Abstract
Objective
The North American coronavirus disease-2019 (COVID-19) epidemic exhibited distinct early trajectories. In Canada, Quebec had the highest COVID-19 burden and its earlier March school break, taking place two weeks before those in other provinces, could have shaped early transmission dynamics.
Methods
We combined a semi-mechanistic model of SARS-CoV-2 transmission with detailed surveillance data from Quebec and Ontario (initially accounting for 85% of Canadian cases) to explore the impact of case importation and timing of control measures on cumulative hospitalizations.
Results
A total of 1544 and 1150 cases among returning travelers were laboratory-confirmed in Quebec and Ontario, respectively (symptoms onset ≤03-25-2020). Hospitalizations could have been reduced by 55% (95% CrI: 51%–59%) if no cases had been imported after Quebec’s March break. However, if Quebec had experienced Ontario’s number of introductions, hospitalizations would have only been reduced by 12% (95% CrI: 8%–16%). Early public health measures mitigated the epidemic spread as a one-week delay could have resulted in twice as many hospitalizations (95% CrI: 1.7–2.1).
Conclusion
Beyond introductions, factors such as public health preparedness, responses and capacity could play a role in explaining interprovincial differences. In a context where regions are considering lifting travel restrictions, coordinated strategies and proactive measures are to be considered.
Keywords: Case introductions, Epidemiology, Infectious diseases, Public health, Travel
Introduction
Both American and Canadian epidemics of coronavirus disease-2019 (COVID-19) are marked by stark geographic heterogeneities (Mishra et al., 2020). Despite reporting its first case on February 28, 2020—close to a month after Ontario on January 25th and British Columbia on January 28th—Quebec quickly became the epicenter of the Canadian COVID-19 epidemic. The disease’s mortality burden in that province, at 653 per million population, was 3.5 times as high as neighboring Ontario (186 per million) and 19 times that of British Columbia (at 35 per million) at the end of the first spring 2020 wave (Groupe de surveillance provinciale de la, 2020). Quebec also had one of the highest ratios of confirmed cases per million population in the world in the spring of 2020 (Figure S1) (Dong et al., 2020) albeit these comparisons are caveated by the underlying testing efforts.
Such interprovincial epidemic differences across Canada are puzzling, given general similarities in age structure, universal health care systems, and the timing of local physical distancing measures. For example, British Columbia and Ontario’s ratios of confirmed cases per population are 11 and 2.6 times lower, respectively, than that recorded in Québec (as of July 5th, 2020). One hypothesis for these differences, is that the timing of the “semaine de relâche”, or March break, played a major role in seeding the Quebec epidemic. This March break took place the week of March 2nd in Quebec—up to 2 weeks earlier than other Canadian provinces. Increased travel over that week could have led to a sudden and high number of introductions—at a time when physical distancing measures had yet to be enacted and implemented. In other provinces, public health authorities recommended against travel during their later March break (Holliday, 2020).
A systematic examination of the impact of imported cases on observed heterogeneities in COVID-19 burden has yet to be conducted. In this study, we analyze and compare surveillance data from Quebec and Ontario, the two largest provinces in Canada, regarding the number and timing of imported cases. Using a dynamic mathematical model of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) transmission, we then estimate the impact of reducing case importation on the size of the epidemic in Quebec as of July 1st, 2020. In addition, we contrast what would have been observed if Quebec had experienced the same number and timing of imported cases as Ontario. Finally, we examined how the timing of public health measures with respect to the observed case importation would have affected COVID-19 hospitalizations.
Methods
Mathematical model
A deterministic SEIR-type of compartmental model of SARS-CoV-2 transmission was developed based on similar validated frameworks (Davies et al., 2020, Kucharski et al., 2020). The model is semi-mechanistic in the sense that it does not explicitly model interventions. Rather, the impact of those interventions is captured by a transmission rate that varies with time. The model can be described (Figure S2) using ordinary differential equations (Text S1) and the main model parameters are described in Table S1 (see supplementary data). Cases that have acquired SARS-CoV-2 outside the province are directly imported into the infectious compartments one day before their date of symptom onset. As only symptomatic cases could have been detected upon arrival, we accounted for underreporting of asymptomatic cases by importing infectious individuals in the homonymous compartment proportionally to the fraction of cases that are asymptomatic (Table S1). The transmission rate is calibrated and varies with time with a weekly time step, which reflects the different interventions implemented (or de-escalated) over time.
