Abstract
Although recombinant adeno-associated virus serotype 8 (AAV8) and serotype 5 (AAV5) vectors have shown efficacy in Phase 1 clinical trials for gene therapy of hemophilia B, it has become increasingly clear that these serotypes are not optimal for transducing primary human hepatocytes. We have previously reported that among the 10 most commonly used AAV serotypes, AAV serotype 3 (AAV3) vectors are the most efficient in transducing primary human hepatocytes in vitro as well as in “humanized” mice in vivo, and suggested that AAV3 vectors expressing human coagulation factor IX (hFIX) may be a more efficient alternative for clinical gene therapy of hemophilia B. In the present study, we extended these findings to develop an AAV3 vector incorporating a compact yet powerful liver-directed promoter as well as optimized hFIX cDNA sequence inserted between two AAV3 inverted terminal repeats. When packaged into an AAV3 capsid, this vector yields therapeutic levels of hFIX in hemophilia B and in “humanized” mice in vivo. Together, these studies have resulted in an AAV3 vector predicted to achieve clinical efficacy at reduced vector doses, without the need for immune-suppression, for clinical gene therapy of hemophilia B.
Keywords: recombinant adeno-associated virus serotype 3 vector, gene therapy, hemophilia B
Introduction
The liver is a common target for gene therapy of a wide variety of human diseases. While multiple clinical trials targeting adeno-associated virus (AAV) vectors to the liver have shown the potential of this approach, the high vector doses needed to achieve clinical efficacy routinely evoke an immune response directed against the vector capsid. This predictable response has made clear the need to develop a new generation of highly potent vectors that achieve efficacy at low vector doses, which remains a crucial challenge in the field of AAV gene therapy. We previously reported that although liver was the predominant target of AAV2 vectors injected intravenously in mice, transgene expression occurred in fewer than 5% of hepatocytes.1 Similar observations were made by other investigators.2
In subsequent studies, persistent expression of human clotting factor IX (FIX) was reported following intravenous injection in mice,3–5 and canine FIX in hemophilia B dogs.6,7 Based on these encouraging preclinical studies, a Phase 1 clinical trial for hemophilia B was performed with AAV2 vectors expressing human coagulation FIX (hFIX).8 Unfortunately, although preclinical studies with both hemophilic mice and dog models using AAV2-FIX vectors provided complete phenotypic correction of the disease, the low and medium vector doses failed to express FIX in two patients. At a high dose of AAV2 vector, production of the therapeutic level of FIX was observed in one patient, but it was short-lived due to induction of AAV2 capsid-specific CD8+ memory T cell response, leading to the destruction of transduced hepatocytes.8,9 Thus, AAV2 vectors, although effective in mice and dogs, failed to provide durable clinical efficacy for hemophilia B in humans.
In the years shortly following these initial studies, additional AAV vector serotypes became available, and among the 10 most commonly used serotype vectors, AAV8 vectors were reported by several investigators to be highly efficient in transducing the mouse liver.10,11 A subsequent clinical trial with AAV8 vectors yielded encouraging results in that therapeutic levels of FIX were sustained over a 3-year follow-up period12,13 and more recently, up to a 7-year follow-up.14 However, a similar immune response, as seen previously with high vector doses, was again observed. With greater understanding of the predicted immunological mechanisms informed by previous trials, in this trial the response was successfully mitigated with the use of prednisolone.12,13 In more recently published studies, clinical trials of AAV8 and AAV5 vectors have been reported to lead to clinical efficacy in patients with hemophilia B,15,16 although with immune-suppression being necessary in most patients administered high vector doses.
Despite these remarkable advances, the following observations have emerged: (1) The host immune response to AAV vectors correlates directly with the vector dose. Thus, it is imperative that the vector dose be kept to a minimum without compromising the FIX levels. (2) Our recently published studies strongly suggest that AAV8 and AAV5 serotype vectors may not also be optimal in transducing human hepatocytes. In a head-to-head comparison, both in primary human hepatocytes in vitro17 and in a “humanized” mouse model in vivo,18 we have reported that AAV3 vectors are more efficient than AAV8 and AAV5 vectors in human hepatocytes. (3) Despite the canonical recognition of AAV vectors as nonintegrating, a growing body of evidence has raised concerns of aberrant vector genome integration into the host cell chromosomal DNA.19,20
These findings support the use of more potent vector transgene designs to lower the genetic burden of gene therapies by achieving efficacy at lower viral vector genome copy numbers per cell. (4) Finally, the possibility remains that the promoter and the FIX expression cassette can be optimized to achieve higher levels of FIX expression such that the effective vector dose can be lowered, and that the use of immune-suppression therapy can be completely avoided while maintaining the highest achieved clotting factor levels.
In the present study, we undertook a systematic approach to further optimize the promoter as well as the FIX expression cassette. We also developed a modified AAV3 vector and evaluated its efficacy in hemophilia B as well as in “humanized” mouse models. We report here that the combination of these approaches has resulted in a clinical candidate AAV3-FIX vector that predicts to be safer and more effective in clinical gene therapy of hemophilia B than the current generation of AAV clinical candidates.
