Abstract
Liver fibrosis constitutes a significant health problem worldwide due to its rapidly increasing prevalence and the absence of specific and effective treatments. Growing evidence suggests that apoptosis-signal regulating kinase 1 (ASK1) is activated in oxidative stress, which causes hepatic inflammation and apoptosis, leading to liver fibrogenesis through a mitogen-activated protein kinase (MAPK) downstream signals. In this study, we investigated whether selonsertib, a selective inhibitor of ASK1, shows therapeutic efficacy for liver fibrosis, and elucidated its mechanism of action in vivo and in vitro. As a result, selonsertib strongly suppressed the growth and proliferation of hepatic stellate cells (HSCs) and induced apoptosis by increasing Annexin V and TUNEL-positive cells. We also observed that selonsertib inhibited the ASK1/MAPK pathway, including p38 and c-Jun N-terminal kinase (JNK) in HSCs. Interestingly, dimethylnitrosamine (DMN)-induced liver fibrosis was significantly alleviated by selonsertib treatment in rats. Furthermore, selonsertib reduced collagen deposition and the expression of extracellular components such as α-smooth muscle actin (α-SMA), fibronectin, and collagen type I in vitro and in vivo. Taken together, selonsertib suppressed fibrotic response such as HSC proliferation and extracellular matrix components by blocking the ASK1/MAPK pathway. Therefore, we suggest that selonsertib may be an effective therapeutic drug for ameliorating liver fibrosis.
Keywords: Liver fibrosis, ASK1, Selonsertib, MAPK
INTRODUCTION
Liver fibrosis is the most common chronic liver disease, which occurs due to various reasons such as viral infection, alcohol abuse, and nonalcoholic steatohepatitis (NASH) (Bataller and Brenner, 2005). These factors give rise to hepatocyte injury and subsequently, the deposition of extracellular matrix components (ECM) (Arriazu et al., 2014). In particular, hepatic stellate cells (HSCs) are the major cell type responsible for the increased deposition of ECM during liver fibrosis (Higashi et al., 2017). Activated HSCs with a high proliferative index release pro-fibrogenic cytokines, and, consequently produce ECM-related molecules such as α-smooth muscle actin (α-SMA) and, collagen type I. Eventually, the progression of liver fibrosis through activation of HSCs results in liver cirrhosis and hepatocellular carcinoma (HCC), which are significant causes of mortality and morbidity (Kanda et al., 2019). However, despite many efforts to manage chronic liver diseases such as liver fibrosis, the development of effective anti-fibrotic therapies remains clinically relevant issue. Furthermore, considering that the molecular mechanism of hepatic fibrosis is poorly understood, effective therapies to target molecules that cause hepatic fibrosis are urgently needed.
Apoptosis-signal regulating kinase 1 (ASK1) is known as a member of mitogen-activated protein kinase family (MAPK), which is involved in severe human diseases including inflammatory diseases, cancer, and liver diseases (Noureddin et al., 2016). ASK1 can be activated by various stimuli, including reactive oxygen species (ROS), lipopolysaccharides (LPS), and cytokines (Hayakawa et al., 2012). Subsequently, activated ASK1 selectively activates the c-Jun N-terminal kinase (JNK) and p38 signaling pathways (Ichijo et al., 1997). In turn, activated JNK and p38 MAPK modulate proliferation and inflammation to maintain cellular integrity (Tobiume et al., 2001). Particularly, ASK1 is proved to be a major upstream player in the regulation of JNK and p38 activities (Amos et al., 2018). Recently, ASK1 has been reported to play a role as a pathological contributor in various diseases, especially liver diseases, attracting attention as a therapeutic target. For instance, ASK1 increases acetaminophen-induced liver injury by inducing JNK activation (Nakagawa et al., 2008). Further, ASK1 accelerates liver fibrosis and hepatocyte apoptosis in the NASH model (Kanda et al., 2018). Moreover, overexpression of ASK1 induces activation and proliferation of HSCs by increasing the phosphorylation of p38 and JNK (Cuenda and Rousseau, 2007). As ASK1 has been known to be a critical driving force for the progression of liver fibrosis in clinical investigations, drug development aiming to target ASK1 for the treatment of advanced liver diseases is of significant importance.
Selonsertib is a first-in-class, small molecule inhibitor of ASK1, which is characterized by anti-fibrotic and anti-inflammatory properties, was in clinical trials for the treatment of NASH (Loomba et al., 2018). It competitively attaches to the catalytic domain of ATP and thereby inhibits kinase activities of ASK1. Currently, studies on the effects and functions of selonsertib in the liver fibrosis model are not available, although ASK1 is an attractive target molecule to be involved in liver diseases. Accordingly, in this study, we evaluated the anti-fibrotic effect of selonsertib in liver fibrosis model along with the underlying mechanisms in vitro and in vivo.
MATERIALS AND METHODS
Cell culture
HSC-T6 and LX-2 cell lines, immortalized human HSCs were donated from Professor S. L. Friedman (Liver Disease Research Center of San Francisco General Hospital, CA, USA). The cells were routinely cultured with complete Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Rockville MD, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco) and 1% antibiotic/antimycotic solution. The cultures were maintained at 37°C in a CO2 incubator with a controlled humidified atmosphere composed of 95% air and 5% CO2.
