Abstract
Stroke is a leading cause of long-term disability in ischemic survivors who are suffering from motor, cognitive, and memory impairment. Previously, we have reported suppressing LPA5 activity with its specific antagonist can attenuate acute brain injuries after ischemic stroke. However, it is unclear whether suppressing LPA5 activity can also attenuate chronic brain injuries after ischemic stroke. Here, we explored whether effects of LPA5 antagonist, TCLPA5, could persist a longer time after brain ischemic stroke using a mouse model challenged with tMCAO. TCLPA5 was administered to mice every day for 3 days, starting from the time immediately after reperfusion. TCLPA5 administration improved neurological function up to 21 days after tMCAO challenge. It also reduced brain tissue loss and cell apoptosis in mice at 21 days after tMCAO challenge. Such long-term neuroprotection of TCLPA5 was associated with enhanced neurogenesis and angiogenesis in post-ischemic brain, along with upregulated expression levels of vascular endothelial growth factor. Collectively, results of the current study indicates that suppressing LPA5 activity can provide long-term neuroprotection to mice with brain ischemic stroke.
Keywords: Ischemic stroke, LPA5, TCLPA5, Long-term neuroprotection, Neurogenesis, Angiogenesis
INTRODUCTION
Stroke is a leading cause of death and long-term disability globally. Stroke survivors suffer from motor, cognitive, and memory impairment. They face various difficulties in their daily lives. To date, many drugs and strategies have been successful in experimental models for acute ischemic stroke. However, they have failed in clinical translation. Most of previous studies that find effective treatments have focused on whether these drugs can work for acute injury after ischemic stroke (around 24 h after challenge) by determining brain infarction volume and neurological score with little consideration of their long-term neuroprotection. The importance for long-term protection has been emerged in drug development to treat ischemic stroke (Gladstone et al., 2002; Girard et al., 2014; Schmidt-Pogoda et al., 2020).
Lysophosphatidic acid (LPA) affects six G protein-coupled receptors (LPA1-LPA6) and influences various biological functions (Choi and Chun, 2013). Among these receptors, LPA1 (Gaire et al., 2019) and LPA5 (Sapkota et al., 2020) have been identified as pathogenic factors in acute ischemic injury. In particular, the latter is upregulated in injured brain after acute ischemic stroke and suppressing its activity can successfully attenuate acute brain injuries (i.e., brain injuries at 1 or 3 days after ischemic challenge) by reducing inflammatory responses in the injured brain (Sapkota et al., 2020). However, it remains unknown whether suppressing LPA5 activity can also be effective for a longer time (up to a few weeks).
The main objective of the current study was to address whether suppressing LPA5 activity with TCLPA5, an LPA5 antagonist, could lead to long-term protection after ischemic challenge using a mouse model of stroke with a transient middle cerebral artery occlusion (tMCAO). Effects of TCLPA5 administration were determined up to 21 days after tMCAO challenge. To determine underlying mechanisms associated with long-term neuroprotection, we determined whether TCLPA5 administration could promote neurogenesis and angiogenesis in the post-ischemic brain of mouse at 21 days after challenge and upregulate vascular endothelial growth factor (VEGF) upregulation, a core factor for both neurogenesis and angiogenesis.
MATERIALS AND METHODS
Animals
Male ICR mice (7 weeks old) were obtained from the Orient Bio (Seongnam, Korea). All animal experiments were performed following Center of Animal Care and Use (CACU) guidelines (approved animal protocol number: LCDI-2019-0027) of Lee Gil Ya Cancer and Diabetes Institute (LCDI) at Gachon University (Incheon, Korea). Mice were housed in controlled temperature and humidity condition with a 12 h light cycle. Mice had free access to food and water ad libitum.
Challenge with tMCAO
Mice were challenged with tMCAO as previously described (Sapkota et al., 2020). Briefly, mice were anesthetized with 3% isoflurane for induction and 1.5% for maintenance in oxygen and nitrogen (30:70% ratio, respectively). Mice were kept in a supine position on an operation plate. The right common carotid artery was separated carefully from the vagus nerve. A small hole in bifurcation was then made. The middle cerebral artery was occluded by inserting monofilaments coated with silicon (9-mm-long 5-0) monofilament. Blood flow was restored at 90 min after occlusion by withdrawing the monofilament. For the sham group, the same surgical procedure was performed without MCAO.
TCLPA5 administration
After tMCAO challenge, mice were randomly divided into either a vehicle group (10% cremophor EL and 10% ethanol in distilled water)- or a TCLPA5-administered group. Vehicle or TCLPA5 (10 mg/kg, i.p., Tocris Bioscience, Bristol, UK) was administered to mice at 0 (immediately after reperfusion), 1, 2, and 3 days after tMCAO challenge. In this study, TCLPA5 was used at 10 mg/kg because the neuroprotective effects of TCLPA5 against an acute brain injury following ischemic challenge were most pronounced at 10 mg/kg in our previous study (Sapkota et al., 2020).