Materials and Methods
Cells and plasmids
Human embryonic kidney (HEK293) and hepatocellular carcinoma (HepG2) cells were purchased from American Type Culture Collection (Manassas, VA), and were maintained in complete Dulbecco's modified Eagle's medium (C-DMEM; Mediatech, Inc., Manassas, VA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO) and 1% penicillin and streptomycin (Lonza, Walkersville, MD) as described previously.21 Cells were grown in adherent culture in a humidified atmosphere at 37°C in 5% CO2 and were subcultured after treatment with trypsin/versene mixture (Lonza) for 2–5 min at room temperature (RT), washed, and resuspended in C-DMEM. FIX was liver codon optimized (LCO) as previously described.22 Promoter and FIX cDNA sequences were synthesized as gBlocks (IDT, Coralville, IA).
Plasmid transfections
HepG2 cells were plated at a density of 200,000 cells per well in 500 μL DMEM/F12 supplemented with 10% FBS and 1% penicillin/streptomycin in six-well plates. Twenty-four hours after seeding, cells were transfected using 1.5 mL Bio TransIT-X2 Transfection Reagent (Mirus Bio, Madison, WI) and 500 ng plasmid according to the manufacturer's instructions. For FIX expression studies, media were supplemented with vitamin K1 (Sigma Aldrich) 24 h before FIX activity assay.
Hydrodynamic injections
Plasmid DNA (20 μg) was diluted into a volume of Transit-EE Hydrodynamic Delivery Solution (Mirus Bio) equivalent to 1/10th the mass of the recipient mouse. Plasmid was delivered as a single bolus into the tail vein over 5–10 s using 3 mL syringes and 26 5/8G needles. Mice were allowed to recover on heating pads for 30 min. Blood was drawn by the retro-orbital method into citrated tubes beginning at 24 h after injections.
FIX activity measurements
FIX activity in conditioned supernatant was performed by activated partial thromboplastin time (aPTT)-based one-stage coagulation assay, as previously described.23 Briefly, 5 μL conditioned supernatant was diluted in 50 μL FIX-deficient plasma. aPTT (50 μL) was then added, and samples were incubated at 37°C for 3 min. Following incubation, 50 μL of 20 mM calcium was added. Time to clot formation was determined by automated viscometric measurement. FIX activity was determined by linear regression analysis of the clotting time versus the logarithm of the reciprocal plasma dilution. FIX levels in plasma samples were measured by human FIX-specific enzyme-linked immunosorbent assay (ELISA) using the Abcam—Human Factor IX SimpleStep ELISA Kit, according to the manufacturer's instructions.
Briefly, citrated plasma samples were collected by the retro-orbital bleeding method and quantified by colorimetric sandwich ELISA against a standard curve prepared from lyophilized native human FIX protein using a SpectraMax 96-well plate reader (Molecular Devices, San Jose, CA). FIX levels in test samples were quantified by a 4-parameter curve fit to the standard curve using the Softmax Pro analysis software (Molecular Devices).
Recombinant AAV vectors
Highly purified stocks of AAV8 vectors were produced by the triple plasmid transfection method.24 Briefly, HEK293 cells were cotransfected with three plasmids by the polyethyleneimine (PEI) transfection protocol. Medium was replaced 6 h post-transfection. Cells were harvested 72 h post-transfection, subjected to three rounds of freeze/thaw and then digested with 50 U/mL Benzonase (Sigma-Aldrich) and 50 U/mL Pierce Universal Nuclease (Thermo Fisher Scientific, Grand Island, NY) at 37°C for 1 h. Viral vectors were purified by iodixanol (Sigma) gradient ultracentrifugation followed by ion exchange chromatography using HiTrap Q HP (GE Healthcare, Piscataway, NJ), washed with PBS, and concentrated by centrifugation using centrifugal spin concentrators with 150 K molecular-weight cutoff. Viral vectors were resuspended in PBS, and titers were determined by qPCR as described previously.25 Highly purified stocks of scAAV3-FIX vectors were generated by SAB Tech (Philadelphia, PA).
AAV vector transduction in mouse models
F9+/+ 129S4/SvJae; C57BL/6 mice (wild-type FIX) were bred at Emory University. Mouse studies were performed using procedures approved by the Emory University Institutional Animal Care and Use Committee and the Emory University Division of Animal Resources (DAR).
Hemophilia B mice (C3H/HeJ-F9-/Y) with a targeted deletion of murine F9 have been bred on the C3H/HeJ background for >20 generations.26 Hemophilia B mice were injected with 1 × 109–1 × 1010 vgs of an scAAV8-F9-Padua vector in the tail vein and followed over time as indicated. Plasma was collected from retro-orbital capillary collection of blood into 3.8% citrate buffer (9:1). FIX levels were determined at various time points postvector administration by ELISA. FIX activity was determined using the FIX chromogenic two-stage assay from Diapharma ROX Factor IX (900020), West Chester, OH. All animal experiments were approved by the Institutional Animal Care and Use Committee and were performed according to the guidelines for animal care specified by the Animal Care Services at the University of Florida, and maintained by the Animal Care Services at the University of Florida College of Medicine (Gainesville, FL).
TK-NOG mice (NOD.Cg-Prkdcscid Il2rgtm1SugTg(Alb−UL23)7-2/ShiJic NOG-Tg(Alb-UL23)7-2/ShiJic), with and without transplantation with primary human hepatocytes from male donors, were purchased from In-Vivo Biosciences (Kawasaki, Japan). All animal experiments were approved by the Institutional Animal Care and Use Committee and were performed according to the guidelines for animal care specified by the Animal Care Services at the University of Florida, and maintained by the Animal Care Services at the University of Florida College of Medicine. Mice were injected with 5 × 1011 vgs of scAAV3-FIX vectors via the tail vein, and FIX levels and FIX activities were determined at various time points postvector administration as described above.