Assessment of the cell viability using an MTT assay
Cells were seeded at a confluence of 3−5×103 cells/well in 96-well plates and incubated for 24 h. Subsequently, the media were discarded and the cells were treated with either DMSO or diverse concentrations (0.5-100 μM) of selonsertib. After 48 h, 10% of an MTT solution (2 mg/mL) was added to each well, and then the cells were incubated for another four h at 37°C. The formed formazan crystals were dissolved in DMSO (200 μL/well) shaking for 5-10 min. Absorbance was then measured by a microplate reader at 540 nm. Quadruplet replicated wells were evaluated for each analysis.
Cell proliferation assay
Proliferation of cells was demonstrated by growth curves using JULITM Stage real-time image recording system. This program automatically captured cell morphologies in every configured time point through equipped camera in the CO2 incubator, which quantified and graphed data.
Annexin V and PI staining
Annexin V and propidium iodide (PI) staining was carried out using Annexin V-APC apoptosis detection kit I (BD pharmingenTM, San Diego, CA, USA). HSC-T6 and LX-2 cells (2×105 cells/mL) were exposed to selonsertib (10-50 μM) for 48 h, and then cellular apoptosis was detected by fluorescent staining. Prepared cells were incubated in Annexin V-APC and PI solution for 15 min in the dark, and the stained cells were instantly analyzed using a FACS verse flow cytometer (BD Biosciences, San Jose, CA, USA).
Terminal deoxynucleotidyl transferase dUTP mediated nick end labeling (TUNEL) assay
Assays were conducted with the ApopTag® peroxidase in situ apoptosis detection kit (Merck Millipore, Burlington, MA, USA). HSC-T6 and LX-2 cells were seeded on 18-mm cover glasses as 70% confluence in DMEM for 24 h. The cells were treated with selonsertib (10-50 μM) for 24 or 48 h, then fixed in ice-cold acetic acid, ethanol mixture, and washed thrice with PBS. According to the kit protocol, the terminal deoxynucleotidyl transferase (TdT) enzyme was activated, followed by stopping the reaction and the attaching digoxigenin-conjugate antibody overnight. After washing, 3,3’-diaminobenzidine (DAB) staining was applied to visualize the apoptotic cell population.
Western blotting
HSC-T6 and LX-2 cells were washed with DPBS and lysed with RIPA buffer (Biosesang, Seongnam, Korea) containing 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl (pH 7.5), and 2 mM EDTA (pH 8.0). Additionally, Xpert protease inhibitor and phosphatase inhibitor Cocktail (genDEPOT, Katy, TX, USA) were mixed with the buffer, followed by centrifugation at 15,000 rpm for 30 min. Approximately 30 μg of total protein lysate was separated in SDS-polyacrylamide gel electrophoresis (PAGE), which was then transferred to polyvinylidene fluoride (PVDF) membrane using the wet transfer kit (Bio-Rad Laboratories, Berkeley, CA, USA). The membrane was blocked with 5% skim milk in PBS and 0.5% of Tween 20 (PBS-T), followed by overnight incubation at 4°C with primary antibodies that were diluted in PBS-T containing 5% BSA. Anti-p-ASK1 (catalog no. 3765S), anti-p-p38 (catalog no. 4631S), anti-p-JNK (catalog no. 9251S), and anti-JNK (catalog no. 9252S) were purchased from Cell Signaling Technology (Beverly, MA, USA); anti-ASK1 (catalog no. 107921) from Genetex (Alton Pkwy Irvine, CA, USA); anti-p38 (catalog no. 4631S) from Santa Cruz Biotechnology (Dallas, TX, USA); anti-collagen I (catalog no. ab34710), anti-fibronectin (catalog no. ab2413) from Abcam (Cambridge, UK); anti-α-SMA (catalog no. F3777), anti-β-Actin (catalog no. A5441) from Sigma-Aldrich (St. Louis, MO, USA). The secondary antibodies were from Cell Signaling Technology, and the blots were visualized using an enhanced chemiluminescence system (Bio-Rad Laboratories).
Immunofluorescence assay
HSC-T6 and LX-2 cells were seeded on 18-mm cover glasses in DMEM at confluence of 1×105 cells/well and incubated for 24 h. The cells were then treated with selonsertib for 12-24 h and then rinsed with PBS, followed by fixation in ice-cold acetic acid/ethanol mixture. After washing thrice, the fixed cells were incubated for 1 h in CAS blocking solution (Life Technology, Seoul, Korea) to prevent non-specific binding of antibodies, and then subjected to the incubation with indicated primary antibodies at 4°C overnight. Next, after washing, the cells were cross-reacted with Alexa 488 and 594-conjugated goat anti-mouse or anti-rabbit secondary antibodies for 1 h at room temperature. Nuclei were counterstained with 4’,6’-diamidino-2-phenylindole (DAPI) in the dark for 30 min at room temperature. The slides were sufficiently rinsed, then mounted with fluorescence mounting medium (DAKO, CA, USA). All the samples were examined with a confocal laser microscope (Olympus, Tokyo, Japan) at 488 and 568 nm.
Animal study
Animal care and experiments were accomplished in compliance with the Guide for Animal Experiments edited by the INHA Institutional Animal Care and Use Committee (INHA IACUC 190315-628) of the Medical School of Inha University. The animals were housed in an air-conditioned room at 25°C with a dark/light cycle for 12 h. All animals received humane care during the study with unlimited access to food and water. Forty male six week-old Sprague-Dawley rats were obtained from Orient-Bio Laboratory Animal Research Center Co., Ltd. (Seongnam, Korea), and were divided into four groups (control, dimethylnitrosamine (DMN), selonsertib 10 or 50 mg/kg). Liver fibrosis was induced by intraperitoneal injections of 10 mg/kg DMN thrice a week for four weeks. Selonsertib dissolved in 0.5% methylcellulose (MC) was administered orally for five days a week for three weeks. Control animals were treated with only vehicles; saline and 0.5% MC solution equivalently. After the final administration, all rats were sacrificed under anesthesia with ketamine.