Determination of functional neurological deficit score
Modified neurological severity score (mNSS) scale was used for assessing the functional neurological deficit score in the motor, sensory, reflex, and balance tests every day for 21 days. The functional neurological deficit score ranged from 0 to 18 points (0 point for normal and 18 points for maximal deficit mice) as previously reported (Chen et al., 2001).
Histological assessment
Measurement of brain tissue loss and preparation of brain sections: To determine brain tissue loss at the end of experiment, whole brain was used and the area of viable tissues was calculated as previously described (Wang et al., 2017). Briefly, mice were anesthetized with a mixture of Zoletil 50® (10 mg/kg, i.m., Virbac Laboratories, Carros, France) and Rompun® (3 mg/kg, i.m., Bayer HealthCare LLC, KS, USA), perfused with ice-cold PBS, and fixed with 4% paraformaldehyde (PFA). Brains were collected and post-fixed with 4% PFA overnight. Surface water of brains was removed using tissue paper. Brains were photographed. Area of the brain was measured using Image J software (National Institute of Mental Health, MD, USA) and calculated according to the following formula: (Contralateral area - ipsilateral are)/Contralateral area×100%.
To prepare brain sections for histological assessment, post-fixed brains were cryoprotected with 30% sucrose, embedded in Tissue-Tek® Optimal Cutting Temperature compound, and sectioned (20 µm in thickness) using a cryostat (RD-2230, Roundfin, Liaoning, China).
Determination of cell apoptosis: Cell apoptosis in post-ischemic brain was analyzed through TUNEL assay. Brain sections were post-fixed with 4% PFA, washed with PBS, and stained with a mixture of Enzyme solution and Label solution (In Situ Cell Death Detection Kit, Roche, Mannheim, Germany) for 1 h at room temperature. These sections were rinsed with PBS and distilled water and then mounted with VECTASHIELD® (Vector Laboratories, Burlingame, CA, USA).
Determination of neurogenesis and angiogenesis: Neurogenesis and angiogenesis were determined by double immunofluorescence staining against a cell-specific marker (DCX or CD31) and 5-Bromo-2’-deoxyuridine (BrdU), respectively, as previously described (Sapkota et al., 2020). Briefly, BrdU (50 mg/kg, i.p., Sigma-Aldrich, St. Louis, MO, USA) was given to mice for 2 days at 12 h intervals, starting from 19 days after tMCAO or sham operation. Coronal brain sections were post-fixed with 4% PFA, incubated with 2N HCl at 37°C for DNA denaturation, neutralized with borate buffer (0.1 M, pH 8.5), blocked with 1% fetal bovine serum (FBS), and incubated with proper primary antibodies including rat anti-BrdU (1:200, Abcam, Cambridge, UK), mouse anti-BrdU (1:200, ImmunoBioScience Corp., Mukilteo, WA, USA), goat anti-DCX (1:200, Santa Cruz Biotechnology, Dallas, TX, USA), and rat anti-CD31 (1:300, Dianova, Hamburg, Germany) overnight at 4°C. Sections were further incubated with secondary antibodies conjugated with Cy3 or AF488 (1:1,000, Jackson ImmunoResearch, West Grove, PA, USA) and coverslipped using VECTASHIELD® (Vector Laboratories).
Determination of VEGF expression: Brain sections were post-fixed with PFA 4%, subjected to antigen retrieval by heating them in a solution containing sodium citrate (0.01M) at 90-100°C for 30 min, and blocked with 1% FBS. Sections were then incubated with a primary antibody against VEGF (1:200, Santa Cruz Biotechnology) overnight at 4°C, labeled with an anti-rabbit secondary antibody conjugated with AF488 (1:1,000, Jackson ImmunoResearch) for 2 h at room temperature, counterstained with DAPI, and mounted with VECTASHIELD® (Vector Laboratories).
For double immunofluorescence staining, sections were incubated with goat anti-Iba1 (1:500, Abcam), mouse anti-NeuN (1:200, Millipore, Burlington, MA, USA), mouse anti-GFAP (1:500, Sigma-Aldrich), or rat anti-CD31 (1:300, Dianova) primary antibody overnight at 4°C. These sections were then incubated with Cy3- or AF488-conjugated (1:1,000, Jackson ImmunoResearch) secondary antibodies for 2 h at room temperature and mounted with VECTASHIELD® (Vector Laboratories).
