Abstract
As the incidence of traumatic spinal cord injury (tSCI) in the elderly rises, clinicians are increasingly faced with difficult discussions regarding aggressiveness of management, likelihood of recovery, and survival. Our objective was to outline risk factors associated with in-hospital mortality in elderly surgical and non-surgical patients following tSCI and to determine those unlikely to have a favorable outcome. Data from elderly patients (≥ 65 years of age) in the Canadian Rick Hansen SCI Registry from 2004 to 2017 were analyzed using descriptive analysis. Survival and mortality groups in each of the surgical and non-surgical group were compared to explore factors associated with in-hospital mortality and their impact, using logistical regression. Of 1340 elderly patients, 1018 had surgical data with 826 having had surgery. In the surgical group, the median time to death post-injury was 30 days with 75% dying within 50 days compared with 7 days and 20 days, respectively, in the non-surgical group. Significant predictors for in-hospital mortality following surgery are age, comorbidities, neurological injury severity (American Spinal Injury Association [ASIA] Impairment Scale [AIS]), and ventilation status. The odds of dying 50 days post-surgery are six times higher for patients ≥77 years of age versus those 65–76 years of age, five times higher for those with AIS A versus those with AIS B/C/D, and seven times higher for those who are ventilator dependent. An expected probability of dying within 50 days post-surgery was determined using these results. In-hospital mortality in the elderly after tSCI is high. The trend with age and time to death and the significant predictors of mortality identified in this study can be used to inform clinical decision making and discussions with patients and their families.
Keywords: elderly, mortality, prognosis, surgery, traumatic spinal cord injury
Introduction
The fastest growing age cohort in North America is the elderly (defined as ≥65 years old), which is attributed both to rising life expectancy, and the wave of “baby boomers” reaching retirement age.1 This wave increased the elderly population by 20%, and it continues to steadily increase.2,3 Forecasts indicate that by 2050, individuals ≥65 years of age will constitute ∼20% of the United States population,4 and with similar growth to 25% of the Canadian population projected.5 Traditionally, traumatic spinal cord injury (tSCI) has been an injury related to high energy mechanisms in a younger male population; however, because of the increasing proportion of the elderly in the population, and increasing life expectancy, falls in the older population are becoming an increasingly common cause.6–8 This is because of a number of factors including: age-related deterioration of mobility and coordination, cognitive impairment, and accumulating medical issues and their associated medications, coupled with increasing rates of cervical stenosis which increases the risk of spinal cord injury.9–12 This has seen the average age of those incurring a tSCI to rise from ∼29 in the 1970s to ≥40 years of age in current estimates,9,13,14 and forecasts indicate that by 2032, ∼ 40% of individuals experiencing a tSCI will be ≥60 years old.14
A tSCI is both a devastating physiological and psychological insult for all patients, particularly in the elderly who often have multiple pre-existing comorbidities. In-hospital mortality rates in elderly patients with tSCI are reported as being 12–40%,15–20 considerably higher than the estimated mortality of <5% in younger patient populations.16 Previously identified risk factors for mortality in the elderly include older age, higher cervical level of injury, neurological severity, and increasing number of comorbidities;17,18,20 however, to our knowledge, no one has developed a clinical decision-making aid using a combination of these factors for in-hospital mortality for this elderly population.
As well as the greater risk of mortality, tSCI and its subsequent treatment are associated with substantial morbidity in the elderly patient population. Aggressive surgical management is often followed by an extended intensive care unit (ICU) stay, tracheostomy and mechanical ventilation, and percutaneous endoscopic gastrostomy (PEG) tube insertion.20–24 This may lead to lengthy hospital admissions, a myriad of complications or adverse events, and, ultimately, in-hospital death.23,25
Knowing that this clinical course is not unusual for elderly patients after tSCI, and given their frailty and limited functional and neurological recovery, clinicians are increasingly faced with the challenge of weighing the risks and anticipated potential benefits of surgery.26 A further challenge is the limited evidence and tools available to facilitate the discussion and education about prognosis with patients and their families making difficult decisions regarding goals of management in severely injured elderly patients.27 Few studies focus on in-hospital mortality of the elderly population, with fewer comparing this outcome following surgical and non-surgical interventions.17,19 Further, most studies compare the elderly with a younger population13,15,16,20 or involve only cervical SCI or spine fractures.15,17,19,20,28–30 Further understanding on the clinical course of elderly patients with tSCI and the specific risk factors for those who die despite aggressive surgical management will inform early communication and shared decision making between clinicians and patients and family.
The objectives of this study were to describe the incidence and pattern of in-hospital mortality following surgery in the elderly after tSCI. We aim to identify factors associated with in-hospital mortality in both surgically and non-surgically treated patients, and to determine their impact on in-hospital mortality.
Methods
The study cohort utilized data from the Rick Hansen Spinal Cord Injury Registry (RHSCIR), which prospectively enrolled adults who were admitted with a new acute tSCI to one of the 18 acute and 12 rehabilitation facilities across Canada. The registry was initiated in 2004 to answer a priori research questions and to facilitate implementation of best practices. All RHSCIR sites obtained local research ethics board approval prior to enrolling participants, and data sharing agreements are in place with each site. Details on the RHSCIR data set have been described elsewhere.31
Analysis cohort
Individuals eligible for this study were patients who were admitted to a RHSCIR acute facility with a reported injury date between May 2004 and February 2017, were ≥65 years of age, and had neurological deficit as described by the American Spinal Injury Association (ASIA) Impairment Scale (AIS) (AIS A–D) affecting any neurological level from C1 to L1 identified at admission using the International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI).32
Study variables
The main outcome of the study was in-hospital mortality (yes; no) in the acute or rehabilitation facility during the initial in-patient stay. Patient factors considered were age at time of injury (65–76; ≥77 for surgical cohort, 65–79; ≥80 for non-surgical cohort), sex (female; male), and pre-existing medical comorbidities assessed by the Charlson Comorbidity Index (CCI). The CCI includes 19 diseases weighted on their seriousness, and is commonly used to predict mortality over 5 years for hospitalized patients.33 Injury factors examined were injury mechanism (falls, sports, transport, other), AOSpine Trauma Classification Systems for subaxial cervical (C3-C7)34 and thoracolumbar (T1-L5)35 spine (Type A, Type B, Type C), Injury Severity Score (ISS) (≤25; >25 indicative of major trauma to region in addition to the spine),36 neurological severity (AIS A, B, C, D) and neurological level at admission (high cervical, C1-C4; low cervical, C5-T1; thoracolumbar, T2-L1). Neurological data as classified by the ISNCSCI was collected and verified using the validated Rick Hansen Institute ISNCSCI Algorithm (v1.0.3)37 to ensure that severity and level were derived accurately from the raw ISNCSCI data. Care management factors included were triage pattern from injury to RHSCIR acute facility (directly, via another facility), time from injury to arrival at RHSCIR acute facility (≤12 h, >12 h), had surgery or not, time from injury to start time of surgical decompression where applicable (≤24 h, >24 h), days in ICU, had received a tracheostomy (yes, no), ventilation (yes, no), and PEG tube (yes, no).