Histopathological analysis
Liver sections were fixed in 10% formaldehyde solution, and then processed using a paraffin embedding manner. Sections about 4 μm thick were stained with hematoxylin and eosin (H&E) for routine histology, and Sirius Red staining for collagen type I/III. The degree of hepatic fibrosis was evaluated by the Knodell scores of fibrosis, which is a four-grade derived from the Metavir scoring method; the area of fibrosis was measured by image analysis. The histopathological scores were as follows: 0, no fibrosis; I, perivenular or pericellular fibrosis; II, septal fibrosis; III, incomplete cirrhosis; and IV, complete cirrhosis. The degree of fibrosis was estimated as the mean of several fields.
Cytokine assay
Fresh sera were immediately separated from the blood of sacrificed rats. Cytokine levels of IFN-γ and TNF-α were measured by sandwich enzyme-linked immunoassay (ELISA). Briefly, this examination was conducted using the appropriate capture and detection antibodies (R&D system, Minneapolis, MN, USA), which is applied with a visualizing system of streptavidin-conjugated HRP and TMB microwell peroxidase substrate (Vector Laboratories, CA, USA).
Immunohistochemistry assay
Immunohistochemical staining analysis was performed using formalin-fixed and deparaffinized tissue sections as previously described (Ikejima et al., 2002; Lee et al., 2011). After blocking for 1 h, the primary antibodies were incubated at 4°C for overnight; anti-p-ASK1, anti-collagen I, anti-fibronectin, anti-α-SMA. Anti-TGF-β (catalog no. SAB4502954) and anti-vimentin (catalog no. V2258) were purchased from Sigma-Aldrich, and MMP-2 (catalog no. SC-13595) was from Santa Cruz Biotechnology. After removing non-specific antibody with PBS, the sections were incubated with the secondary antibody at room temperature for 1 h. The samples were reacted by avidin biotin-peroxidase complex (ABC) solution kit (Vector Laboratories) for 30 min, and then washed in PBS, final visualization was performed using a DAB substrate and counterstaining with hematoxylin.
Statistical analysis
Data were expressed as mean ± SD, and statistical analysis was performed using an ANOVA and an unpaired Students t-test. p-value of <0.05 or <0.01 was taken to represent statistical significance. Statistical calculations were conducted with SPSS software for Windows operating system (version 10.0: SPSS, Chicago, IL, USA).
RESULTS
Selonsertib inhibits the proliferation and activation of HSCs
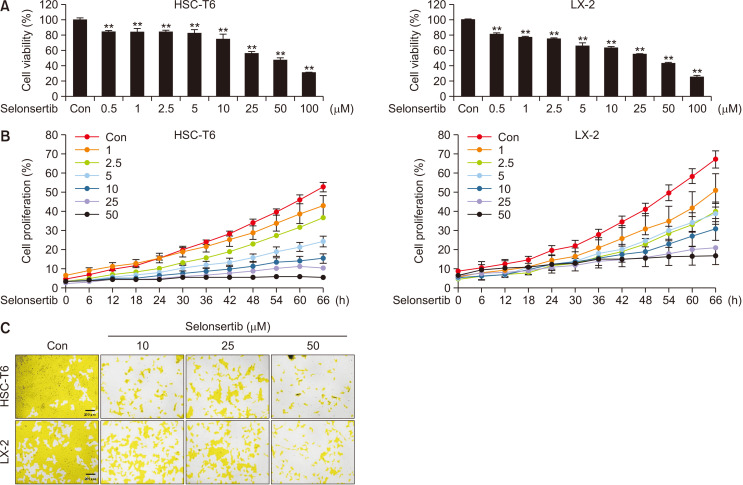
To investigate the effect of seonsertib on the proliferation/growth of HSCs, two cell lines (HSC-T6 and LX-2) were exposed to various concentrations of selonsertib for 48 h. Selonsertib treatment decreased cell viability in both HSC lines in a dose-dependent manner (Fig. 1A). Furthermore, the selonsertib treatment significantly and dose-dependently decreased the proliferative ability of HSCs and caused changes in the cell morphologies. Notably, it inhibited cell growth by 80-90% at a concentration of 50 μM (Fig. 1B, 1C).
Fig. 1.
Effect of selonsertib on the proliferation of hepatic stellate cells (HSCs). (A) Cytotoxic effect of selonsertib on HSC-T6 and LX-2 cells was assessed using an MTT assay. The HCSs were seeded in 96-well culture plates. After incubating for 1 day, HSCs were treated with various concentrations of selonsertib (0.5-100 μM) for 24 or 48 h and then MTT assay was performed. Each value is represented as the mean ± SD (n=4). (B, C) Effect of selonsertib on HSC proliferation was estimated using JULITM stage real-time cell recorder. Distinct areas in each group were imaged and counted in quadruplicate and expressed as a mean percentage of cell density. **p<0.01 versus the control.
