
Both diagnosis and treatment of breast cancer are already stressful life events. However, during the COVID-19 pandemic, changes in the primary treatment of breast cancer and exposure to SARS-CoV-2 infection lead to an even more stressful situation in these patients. A recent survey of increased COVID-19 distress among patients awaiting surgery detected fear that the pandemic could cause a delay in their oncologic treatments and fear they could be more vulnerable to the infection, in women with invasive cancer.21
In addition, there is a direct relation between care satisfaction levels and adherence to medical recommendations, adherence of oncologic treatment regime, and health condition improvement.22 In the present study, we considered the psychological impact after surgical procedures. We found that the COVID-19 pandemic required rapid changes in relation to outpatient consultations, information, and interventions. Our results show that all medical and nursing scales got a final “very good” evaluation, tending to “excellent.” The lowest score was hospital accessibility, which the test itself recognizes has lower internal consistency because it includes 2 related elements but is totally different in concept (ease of access to the hospital itself–transport and parking lots–and ease of access to services inside the hospital).
Conclusion
In conclusion, in the context of the COVID-19 pandemic, it is our role to provide breast cancer care and a surgical strategy that optimizes resources. The quality of care of our Breast Cancer Unit was perceived by patients as good; this has a potential relevance in the absence of outcome data. In this paper, we also share an approach that provides physicians with a visual and dynamic tool for prioritizing surgical time.
Further investigation and different points must be considered in breast cancer care and surgery over the following months and particularly should a second wave occur.
Clinical Practice Points
-
•
We present local management for adapting breast cancer care in a large Madrid hospital during and following the COVID-19 spring peak.
-
•
Treatment decision-making should balance risk and benefits of the surgical time based on a practical “traffic light” tool.
-
•
A preoperative COVID-19 protocol is necessary for a safe surgical procedure.
-
•
Patients’ perception of care quality has potential relevance in the absence of outcome data.
Disclosure
The authors have stated that they have no conflicts of interest.
Footnotes
Supplemental Appendix accompanying this article can be found in the online version at https://doi.org/10.1016/j.clbc.2020.10.006.
Supplemental Appendix 1. COVID-19 Surgery Protocol
Phone call date __/__/____
-
1.Epidemiological Checklist
-
1.1Have you been diagnosed with a probable, possible or confirmed case of COVID19? Yes No
-
1.2Have you been exposed to a probable, possible or confirmed case of COVID19 in the last 14 days? Yes No
-
1.1
-
2.
Clinical Checklist
In the last 14 days have you noticed any of the following symptoms?
-
❏
Fever or light fever
-
❏
Cough
-
❏
Dyspnea
-
❏
Discomfort
-
❏
Headache
-
❏
Muscle or joint pain
-
❏
Shivers
-
❏
Fatigue
-
❏
Odynophagia
-
❏
Nausea or vomiting
-
❏
Diarrhea
-
❏
Anosmia or ageusia
-
❏
Other: __________
Was it delayed for a clinic criterion? Yes No (reevaluate 14 days after the beginning of the symptomatology)
Was it delayed for an epidemiological criterion? Yes No (reevaluate 14 days after the last certified contact with the subject or 14 days after the last day of domestic isolation if the contact was with someone from the same household)
Re phone call date: ___/___/_____
(remember to remain in preventive domestic isolation until the following hospital appointment)
-
3.Lab and image Checklist
-
3.1PCR results: Positive Negative Inconclusive
-
3.2Serology results: Positive Negative Inconclusive
-
3.3COVID 19 suggestive blood test? Yes No
-
3.4COVID19 pneumonia suggestive imaging test? Yes No
-
3.5Oxygen saturation <94%? Yes No
-
3.1
Final Evaluation: Planned Delayed
Signature: Employee number: _____________
References
- 1.Coronavirus disease 2019 (COVID-19) Situation Report – 92. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200421-sitrep-92-covid-19.pdf?sfvrsn=38e6b06d_8 Available at:
- 2.Tasouli M.-K., Roche N., McNeill F. Rationalizing breast cancer surgery during the COVID-19 pandemic. Eur J Surg Oncol. 2020;46:1192–1193. doi: 10.1016/j.ejso.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietz J.R., Moran M.S., Isakoff S.J., et al. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 Pandemic Breast Cancer Consortium. Breast Cancer Res Treat. 2020;181:487–497. doi: 10.1007/s10549-020-05644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bear H.D., Wan W., Robidoux A., et al. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: a multicenter trial. J Surg Oncol. 2017;115:917–923. doi: 10.1002/jso.24610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwata H., Masuda N., Yamamoto Y., et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+ HER2-negative breast cancer: the TransNEOS study. J Breast Cancer Res Treat. 2019;173:123–133. doi: 10.1007/s10549-018-4964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pérez Ramírez S., Del Monte-Millán M., López-Tarruella S., et al. Prospective, multicenter study on the economic and clinical impact of gene-expression assays in early-stage breast cancer from a single region: the PREGECAM registry experience. Clin Transl Oncol. 2020;22:717–724. doi: 10.1007/s12094-019-02176-x. [DOI] [PubMed] [Google Scholar]
- 7.Brédart A., Bottomley A., Blazeby J.M., et al. European Organisation for Research and Treatment of Cancer Quality of Life Group and Quality of Life Unit An international prospective study of the EORTC cancer in-patient satisfaction with care measure (EORTC IN-PATSAT32) Eur J Cancer. 2005;41:2120–2131. doi: 10.1016/j.ejca.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Azambuja E., Trapani D., Loibl S., et al. ESMO management and treatment adapted recommendations in the COVID-19 era: breast cancer. ESMO Open. 2020;5:e000793. doi: 10.1136/esmoopen-2020-000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curigliano G., Cardoso M.J., Poortmans P., et al. editorial board of The Breast. Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast. 2020;52:8–16. doi: 10.1016/j.breast.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vicini E., Galimberti V., Naninato P., Vento A.R., Ribiero Fontana S.K., Veronisi P. COVID-19: the European Institute of Oncology as a “hub” centre for breast cancer surgery during the pandemic in Milan (Lombardy region, northern Italy) - A screenshot of the first month. Eur J Surg Oncol. 2020;46:1180–1181. doi: 10.1016/j.ejso.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viale G., Licata L., Sica L., et al. Personalized risk-benefit ratio adaptation of breast cancer care at the epicentre of COVID-19 outbreak. Oncologist. 2020;25:e1013–e1020. doi: 10.1634/theoncologist.2020-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson C.K., Lee M.K., Baker J.L., Attai D.J., DiNome M.L. Taking a second look at neoadjuvant endocrine therapy for the treatment of early stage estrogen receptor positive breast cancer during the COVID-19 outbreak. Ann Surg. 2020;272:e96–e97. doi: 10.1097/SLA.0000000000004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L., Zhu F., Xie L., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee L.Y.W., Cazier J.B., Starkey T., et al. UK Coronavirus Cancer Monitoring Project Team COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grasselli G., Zangrillo A., Zanella A., et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vuagnat P., Frelaut M., Ramtohul T., et al. COVID-19 in breast cancer patients: a cohort at the Institut Curie hospitals in the Paris area. Breast Cancer Res. 2020;22:55. doi: 10.1186/s13058-020-01293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jindal V., Sahu K.K., Gaikazian S., Siddiqui A.D., Jaiyesimi I. Cancer treatment during COVID-19 pandemic. Med Oncol. 2020;37:58. doi: 10.1007/s12032-020-01382-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipe M.D., van Deukeren D., Kip M., et al. Impact of the COVID-19 pandemic on surgical breast cancer care in the Netherlands: a multicentre restrospective cohort study. Clin Breast Cancer. 2020 doi: 10.1016/j.clbc.2020.08.002. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang L.V., Hu Y. Poor clinical outcomes for patients with cancer during the COVID-19 pandemic. Lancet Oncol. 2020;21:862–864. doi: 10.1016/S1470-2045(20)30311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magno S., Linardos M., Carnevale S., Dilucca M., Di Leone A., Terribile D.A. The impact of the COVID-19 pandemic on breast cancer patients awaiting surgery: observational survey in an Italian University hospital. Breast J. 2020;26:1597–1602. doi: 10.1111/tbj.13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trapani D., Marra A., Curigliano G. The experience on coronavirus disease 2019 and cancer from an oncology hub institution in Milan, Lombardy region. Eur J Cancer. 2020;132:199–206. doi: 10.1016/j.ejca.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]