Time from injury/surgery to death (days), acute length of stay (LOS), and discharge destination from initial in-patient stay (acute or rehabilitation care) were also reported.
Statistical analysis
Descriptive statistics were used to describe the patient, injury, and care management factors of the cohort. Further analysis was performed to compare those who survived with those who did not in both the “surgical” and the “non-surgical” groups, to explore factors associated with in-hospital mortality. The comparison was made using the t test or the Wilcoxon rank sum test for normally distributed or non-normally distributed continuous variables, respectively; if the expected cell counts were five or less, χ2 test or Fisher's exact test was used for categorical variables. Data visualization was performed to understand the pattern of age and time to death for in-hospital mortality and to determine any threshold of these two variables to inform the logistical regression modeling. To determine the impact of risk factors on in-hospital mortality, logistical regression model was performed adjusting for potential confounding variables that are deemed clinically relevant and informed by the literature. Variables examined were age at injury, neurological injury severity and level, comorbidities, and ventilation dependence. Status on tracheostomy and PEG were considered clinically relevant but were omitted from the model because they were not significant in the final model and also because they were related to ventilation status, which would cause collinearity in the model. For the surgical group, the model included only patients who died before the threshold (50 days post-surgery), to have a homogenous sample. A similar threshold was not used for the non-surgical group given the smaller sample size. Using the parameter estimates from the final model, expected probabilities of in-hospital mortality were obtained for several scenarios of different patient characteristics. Goodness-of-fit tests were performed for all regression models. Associations with a p value <0.05 were considered statistically significant. All analyses were performed using SAS software, Version 9.4 of the SAS System for Windows. ©2013, SAS Institute Inc., Cary, NC, USA.
Results
Analysis cohort
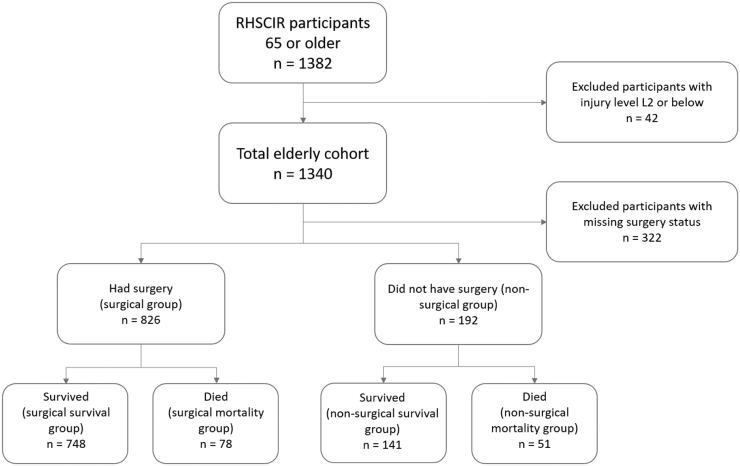
The total cohort consisted of 1340 patients with tSCI who were ≥65 years of age; 24% did not have any data on surgery, 62% had surgery, and 14% did not have surgery (Fig 1). The patient, injury, and management characteristics for the groups are presented in Table 1. The surgical and the non-surgical groups were significantly different regarding many factors examined including age at injury, sex, and CCI, as well as injury factors (neurological injury severity and level, AOSpine classification), and care management factors (triage pattern, ICU stay, treatment with tracheostomy, ventilator, PEG, rehabilitation, and acute LOS) (Table 1). Of the total cohort, 43% went home, 41% were discharged to another hospital or long-term care, and 16% died (10% for the surgical group and 27% for the non-surgical group).
FIG. 1.
Flow and selection of participants for the analysis. RHSCIR, Rick Hansen Spinal Cord Injury Registry; L2, second lumbar spinal vertebra.
Table 1.
Characteristics for the Total Study Cohort and Comparison of Those Who Had Surgery with Those Who Did Not
| Variable | Total elderly cohort |
Surgical group |
Non-surgical group |
p-value |
|---|---|---|---|---|
| n = 1340 | n = 826 | n = 192 | ||
| Age at injury (years); mean (SD) | 74.6 (7.1) | 73.6 (6.5) | 76.2 (8.1) | 0.0002 |
| Male; n (%) | 957 (71.4) | 605 (73.2) | 124 (64.6) | 0.0165 |
| Charlson Comorbidity Index; mean (SD) | 1.0 (2.0) | 1.4 (1.5) | 1.7 (1.7) | 0.0122 |
| Mechanism of injury; n (%) | 0.1377 | |||
| Falls Transport Sports Other |
1018 (77.4) 213 (16.2) 31 (2.4) 53 (4.0) |
629 (76.7) 136 (16.6) 21 (2.6) 34 (4.1) |
160 (83.8) 24 (12.6) 4 (2.1) 3 (1.6) |
|
| Injury Severity Score; n (%) | 0.1871 | |||
| ≤25 >25 |
428 (72.7) 161 (27.3) |
263 (69.4) 116 (30.6) |
93 (75.6) 30 (24.4) |
|
| Neurological injury severity (AIS); n (%) | 0.0304 | |||
| AIS A AIS B AIS C AIS D |
234 (22.2) 72 (6.8) 252 (23.9) 496 (47.1) |
148 (22.2) 56 (8.4) 168 (25.3) 293 (44.1) |
27 (17.9) 7 (4.6) 31 (20.5) 86 (60.0) |
|
| Neurological injury level; n (%) | 0.0009 | |||
| High cervical (C1-C4) Low cervical (C5-T1) Thoracolumbar (T2-L1) |
461 (46.4) 402 (40.5) 130 (13.1) |
265 (42.9) 260 (42.1) 93 (15.0) |
86 (59.3) 48 (33.1) 11 (7.6) |
|
| AOSpine classification; n (%) | <0.0001 | |||