Statistical analysis
Results are shown as mean ± standard deviation. Differences between groups were determined using a grouped-unpaired two-tailed distribution of Student's t-test. p-Values <0.05 were considered statistically significant.
Results
Liver codon-optimization of, and inclusion of Padua mutation in, the transgene expression cassette increases FIX activity
In addition to the use of an AAV3 vector design, we used several other state-of-the-art technologies to our FIX transgene design. We have previously described a codon optimization strategy that seeks to optimize the codon adaptive index (CAI) of a transgene as a function of the predicted tRNA milieu present in the target tissue rather than the standard method, which seeks to optimize according to the overall CAI of the host organism's genomic coding sequence. Our previous work has shown this approach to increase heterologous in vitro protein expression two to threefold over standard human codon optimization approaches in both factor VIII and FIX cDNA designs.27 In the present study, the previously described LCO human FIX cDNA design was implemented in the context of a novel, clinically compatible AAV vector expression system. LCO FIX was tested with and without R338L (Padua) substitution that has previously been shown to increase the specific activity of FIX by approximately eightfold.
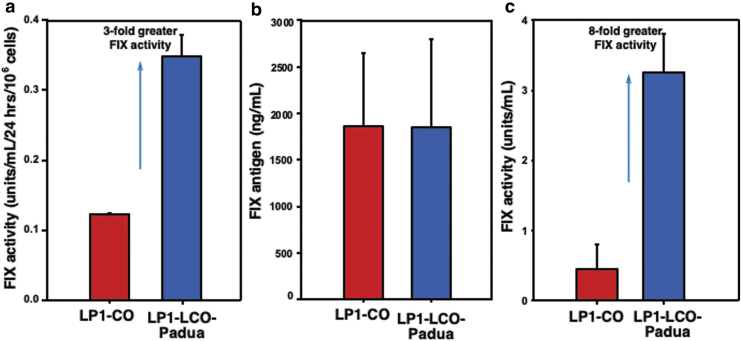
Comparison of identical plasmid expression cassettes differing only in the inclusion of the Padua mutation following plasmid DNA transfection in human HepG2 cells showed threefold improvement in FIX activity in vitro (Fig. 1a) and eightfold benefit in vivo (Fig. 1c). To confirm this benefit was conferred by increased specific activity of the FIX molecule, and not due to increased levels of plasma protein, we quantified FIX levels by ELISA (Fig. 1b). Together, these findings show that the combination of human liver codon optimization and Padua mutation produces a vector transgene design with high in vivo expressivity in the context of liver-directed gene therapy.
Figure 1.
Optimization of the FIX expression cassette. (a) The two identical plasmid expression cassettes, differing only in the indicated FIX cDNAs (LP1 promoter-driven codon-optimized and human liver codon-optimized with the Padua mutation), were transfected into human hepatoma cells, HepG2, and FIX activity was determined by one-stage clot assay 24 h post-transfections. (b) Twenty micrograms of the indicated plasmid DNA was hydrodynamically injected separately into FIX wild-type mice, and total FIX antigen levels were detected in sera by ELISA 24 h postinjections. (c) FIX activity in sera from hydrodynamically injected wild-type FIX mice was detected by ELISA 24 h postinjections. ELISA, enzyme-linked immunosorbent assay; FIX, factor IX; HepG2, hepatocellular carcinoma.
Inclusion of SERPINA1 enhancer derivatives significantly enhances the promoter activity
The choice of a transgene promoter is a crucial element in designing a potent and efficacious gene therapy vector. In the context of an AAV vector design, which has an approximate packaging capacity of 5.0 kb, the choice of promoter must balance the strength of expression with the size constraints imposed by the packaging capacity of the AAV vector. While the FIX cDNA (1,383 bp) does not face the same implicit size constraints as B-domain deleted factor VIII cDNAs (4,374 bp), accommodating a self-complementary (sc) vector genome design, which has been shown to increase the efficiency of AAV vectors in other FIX transgene designs, effectively reduces the packaging capacity of the virus by 50%. We therefore sought to identify or design a promoter that would confer high-level FIX expression while remaining compatible with an scAAV vector genome design.
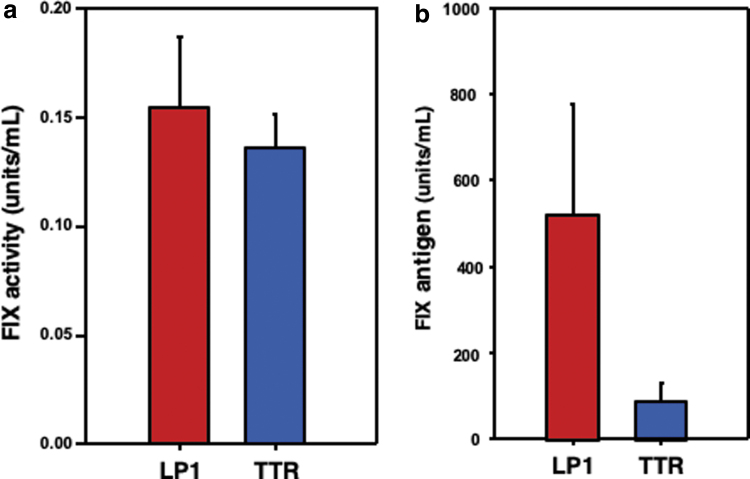
We began by characterizing the strength of the minimally sized murine transthyretin (TTR) promoter (223 bp) against that of the LP1 promoter (447 bp). LP1 is generated from the fusion of the human apolipoprotein E hepatic control region HCR-2, but its large size limits compatibility with the scAAV- FIX vector genome design. In vitro transfection studies in HepG2 cells showed that the TTR promoter drove FIX expression at comparable levels with that of LP1 (Fig. 2a). However, when these plasmids were delivered to the livers of mice by hydrodynamic injection, the LP1 promoter demonstrated threefold higher levels of FIX expression than conferred by a TTR design (Fig. 2b).