Image preparation and quantification: Fluorescent images were captured with a fluorescence microscope equipped with a DP72 camera (BX53T, Olympus, Tokyo, Japan) or a laser scanning confocal microscope (Eclipse A1 Plus, Nikon, Tokyo, Japan). All final images were prepared using Adobe Photoshop Elements 8 (Adobe, San Jose, CA, USA). The number of immunopositive cells was counted from three different regions of each brain region. The number of immunopositive cells was manually counted and presented as the number of cells per unit area.
Statistical analysis: All data are presented as mean ± standard error of the mean (SEM). All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA). Statistical data from different groups were analyzed using one-way analysis of variance (ANOVA) followed by Newman–Keuls test for multiple comparisons. Differences between the two groups were analyzed with Student’s t-test. Survival rate was analyzed with Log-rank (Mantel-Cox) test. For all analyses, statistical significance was set at p<0.05.
RESULTS
TCLPA5 administration exerts long-term neuroprotective effects in mice with tMCAO challenge
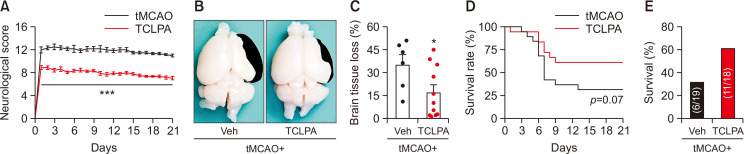
To investigate whether suppressing LPA5 activity with TCLPA5 administration could lead to long-term protection against tMCAO challenge, vehicle or TCLPA5 (10 mg/Kg/i.p.) was administered to mice every day for 3 days, starting immediately after reperfusion. TCLPA5 administration significantly improved neurological deficit scores of mice at all determined time points (from day 1 to day 21) compared to vehicle-administration control mice (Fig. 1A). Next, brain tissue loss was measured at 21 days after tMCAO challenge. In the vehicle-administered tMCAO group, a significant brain tissue loss was observed (Fig. 1B, 1C). This brain tissue loss was significantly attenuated by TCLPA5 administration (Fig. 1B, 1C). In addition, we determined whether suppressing LPA5 activity with TCLPA5 could affect survival of mice with tMCAO challenge by analyzing survival rate with a log-rank test. Although the survival rate was not significantly different between vehicle and TCLPA5 administration groups, the survival rate in the latter group was overall higher than that in the former group (Fig. 1D). Moreover, cohorts exposed to tMCAO challenge with vehicle administration showed survival of 31.58% (6 of 19 mice survived), whereas those with TCLPA5 administration showed survival of 61.11% (11 of 18 mice survived) (Fig. 1E). These results indicate that suppressing LPA5 activity can provide long-term neuroprotection in mice after ischemic challenge, along with a slight beneficial effects on survival of these mice.
Fig. 1.
Neuroprotective effects of LPA5 antagonism persist up to 21 days after tMCAO challenge. Mice were challenged with tMCAO. TCLPA5 (TCLPA: 10 mg/kg, i.p.) was administered daily to mice for 3 days (administered at 0, 1, 2, and 3 days after reperfusion). (A) Effects of TCLPA5 administration on neurological deficit score were assessed daily up to 21 days after tMCAO challenge. (B, C) Effects of TCLPA5 administration on brain tissue loss were assessed at 21 days after tMCAO challenge. Representative images for brain tissue loss (B) and quantification (C) are shown. The colored region in black indicates lost area. (D) Effects of TCLPA5 administration on survival rate were assessed daily up to 21 days after tMCAO challenge. (E) Survival of mice was quantified for each group; Numbers in parentheses indicate the number of survived mice/cohort. n=6 for tMCAO+Veh and n=11 for tMCAO+TCLPA. *p<0.05 and ***p<0.001 vs. vehicle-administered tMCAO group.
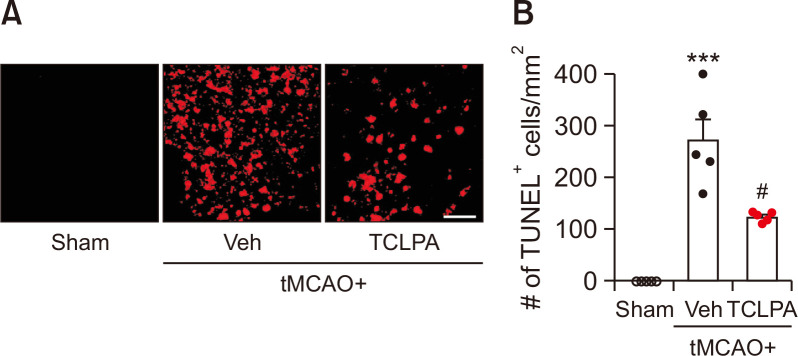
Next, we determined whether suppressing LPA5 activity with TCLPA5 could attenuate cell apoptosis in the injured brain at a chronic phase after ischemic challenge. The extent of cell apoptosis was assessed by TUNEL assay at 21 days after tMCAO challenge (Fig. 2). In vehicle-treated tMCAO mice, the number of apoptotic cells was markedly increased in the penumbra and the ischemic core regions after ischemic challenge, whereas such apoptotic cell death was significantly attenuated by TCLPA5 administration (Fig. 2). These results suggest that suppressing LPA5 activity may suppress cell apoptosis in post-ischemic brain at a chronic phase.