Data sources
COVID-19 case surveillance data in Quebec are collected for both laboratory-confirmed cases and cases confirmed through epidemiological links by public health departments and recorded in the “Fichier V10/COVID-19”. For each confirmed case, basic sociodemographic information is collected in addition to epidemiological characteristics. For cases with a travel history, date of arrival in Quebec, and travel destination(s) are also registered. Hospitalization records are transmitted from each hospital daily with admission date, transfer and discharge information, and the use of intensive care and ventilators.
In Ontario, information on COVID-19 cases is reported by each public health unit to the Ministry of Health and Long-Term Care through the Integrated Public Health Information System (iPHIS). In this database, cases with a travel history (i.e., imported cases) lack the date of return travel and, to enable comparisons with Quebec, we used the date of symptoms onset.
Model calibration
Because of important changes over time in testing criteria and COVID-19 testing efforts, we did not calibrate the model to case surveillance data. Instead, it was calibrated to daily incident hospitalizations (King et al., 2015) using a negative binomial likelihood. The model was initialized on February 27th, when the first case of COVID-19 was notified in Quebec, and calibrated using Bayesian methods. All details regarding model calibration are provided in Text S2 (code is available at https://github.com/pop-health-mod/covid19-release).
Analyses
Following model calibration, we estimated the impact of case importation on SARS-CoV-2’s transmission dynamics by modeling counterfactual scenarios where the number of daily imported cases would have been different. The first scenario corresponds to no imported cases after March 8th (the end of March break in Quebec). In this scenario, we included cases that returned before March 8th but had onset of symptoms at a later date. The second counterfactual scenario considered what would have happened if Quebec had experienced the same daily imported case counts as Ontario. The third scenario applied Quebec’s daily imported cases before March 8th and Ontario’s thereafter. In addition to these analyses, we examined the timing of implementation of public health measures on COVID-19 hospitalizations. We first investigated what would have happened in Quebec if control measures and physical distancing restrictions had been implemented one week earlier. Then, we examined how hospitalizations would have been affected had these measures been delayed by one week. These two scenarios were implemented assuming that the population would have experienced the estimated transmission rates one week earlier or later, respectively.
Results
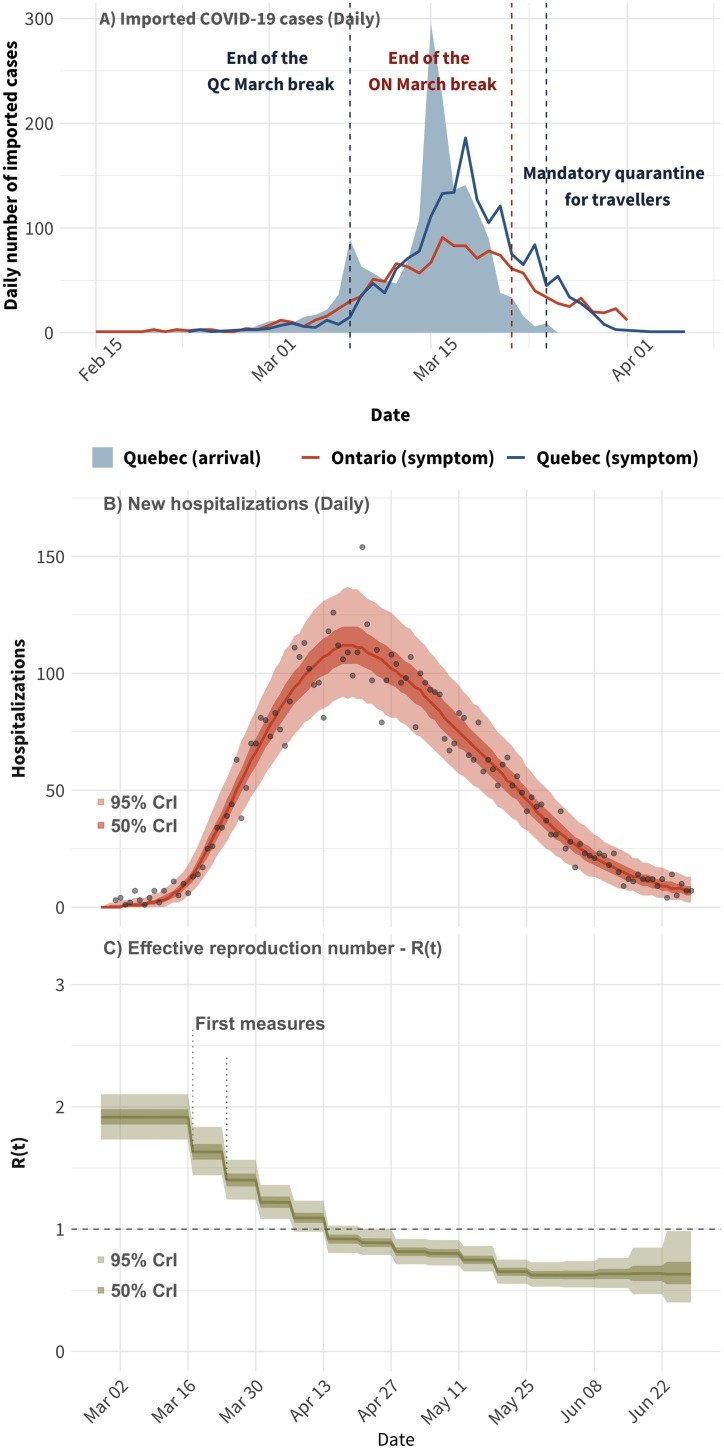