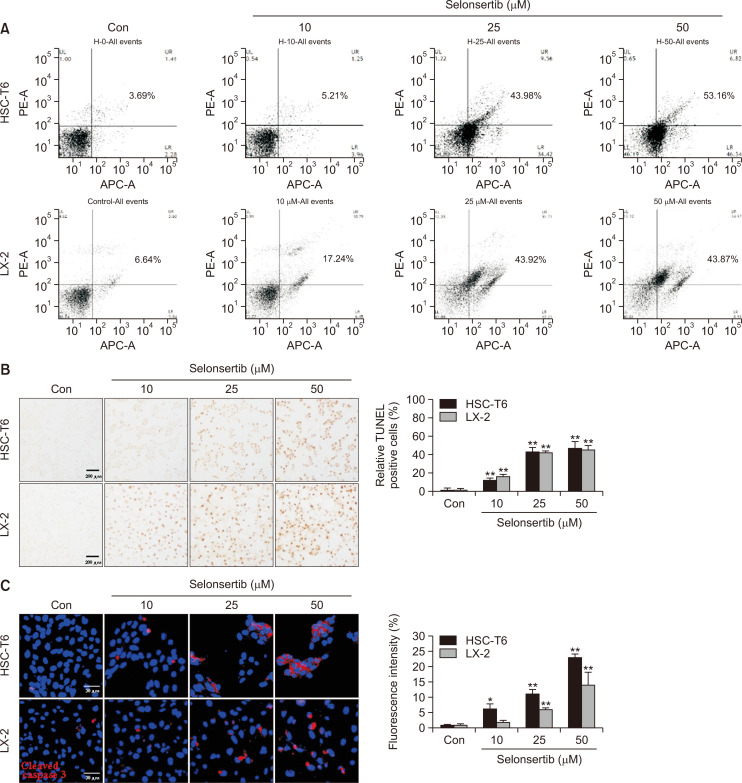
Selonsertib induces HSC apoptosis
Apoptotic cell death by selonsertib was evaluated by double staining with Annexin V-APC and PI using flow cytometry. Subsequent detection of cellular changes was analyzed after treatment with 10, 25, and 50 µM of selonsertib. As shown in Fig. 2A, the distribution of the cell population toward apoptosis was gradually increased in proportion to the concentration. We also identified the apoptotic effect of selonsertib by characterizing nuclear morphology with TUNEL staining. Selonsertib induced acquisition of the morphological features of apoptotic cells such as nuclear condensation and the formation of perinuclear apoptotic bodies in a dose-dependent manner (Fig. 2B). Additionally, the expression of cleaved caspase-3 (red) was increased by selonsertib treatment in both cell lines, which was assessed by quantification (Fig. 2C). These results indicated that selonsertib prevented HSC activation through induction of apoptotic cell death.
Fig. 2.
Effect of selonsertib on HSC apoptosis. (A) HSC-T6 and LX-2 cells were treated with various concentrations of selonsertib (10-50 μM) for 48 or 72 h. The HSCs were double-stained with annexin V-APC and propidium iodide (PI) and analyzed by the FACS-verse flow cytometer. Annexin V-APC events represent apoptotic cells for early stage (low right quadrants) and late-stage (upper right quadrants). The analysis was conducted with two independent experiments. (B) The induction of apoptosis by selonsertib (10-50 μM) was observed by TUNEL staining (200× magnification). Data are represented by fold changes of TUNEL-positive cells. (C) Red fluorescence intensity from cleaved caspase-3 staining after the treatment of selonsertib. Data are shown as the fold changes compared to the control cells. All the values are presented as mean ± SD. *p<0.05 and **p<0.01 versus the control.
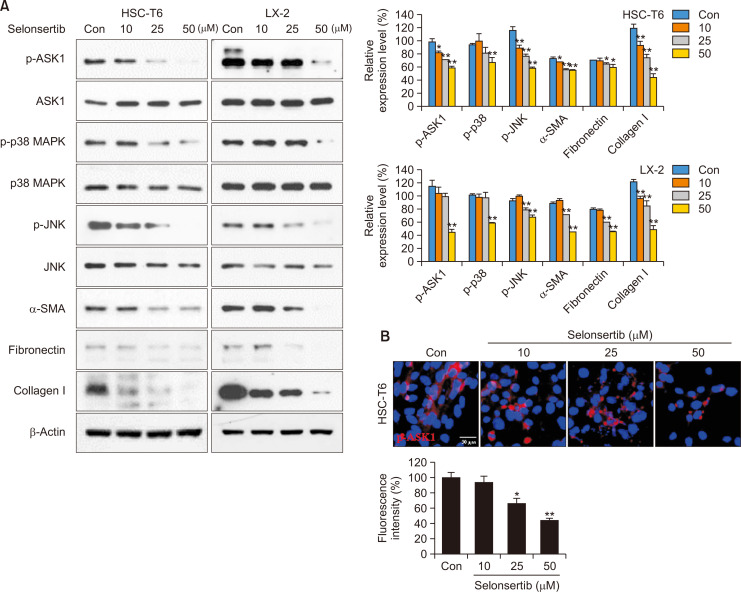
Selonsertib inhibits fibrotic response by ASK1/MAPK signaling
ASK1 is closely related to the induction of liver disease through activation of the MAPK signaling pathway, including p38 and JNK (Kovalic et al., 2018). In particular, p38 and JNK accelerate hepatic fibrosis by increasing ECM accumulation (Kluwe et al., 2010). Therefore, we investigated whether selonsertib, ASK1 selective inhibitor, would decrease the expression of fibrosis-related molecules via MAPK signaling in HSCs. As shown in Fig. 3A, selonsertib suppressed phosphorylation of ASK1 and its downstream p38/JNK MAPK signaling in HSCs, which was confirmed by immunofluorescence assay (Fig. 3B). Additionally, selonsertib inhibited the expression of α-SMA, collagen I, and fibronectin.
Fig. 3.