Fig. 2.
Suppressing LPA5 activity with TCLPA5 reduces cell apoptosis in injured brains at 21 days after tMCAO challenge. Mice were challenged with tMCAO. TCLPA5 was administered daily to mice for 3 days. Effects of TCLPA5 on cell apoptosis were assessed at 21 days after tMCAO challenge. Representative images of TUNEL-positive cells in the ischemic core region (A) and quantification of the number of TUNEL-positive cells in the penumbra and the ischemic core regions (B) are shown. Scale bar, 50 µm. n=5 mice per group. ***p<0.001 vs. sham; #p<0.05 vs. vehicle-administered tMCAO group.
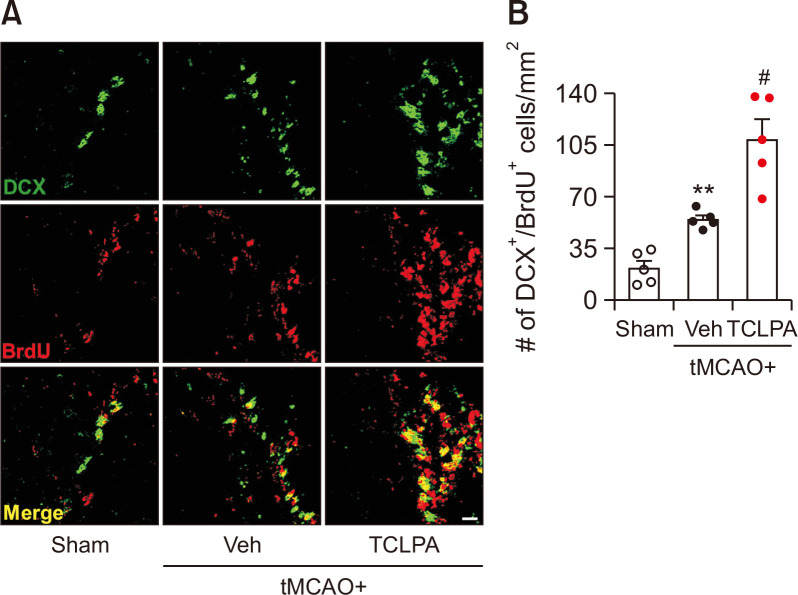
TCLPA5 administration enhances neurogenesis in the injured brain of mouse with tMCAO challenge
Neurogenesis has been regarded as an important event for brain repair following ischemic injury (Koh and Park, 2017; Palma-Tortosa et al., 2017). It occurs mainly in the subventricular zone (SVZ) of the neurogenic niche of the brain (Koh and Park, 2017; Palma-Tortosa et al., 2017). To determine whether suppressing LPA5 activity with TCLPA5 could promote neurogenesis in injured brain after ischemic challenge, the number of DCX and BrdU double-immunopositive cells was analyzed in the SVZ of post-ischemic brain at 21 days after tMCAO challenge. In the vehicle-administered group, the number of new-born neurons (DCX+/BrdU+) was significantly increased in the SVZ of post-ischemic brain (Fig. 3). Interestingly, in the TCLPA5-adminstered group, the number of new-born neurons was significantly higher than that in the vehicle-administered group (Fig. 3). These results indicate that suppressing LPA5 activity can enhance neurogenesis in the post-ischemic brain after tMCAO challenge, possibly leading to its long-term neuroprotection.
Fig. 3.
Suppressing LPA5 activity with TCLPA5 promotes angiogenesis in injured brains after tMCAO challenge. Mice were challenged with tMCAO. TCLPA5 was administered daily to mice for 3 days. Effects of TCLPA5 on angiogenesis were determined by CD31 and BrdU double immunofluorescence staining at 21 days after tMCAO challenge. (A) Representative images of CD31-immunopositive cells in the penumbra region of post-ischemic brain. (B) Quantification of the length of CD31-immunopositive cells. Scale bar, 20 µm. n=5 mice per group. (C) Representative images of CD31 and BrdU double-immunopositive cells in the penumbra region of post-ischemic brain. (D) Quantification of the number of double-immunopositive cells. Scale bar, 10 µm. n=5 mice per group. *p<0.05 and **p<0.01 vs. sham; #p<0.05 vs. vehicle-administered tMCAO group.