| Type A Type B Type C |
330 (43.3) 243 (31.9) 189 (24.8) |
237 (40.6) 179 (30.7) 167 (28.6) |
88 (52.7) 59 (35.3) 20 (12.0) |
|
| Arrival at RHSCIR facility; n (%) | <0.0001 | |||
| Directly Via another facility |
302 (39.8) 457 (60.2) |
206 (34.7) 387 (65.3) |
86 (57.0) 65 (43.0) |
|
| Time from injury to RHSCIR facility; n (%) | 0.0931 | |||
| ≤12h >12h |
621 (52.1) 571 (47.9) |
396 (52.4) 360 (47.6) |
100 (59.5) 68 (40.5) |
|
| Time from injury to surgery; n (%) | N/A | |||
| ≤24h | 200 (28.7) | 200 (28.7) | N/A | |
| >24h | 497 (71.3) | 497 (71.3) | N/A | |
| Had ICU stay; n (%) | 605 (58.7) | 431 (63.4) | 82 (53.6) | 0.0245 |
| ICU stay (days); median (IQR) | 3.0 (12.0) | 4.0 (14.0) | 1.2 (6.0) | 0.0001 |
| Had tracheostomy; n (%) | 195 (17.3) | 158 (19.6) | 10 (5.6) | <0.0001 |
| Had ventilator; n (%) | 379 (34.1) | 290 (36.4) | 34 (19.1) | <0.0001 |
| Had PEG tube; n (%) | 92 (8.3) | 77 (9.8) | 11 (6.2) | <0.0001 |
| Acute LOS (days); median (IQR) | 24.0 (35.0) | 27.5 (36.5) | 14.0 (24.5) | <0.0001 |
| Attended rehabilitation; n (%) | 663 (49.5) | 468 (56.7) | 83 (43.2) | 0.0008 |
| Discharge destination; n (%) | <0.0001 | |||
| Home Long-term care Hospital Morgue |
569 (43.0) 155 (11.7) 385 (29.1) 215 (16.2) |
388 (47.2) 105 (12.8) 251 (30.5) 78 (9.5) |
79 (42.0) 19 (10.1) 39 (20.7) 51 (27.1) |
|
| Time from injury to death (days); median (IQR) | 15.0 (41.0) | 30.0 (60.0) | 7.0 (16.0) | <.0001 |
| Time from admission to death (days); median (IQR) | 14.0 (37.0) | 26.5 (48.0) | 6.0 (12.0) | <.0001 |
| Time from surgery to death (days); median (IQR) | 24.5 (50.0) | 24.5 (50.0) | N/A | N/A |
p values in bold indicate statistical significance.
SD, standard deviation; AIS, American Spinal Injury Association (ASIA) Impairment Scale; RHSCIR, Rick Hansen Spinal Cord Injury Registry; ICU, intensive care unit; IQR, interquartile range; PEG, percutaneous endoscopic gastrostomy; LOS, length of stay.
In-hospital mortality incidence and pattern
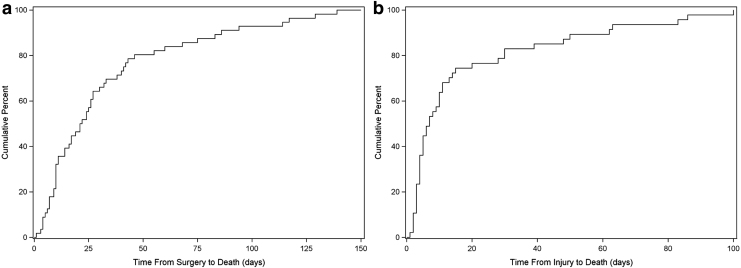
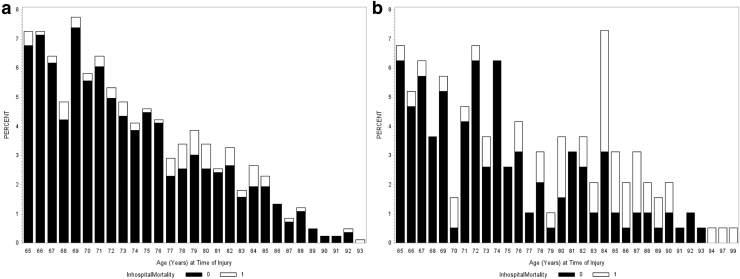
For the surgical group, the median time to death post-injury was 30 days, but post-surgery, the median time to death was 24 days. The majority of patients (75%) died within the first 50 days after surgery and then the rate of mortality slowed down afterward (Fig. 2a). Sixteen percent of the surgical mortality group died within 1 week of surgery, 60% died within 1 month, 76% died within 2 months, and 97% died by 6 months. The age threshold was 77, with the highest rate of mortality observed in the 77–85 age group (20%) compared with 5% in the 65–76 group, and 10% in the ≥86 group (Fig. 3a). For the 77–85 age group, their median time to death was 26 days from surgery and 32 days from injury.
FIG. 2.
Cumulative percent of participants who died in-hospital for the (a) surgical group and (b) non-surgical group.
FIG. 3.
Percent of participants who survived (black) and who died in-hospital (white) by each age year for the (a) surgical group and (b) non-surgical group.
For the non-surgical group, the median time to death post-injury was 7 days. The majority of patients (75%) died within 20 days post-injury (Fig. 2b). Within 1 week of injury, 53% of the non-surgical mortality group had died, 83% died within 1 month, and 91% died by 2 months. The age threshold was 80 with the highest rate of mortality in the 80–90 age group (51%) compared with 12% in the 65–79 group (Fig 3b). For the ≥80 group, the median time to death from injury was 9 days.
Factors associated with in-hospital mortality
In the surgical group, patients who died were significantly older, had higher comorbidities, and more had AIS A compared with those who survived (Table 2). More patients in the mortality group also had an ISS >25 and more had an AO Spine Classification Type C (AOC) type injury than the survival group, but data were not available for 46% and 30% of patients for these two variables, respectively. For the care management factors, significantly more patients in the mortality group had ICU stays (79% vs. 61%, p = 0.0026) and their stays were significantly longer (median 11 days vs. 3 days, p < 0.0001) compared with the survival group. Patients who died were more likely to have received a tracheostomy (38% vs. 18%), have been ventilator dependent (76% vs. 32%), and have required a PEG tube (23% vs. 9%) than those who survived (Table 2). Comparing the 77–85 age group that had the highest mortality rate among the surgical group with other age groups, patient and injury factors were not different between those who died in these age groups.
Table 2.