Effect of selonsertib on ASK1/MAPK signaling and expression of fibrogenic proteins in HSCs. (A) HSCs were treated with selonsertib at various concentrations (10-50 μM) for 24 or 48 h. Western blotting for ASK1/MAPKs and ECM-related signals was performed with cell lysates obtained from selonsertib-treated cells. (B) Immunofluorescence staining to examine the effect of selonsertib on the expression of p-ASK1. The images are presented at 400× magnification. Data are represented as the mean ± SD (n=4). *p<0.05 and **p<0.01 versus the control.
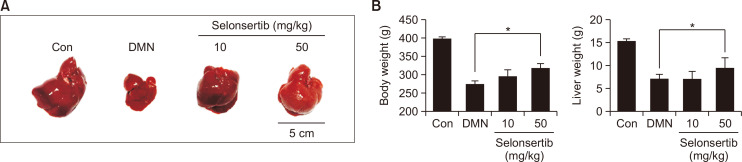
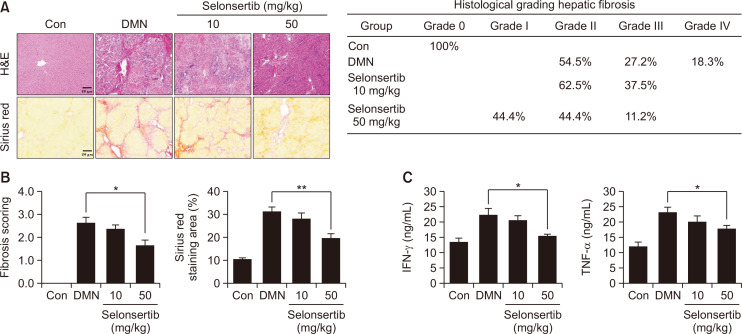
Selonsertib improves DMN-induced liver fibrosis in rats
The effects of selonsertib on the body weight and liver weight of the rats are shown in Fig. 4. Treatment with DMN caused a significant decrease in both body weight and liver weight compared to control group. However, oral administration of selonsertib (50 mg/kg) decreased the loss by 20-30% (Fig. 4). Moreover, liver fibrosis induced by DMN in rats was accessed by two histological methods: H&E and Picro-Sirius Red staining. Liver tissues of the control group showed typical architecture, whereas the DMN group displayed extensive and severe hemorrhagic necrosis, disruption of tissue architecture, along with infiltration of inflammatory cells. These alterations were remarkably reduced in the selonsertib group. Moreover, Picro-Sirius Red staining was conducted to identify collagen accumulation. In the control group, collagen was observed only in the periportal area, but the DMN group showed increased collagen and displayed bundles of collagen fibers surrounding the lobules, forming large fibrous septa. The thickening of these collagen fiber bundles was markedly reduced in the selonsertib group (Fig. 5A, 5B). The histopathological fibrosis scores and Sirius red staining index confirmed that the liver fibrosis was significantly reduced by selonsertib treatment, as compared to those in the DMN group. Besides, selonsertib decreased the levels of IFN-γ and TNF-α in the serum of DMN-treated rats (Fig. 5C).
Fig. 4.
Effect of selonsertib on DMN-induced liver fibrosis rat models. Selonsertib was administered orally five times to the rats with DMN-induced liver fibrosis (each group, n=10). (A) Macroscopic appearance of liver; control (CON) group, Sprague-Dawley (SD) rats were injected with PBS intraperitoneally; DMN-induced fibrosis (DMN) group, SD rats were injected with DMN (10 mg/kg) intraperitoneally thrice a week for three weeks; Selonsertib group, SD rats were injected with DMN and administered with selonsertib (10 or 50 mg/kg) containing 0.5% methylcellulose. (B) The body weight gain (g) was measured daily. Relative liver weight (g/100 g of body weight) was examined after sacrifice. All data are presented as the mean ± SD. (n=10). *p<0.05 versus the control.
Fig. 5.
Effect of selonsertib on fibrogenic progression in rat models. (A) Liver fibrosis was assessed by H&E and Sirius Red staining (200× magnification). (B) The data are expressed statistically from Sirius Red staining area and decision for the official scoring criteria of liver fibrosis. (C) Effects of selonsertib on inflammatory markers in rats with DMN-induced liver injury. Data are presented as the mean ± SD. (n=10). *p<0.05 and **p<0.01 versus the DMN group.