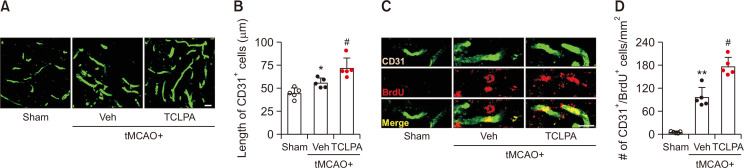
TCLPA5 administration enhances angiogenesis in the injured brain of mouse with tMCAO challenge
Angiogenesis is another important event for brain repair following ischemic injury (Ergul et al., 2012). To investigate whether suppressing LPA5 activity with TCLPA5 could increase angiogenesis in injured brain after ischemic challenge, the number of CD31 and BrdU double-immunopositive cells in the penumbra region of post-ischemic brain at 21 days after ischemic challenge was analyzed. In the vehicle-administered group, lengths of endothelial cells (CD31+) in the penumbra region of post-ischemic brain were longer than those in the sham group (Fig. 4A, 4B). The number of new-born endothelial cells (CD31+/BrdU+) was also significantly increased in the penumbra region of post-ischemic brain than that in the sham group (Fig. 4C, 4D). Intriguingly, in the TCLPA5-adminstered group, lengths of endothelial cells (Fig. 4A, 4B) and the number of new-born endothelial cells (Fig. 4C, 4D) were significantly longer and higher than those in the vehicle-administered group, respectively. These results indicate that suppressing LPA5 activity can enhance angiogenesis in post-ischemic brain after tMCAO challenge, possibly leading to its long-term neuroprotection in post-ischemic brain.
Fig. 4.
Suppressing LPA5 activity with TCLPA5 promotes neurogenesis in injured brains after tMCAO challenge. Mice were challenged with tMCAO. TCLPA5 was administered daily to mice for 3 days. Effects of TCLPA5 on neurogenesis were determined by DCX and BrdU double immunofluorescence staining at 21 days after tMCAO challenge. Representative images of DCX and BrdU double-immunopositive cells in the SVZ (A) and quantification (B) are shown. Scale bar, 20 µm. n=5 mice per group. **p<0.01 vs. sham; #p<0.05 vs. vehicle-administered tMCAO group.
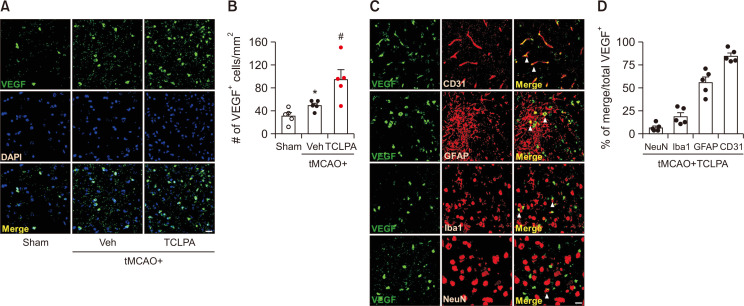
VEGF signaling participates in long-term neuroprotection provided by TCLPA5 administration in tMCAO-challenged mice
VEGF signaling plays a key role in angiogenesis and neurogenesis in post-ischemic brain (Sun et al., 2003). To address the link between VEGF signaling and long-term neuroprotection provided by TCLPA5 in stroke, we determined VEGF expression levels in the penumbra region at 21 days post-tMCAO challenge by VEGF immunofluorescence staining. The number of VEGF-immunopositive cells was significantly increased in the vehicle-administered group compared to the sham group (Fig. 5A, 5B). This number was significantly higher in the TCLPA5-administered group than in the vehicle-administered group (Fig. 5A, 5B). Next, we specified cell types responsible for the upregulated VEGF expression in the brain through double immunofluorescence staining for VEGF combined with specific cell markers: NeuN (neuron), Iba1 (activated microglia), GFAP (activated astrocyte), or CD31 (endothelial cell). Double immunofluorescence staining was performed for brain sections obtained from TCLPA5-treated mice at 21 days after tMCAO challenge. VEGF was expressed in endothelial cells at the highest level. VEGF signals were observed in approximately 85% of CD31-immunopositive cells (Fig. 5C, 5D). They were also observed in GFAP- (56%), Iba1- (19%), and NeuN-immunopositive cells (7%) (Fig. 5C, 5D). These results indicate that suppressing LPA5 activity can regulate VEGF expression in post-ischemic brain, mostly in endothelial cells and activated astrocytes.
Fig. 5.