From February 28th to March 25th, the date at which Canada implemented mandatory quarantine of returning travelers, 1544 out of 4859 (32%) COVID-19 confirmed cases with symptom onset were returning travelers to Quebec as compared to 1150 out of 3719 (31%) in Ontario (Figure 1 ). The majority of those imported cases in Quebec were aged 26–65 years and the top 3 countries visited are the United States (24%), France (10%), and Mexico (7%). In Ontario, the top 3 countries visited by travel-related cases are the United States (48%), the United Kingdom (9%), and Mexico (5%).
Figure 1.
Panel (A): Number of daily imported COVID-19 cases by the date of return travel (shaded area; Quebec only) and imported cases by the date of symptoms onset in Quebec and Ontario (lines). Panel (B): Model fit to the time series of daily hospitalizations in Quebec. Panel (C): the estimated time-varying effective reproduction number. The points in panel (A) correspond to the observed number of hospitalizations. In both panels, the solid lines correspond to the median of the model posterior distribution for that outcome and the shaded areas to the 50% and 95% credible intervals.
Almost all imported cases returned to Quebec before the federal implemention of mandatory quarantine for travelers with one peak after the main week for March break (March 8th) and another larger peak (March 15th) (Figure 1). Compared to Quebec, Ontario had slightly lower number of imported cases (based on dates of symptom onset) before the mandatory quarantine. The time series of the daily number of imported cases in Ontario is flatter than the one in Quebec, and the peak of the curve occurred before the end of March break in Ontario.
As of July 1st, 2020, a total of 6250 COVID-19-related hospitalizations were recorded in Quebec (excluding transfers from long-term care facilities; LTCF). The daily number of hospitalizations rose rapidly from mid-March to late April, at which point it began to steadily decrease (Figure 1). These dynamics were accurately replicated by the calibrated model. Public health measures imposed in the early stages of the epidemic coincide with decreases in the effective reproduction number below 1 within three to four weeks of implementation.
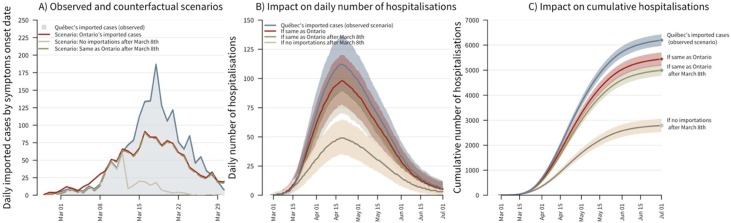
Results suggest that case importation played an important role in driving the initial spread of SARS-CoV-2 transmission in Quebec. Compared to our baseline, using observed daily number of imported cases, the counterfactual scenario with no case importation after March 8th resulted in a cumulative number of hospitalizations that would have been 55% lower (95% credible interval–CrI: 51%–59%; Figure 2 ). However, case importation alone was not sufficient to explain the extent of the epidemic in Quebec. Indeed, when using Ontario’s number of imported cases, the cumulative number of hospitalizations was only reduced by 12% (95% CrI: 8%–16%). If Quebec had experienced the same number of daily imported cases as Ontario after March 8th, the cumulative number of new hospitalizations would have been 19% lower (95% CrI: 16%–23%). All the scenarios presented above suggest a lower daily number of hospitalizations peaking after mid-April. Other factors could help explain the Quebec-Ontario differential in epidemic size given that Ontario had 29% fewer cumulative hospitalizations (4423/6250; as of July 5th).