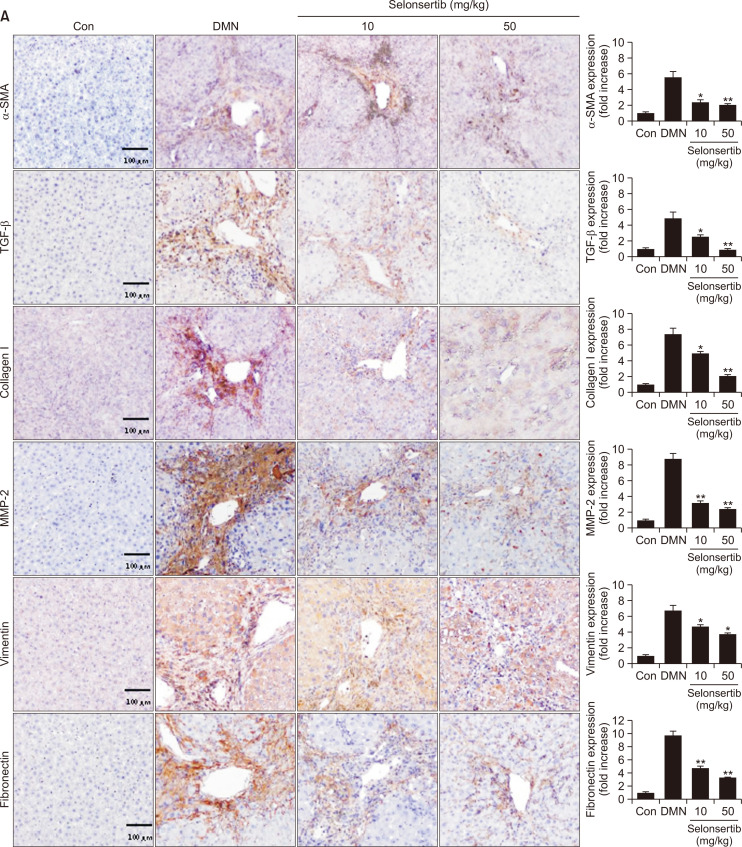
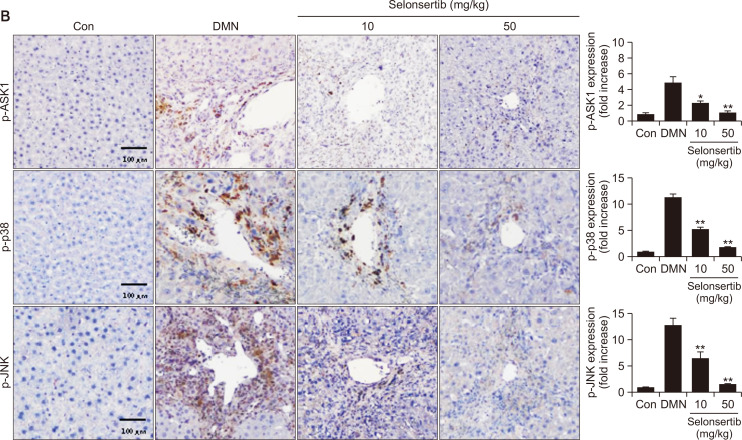
Selonsertib inhibits ECM accumulation and ASK1/MAPK signaling in DMN-induced liver fibrosis model
We further determined whether selonsertib could inhibit the expression of profibrotic mediators such as α-SMA and collagen by suppression of ASK1 phosphorylation in DMN-induced liver fibrosis model (Fig. 6). The DMN group showed high expression levels of α-SMA, collagen, vimentin, and fibronectin in the periportal fibrotic areas, central veins, portal tracks, and fibrous septa. However, these fibrosis-related markers were weakly expressed in the selonsertib-treatment groups. Additionally, selonsertib inhibited the expression of matrix metalloprotease (MMP), which plays an important role in mediating HSC proliferation and activation, potentially by regulating ECM turnover. Furthermore, we investigated whether selonsertib inhibited ASK1/MAPK signaling. Expression of p-ASK1, p-p38, and p-JNK were amplified by DMN induction, whereas it was significantly diminished after selonsertib treatment (10 and 50 mg/kg). Taken together, our results demonstrated that selonsertib exerts potent effects against DMN-induced liver fibrosis models.
Fig. 6.
Effect of selonsertib on expression of fibrogenic proteins and the ASK1/MAPK signaling in rat animal models. (A) Expression of ECM-related proteins in liver tissue of mice with liver fibrosis. (B) Expression of p-ASK1, p-p38, and p-JNK in liver tissue of mice with liver fibrosis. Data are presented as the mean ± SD. (n=4). *p<0.05 and **p<0.01 versus the DMN group.
DISCUSSION
Liver fibrosis is a common consequence of chronic liver injury, leading to cirrhosis and hepatocellular carcinoma (Baffy, 2013). Activation of HSCs plays an essential role in this process, which is characterized by fibrogenic potential and high proliferative index (Zhang et al., 2016). The ASK1 pathway has been shown to mediate and promote chronic liver fibrosis from NASH (Loomba et al., 2018). The importance of such signaling in the development of hepatic fibrogenesis through HSC activation and pro-fibrogenic mediators has been reported (Son et al., 2009). Therefore, inhibition of ASK1 signaling in HSCs has been in the scope of investigations as an emerging target for treating liver fibrosis. In our study, we evaluated the anti-fibrosis effect of an ASK1 inhibitor, selonsertib, and its underlying mechanisms of action in liver fibrosis. The results showed that it inhibited HSC growth and proliferation along with the induction of apoptosis. Moreover, acquired data demonstrated that selonsertib inhibited ASK1/MAPK signaling and fibrogenesis by decreasing ECM-related molecules in HSCs and DMN-induced liver fibrosis models. Our findings suggest that selonsertib is an anti-fibrotic agent that suppresses HSC activation via inhibition of ASK/MAPK signaling in liver fibrosis.
Since HSCs are the primary source of ECM that play a pivotal role in liver fibrogenesis, they attracted attention as critical target cells for antifibrotic therapy (Higashi et al., 2017). Decreasing the number of HSCs is considered as an essential strategy for clinical antifibrotic therapy, which can be achieved by inducing apoptosis of HSCs (Elsharkawy et al., 2005; Pellicoro et al., 2014; de Oliveira da Silva et al., 2017). Moreover, ASK1 is known to be associated with stress-induced apoptosis in inflammatory response (Hatai et al., 2000; Song et al., 2013). In this study, we first discovered that selonsertib significantly suppresses the viability and proliferation of HSCs. Additionally, selonsertib significantly induces the apoptosis of HSCs by increasing the late-phase population and Annexin V-stained cells. Furthermore, it increases expression of cleaved caspase-3, a typical marker of apoptosis. Previous studies have reported that some agents induced apoptosis of HSCs through caspases (Yu et al., 2014; Zhang et al., 2018), which is similar to our result. Accordingly, our study showed that selonsertib is associated with apoptosis, which is an important mechanism that alleviates liver fibrosis.