Characteristics for the Surgical Group and Comparison of Those Who Survived with Those Who Did Not
| Surgical group |
Survival group |
Mortality group |
p value |
|---|---|---|---|
| variable | n = 748 (90.6%) | n = 78 (9.4%) | |
| Age at injury (years); mean (SD) | 73.3 (6.4) | 76.8 (6.6) | <0.0001 |
| 65-76; n (%) ≥77 |
538 (71.9) 210 (28.1) |
31 (39.7) 47 (60.3) |
<0.0001 |
| Male; n (%) | 547 (73.1) | 58 (74.4) | 0.8153 |
| Charlson Comorbidity Index; mean (SD) | 1.3 (1.4) | 1.8 (1.8) | 0.0333 |
| Mechanism of injury; n (%) | 0.3430 | ||
| Falls Transport Sports Other |
575 (77.3) 118 (15.9) 19 (2.6) 32 (4.3) |
54 (71.1) 18 (23.7) 2 (2.6) 2 (2.6) |
|
| Injury Severity Score; n (%) | 0.0129 | ||
| ≤25 >25 |
247 (71.2) 100 (28.8) |
16 (50.0) 16 (50.0) |
|
| Neurological injury severity (AIS); n (%) |
<0.0001 |
||
| AIS A AIS B AIS C AIS D |
120 (19.8) 50 (8.3) 157 (25.9) 279 (46.0) |
28 (47.5) 6 (10.2) 11 (18.6) 14 (23.7) |
|
| Neurological injury level; n (%) | 0.1135 | ||
| High cervical (C1-C4) Low cervical (C5-T1) Thoracolumbar (T2-L1) |
239 (42.5) 234 (41.5) 90 (16.0) |
26 (47.3) 26 (47.3) 3 (5.4) |
|
| AOSpine classification; n (%) | 0.0009 | ||
| Type A Type B Type C |
232 (41.9) 172 (31.1) 150 (27.1) |
5 (17.2) 7 (24.1) 17 (58.6) |
|
| Arrival at RHSCIR facility; n (%) | 0.5017 | ||
| Directly Via another facility |
196 (35.1) 363 (64.9) |
10 (29.4) 24 (70.6) |
|
| Time from injury to RHSCIR facility; n (%) | 0.7731 | ||
| ≤12h >12h |
355 (52.2) 325 (47.8) |
41 (54.0) 35 (46.0) |
|
| Time from injury to surgery; n (%) | |||
| ≤24h | 180 (28.2) | 20 (34.5) | 0.3088 |
| >24h | 459 (71.8) | 38 (65.5) | |
| Had ICU stay; n (%) | 373 (61.5) | 58 (79.5) | 0.0026 |
| ICU stay (days); median (IQR) | 3.0 (130.0) | 11.0 (30.8) | <0.0001 |
| Had tracheostomy; n (%) | 130 (17.7) | 28 (37.8) | <0.0001 |
| Had ventilator; n (%) | 234 (32.4) | 56 (75.7) | <0.0001 |
| Had PEG tube; n (%) | 62 (8.6) | 15 (22.7) | <0.0001 |
| Time from injury to death (days); median (IQR) | N/A | 30.0 (60.0) | N/A |
| Time from admission to death (days); median (IQR) | N/A | 26.5 (48.0) | N/A |
| Time from surgery to death (days); median (IQR) | N/A | 24.5 (50.0) | N/A |
p values in bold indicate statistical significance.
SD, standard deviation; AIS, American Spinal Injury Association (ASIA) Impairment Scale; RHSCIR, Rick Hansen Spinal Cord Injury Registry; ICU, intensive care unit; IQR, interquartile range; PEG, percutaneous endoscopic gastrostomy.
In the non-surgical group, similar factors were found associated with in-hospital mortality including older age, AIS A, ICU stay, and ventilator dependency, but comorbidities, length of ICU stay, tracheostomy, and PEG status were not significantly different between those who survived and who did not (Table 3). Comparing the patient and injury factors between the ≥80 age group that had the highest mortality rate and the other age group, the only difference observed was the higher proportion of high cervical injuries in the ≥80 group (p = 0.0023).
Table 3.
Characteristics of the Non-Surgical Group and Comparison of Those Who Survived with Those Who Did Not
| Non-surgical group |
Survival group |
Mortality group |
p value |
|---|---|---|---|
| variable | n = 141 (73.4%) | n = 51 (26.6%) | |
| Age at injury (years); mean (SD) | 74.2 (7.3) | 81.9 (7.6) | <0.0001 |
| 65-79; n (%) ≥80 |
105 (74.5) 36 (25.5) |
15 (29.4) 36 (70.6) |
<0.0001 |
| Male; n (%) | 86 (61.0) | 38 (74.5) | 0.0837 |
| Charlson Comorbidity Index; mean (SD) | 1.7 (1.5) | 1.9 (2.0) | 0.5345 |
| Mechanism of injury; n (%) | 0.1840 | ||
| Falls Transport Sports Other |
119 (85.0) 14 (10.0) 4 (2.9) 3 (2.1) |
41 (80.4) 10 (19.6) 0 (0) 0 (0) |
|
| Injury Severity Score; n (%) | <0.0001 | ||
| ≤25 >25 |
76 (90.5) 8 (9.5) |
17 (43.6) 22 (56.4) |
|
| Neurological injury severity (AIS); n (%) | <0.0001 | ||
| AIS A AIS B AIS C AIS D |
10 (8.5) 3 (2.5) 24 (20.3) 81 (68.6) |
17 (51.5) 4 (12.1) 7 (21.2) 5 (15.2) |
|
| Neurological injury level; n (%) | 0.1158 | ||
| High cervical (C1-C4) Low cervical (C5-T1) Thoracolumbar (T2-L1) |
61 (56.0) 41 (37.6) 7 (6.4) |
25 (69.4) 7 (19.4) 4 (11.1) |
|
| AOSpine classification; n (%) | 0.0002 | ||
| Type A Type B Type C |
76 (57.1) 48 (36.1) 9 (6.8) |
12 (35.3) 11 (32.3) 11 (32.3) |
|
| Arrival at RHSCIR facility; n (%) | 0.7553 | ||
| Directly Via another center |
67 (56.3) 52 (43.7) |
19 (59.4) 13 (40.6) |
|
| Time from injury to RHSCIR facility; n (%) | 0.6192 | ||
| ≤12h >12h |
70 (58.3) 50 (41.7) |
30 (62.5) 18 (37.5) |
|
| Had ICU stay; n (%) | 48 (46.6) | 34 (68.0) | 0.0128 |
| ICU stay (days); median (IQR) | 0.0 (6.0) | 3.0 (6.0) | 0.0626 |
| Had tracheostomy; n (%) | 7 (5.2) | 3 (6.8) | 0.7102 |
| Had ventilator; n (%) | 13 (9.2) | 21 (47.7) | <0.0001 |
| Had PEG tube; n (%) | 6 (4.3) | 5 (12.8) | 0.0635 |
| Time from injury to death (days); median (IQR) | N/A | 7.0 (16.0) | N/A |
| Time from admission to death (days); median (IQR) | N/A | 6.0 (12.0) | N/A |
p values in bold indicate statistical significance.