Figure 2.
Comparative analysis of FIX expression from LP1 and TTR promoters. (a) Two plasmids containing identical FIX expression cassettes, differing only in the promoters (LP1 and TTR), were transfected into HepG2 cells, and FIX activity was determined by one-stage clot assay 24 h post-transfections. (b) Twenty micrograms of the indicated plasmid DNA was hydrodynamically injected separately into FIX wild-type mice, and total FIX antigen levels were detected in sera by ELISA 24 h postinjections. TTR, transthyretin.
We therefore sought to augment the strength of the TTR promoter with the addition of a liver-directed enhancer element while retaining a design compact enough to retain compatibility with an scAAV vector genome. The human 5′ SERPINA1 untranslated region (UTR) has been predicted to contain a potent enhancer element present in a variety of genetic orthologs contained within a 71 bp region designated HS-CRM8, which has been characterized as an enhancer element generated from the consensus sequence of the orthologous genomic regions used to identify this region.28 Building on these findings, we tested combinations of TTR in conjunction with variants of the human SERPINA1 HS-CRM8 (HSCRM8h) enhancer region along with fusions of this enhancer with the HNF6 transcription factor binding motif. We also wished to identify the elements in the LP1 promoter that may confer additional expression benefit by modifying the LP1 sequence to include consensus HNF4 and HNF1 transcription factor binding sites (LP1mod) as well as reverting four nucleotide residues in the pan-ortholog consensus HS-CRM8 sequence to the human consensus sequence within or proximal to the predicted transcription factor binding sites (4bp-HSCRM8).
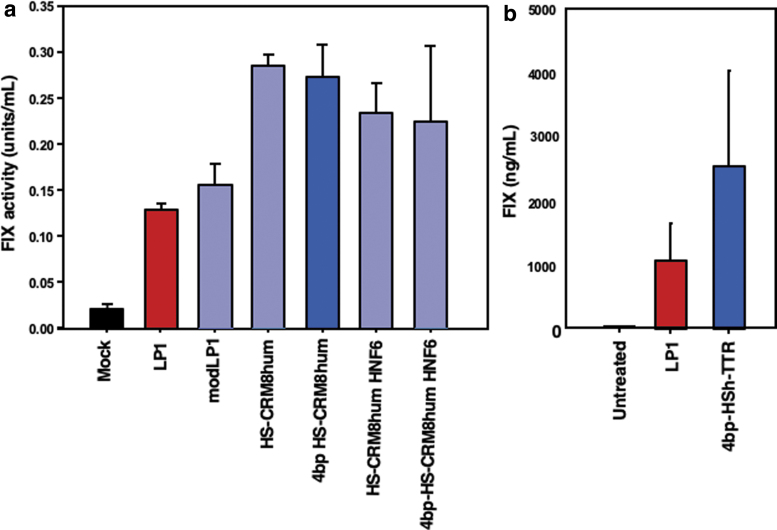
When placed into an hFIX expression cassette, we found that the addition of the HNF1 and HNF4 (LP1mod) or HNF6 (HSCR8h) did not impact expression in transient transfection in HepG2 cells. However, when placed in front of the TTR promoter, all tested variants of the HSCRM8 promoter resulted in approximately a twofold enhancement in FIX expression over the LP1 comparator promoter while retaining a more compact design than that of LP1 (Fig. 3a). When delivered to the liver of wild-type mice by hydrodynamic injection, the 4bp-HSCRM8 design (hereafter designated HSh) expressed two- to threefold higher than an otherwise identical cassette driven by the LP1 promoter (Fig. 3b). Due to its compact yet powerful design, we therefore designated the TTR promoter augmented with the HSh enhancer (HSh-TTR) as the lead candidate design in further studies for benchmark comparison with other clinically relevant vector designs, including the LP1 and alpha 1 antitrypsin (AAT) promoter-driven FIX vector designs.
Figure 3.
Optimization of the TTR promoter. (a) Promoters with several indicated derivatives of SERPINA1 enhancers in identical plasmid FIX expression cassettes FIX cDNAs were transfected into HepG2 cells, and FIX activity was determined 24 h post-transfections. (b) Twenty micrograms of identical FIX plasmid DNA expression cassettes differing only in their promoters/enhancers was hydrodynamically injected separately into FIX wild-type mice, and total FIX antigen levels were detected in sera by ELISA 24 h postinjection.