Suppressing LPA5 activity with TCLPA5 enhances VEGF upregulation in injured brains after tMCAO challenge. Mice were challenged with tMCAO. TCLPA5 was administered daily to mice for 3 days. (A, B) Effects of TCLPA5 on VEGF expression were determined in injured brains at 21 days after tMCAO challenge by VEGF immunofluorescence. Representative images of VEGF-immunopositive cells in the penumbra region (A) and quantification (B) are shown. (C, D) Cellular localization of upregulated VEGF was determined in the penumbra regions of brains from TCLPA5-treated mice at 21 days after tMCAO challenge through double immunofluorescence staining. Representative images for VEGF expression in endothelial cells (CD31), reactive astrocytes (GFAP), activated microglia (Iba1), or neurons (NeuN) (C) and quantification (D) are shown. Arrowheads indicate VEGF and brain cell markers double-immunopositive cells. Scale bars, 20 µm. n=5 mice per group. *p<0.05 vs. sham; #p<0.05 vs. vehicle-administered tMCAO group.
DISCUSSION
LPA5 has been recently identified as a novel pathogenic factor for acute brain injuries following ischemic stroke (Sapkota et al., 2020). Its antagonism can lead to neuroprotection for acute brain injuries, along with attenuation of neuroinflammatory responses including activation of microglia and production of pro-inflammatory cytokines in post-ischemic brains (Sapkota et al., 2020). In the current study, we demonstrated that suppressing LPA5 activity with TCLPA5 could provide long-term neuroprotection against chronic brain injuries following ischemic stroke based on the findings that TCLPA5 administration significantly attenuated neurological functional deficit, brain tissue loss, and neural cell death at 21 days after tMCAO challenge. In fact, validation of long-term neuroprotection in experimental models of ischemic stroke is believed to be important for developing drugs to treat ischemic stroke. Although most of neuroprotective compounds have demonstrated their effects in experimental models of ischemic stroke, they are unexpectedly less effective in clinical settings (Gladstone et al., 2002; Schmidt-Pogoda et al., 2020). This discrepancy might due to various factors, including efficacy validation only at an acute phase of ischemic stroke, drug treatment strategy, and publication bias (Frechou et al., 2019; Schmidt-Pogoda et al., 2020). In particular, efficacy validation at a chronic phase of ischemic stroke must be done to address whether the neuroprotective effects observed at the acute phase with experimental treatments could persist for a long time after cerebral ischemic challenge. Results of a previous study (Sapkota et al., 2020) and the current study strongly indicate that suppressing LPA5 activity is neuroprotective in mice challenged with ischemic stroke.
Accumulating evidences have suggested that neurogenesis and angiogenesis can improve neurological dysfunction, including motor function, cognitive impairment, and memory in the chronic phase of ischemic stroke (Freret et al., 2006; Luo et al., 2007). It is well-defined that the number of new-born neurons could be increased in the SVZ of the lateral ventricle and the dentate gyrus of the hippocampus after an ischemic stroke and then migrate to injured areas for self-repair of injured areas following an ischemic challenge (Arvidsson et al., 2002). In addition, angiogenesis is regarded as a self-repair event in an injured brain following an ischemic challenge (Ergul et al., 2012). Therefore, it has been suggested that promoting neurogenesis and angiogenesis could slow down disease progression and improve symptoms of ischemic stroke (Sun et al., 2003; Koh and Park, 2017). In fact, improving neurological functions has become an ultimate goal in the development of therapeutics to treat ischemic stroke. In the present study, long-term neuroprotective effects of LPA5 antagonism were demonstrated to be possibly associated with enhanced neurogenesis and angiogenesis.
The current study also demonstrated that suppressing LPA5 activity with TCLPA5 could upregulate VEGF in the injured brain at 21 days after tMCAO challenge. VEGF is a well-known growth factor that is present in different brain cells, including endothelial cell, astrocyte, neuron, and microglia (Plate et al., 1999; Lee et al., 2009; Huang et al., 2016; Shen et al., 2016). It exerts neuroprotective activity against ischemic stroke by reducing brain infarct size, improving neurological functions, and enhancing brain recovery through multiple mechanisms (Zhang et al., 2000; Sun et al., 2003; Greenberg and Jin, 2013). In particular, enhanced brain recovery by VEGF can be closely associated with neurogenesis and angiogenesis in a post-ischemic brain (Sun et al., 2003; Greenberg and Jin, 2013). VEGF can regulate angiogenesis by different actions such as enhancing proliferation, migration, and survival of new-born endothelial cells in neonatal ischemic stroke and other experimental models for studying angiogenesis (Flamme et al., 1995; Caron et al., 2009; Shimotake et al., 2010; Kinugasa et al., 2012; Claesson-Welsh and Welsh, 2013). VEGF can also regulate neurogenesis by enhancing axonal outgrowth, proliferation of progenitor cells and their survival in the SVZ, and migration of new-born neurons in various experimental models including ischemic stroke (Jin et al., 2002; Sun et al., 2003; Jin et al., 2006; Li et al., 2011). Therefore, the long-term efficacy of TCLPA5 in tMCAO-challenged mice might be due to production of VEGF which could then lead to increased functional recovery via enhanced neurogenesis and angiogenesis in ischemic stroke. Other than VEGF, it might be possible that other types of neurotrophic factors, such as nerve growth factor, insulin growth factor, and brain-derived neurotrophic factor, contribute to the observed TCLPA5-enhaced neurogenesis and/or angiogenesis because they have also been reported as regulators of, at least, neurogenesis in post-ischemic brain (Schabitz et al., 2007; Zhu et al., 2009; Yang et al., 2020). Therefore, such issue might be tempting to be addressed in the future independent studies.