SD, standard deviation; AIS, American Spinal Injury Association (ASIA) Impairment Scale; RHSCIR, Rick Hansen Spinal Cord Injury Registry; ICU, intensive care unit; IQR, interquartile range; PEG, percutaneous endoscopic gastrostomy.
Impact of patient, injury, and management factors on in-hospital mortality
The significant predictors for in-hospital mortality for patients who had undergone surgery were age, comorbidities (CCI), neurological injury severity (AIS), and ventilation status (Table 4). In particular, the odds of dying 50 days post-surgery is six times higher for those 77 years of age than for the 65–76 year age group (95% confidence interval [CI] 3–15), five times higher for patients with AIS A than for those with AIS B, C, or D injury (95% CI 2–11), seven times higher for patients who are ventilated than for those who are not (95% CI 3–17), and the odds are also higher with more comorbidities (95% CI 1.1–1.7). Considering all these predictors together to give a patient's perspective, the expected probability of dying within 50 days of surgery for an individual who is 77 years old, has a high cervical AIS A injury, a CCI of 2, and is ventilated, is 66% compared with 3% for someone with a similar injury but who is younger with fewer comorbidities and does not require a ventilator (Table 5). The model was significant as a whole (p < 0.0001) and there was no lack of fit (Hosmer and Lemeshow Goodness-of-Fit Test, p = 0.6642), meaning the model was robust, and the area under the curve (AUC) value was 0.876.
Table 4.
Multivariable Logistical Regression Model Analyzing Impact of Variables on In-Hospital Mortality 50 Days Post-Surgery for the Surgical Group
| Outcome: In-hospital mortality 50 days post-surgery | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | Estimate | Standard error | Odds ratio | 95% confidence interval | p value | ||
| Intercept | -6.54 | 0.86 | <.0001 | ||||
| Age at injury | ≥77 | 1.91 | 0.41 | 6.76 | 3.04 | 15.05 | <0.0001 |
| Neurological injury level | High cervical | 1.15 | 0.70 | 3.14 | 0.79 | 12.47 | 0.1032 |
| Low cervical | 1.11 | 0.70 | 3.02 | 0.76 | 11.99 | 0.1157 | |
| Neurological injury severity | AIS A | 1.59 | 0.43 | 4.90 | 2.13 | 11.29 | 0.0002 |
| Charlson Comorbidity Index | Continuous | 0.29 | 0.11 | 1.34 | 1.07 | 1.66 | 0.0093 |
| Ventilation dependence | Yes | 1.98 | 0.45 | 7.21 | 2.97 | 17.51 | <0.0001 |
p values in bold indicate statistical significance.
Table 5.
Expected Probabilities for In-Hospitality Mortality 50 Days Post-Surgery Derived from Logistical Regression Model
| Age (years) | Neurological injury level | Neurological injury severity | Charlson Comorbidity Index | Ventilation dependence | Expected probability of in-hospital mortality |
|---|---|---|---|---|---|
| ≥77 | High cervical | A | 2 | Yes | 66% |
| ≥77 | High cervical | A | 1.4 | Yes | 62% |
| ≥77 | Low cervical | B/C/D | 1.4 | Yes | 24% |
| ≥77 | High cervical | A | 1.4 | No | 18% |
| 65-76 | High cervical | A | 1.4 | Yes | 19% |
| 65-76 | High cervical | A | 1.4 | No | 3% |
| 65-76 | Low cervical | B/C/D | 1.4 | Yes | 5% |
For the non-surgical group, the significant predictors for in-hospital mortality were age and neurological injury severity (Table 6). The odds of dying for a patient in the non-surgical group is 14 times higher for those ≥80 years of age than for those 65–79 (95% CI 4–47) and 12 times higher for those with an AIS A injury than for those with an AIS B, C, or D injury (95% CI 3–44). Comorbidities, status on tracheostomy, ventilation, and PEG were not significant and were therefore omitted from the final model. The expected probability of dying for an elderly patient without surgical treatment who is 80 years old and has a high cervical AIS A is 86% compared with 25% for someone who is of similar age but with an incomplete injury (Table 7). This final model was significant as a whole (p < 0.001) with no lack of fit (Hosmer and Lemeshow Goodness-of-Fit Test, p = 0.7889) and an AUC of 0.872.
Table 6.
Multivariable Logistical Regression Model Analyzing Impact of Variables on In-Hospital Mortality for the Non-Surgical Group
| Outcome: In-hospital mortality | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | Estimate | Standard error | Odds ratio | 95% confidence interval | p value | ||
| Intercept | -2.93 | 0.89 | - | - | - | 0.0010 | |
| Age at injury (years) | ≥80 | 2.64 | 0.62 | 14.04 | 4.15 | 47.51 | <0.0001 |
| Neurological injury level | High cervical | -0.36 | 0.84 | 0.69 | 0.14 | 3.59 | 0.6644 |
| Low cervical | -0.81 | 0.94 | 0.45 | 0.07 | 2.84 | 0.3920 | |
| Neurological injury severity | AIS A | 2.49 | 0.66 | 12.03 | 3.27 | 44.24 | 0.0002 |
p values in bold indicate statistical significance.
Table 7.
Expected Probabilities for In-Hospitality Mortality Derived from Logistical Regression Model for Elderly Who Did Not Have Surgery
| Age (years) | Neurological injury level | Neurological injury severity | Expected probability of in-hospital mortality |
|---|---|---|---|
| ≥80 | High cervical | A | 86% |
| ≥80 | High cervical | B/C/D | 34% |
| ≥80 | Low cervical | B/C/D | 25% |
| 65-79 | High cervical | A | 31% |
| 65-79 | High cervical | B/C/D | 4% |
| 65-79 | Low cervical | B/C/D | 2% |
Discussion
Key findings and relevance
This study describes an overall in-hospital mortality rate of 16% for the whole cohort of patients >65 years of age with tSCI in the RHSCIR; 10% for the surgical group and 27% for the non-surgical group. There was a dramatic difference in the time to death between the surgical and non-surgical groups, with those managed surgically having a median time to death of 30 days and >90% dying within 6 months, compared with 7 days and 2 months respectively in the non-surgical group. Our analysis has shown the factors associated with in-hospital mortality in both groups are age, neurological severity of injury, concomitant comorbidities, and need for ventilation in the surgical group.