Generation of an optimized AAV3-FIX vector
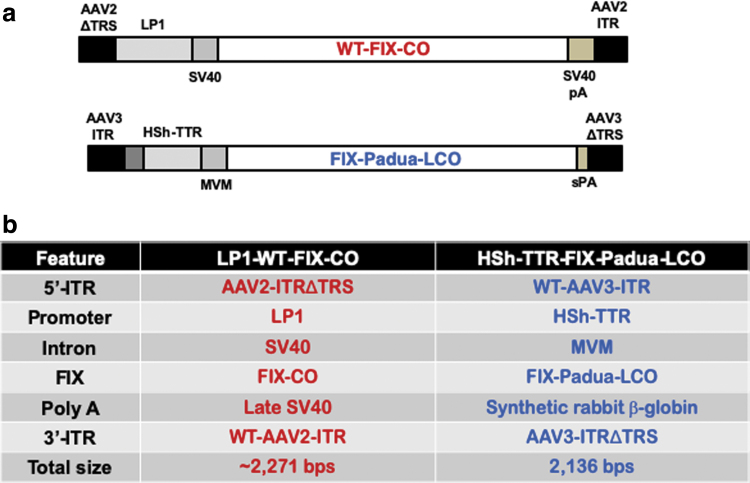
We next synthesized an scAAV vector in which the optimized HSh-TTRp-FIX-Padua-LCO expression cassette was flanked by a wild-type (WT) AAV3 inverted terminal repeat (ITR) at the 5′-end, and the D-sequence-deleted AAV3-ITR (AAV3ΔTRS) at the 3′-end. AAV3-ITRs were used instead of the commonly used AAV2-ITRs because of our ultimate objective to encapsidate the optimized FIX expression cassette into AAV3 capsids, since we have previously reported that AAV3 vectors composed of expression cassettes containing AAV3-ITRs and AAV3 capsids are packaged at higher titers and with higher potency.24 The schematic structures of FIX expression cassettes used in the first successful clinical trial for hemophilia B (scAAV8-LP1-FIX-WT-CO), and the one used in the current studies (scAAV3-HSh-TTR-Padua-FIX-LCO), are shown in Fig. 4a, and the salient features of various components of the two vectors are detailed in Fig. 4b. While conceptually similar, the two vectors share none of the same components.
Figure 4.
Schematic structures and salient features of AAV-FIX vectors. (a) Schematic structures of FIX expression cassettes used in the first successful clinical trial for hemophilia B (scAAV8-LP1-FIX-WT-CO), and the one used in the current studies (scAAV3-HSh-TTR-Padua-FIX-LCO). (b) Salient features of various components of the two vectors are detailed. AAV, adeno-associated virus; AAV2-ITR, adeno-associated virus serotype 2 inverted terminal repeat; AAV3-ITR, AAV3 serotype ITR; AAV2- and AAV3-ITRΔtrs, terminal resolution site-deleted AAV2- and AAV3-ITRs; FIX-CO, codon-optimized, CpG-depleted human FIX; HSh-TTR, modified variant of human SERPINA1 enhancer/transthyretin promoter; LP1, human apolipoprotein enhancer/α1 antitrypsin-based liver-specific promoter; MVM, minute virus of mice intron; Padua-FIX-LCO, FIX gene containing the Padua mutation, CpG-free, human liver codon-optimized, 148T isoform; s-β-globin pA, synthetic β-globin polyA signal; SV40, simian vacuolating virus intron; SV40 pA, SV40 polyadenylation signal; WT, wild type.
Optimized FIX expression cassette encapsidated in AAV8 vectors leads to phenotypic correction in hemophilia B mice
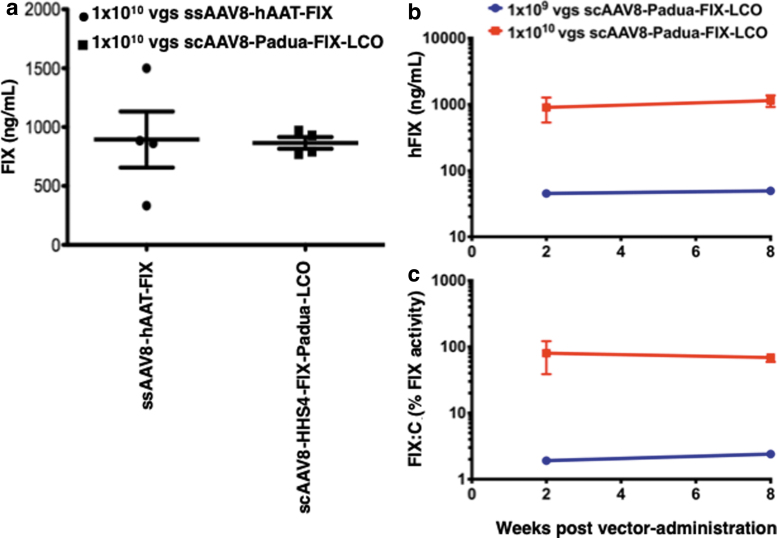
We next wished to evaluate the transduction efficiency of our optimized AAV3-FIX vector in a murine model in vivo. However, since AAV3 vectors do not transduce mouse hepatocytes,29,30 the optimized genomes were packaged into AAV8 capsids, a serotype known to be highly liver-tropic.10,11 ssAAV8-hAAT-FIX-CO vectors were used as an appropriate control. Hemophilia B mice were injected via the tail vein with 1 × 1010 vgs each of the two vectors, and serum FIX levels were evaluated 3 weeks postvector administration. These results are shown in Fig. 5a. As can be seen, both vectors produced very similar levels of FIX protein.