Figure 2.
The impact of case importation on incident and cumulative hospitalizations. Panel (A): The observed number of imported cases by date of symptom onsets in Quebec (shaded area) and three counterfactual scenarios corresponding to: (1) Ontario’s daily number of imported cases, (2) no case importation after March 8th (cases imported before that date can still become infectious later on), and (3) Quebec’s observed daily imported cases up to March 8th, after which, Ontario’s daily imported cases are used. For all scenarios, imported cases are assumed to become infectious 1 day prior to symptom onset. Panel (B): The daily number of COVID-19 hospitalizations in Quebec for the observed scenario and the three counterfactual scenarios. Panel (C): The cumulative number of COVID-19 hospitalizations in Quebec for the observed scenarios and the three counterfactual scenarios. The solid lines correspond to the median estimates and the shaded areas to the model’s 95% credible intervals.
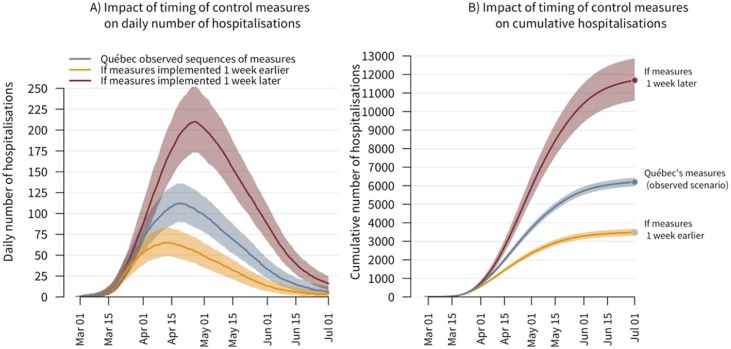
Importantly, the timing of public health control measures had a notable impact on transmission (Figure 3 ). If the whole sequence of measures had taken place one week earlier but the number and timing of imported cases would have remained unchanged, the cumulative number of hospitalizations would have been 44% lower (95% CrI: 40%–47%), and the peak in incident hospitalizations would have decreased earlier. However, had the implementation of these measures been delayed by one week, COVID-19 burden could have been much higher. The daily number of new hospitalizations would have risen more sharply and taken more time to decline, whereas the cumulative number of hospitalizations could have been close to twice as high (11,700 hospitalizations; 95% CrI: 10,600-12,900).
Figure 3.
The impact of earlier or delayed implementation of control measures on incident and cumulative hospitalizations (with Quebec’s observed number/timing of imported cases). Panel (A): The daily number of incident COVID-19 hospitalizations in Quebec under (1) the observed scenario with first measures announced on March 13th, (2) if the whole sequence of measures would have been implemented one week earlier, and (3) if the sequence of measures would have been delayed by one week. Panel (B): The cumulative number of COVID-19 hospitalizations in Quebec for the observed scenario and the two alternative ones, where control measures would have been implemented earlier or delayed. The solid lines correspond to the median estimates and the shaded areas to the model’s 95% credible intervals.
Discussion
This study examined the role of case importation following Quebec’s March break in making that province the epicenter of the Canadian epidemic during the spring of 2020. Using a model of SARS-CoV-2 transmission dynamic calibrated to detailed surveillance data, we found that cases acquired outside the province played an important role in the initial spread of COVID-19 in Quebec and that early interventions had a strong impact on transmission. Had Quebec not experienced any imported cases after its March break, but nonetheless implemented the same interventions, the cumulative number of hospitalizations could have been halved. However, case importation alone is not sufficient to fully explain why Quebec suffered a much more severe epidemic as compared to its close neighbor, Ontario. That province also had many travel-related cases and, had Quebec received Ontario’s daily number of imported cases, the epidemic could have been 12%–19% smaller in terms of hospitalizations.
In Quebec, a public health state of emergency was declared on March 13th by the Ministry of Health and Social Services, followed by a range of physical distancing recommendations, restrictions, and closures (CIHI, 2020). Ontario followed Quebec’s path and declared a state of public health emergency four days later. In Quebec, had this sequence of restrictions and public health measures been implemented one week later, the number of cumulative hospitalizations could have been twice as high during this first wave. Our results highlight the importance of early interventions to rapidly control transmission.