In summary, we demonstrated that suppressing LPA5 activity with TCLPA5 could provide long-term neuroprotection to mice challenged with ischemic stroke based on the findings that neuroprotective effects of LPA5 antagonism could persist up to 21 days after tMCAO challenge. Regarding its underlying mechanisms, we demonstrated that TCLPA5 administration could enhance neurogenesis and angiogenesis in post-ischemic brain possibly via VEGF upregulation. Considering that suppressing LPA5 activity with TCLPA5 can provide neuroprotection against both acute (Sapkota et al., 2020) and chronic (the current study) phases of ischemic stroke, targeting LPA5 could be a promising strategy for drug development to treat ischemic stroke.
ACKNOWLEDGMENTS
This work was supported by grants from the National Research Foundation (NRF) funded by the Korean government to JWC [NRF-2020R1F1A1067154 and NRF-2020R1A6A1A03043708]. We thank CH Lee for providing assistance for a blind determination.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interests.
REFERENCES
- Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Caron C., Spring K., Laramee M., Chabot C., Cloutier M., Gu H., Royal I. Non-redundant roles of the Gab1 and Gab2 scaffolding adapters in VEGF-mediated signalling, migration, and survival of endothelial cells. Cell. Signal. 2009;21:943–953. doi: 10.1016/j.cellsig.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Chen J., Sanberg P. R., Li Y., Wang L., Lu M., Willing A. E., Sanchez-Ramos J., Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Choi J. W., Chun J. Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta. 2013;1831:20–32. doi: 10.1016/j.bbalip.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L., Welsh M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013;273:114–127. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- Ergul A., Alhusban A., Fagan S. C. Angiogenesis: a harmonized target for recovery after stroke. Stroke. 2012;43:2270–2274. doi: 10.1161/STROKEAHA.111.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamme I., Breier G., Risau W. Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev. Biol. 1995;169:699–712. doi: 10.1006/dbio.1995.1180. [DOI] [PubMed] [Google Scholar]
- Frechou M., Margaill I., Marchand-Leroux C., Beray-Berthat V. Behavioral tests that reveal long-term deficits after permanent focal cerebral ischemia in mouse. Behav. Brain Res. 2019;360:69–80. doi: 10.1016/j.bbr.2018.11.040. [DOI] [PubMed] [Google Scholar]
- Freret T., Chazalviel L., Roussel S., Bernaudin M., Schumann-Bard P., Boulouard M. Long-term functional outcome following transient middle cerebral artery occlusion in the rat: correlation between brain damage and behavioral impairment. Behav. Neurosci. 2006;120:1285–1298. doi: 10.1037/0735-7044.120.6.1285. [DOI] [PubMed] [Google Scholar]
- Gaire B. P., Sapkota A., Song M. R., Choi J. W. Lysophosphatidic acid receptor 1 (LPA1) plays critical roles in microglial activation and brain damage after transient focal cerebral ischemia. J. Neuroinflammation. 2019;16:170. doi: 10.1186/s12974-019-1555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard S., Murray K. N., Rothwell N. J., Metz G. A., Allan S. M. Long-term functional recovery and compensation after cerebral ischemia in rats. Behav. Brain Res. 2014;270:18–28. doi: 10.1016/j.bbr.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone D. J., Black S. E., Hakim A. M. Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery , author. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.STR.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Greenberg D. A., Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell. Mol. Life Sci. 2013;70:1753–1761. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Cao W., Deng Y., Zhu G., Han Y., Zeng H. Hypertonic saline alleviates experimentally induced cerebral oedema through suppression of vascular endothelial growth factor and its receptor VEGFR2 expression in astrocytes. BMC Neurosci. 2016;17:64. doi: 10.1186/s12868-016-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Mao X. O., Greenberg D. A. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J. Neurobiol. 2006;66:236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- Jin K., Zhu Y., Sun Y., Mao X. O., Xie L., Greenberg D. A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinugasa M., Amano H., Satomi-Kobayashi S., Nakayama K., Miyata M., Kubo Y., Nagamatsu Y., Kurogane Y., Kureha F., Yamana S., Hirata K., Miyoshi J., Takai Y., Rikitake Y. Necl-5/poliovirus receptor interacts with VEGFR2 and regulates VEGF-induced angiogenesis. Circ. Res. 2012;110:716–726. doi: 10.1161/CIRCRESAHA.111.256834. [DOI] [PubMed] [Google Scholar]