Based on these results, the effect of selonsertib on apoptosis was anticipated in HSCs and animal models with DMN-induced liver fibrosis. Several studies have reported that activated HSCs increase ECM-related proteins, and in turn, the ECM itself regulates cell proliferation and apoptosis (Priya and Sudhakaran, 2008; Wang et al., 2010), resulting in development of liver fibrosis. Among those, α-SMA, fibronectin and collagen type I are known as pro-fibrotic hallmarks, which play a key role in collagen synthesis and ECM deposition (Carpino et al., 2005). Additionally, recent studies have reported that ASK1 knock-out mice are protected from pathological organ remodeling and fibrosis by inhibiting ECM-related molecules (Liles et al., 2018). Therefore, we therefore determined whether selonsertib inhibited the expression of profibrotic factors such as α-SMA, vimentin, fibronectin, collagen I, MMP-2, and TGF-β. Selonsertib reduced the expression of these mediators in both HSCs and animal models with liver fibrosis. This is in line with previous study that ASK1 inhibition reduced expressions of fibrotic-related genes such as α-SMA in pulmonary fibrosis (Budas et al., 2018).
Our data also revealed that selonsertib inhibited the ASK1/MAPK signaling pathway by decreasing the expression of p-p38 and p-JNK in both HSCs and DMN-induced liver fibrosis models. Given that phosphorylated p38 and JNK are known to increase expression of collagen (Tsukada et al., 2005), it seems that selonsertib improved liver fibrosis by decreasing ECM production through inhibition of the ASK1/MAPK signaling pathway. These results were consistent with the study showing that GS-444217, another novel ASK1 inhibitor, blocks fibrogenic factors in kidney fibrosis models via inhibition of the ASK1/MAPK signaling pathway (Liles et al., 2018). Collectively, our results imply that the anti-fibrotic effect of selonsertib is mediated by ASK1/MAPK signaling. Furthermore, selonsertib is expected to confer anti-fibrotic and anti-inflammatory effects in various diseases with fibrosis.
In conclusion, the current study demonstrated that selonsertib markedly attenuated the development of liver fibrosis by blocking ASK1/MAPK signaling both in vitro and in vivo. Moreover, this inhibition is critical for the induction of HSC apoptosis, leading to the elimination collagen-producing cells in liver fibrosis. Therefore, we suggest that selonsertib treatment may be an efficient therapeutic approach for chronic hepatic fibrosis by decreasing amounts of ECM deposition.
ACKNOWLEDGMENTS
This research was supported by National Research Foundation of Korea (2018R1A2A1A05077263, 2019M3E5D1A020 69621, 2014009392).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
- Amos L. A., Ma F. Y., Tesch G. H., Liles J. T., Breckenridge D. G., Nikolic-Paterson D. J., Han Y. ASK1 inhibitor treatment suppresses p38/JNK signalling with reduced kidney inflammation and fibrosis in rat crescentic glomerulonephritis. J. Cell. Mol. Med. 2018;22:4522–4533. doi: 10.1111/jcmm.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriazu E., Ruiz, de Galarreta M., Cubero F. J., Varela-Rey M., Perez, de Obanos M. P., Leung T. M., Lopategi A., Benedicto A., Abraham-Enachescu I., Nieto N. Extracellular matrix and liver disease. Antioxid. Redox Signal. 2014;21:1078–1097. doi: 10.1089/ars.2013.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffy G. Hepatocellular carcinoma in non-alcoholic fatty liver disease: epidemiology, pathogenesis, and prevention. J. Clin. Transl. Hepatol. 2013;1:131–137. doi: 10.14218/JCTH.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R., Brenner D. A. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas G. R., Boehm M., Kojonazarov B., Viswanathan G., Tian X., Veeroju S., Novoyatleva T., Grimminger F., Hinojosa-Kirschenbaum F., Ghofrani H. A., Weissmann N., Seeger W., Liles J. T., Schermuly R. T. ASK1 inhibition halts disease progression in preclinical models of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2018;197:373–385. doi: 10.1164/rccm.201703-0502OC. [DOI] [PubMed] [Google Scholar]
- Carpino G., Morini S., Ginanni Corradini S., Franchitto A., Merli M., Siciliano M., Gentili F., Onetti Muda A., Berloco P., Rossi M., Attili A. F., Gaudio E. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig. Liver Dis. 2005;37:349–356. doi: 10.1016/j.dld.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Cuenda A., Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- De Oliveira, da Silva B., Ramos L. F., Moraes K. C. M. Molecular interplays in hepatic stellate cells: apoptosis, senescence, and phenotype reversion as cellular connections that modulate liver fibrosis. Cell Biol. Int. 2017;41:946–959. doi: 10.1002/cbin.10790. [DOI] [PubMed] [Google Scholar]
- Elsharkawy A. M., Oakley F., Mann D. A. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10:927–939. doi: 10.1007/s10495-005-1055-4. [DOI] [PubMed] [Google Scholar]