The large difference in epidemic sizes of COVID-19 between Quebec and Ontario remains puzzling. Health system preparedness and capacity to respond to emerging epidemics could have played a role. For instance, Ontario recorded its first SARS-CoV-2 case close to a month before Quebec. As such, the largest Canadian province could have been better prepared for tracing contacts and isolating imported cases—important nonpharmaceutical interventions to prevent onward transmission (Davies et al., 2020, Kucharski et al., 2020). While public health capacity itself remains difficult to evaluate, Quebec’s regional public health agencies have been subjected to reforms in 2015, with budget decreases of 30% and changes in the organizational structure that could have made coordination more challenging (Guyon and Perreault, 2016, Poirier et al., 2019). Other factors such as suboptimal surveillance systems for early outbreak detections, bottlenecks in testing capacity, initial unavailability of personal protective equipment, and shortages of healthcare personnel, among other things, could have negatively affected transmission in Quebec.
These results need to be interpreted considering certain limitations. First, our model mainly reflects transmission in the community and outbreaks occurring in LTCF are not modeled explicitly. As such, it is unclear if the modeled proportional reductions in cumulative hospitalizations for our different scenarios could have directly translated in reduced deaths as most of them occurred in LTCF. Second, the validity of our provincial comparison hinges on the assumption that a comparable fraction of all symptomatic travel-related COVID-19 cases were detected by surveillance systems in both provinces. This assumption could be violated if, for example, more travelers in one province would be returning from countries, which were deemed to be at a low risk of COVID-19 (hence less likely to be tested). Given the reasonably low positivity of 5% before March 25th in Quebec, however, incomplete case detection could have been limited. This is also supported by a retrospective analysis conducted on more than 1000 samples collected by the National Public Health Laboratory of Quebec from patients with flu-like symptoms between November 2019 and early March 2020 that did not detect SARS-CoV-2 (Paré, 2020).
Alternatively, more nonresident tourists from high-risk areas could have visited one province, which would have lead to local chains of transmission, and these tourists being missed by case surveillance systems. Assessing the levels of SARS-CoV-2 introduction by nonresident tourists is challenging, but we note that Ontario received twice as many nonresident tourists from Europe and the United States during February and March of 2020 (521,081 and 245,521 in Ontario and Quebec, respectively), albeit Quebec had a higher number of visitors from France (Statistique, 2020a, Statistique, 2020b). A recent genomic analysis provides credence to our assumptions. Reporting on 734 high-quality SARS-CoV-2 consensus sequence from March 2020, the authors found that most case importations occurred through Europe and the Americas around March Break and that there were very few cryptic transmission chains before early March (Murall et al., 2020), as suggested by the surveillance data.
Our analytical framework has several strengths. First, we used detailed surveillance data to inform model development, parameterization, and calibration. Second, the model was calibrated to hospitalization data which is believed to be more robust than case surveillance data, which are affected by time-varying COVID-19 testing efforts, testing protocols, and health-seeking behaviors. Third, we contrasted the experience of Quebec and Ontario in terms of imported cases. These two provinces experienced different epidemic trajectories but otherwise share many similarities.
In conclusion, the rapid importation of more than 1500 COVID-19 cases in Quebec resulted in major outbreaks with more than six thousand cumulative hospitalizations by the beginning of the summer of 2020. Although the size of the Quebec epidemic would have been drastically reduced if case importation could have been decreased in early March, comparisons with neighboring Ontario suggest that other factors could explain part of the observed provincial heterogeneity in epidemic size. As different countries contemplate when and how to lift travel restrictions, it is imperative to implement coordinated strategies that would prevent within and between-country case importation of this magnitude in the future (Tuite et al., 2020, Ruktanonchai et al., 2020). Granular surveillance systems and epidemic intelligence, coupled with early intervention, will be key to mitigate and control SARS-CoV-2 resurgence.
Ethics
Ethical approval for secondary analyses of provincial surveillance data was obtained from McGill University’s Institutional Review Board. Secondary analyses of iPHIS data, through the Ontario COVID-19 Modelling Consensus Table, were conducted with approval from the University of Toronto Health Sciences’ Research Ethics Board (protocol No. 39253).
Authors’ contributions
DB, AG, MB, MM-G, and YX conceptualized and designed the study; AG, YX, YS, ML, CB, and MD performed data analyses; DD-S, MM-G, ML, AG, and AMS performed model calibration; all authors interpreted the data. AG, MM-G, and YX wrote the first draft of the manuscript and all authors revised it for important intellectual content. All authors have approved the final version for publication and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. We thank David Landsman (University of Toronto) for helping with data cuts of the Ontario surveillance data.