These findings are highly relevant and reinforce benefits that may arise from the development of a predictive clinical tool to assist with up-front decision making around early management of elderly patients with tSCI and discussions with them and their families. The results also provide information on the course of care of these patients in terms of ICU admission and interventions such as tracheostomy and PEG tube insertion, to help inform discussion around the morbidity associated with surgery.
Rationale for surgical and non-surgical groups
It was not the objective of this study to examine the impact of surgery on mortality, but the surgical and non-surgical groups were examined separately because there are key differences between the two regarding their course of care and early decision making by patients, families, and their surgeons. Treatment decision making in the elderly is less clear than in younger individuals given the elderly are less likely to withstand the physiological insult of surgery and more likely to have potential complications. They often have spine instability requiring extensive surgery with a poor neurological prognosis, and subsequent healthcare issues associated with living with more significant neurological deficit. This reflects clinical decisions to “watch and wait,” or clinical preference in determining how aggressively to manage. For those older and more severely injured, there is less push toward operative intervention and an acceptance of a conservative “comfort care” approach by patients and their families. Early decisions might also come from individuals who have advanced care directives to forego any ag- gressive medical and surgical care for serious medical issues requiring aggressive management, including resuscitation and intubation.
In addition, little is known about the details of the course of injury when a patient's injury may be so severe that it leads to death prior to surgery, or the patient has significant co-morbidities that preclude operative intervention. These details and the early decisions, neither of which are captured in the registry, might explain the different characteristics observed in these two groups, as well as the faster and higher rate of mortality in the non-surgical group. Subsequently the surgical group may have been biased, representing a group of more robust surgical candidates.
Relation to literature
Concordant with previous literature, we note that the in-hospital mortality rate increases with age, with a significantly higher rate observed in those ≥77 years of age for the surgical group and in those ≥80 years of age in the non-surgical group. This is consistent with the findings by Martin and coworkers,20 who described an “inflection point” at the age of 75 for in-hospital mortality following cervical SCI. Our overall in-hospital mortality rate of 16% is slightly lower than the 12–40% reported by others,15–20 but our data may underestimate the rate, as some patients who died shortly after admission may not have been enrolled in the registry.
The risk factors for in-hospital mortality found in our study are similar to those reported in the literature.17,18,20 With the largest Canadian tSCI registry, the sample size was big enough to allow for robust multivariable analysis and for examining the impact of care management such as surgical intervention, days in ICU, tracheostomy, and ventilatory and nutritional support. This enabled the estimation of in-hospital mortality within 50 days, allowing identification of the subset of patients unlikely to have a favorable outcome to guide the need for discussions with patients and families around acute management.
Clinical implication
For this certain subset of patients, this study raises the question of whether surgery simply delays the inevitable. The median time to death post-injury was 7 days in the non-surgical group versus 30 days in those post-surgery; 75% of non-surgical patients died within the first 20 days and 91% died by 2 months versus 75% dying within 50 days and 97% dying by 6 months for surgical patients.
Does an extension of life by 4 months justify the morbidity associated with surgery? Post-surgical patients who died in-hospital were more likely to have received a tracheostomy, been ventilated, and have had a PEG tube inserted. They were more likely to be admitted to the ICU and they stayed longer. In contrast, very few patients in the non-surgical group had a tracheostomy or PEG tube although 48% received ventilation. Once a decision to surgically intervene is made, it becomes difficult to pull back and withdraw active treatment. These may represent futile interventions in patients with a high likelihood of mortality imposing substantial morbidity on the individual without altering the final outcome, when an earlier decision toward comfort care may have been more appropriate. A better understanding of when treatment may be ineffective could help in determining a more comfortable course of care.
Next steps
Conversations on course of care with surgeons early after injury may be difficult for patients and families who have expressed concern regarding confusing terminology, false hope, and communication on prognosis at odds with the aggressiveness of treatment.27 Development of tools supported by evidence would assist in conversations and decision making relating to comfort versus more aggressive care. With the demographic shifting toward a greater proportion of older individuals both in the general population and among those experiencing tSCI, such a tool would require more person and injury factors specific to this population to guide shared decision making. Further, closer examination is also warranted to better understand the impact of surgery on in-hospital mortality by carefully matching those who had surgery with those who did not, to account for the early decisions made by surgeons and patients. In consideration of having the results useful for clinicians to support their early decision making, only variables readily available at time of a patient's admission were examined in our model. But the aging process, in which patient's physiological reserve declines,38 or frailty as measured by the Frailty Index,39 have previously been related to outcomes in elective spine surgery cohorts,40 highlighting that more physiological indicators also need to be evaluated given the variability in deficits within the elderly population.
These future studies, along with assessing generalizability in the wider tSCI population such as testing the recently developed SCI Risk Score Model for mortality in the general tSCI population will be important steps toward our goal of developing a simple, evidence-informed tool for clinical use in early prognostication and management decisions for tSCI.
Limitations
Our study considered all tSCIs including central cord syndrome which is highly prevalent in the elderly population. In fact, patients with central cord syndrome may well account for this 6-day discrepancy observed between the time to death post-injury and the time to death post-surgery, when surgical intervention was often delayed until the patient's neurological improvement had plateaued. Separate analysis on this subgroup would have been beneficial, but would require imaging to confirm the diagnosis of central cord syndrome. However, as the data for this study come from an observational registry that does not include magnetic resonance imaging (MRI) data yet, our analysis is limited to the data currently collected. This also prevented the investigation of MRI signal characteristics such as the degree of spinal cord edema and hemorrhage, which have been associated with poorer functional outcome.41 Whether MRI is also helpful in predicting survival remains to be determined.
Surgical data were missing on 24% of patients who were excluded from further analysis, leading to potential bias in the analysis. Limited data availability on injury factors including ISS and AOSpine classification might have underestimated the role of multiple traumas on outcome. ISS is obtained from the trauma registry, and usually only patients with more severe injuries are included in the registry. Neurology data were not available for all patients, partly because neurological examinations at admission are not feasible in patients who are unconsciousness and have concurrent injury such as traumatic brain injury. Further, our in-hospital mortality rates do not include pre-hospital deaths, because of lack of data availability, which may also fail to identify the presence of tSCI.
Conclusion
In-hospital mortality is significant in both surgical and non-surgical groups after tSCI in elderly patients. Increasing age, neurological injury severity, and comorbidities are significant predictors of mortality and can be used to inform clinical decision making and discussions with patients and their families. Further delineating the factors associated with inevitable in-hospital mortality versus an acceptable quality of life either as in-patients or in the community would assist clinicians in these situations.