Figure 5.
Transduction efficiency of ssAAV8-FIX and scAAV3-FIX vectors in hemophilia B mice. (a) Four hemophilia B mice per group were injected with 1 × 1010 vgs of ssAAV8-hAAT-FIX, or scAAV8-HSh-TTR-Padua-FIX-LCO vectors via the tail-vein, and serum FIX levels were determined by ELISA 3 weeks postvector administration. Hemophilia B mice (n = 3 per group) were injected with 1 × 109 or 1 × 1010 vgs/mouse of scAAV8-HSh-TTR-Padua-FIX-LCO vectors via the tail-vein, and (b) FIX levels and (c) two-stage FIX activity were determined at 2 and 8 weeks postvector administration.
In a second set of experiments, we examined functional correction of hemostasis in hemophilia B mice using two vector doses of 1 × 109 and 1 × 1010 vgs of the scAAV8-Padua-FIX LCO vector. Three mice per group were injected with the indicated vector doses and followed over time for human FIX expression in the plasma by ELISA and restoration of coagulation function using a two-stage chromogenic assay. Mice that received 1 × 1010 vgs had complete normalization of FIX activity while expressing ∼20% of normal levels of hFIX protein in the plasma (Fig. 5b, c).
Optimized FIX expression cassette encapsidated in AAV3 vectors leads to production of therapeutic levels of FIX in “humanized” mice
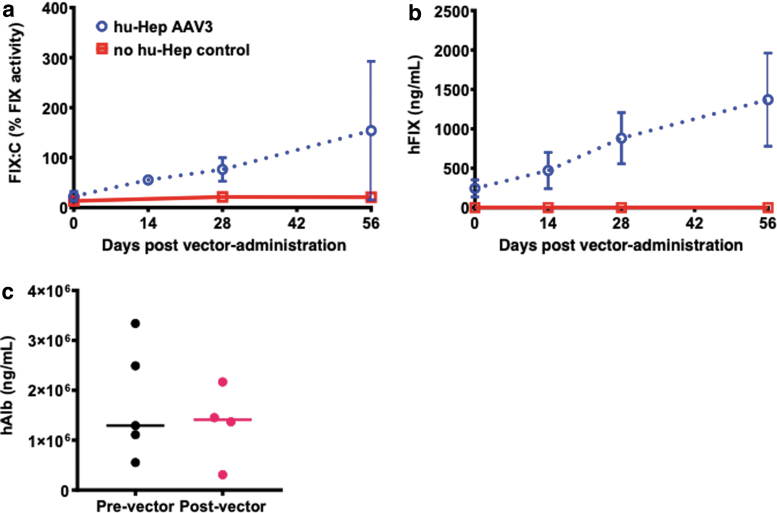
Finally, we evaluated the transduction efficiency of our optimized AAV3-FIX vector in a “humanized” mouse model. TK-NOG mice, transplanted with and without primary human hepatocytes, were injected with 5 × 1011 vgs of the scAAV3-HSh-TTR-Padua-FIX-LCO vector via the tail-vein, and FIX activity and plasma FIX levels were determined at 0, 2, 4, and 8 weeks postvector administration. As expected, background FIX activity before vector treatment was measured from both murine FIX protein and hFIX protein produced from the transplanted human hepatocytes (Fig. 6a). However, FIX activity increased in a time-dependent manner in vector-treated TK-NOG hum hepatocytes (Fig. 6a, b), which correlated well with serum FIX levels (Fig. 6b). To ensure that increased FIX levels were due to AAV-mediated gene transfer and not expansion of the human hepatocytes, levels of human albumin were measured before vector administration and again at the termination of the study.
Figure 6.
Transduction efficiency of scAAV3-FIX vectors in “humanized” mice. Five TK-NOG mice transplanted with primary human hepatocytes and were injected with 5 × 1011 vgs of the scAAV3-HSh-TTR-Padua-FIX-LCO vector via the tail-vein. (a) Plasma FIX activity was determined before and 2, 4, and 8 weeks postvector administration in vector-treated mice and control mice that did not receive human hepatocytes nor vector. (b) Plasma FIX levels were also determined by ELISA before and 2, 4, and 8 weeks postvector administration in vector-treated mice and control mice that did not receive human hepatocytes nor vector. (c) Human albumin levels in plasma were measured by ELISA before and at the end of study to evaluate the stability of human hepatocytes in TK-NOG mice. Control mice were included to provide background FIX activity resulting from murine FIX protein.
While we noted some intra-animal variability in human albumin levels, these levels remained unchanged before and after vector administration, suggesting that the level of engraftment in each animal remained constant throughout the study and that the increase in human FIX activity was from transduced hepatocytes expressing the Padua-FIX protein and not from expansion of human hepatocytes since no significant change in plasma human albumin levels was observed between before vector administration and at the end of the study (Fig. 6c).
Taken together, these data suggest that a clinical candidate AAV3-FIX vector described here may be more efficacious for gene therapy of hemophilia B in humans than the AAV serotype vectors currently being used.
Discussion
Successful gene therapy of hemophilia has long been a desirable goal. Indeed, in the first liver-directed Phase 1 clinical trial with the first generation of AAV2 vectors, therapeutic levels of FIX were achieved, although at a high vector dose.8 However, FIX gene expression was short-lived due to the anti-AAV2 capsid protein-induced host CD8+ T cell response.9 In a subsequent trial with scAAV8 vectors, FIX levels ranging from ∼8–12% could be achieved.12 Over a 3-year follow-up period, the FIX levels declined to ∼5%,13 but have since been maintained at the same level over the next 4 years,14 showing that with immune responses out of the way, AAV8 vector-based gene therapy for hemophilia B can provide stable expression over many years.