Funding statement
We acknowledge financial support from the McGill Interdisciplinary Initiative in Infection and Immunity (MI4; to MM-G), with seed funding from the MUHC Foundation, and a Canadian Institutes of Health Research (CIHR) grant (to SM). MM-G’s research program is supported by a Canada Research Chair (Tier 2) in Population Health Modeling. SM’s research program is supported by a Canada Research Chair (Tier 2) in Mathematical Modeling and Program Science.
Conflict of interest
MM-G report contractual agreements with the Institut national de santé publique du Québec (INSPQ) and the Institut d’excellence en santé et en services sociaux (INESSS). In addition, MM-G discloses an investigator-sponsored research grant from Gilead Sciences Inc., and funding from both the World Health Organization and the Joint United Nations Programme on HIV/AIDS (UNAIDS), outside of the submitted work. DLB discloses a contractual agreement with INESSS and MB reports research funding from INSPQ.
Declaration of Competing Interest
The authors report no declarations of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.10.046.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- CIHI . 2020. COVID-19 Intervention Timeline in Canada. Available at:https://www.cihi.ca/en/covid-19-intervention-timeline-in-canada[Accessed 13 October 2020] [Google Scholar]
- Davies N., Kucharski A., Eggo R., Gimma A., Group CMMID COVIDW, Edmunds W. Centre for Mathematical Modelling of Infectious Diseases. LSHTM; 2020. The Effect of Non-Pharmaceutical Interventions on COVID-19 Cases, Deaths and Demand for Hospital Services in the UK: a Modelling Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groupe de surveillance provinciale de la C. Institut national de santé publique du Québec; 2020. Vigie Quotidienne de la COVID-19 au Québec: Épidémiologie Descriptive Rapport du 14 juin 2020; p. 53. [Google Scholar]
- Guyon A., Perreault R. Public health systems under attack in Canada: evidence on public health system performance challenges arbitrary reform. Can J Public Health. 2016;107:e326–e329. doi: 10.17269/CJPH.107.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday I. 2020. B.C. Health Officials Discouraging ‘All Non-Essential Travel’ Outside Canada. British Columbia. [Google Scholar]
- King A.A., Celles M., Magpantay F.M., Rohani P. Avoidable errors in the modelling of outbreaks of emerging pathogens, with special reference to Ebola. Proc Biol Sci. 2015:282. doi: 10.1098/rspb.2015.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharski A.J., Russell T.W., Diamond C., Liu Y., Edmunds J., Funk S. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S., Kwong J.C., Chan A.K., Baral S.D. Understanding heterogeneity to inform the public health response to COVID-19 in Canada. CMAJ. 2020 doi: 10.1503/cmaj.201112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murall C., Fournier E., Galvez J. 2020. Genomic Epidemiology of Early Introductions of SARS-CoV-2 into the Canadian Province of Québec. Available at:https://virological.org/t/genomic-epidemiology-of-early-introductions-of-sars-cov-2-into-the-canadian-province-of-quebec/553[Accessed 12 October 2020] [Google Scholar]
- Paré I. Le Devoir; Montréal: 2020. L’épidémie a Commencé Autour de la Relâche Scolaire. [Google Scholar]
- Poirier L.-R., Pineault R., Guitèrrez M., Vien L.-P., Morisset J. Institut national de santé publique du Québec (INSPQ), Direction de la valorisation scientifique, des communications et de la performance organisationnelle; Ville de Québec, QC: 2019. Évaluation de la Mise en Œuvre du Programme National de Santé Publique 2015-2025: Analyse de l’impact des Nouveaux Mécanismes de Gouvernance; p. 51. [Google Scholar]
- Ruktanonchai N.W., Floyd J.R., Lai S. Assessing the impact of coordinated COVID-19 exit strategies across Europe. Science. 2020 doi: 10.1126/science.abc5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistique C. Tableau 24-10-0043-01 Touristes Internationaux Entrant ou Revenant au Canada Selon la Province d’entrée.
- Statistique C. Tableau 24-10-0003-01 Voyageurs non Résidents Entrant au Canada Selon le Pays de Résidence (sauf les États-Unis).
- Tuite A.R., Fisman D.N., Greer A.L. Mathematical modelling of COVID-19 transmission and mitigation strategies in the population of Ontario, Canada. Can Med Assoc J. 2020;192:E497–E505. doi: 10.1503/cmaj.200476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.