Acknowledgments
The authors thank the RHSCIR network and all the participating local RHSCIR facilities: GF Strong Rehabilitation Centre, Vancouver General Hospital, Foothills Hospital, Glenrose Rehabilitation Hospital, Royal Alexandra Hospital, University of Alberta Hospital, Royal University Hospital, Saskatoon City Hospital, Winnipeg Health Sciences Centre, Toronto Western Hospital, Toronto Rehabilitation Institute, St. Michael's Hospital, Sunnybrook Health Sciences Centre, Hamilton General Hospital, Hamilton Health Sciences Regional Rehabilitation Centre, Victoria Hospital (London), University Hospital (London), Parkwood Hospital (London), The Ottawa Hospital Rehabilitation Centre, The Ottawa Hospital Civic Campus, Hôpital de l'Enfant Jésus, Institut de réadaptation en deficience physique de Quebec, Centre de réadaptation Lucie-Bruneau, Institut de réadaptation Gingras-Lindsay-de-Montréal, Hôpital du Sacre Cœur de Montréal, Nova Scotia Rehabilitation Centre, QEII Health Sciences Centre, Saint John Regional Hospital, Stan Cassidy Centre for Rehabilitation, St. John's Health Sciences Centre, and L.A. Miller Rehabilitation Centre.
Contributor Information
Collaborators: The Rick Hansen Spinal Cord Injury Registry Network
Funding Information
The RHSCIR and this work are supported by funding from the Praxis Spinal Cord Institute, Health Canada, Western Economic Diversification Canada, and the Governments of Alberta, British Columbia, Manitoba, and Ontario.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Dion P., and Galbraith N. (2015). Back to the future: a review of forty years of population projections at Statistics Canada. Can. Stud. Popul. 42, 102 [Google Scholar]
- 2. Etzioni D.A., Liu J.H., Maggard M.A., Ko C.Y., and Geffen D. (2003). The aging population and its impact on the surgery workforce. Ann. Surg. 238, 170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Statistics Canada (2017). Age and sex, and type of dwelling data: key results from the 2016 Census. https://www150.statcan.gc.ca/n1/en/daily-quotidien/170503/ dq170503a-eng.pdf?st = rYAR6YeG. (last accessed February, 2019)
- 4. Arul K., Ge L., Ikpeze T., Baldwin A., and Mesfin A. (2019). Traumatic spinal cord injuries in geriatric population: etiology, management, and complications. J. Spine Surg. 5, 38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Statistics Canada. (2010). Population Projections for Canada, Provinces and Territories 2009 to 2036. Available from: https://www150.statcan.gc.ca/n1/en/pub/91-520-x/91-520-x2010001-eng.pdf?st=6t1ZcDCg
- 6. Chen Y., He Y., and DeVivo M.J. (2016). Changing demographics and injury profile of new traumatic spinal cord injuries in the United States, 1972–2014. Arch. Phys. Med. Rehabil. 97, 1610–1619 [DOI] [PubMed] [Google Scholar]
- 7. Chamberlain J.D., Deriaz O., Hund-Georgiadis M., Meier S., Scheel-Sailer A., Schubert M., Stucki G., and Brinkhof M.W.G. (2015). Epidemiology and contemporary risk profile of traumatic spinal cord injury in Switzerland. Inj. Epidemiol. 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jain N.B., Ayers G.D., Peterson E.N., Harris M.B., Morse L., O'Connor K.C., and Garshick E. (2015). Traumatic spinal cord injury in the United States, 1993–2012. JAMA 313, 2236–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jackson A.B., Dijkers M., Devivo M.J., and Poczatek R.B. (2004). A demographic profile of new traumatic spinal cord injuries: change and stability over 30 years. Arch. Phys. Med. Rehabil. 85, 1740–1748 [DOI] [PubMed] [Google Scholar]
- 10. Ronzi Y., Perrouin-Verbe B., Hamel O., and Gross R. (2018). Spinal cord injury associated with cervical spinal canal stenosis: outcomes and prognostic factors. Ann. Phys. Rehabil. Med. 61, 27–32 [DOI] [PubMed] [Google Scholar]
- 11. Ferro S., Cecconi L., Bonavita J., Pagliacci M.C., Biggeri A., and Franceschini M. (2017). Incidence of traumatic spinal cord injury in Italy during 2013–2014: a population-based study. Spinal Cord 55, 1103–1107 [DOI] [PubMed] [Google Scholar]
- 12. Chen Y., Tang Y., Allen V., and De Vivo M.J. (2016). Fall-induced spinal cord injury: External causes and implications for prevention. J. Spinal Cord Med. 39, 24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahn H., Bailey C.S., Rivers C.S., Noonan V.K., Tsai E.C., Fourney D.R., Attabib N., Kwon B.K., Christie S.D., Fehlings M.G., Finkelstein J., Hurlbert R.J., Townson A., Parent S., Drew B., Chen J., Dvorak M.F., and Rick Hansen Spinal Cord Injury Registry Network. (2015). Effect of older age on treatment decisions and outcomes among patients with traumatic spinal cord injury. CMAJ 187, 873–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahn H., Lewis R., Santos A., Cheng C.L., Noonan V.K., Dvorak M.F., Singh A., Linassi A.G., Christie S., Goytan M., and Atkins D. (2017). Forecasting financial resources for future traumatic spinal cord injury care using simulation modeling. J. Neurotrauma 34, 2917–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson A.P., Haak M.H., Khan N., and Meyer P.R. (2005). Cervical spine injuries in the elderly: Acute postoperative mortality. Spine (Phila. Pa. 1976). 30, 1524–1527 [DOI] [PubMed] [Google Scholar]
- 16. Lau D., Dalle Ore C.L., Tarapore P.E., Huang M., Manley G., Singh V., Mummaneni P. V., Beattie M., Bresnahan J., Ferguson A.R., Talbott J.F., Whetstone W., and Dhall S.S. (2019). Value of aggressive surgical and intensive care unit in elderly patients with traumatic spinal cord injury. Neurosurg. Focus 46, E3. [DOI] [PubMed] [Google Scholar]
- 17. Bokhari A.R., Sivakumar B., Sefton A., Lin J.-L., Smith M.M., Gray R., and Hartin N. (2018). Morbidity and mortality in cervical spine injuries in the elderly. ANZ J. Surg. 89, 412–417 [DOI] [PubMed] [Google Scholar]
- 18. Fassett D.R., Harrop J.S., Maltenfort M., Jeyamohan S.B., Ratliff J.D., Anderson D.G., Hilibrand A.S., Albert T.J., Vaccaro A.R., and Sharan A.D. (2007). Mortality rates in geriatric patients with spinal cord injuries. J. Neurosurg. Spine 7, 277–281 [DOI] [PubMed] [Google Scholar]