- Koh S. H., Park H. H. Neurogenesis in stroke recovery. Transl. Stroke Res. 2017;8:3–13. doi: 10.1007/s12975-016-0460-z. [DOI] [PubMed] [Google Scholar]
- Lee H. T., Chang Y. C., Tu Y. F., Huang C. C. VEGF-A/VEGFR-2 signaling leading to cAMP response element-binding protein phosphorylation is a shared pathway underlying the protective effect of preconditioning on neurons and endothelial cells. J. Neurosci. 2009;29:4356–4368. doi: 10.1523/JNEUROSCI.5497-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. L., Fraser J. L., Yu S. P., Zhu J., Jiang Y. J., Wei L. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exp. Brain Res. 2011;214:503–513. doi: 10.1007/s00221-011-2849-y. [DOI] [PubMed] [Google Scholar]
- Luo C. X., Jiang J., Zhou Q. G., Zhu X. J., Wang W., Zhang Z. J., Han X., Zhu D. Y. Voluntary exercise-induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from stroke. J. Neurosci. Res. 2007;85:1637–1646. doi: 10.1002/jnr.21317. [DOI] [PubMed] [Google Scholar]
- Palma-Tortosa S., Garcia-Culebras A., Moraga A., Hurtado O., Perez-Ruiz A., Duran-Laforet V., Parra J., Cuartero M. I., Pradillo J. M., Moro M. A., Lizasoain I. Specific features of SVZ neurogenesis after cortical ischemia: a longitudinal study. Sci. Rep. 2017;7:16343. doi: 10.1038/s41598-017-16109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate K. H., Beck H., Danner S., Allegrini P. R., Wiessner C. Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J. Neuropathol. Exp. Neurol. 1999;58:654–666. doi: 10.1097/00005072-199906000-00010. [DOI] [PubMed] [Google Scholar]
- Sapkota A., Lee C. H., Park S. J., Choi J. W. Lysophosphatidic acid receptor 5 plays a pathogenic role in brain damage after focal cerebral ischemia by modulating neuroinflammatory responses. Cells. 2020;9:1446. doi: 10.3390/cells9061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabitz W. R., Steigleder T., Cooper-Kuhn C. M., Schwab S., Sommer C., Schneider A., Kuhn H. G. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- Schmidt-Pogoda A., Bonberg N., Koecke M. H. M., Strecker J. K., Wellmann J., Bruckmann N. M., Beuker C., Schabitz W. R., Meuth S. G., Wiendl H., Minnerup H., Minnerup J. Why most acute stroke studies are positive in animals but not in patients: a systematic comparison of preclinical, early phase, and phase 3 clinical trials of neuroprotective agents. Ann. Neurol. 2020;87:40–51. doi: 10.1002/ana.25643. [DOI] [PubMed] [Google Scholar]
- Shen S. W., Duan C. L., Chen X. H., Wang Y. Q., Sun X., Zhang Q. W., Cui H. R., Sun F. Y. Neurogenic effect of VEGF is related to increase of astrocytes transdifferentiation into new mature neurons in rat brains after stroke. Neuropharmacology. 2016;108:451–461. doi: 10.1016/j.neuropharm.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Shimotake J., Derugin N., Wendland M., Vexler Z. S., Ferriero D. M. Vascular endothelial growth factor receptor-2 inhibition promotes cell death and limits endothelial cell proliferation in a neonatal rodent model of stroke. Stroke. 2010;41:343–349. doi: 10.1161/STROKEAHA.109.564229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Jin K., Xie L., Childs J., Mao X. O., Logvinova A., Greenberg D. A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest. 2003;111:1843–1851. doi: 10.1172/JCI200317977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Li J., Duan Y., Tao Z., Zhao H., Luo Y. Effects of erythropoietin on gliogenesis during cerebral ischemic/reperfusion recovery in adult mice. Aging Dis. 2017;8:410–419. doi: 10.14336/AD.2016.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Wu S., Hou L., Zhu D., Yin S., Yang G., Wang Y. Therapeutic effects of simultaneous delivery of nerve growth factor mrna and protein via exosomes on cerebral ischemia. Mol. Ther. Nucleic Acids. 2020;21:512–522. doi: 10.1016/j.omtn.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. G., Zhang L., Jiang Q., Zhang R., Davies K., Powers C., Bruggen N., Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Fan Y., Hao Q., Shen F., Hashimoto T., Yang G. Y., Gasmi M., Bartus R. T., Young W. L., Chen Y. Postischemic IGF-1 gene transfer promotes neurovascular regeneration after experimental stroke. J. Cereb. Blood Flow Metab. 2009;29:1528–1537. doi: 10.1038/jcbfm.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]