However, although the superior performance of AAV8 vectors in humans appeared to parallel that observed in murine models, we have argued that the use of scAAV genome, rather than the AAV8 serotype, was responsible for the higher levels of transgene expression31 since AAV8 vectors transduce human hepatocytes less efficiently than mouse hepatocytes.17,32 This was further corroborated in the next trial, sponsored by Baxalta (now Takeda), also with scAAV8 vectors, which yielded inconsistent results, and the trial was halted because of the inconsistent results as well as immune responses. Interestingly, however, the trial sponsored by Spark Therapeutics, using capsid-modified ssAAV8 vectors, led to higher therapeutic levels of hFIX at a relatively lower dose.15 The trial sponsored by Dimension Therapeutics using ssAAVrh10 vectors resulted in <2% levels of hFIX, and this trial was also halted. More recently, UniQure has reported modest levels of expression of hFIX using scAAV5 vectors, however, enormously high vector doses were required, and they subsequently transitioned to incorporation of the FIX Padua mutation.16
Taken together, the following conclusions have emerged: (1) studies from animal models do not necessarily translate well in humans with reference to the tropism, safety, and efficacy of AAV serotype vectors; (2) there is a direct correlation between the AAV vector dose and the host immune response; and (3) since the host immune system cannot distinguish between AAV as a virus versus AAV as a vector, all AAV serotype vectors composed of the naturally occurring capsids are likely to induce an immune response, especially at high doses.
More than a decade ago, we first reported that among the 10 most commonly used AAV serotype vectors, AAV3 vectors are by far the most efficient in transducing primary human hepatocytes in vitro.33 We also reported that the tropism of AAV3 vectors for human hepatic cells was due to the fact that AAV3 uses the human hepatocyte growth factor receptor (huHGFR) as a cellular coreceptor for cellular entry.29 Our subsequent studies corroborated the remarkable specificity, efficacy, and safety of AAV3 vectors in xenograft and in “humanized” murine models in vivo.18 Since human and nonhuman primate (NHP) HGFRs share 99% identity, we also documented selective tropism and efficient liver transduction in NHPs,17 an observation that was corroborated by others.32
More recently, in a head-to-head comparison, we also reported that AAV3 vectors are superior to AAV5 and AAV8 vectors in “humanized” mice in vivo.18
As stated above, since the host immune response is directly correlated with the AAV vector dose, it stands to reason that every attempt be made to achieve therapeutic levels of FIX at the lowest possible vector dose. Thus, in addition to the use of human hepatotropic AAV3 serotype, we have also expended extensive efforts to further optimize the promoter and the FIX expression cassette, as described here. While an efficient vector serotype can help ease the antigenic burden imposed by bolus infusion of vector by reducing the number of vector particles needed to deliver sufficient vector copy numbers per cell to achieve efficacy, a more potent transgene design can similarly reduce the per-cell genetic burden imposed by the introduction of exogenous therapeutic DNA by reducing the number of steady-state vector genome concatemers needed to achieve durable efficacy.
Transgene design elements such as strong promoters and optimized DNA sequences produce a greater number of mRNA transcripts per vector copy number, reducing the total number of vector genomes carried by the host cells. As integration into the host cell genomic DNA remains a possibility through any means of gene transfer, reducing this burden represents a proactive mechanism of improving the safety profile of the vector while additionally lowering the antigenic burden of therapy by reducing the total number of viral particles infused during therapy.
Although we did not directly compare the efficacy with other AAV serotype vectors in the current study, it is likely that the clinical candidate AAV3 vector described here will be more efficacious at relatively lower doses, in contrast to the high doses that are currently being used with AAV5 and AAV8 vectors for gene therapy of hemophilia B. This assumption is based on our observation that a capsid-modified AAV3 vector was found to be approximately eightfold more efficient than AAV8 vectors, and ∼82-fold more efficient than AAV5 vectors in a “humanized” mouse model.18
Plans are currently underway to initiate a Phase I/II clinical trial for the potential gene therapy of hemophilia B at lower AAV3 vector doses without the use of immune-suppression, in the near future. Future clinical trials will also witness the use of further capsid- and genome-modified AAV3 vectors, which have shown promise in murine and NHP models following systemic administration in vivo.25,30,34,35 The use of optimized AAV3 vectors, in addition to being far more efficacious at significantly lower doses, also offers the potential advantages of being less immunogenic, and more cost-effective, thus obviating the need for prophylactic immune-suppression.
Acknowledgments
We thank Brett Palaschak and Irene Zolotukhin for their expert technical assistance.
Author Disclosure
Ar.S. is a cofounder of, and holds equity in, Lacerta Therapeutics and Nirvana Therapeutics, and is an inventor on several issued patents on recombinant AAV vectors that have been licensed to various gene therapy companies. C.B.D. and H.T.S. are cofounders of Expression Therapeutics and own equity in the company. Expression Therapeutics owns the intellectual property associated with the LCO-FIX transgene. H.C.B. is an inventor of the technology and an employee of Expression Therapeutics. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. All other authors declare no conflict of interests.