- 19. Daneshvar P., Roffey D.M., Brikeet Y. a, Tsai E.C., Bailey C.S., and Wai E.K. (2013). Spinal cord injuries related to cervical spine fractures in elderly patients: factors affecting mortality. Spine J. 13, 862–866 [DOI] [PubMed] [Google Scholar]
- 20. Martin N.D., Marks J.A., Donohue J., Giordano C., Cohen M.J., and Weinstein M.S. (2011). The mortality inflection point for age and acute cervical spinal cord injury. J. Trauma 71, 380–386 [DOI] [PubMed] [Google Scholar]
- 21. Campbell P.G., Yadla S., Malone J., Zussman B., Maltenfort M.G., Sharan A.D., Harrop J.S., and Ratliff J.K. (2010). Early complications related to approach in cervical spine surgery: single-center prospective study. World Neurosurg. 74, 363–368 [DOI] [PubMed] [Google Scholar]
- 22. Harrop J.S., Sharan A.D., Scheid E.H., Vaccaro A.R., and Przybylski G.J. (2004). Tracheostomy placement in patients with complete cervical spinal cord injuries: American Spinal Injury Association Grade A. J. Neurosurg. 100, 20–23 [DOI] [PubMed] [Google Scholar]
- 23. Jabbour P., Fehlings M., Vaccaro A.R., and Harrop J.S. (2008). Traumatic spine injuries in the geriatric population. Neurosurg. Focus 25, E16. [DOI] [PubMed] [Google Scholar]
- 24. Needham D.M., Bronskill S.E., Calinawan J.R., Sibbald W.J., Pronovost P.J., and Laupacis A. (2005). Projected incidence of mechanical ventilation in Ontario to 2026: Preparing for the aging baby boomers. Crit. Care Med. 33, 574–579 [DOI] [PubMed] [Google Scholar]
- 25. Melo-Neto J.S. de, Vidotto L.E.L., Gomes F. de C., Morais D.F. de, and Tognola W.A. (2016). Characteristics and clinical aspects of patients with spinal cord injury undergoing surgery. Rev. Bras. Ortop. 52, 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ikpeze T.C., and Mesfin A. (2017). Spinal cord injury in the geriatric population: risk factors, treatment options, and long-term management. Geriatr. Orthop. Surg. Rehabil. 8, 115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krawczyk M., and Gallagher R. (2016). Communicating prognostic uncertainty in potential end-of-life contexts: experiences of family members. BMC Palliat. Care 15, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graffeo C.S., Perry A., Puffer R.C., Carlstrom L.P., Chang W., Mallory G.W., and Clarke M.J. (2017). Deadly falls: Operative versus nonoperative management of Type II odontoid process fracture in octogenarians. J. Neurosurg. Spine 26, 4–9 [DOI] [PubMed] [Google Scholar]
- 29. Irwin Z.N., Arthur M., Mullins R.J., and Hart R.A. (2004). Variations in injury patterns, treatment, and outcome for spinal fracture and paralysis in adult versus geriatric patients. Spine (Phila. Pa. 1976). 29, 796–802 [DOI] [PubMed] [Google Scholar]
- 30. Dhall S.S., Yue J.K., Winkler E.A., Mummaneni P. V, Manley G.T., and Tarapore P.E. (2017). Morbidity and mortality associated with surgery of traumatic c2 fractures in octogenarians. Neurosurgery 80, 854–862 [DOI] [PubMed] [Google Scholar]
- 31. Noonan V.K., Kwon B.K., Soril L., Fehlings M.G., Hurlbert R.J., Townson A., Johnson M., and Dvorak M.F. (2012). The Rick Hansen Spinal Cord Injury Registry (RHSCIR): a national patient-registry. Spinal Cord 50, 22–27 [DOI] [PubMed] [Google Scholar]
- 32. Kirshblum S.C., Burns S.P., Biering-Sørensen F., Donovan W., Graves D.E., Jha A., Johansen M., Jones L., Krassioukov A. V, Mulcahey M.J., Schmidt-Read M., and Waring W.P. (2011). International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 34, 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Charlson M., Pompei P., Ales K., and MacKenzie R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383 [DOI] [PubMed] [Google Scholar]
- 34. Vaccaro A.R., Koerner J.D., Radcliff K.E., Oner F.C., Reinhold M., Schnake K.J., Kandziora F., Fehlings M.G., Dvorak M.F., Aarabi B., Rajasekaran S., Schroeder G.D., Kepler C.K., and Vialle L.R. (2016). AOSpine subaxial cervical spine injury classification system. Eur. Spine J. 25, 2173–2184 [DOI] [PubMed] [Google Scholar]
- 35. Kepler C.K., Vaccaro A.R., Schroeder G.D., Koerner J.D., Vialle L.R., Aarabi B., Rajasekaran S., Bellabarba C., Chapman J.R., Kandziora F., Schnake K.J., Dvorak M.F., Reinhold M., and Oner F.C. (2016). The thoracolumbar AOSpine injury score. Glob. Spine J. 6, 329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baker S., O'Neill B., Haddon W., and Long W. (1974). The Injury Severity Score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma. 14, 187–196 [PubMed] [Google Scholar]
- 37. Walden K., Bélanger L.M., Biering-Sørensen F., Burns S.P., Echeverria E., Kirshblum S., Marino R.J., Noonan V.K., Park S.E., Reeves R.K., Waring W., and Dvorak M.F. (2016). Development and validation of a computerized algorithm for International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI). Spinal Cord 54, 197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iqbal J., Denvir M., and Gunn J. (2013). Frailty assessment in elderly people. Lancet 381, 1985–1986 [DOI] [PubMed] [Google Scholar]
- 39. Mitnitski A.B., Mogilner A.J., and Rockwood K. (2001). Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 1, 323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moskven E., Bourassa-Moreau, É., Charest-Morin R., Flexman A., and Street J. (2018). The impact of frailty and sarcopenia on postoperative outcomes in adult spine surgery. A systematic review of the literature. Spine J. 18, 2354–2369 [DOI] [PubMed] [Google Scholar]
- 41. Wilson J.R., Grossman R.G., Frankowski R.F., Kiss A., Davis A.M., Kulkarni A. V, Harrop J.S., Aarabi B., Vaccaro A., Tator C.H., Dvorak M., Shaffrey C.I., Harkema S., Guest J.D., and Fehlings M.G. (2012). A clinical prediction model for long-term functional outcome after traumatic spinal cord injury based on acute clinical and imaging factors. J. Neurotrauma 29, 2263–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]