- Hatai T., Matsuzawa A., Inoshita S., Mochida Y., Kuroda T., Sakamaki K., Kuida K., Yonehara S., Ichijo H., Takeda K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J. Biol. Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- Hayakawa R., Hayakawa T., Takeda K., Ichijo H. Therapeutic targets in the ASK1-dependent stress signaling pathways. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012;88:434–453. doi: 10.2183/pjab.88.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T., Friedman S. L., Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo H., Nishida E., Irie K., ten Dijke P., Saitoh M., Moriguchi T., Takagi M., Matsumoto K., Miyazono K., Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Ikejima K., Takei Y., Honda H., Hirose M., Yoshikawa M., Zhang Y. J., Lang T., Fukuda T., Yamashina S., Kitamura T., Sato N. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122:1399–1410. doi: 10.1053/gast.2002.32995. [DOI] [PubMed] [Google Scholar]
- Kanda T., Goto T., Hirotsu Y., Moriyama M., Omata M. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: a review. Int. J. Mol. Sci. 2019;20:1358. doi: 10.3390/ijms20061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Matsuoka S., Yamazaki M., Shibata T., Nirei K., Takahashi H., Kaneko T., Fujisawa M., Higuchi T., Nakamura H., Matsumoto N., Yamagami H., Ogawa M., Imazu H., Kuroda K., Moriyama M. Apoptosis and non-alcoholic fatty liver diseases. World J. Gastroenterol. 2018;24:2661–2672. doi: 10.3748/wjg.v24.i25.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluwe J., Pradere J. P., Gwak G. Y., Mencin A., De Minicis S., Osterreicher C. H., Colmenero J., Bataller R., Schwabe R. F. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347–359. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalic A. J., Satapathy S. K., Chalasani N. Targeting incretin hormones and the ASK-1 pathway as therapeutic options in the treatment of non-alcoholic steatohepatitis. Hepatol. Int. 2018;12:97–106. doi: 10.1007/s12072-018-9854-1. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Lee H., Joung Y. K., Jung K. H., Choi J. H., Lee D. H., Park K. D., Hong S. S. The use of low molecular weight heparin-pluronic nanogels to impede liver fibrosis by inhibition the TGF-beta/Smad signaling pathway. Biomaterials. 2011;32:1438–1445. doi: 10.1016/j.biomaterials.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Liles J. T., Corkey B. K., Notte G. T., Budas G. R., Lansdon E. B., Hinojosa-Kirschenbaum F., Badal S. S., Lee M., Schultz B. E., Wise S., Pendem S., Graupe M., Castonguay L., Koch K. A., Wong M. H., Papalia G. A., French D. M., Sullivan T., Huntzicker E. G., Ma F. Y., Nikolic-Paterson D. J., Altuhaifi T., Yang H., Fogo A. B., Breckenridge D. G. ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J. Clin. Invest. 2018;128:4485–4500. doi: 10.1172/JCI99768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R., Lawitz E., Mantry P. S., Jayakumar S., Caldwell S. H., Arnold H., Diehl A. M., Djedjos C. S., Han L., Myers R. P., Subramanian G. M., McHutchison J. G., Goodman Z. D., Afdhal N. H., Charlton M.R. GS-US-384-1497 Investigators, author. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology. 2018;67:549–559. doi: 10.1002/hep.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H., Maeda S., Hikiba Y., Ohmae T., Shibata W., Yanai A., Sakamoto K., Ogura K., Noguchi T., Karin M., Ichijo H., Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Noureddin M., Anstee Q. M., Loomba R. Review article: emerging anti-fibrotic therapies in the treatment of non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2016;43:1109–1123. doi: 10.1111/apt.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicoro A., Ramachandran P., Iredale J. P., Fallowfield J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- Priya S., Sudhakaran P. R. Cell survival, activation and apoptosis of hepatic stellate cells: modulation by extracellular matrix proteins. Hepatol. Res. 2008;38:1221–1232. doi: 10.1111/j.1872-034X.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- Son G., Hines I. N., Lindquist J., Schrum L. W., Rippe R. A. Inhibition of phosphatidylinositol 3-kinase signaling in hepatic stellate cells blocks the progression of hepatic fibrosis. Hepatology. 2009;50:1512–1523. doi: 10.1002/hep.23186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Cho K. J., Cheon S. Y., Kim S. H., Park K. A., Lee W. T., Lee J. E. Apoptosis signal-regulating kinase 1 (ASK1) is linked to neural stem cell differentiation after ischemic brain injury. Exp. Mol. Med. 2013;45:e69. doi: 10.1038/emm.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada S., Westwick J. K., Ikejima K., Sato N., Rippe R. A. SMAD and p38 MAPK signaling pathways independently regulate alpha1(I) collagen gene expression in unstimulated and transforming growth factor-beta-stimulated hepatic stellate cells. J. Biol. Chem. 2005;280:10055–10064. doi: 10.1074/jbc.M409381200. [DOI] [PubMed] [Google Scholar]
- Wang Y., Gao J., Zhang D., Zhang J., Ma J., Jiang H. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J. Hepatol. 2010;53:132–144. doi: 10.1016/j.jhep.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Yu F. X., Teng Y. Y., Zhu Q. D., Zhang Q. Y., Tang Y. H. Inhibitory effects of capsaicin on hepatic stellate cells and liver fibrosis. Biochem. Cell Biol. 2014;92:406–412. doi: 10.1139/bcb-2014-0036. [DOI] [PubMed] [Google Scholar]
- Zhang C. Y., Yuan W. G., He P., Lei J. H., Wang C. X. Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016;22:10512–10522. doi: 10.3748/wjg.v22.i48.10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. L., Chen Z. N., Huang Q. F., Bai F. C., Nie J. L., Lu S. J., Wei J. B., Lin X. Methyl helicterate inhibits hepatic stellate cell activation through modulation of apoptosis and autophagy. Cell. Physiol. Biochem. 2018;51:897–908. doi: 10.1159/000495390. [DOI] [PubMed] [Google Scholar]