Funding Information
This research was supported, in part, by grants from Public Health Service grants R01 HL-097088, R41 AI-122735, and R21 EB-015684; grants from the Children's Miracle Network and aaVective; and support from the Kitzman Foundation (to Ar.S.), and a grant BT/PR17316/MED/31/326/2015 from the Department of Biotechnology, Government of India (to Al.S.).
References
- 1. Ponnazhagan S, Mukherjee P, Yoder MC, et al. Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene 1997;190:203–210 [DOI] [PubMed] [Google Scholar]
- 2. Snyder RO, Miao CH, Patijn GA, et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet 1997;16:270–276 [DOI] [PubMed] [Google Scholar]
- 3. Koeberl DD, Alexander IE, Halbert CL, et al. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci U S A 1997;94:1426–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herzog RW, Hagstrom JN, Kung SH, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci U S A 1997;94:5804–5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herzog RW, High KA. Adeno-associated virus-mediated gene transfer of factor IX for treatment of hemophilia B by gene therapy. Thromb Haemost 1999;82:540–546 [PubMed] [Google Scholar]
- 6. Chao H, Samulski R, Bellinger D, et al. Persistent expression of canine factor IX in hemophilia B canines. Gene Ther 1999;6:1695–1704 [DOI] [PubMed] [Google Scholar]
- 7. Herzog RW, Yang EY, Couto LB, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med 1999;5:56–63 [DOI] [PubMed] [Google Scholar]
- 8. Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 9. Mingozzi F, Maus MV, Hui DJ, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med 2007;13:419–422 [DOI] [PubMed] [Google Scholar]
- 10. Gao GP, Alvira MR, Wang L, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A 2002;99:11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao G, Vandenberghe LH, Alvira MR, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol 2004;78:6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nathwani AC. Gene therapy for hemophilia. Hematology Am Soc Hematol Educ Program 2019;2019:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. George LA, Sullivan SK, Giermasz A, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med 2017;377:2215–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miesbach W, Meijer K, Coppens M, et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 2018;131:1022–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li S, Ling C, Zhong L, et al. Efficient and targeted transduction of nonhuman primate liver with systemically delivered optimized AAV3B vectors. Mol Ther 2015;23:1867–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vercauteren K, Hoffman BE, Zolotukhin I, et al. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol Ther 2016;24:1042–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donsante A, Miller DG, Li Y, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science 2007;317:477. [DOI] [PubMed] [Google Scholar]
- 20. La Bella T, Imbeaud S, Peneau C, et al. Adeno-associated virus in the liver: natural history and consequences in tumour development. Gut 2020;69:737–747 [DOI] [PubMed] [Google Scholar]
- 21. Yin L, Keeler GD, Zhang Y, et al. AAV3-miRNA vectors for growth suppression of human hepatocellular carcinoma cells in vitro and human liver tumors in a murine xenograft model in vivo. Gene Ther 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown HC, Zakas PM, George SN, et al. Target-cell-directed bioengineering approaches for gene therapy of hemophilia A. Mol Ther Methods Clin Dev 2018;9:57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Markusic DM, Nichols TC, Merricks EP, et al. Evaluation of engineered AAV capsids for hepatic factor IX gene transfer in murine and canine models. J Transl Med 2017;15:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ling C, Yin Z, Li J, et al. Strategies to generate high-titer, high-potency recombinant AAV3 serotype vectors. Mol Ther Methods Clin Dev 2016;3:16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ling C, Wang Y, Lu Y, et al. Enhanced transgene expression from recombinant single-stranded D-sequence-substituted adeno-associated virus vectors in human cell lines in vitro and in murine hepatocytes in vivo. J Virol 2015;89:952–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mingozzi F, Liu YL, Dobrzynski E, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest 2003;111:1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zakas PM, Brown HC, Knight K, et al. Enhancing the pharmaceutical properties of protein drugs by ancestral sequence reconstruction. Nat Biotechnol 2017;35:35–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chuah MK, Petrus I, De Bleser P, et al. Liver-specific transcriptional modules identified by genome-wide in silico analysis enable efficient gene therapy in mice and non-human primates. Mol Ther 2014;22:1605–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ling C, Lu Y, Kalsi JK, et al. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum Gene Ther 2010;21:1741–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ling C, Wang Y, Zhang Y, et al. Selective in vivo targeting of human liver tumors by optimized AAV3 vectors in a murine xenograft model. Hum Gene Ther 2014;25:1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berns KI, Srivastava A. Next generation of adeno-associated virus vectors for gene therapy for human liver diseases. Gastroenterol Clin North Am 2019;48:319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang L, Bell P, Somanathan S, et al. Comparative study of liver gene transfer with AAV vectors based on natural and engineered AAV capsids. Mol Ther 2015;23:1877–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glushakova LG, Lisankie MJ, Eruslanov EB, et al. AAV3-mediated transfer and expression of the pyruvate dehydrogenase E1 alpha subunit gene causes metabolic remodeling and apoptosis of human liver cancer cells. Mol Genet Metab 2009;98:289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng B, Ling C, Dai Y, et al. Development of optimized AAV3 serotype vectors: mechanism of high-efficiency transduction of human liver cancer cells. Gene Ther 2012;19:375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ling C, Li B, Ma W, et al. Development of optimized AAV serotype vectors for high-efficiency transduction at further reduced doses. Hum Gene Ther Methods 2016;27:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]