Abstract
Objectives
The aim was to investigate if there is synergy in HIV infection and COVID-19 in their influence on human immunity, if there is an exacerbation of HIV patients’ immune status caused by SARS-CoV-2; and if HIV infection without antiretroviral therapy (ART) leads to a more serious COVID-19 course than HIV infection with ART.
Design
Anonymised blood samples and clinical data were collected in 47 hospitals, clinics and medical centres in six Russian cities/regions in the period from 20 March to 15 June 2020. Three hundred and seventy-six HIV/COVID-19 patients were studied (171 without ART and 205 with ART). The control group consisted of 382 SARS-CoV-2-positive patients without HIV infection. Lymphocyte and cytokine amounts were measured by flow cytometry and ELISA. This work is a retrospective study.
Results
COVID-19 led to rapid augmentation of the process of T-cell exhaustion initially caused by HIV, and this T cell degradation was most pronounced in patients without ART. A rise in IL-10 and TGFβ serum concentrations was observed. Diminishing CD4+/CD8+ cell and Th1/Th2 cell ratios characteristic for HIV progression were accompanied by a surge in exhausted T cell count with simultaneous exacerbation of COVID-19-related respiratory distress.
Conclusions
HIV infection without ART may be a very serious comorbidity of COVID-19, whereas immunity of HIV/COVID-19 patients with proper ART is not generally affected by SARS-CoV-2. HIV-1 and SARS-CoV-2 are likely to exhibit a synergic effect, and exhausted T lymphocyte dynamics may be its effective marker.
Keywords: HIV-1, SARS-CoV-2, Co-infection, Immune status, T cell, Cytokine
Introduction
Background
HIV-1 and SARS-CoV-2 co-action in humans is a subject of growing research interest and clinical importance, including the problem of searching for the most appropriate and relevant therapy (Calza et al., 2020, Härter et al., 2020, Mascolo et al., 2020). Both HIV-1 infection and COVID-19 (which is caused by SARS-CoV-2) are global pandemics (Patel et al., 2020). As Sassin, 2019a, Sassin, 2019b, Sassin, 2020) notes, the worldwide spread of both diseases is truly global and it is a direct consequence of digital-age globalisation with huge level of cross-border relocation of people, tourist flows and migration on the scale of the entire planet without any cultural, religious, national, racial, mental or other boundaries. The character of the simultaneous effect of these two diseases on people concerns mainly possible deterioration of the human immune system (Neidleman et al., 2020, Silva-Arrieta et al., 2020).
From most of the studies of HIV/SARS-CoV-2 co-infection published thus far, there is still no clear understanding of what may be regarded as the main disease and what is a comorbidity in the co-infection. Some research groups, on the basis of investigating sample sets limited in size, conclude that SARS-CoV-2 infection doses not aggravate the course of HIV infection in people living with HIV (PLWH) nor does HIV infection worsen the COVID-19 course (Pinnetti et al., 2020, Schmidt et al., 2020, Shah et al., 2020). However, there is a contradictory viewpoint. Wang et al. (2020) recently reported a case of one HIV/COVID-19 patient with a low CD4+ T cell count who had a longer COVID-19 course and lower antibody amount. Furthermore, it has been found that HIV/SARS-CoV-2 co-infection may lead to pneumonia complications oftener than COVID-19 itself (Madge et al., 2020, Okoh et al., 2020). The ability of HIV/SARS-CoV-2 co-infection to cause excessive T cell activation has been observed in small sample sets (D’Ettorre et al., 2020, Gao et al., 2020). Besides, it has been found that severe symptomatic manifestations of COVID-19 may lead to a more pronounced T cell response than a mild symptomatic clinical course (Ho et al., 2020, Mondi et al., 2020).
Focus
In the current work, we report T cell profiles as well as levels and lymphocytic producing ability for a number of cytokines involved in T cell regulation of two groups of PLWH who tested positive for SARS-CoV-2, with proper antiretroviral therapy (ART) and without it, as well as a control group of SARS-CoV-2-positive patients without HIV infection.
Specific purpose
The major purpose of the study was to find out on the basis of the T cell counts and cytokine amounts/production detected
-
1.
if there is synergy in HIV infection and COVID-19 in their influence on human immunity;
-
2.
if there is an exacerbation of HIV patients’ immune status caused by SARS-CoV-2; and
-
3.
if HIV infection without ART leads to a more serious COVID-19 course than HIV infection with proper ART.
Methods
Participants
Three hundred and seventy-six PLWH who tested positive for SARS-CoV-2 constituted group 1 (without ART, 171 persons, 22–67 years old) and group 2 (with ART, 205 persons, 18–58 years old). ART was suspended during the COVID-19 pandemic due to antiretroviral medication shortages in group 1 (ART interruption of 1.5–6.5 months, with a median of 3.5 months) but was properly continued in group 2. Group 3 (control group) was represented by 382 SARS-CoV-2-positive persons without HIV infection. Group 3 was composed in such a way to match the demographics (age, sex, social strata, habits) and clinical data (comorbidities, COVID-19 severity) of groups 1 and 2 as closely as possible. No asymptomatic SARS-CoV-2 carriers were considered in the current research.
Geographical distribution
The information was collected in 47 hospitals, clinics and medical centres in six Russian regions studied in the current research (Moscow, Moscow region, St Petersburg, Nizhny Novgorod, Murmansk, Krasnodar). The number of PLWH positive for SARS-CoV-2 and residing in the six aforementioned Russian regions was some 3000–3500 persons. This figure can be obtained by multiplying official statistics for PLWH in these regions published by the Russian Statistical Agency (Rosstat) by the average population infection rate measured by us for these regions. Such a method has some deficiencies. First, we assume that the population infection rate in the PLWH group is the same as in the general population, but that can be an incorrect or not always correct premise. Second, official governmental statistics do not count positive results of anonymised HIV tests, as according to Russian law, medical centres and clinics have a right not to disclose the results of such tests taken on an anonymous basis. However, despite the flaws described, such a simple approach allows us to estimate the overall number of PLWH in different geographical regions in Russia, at least roughly.
Time of study
The work was conducted from 20 March to 15 June 2020.
Ethical guidelines
Reporting of the study conforms to broad EQUATOR and STROBE-NI cohort study guidelines. No identifying information accompanied the clinical data. In hospitals, government-sponsored medical centres and private clinics involved in treating the patients or surveillance, written informed consent for use of anonymised data for scientific research and publications was obtained from every patient/carrier concerned. All written informed consent forms duly signed are kept in the hospitals, clinics and medical centres. The Ethical Committee of Koltzov Institute of Developmental Biology of the Russian Academy of Sciences gave its approval for the conduct of the study (protocol no. 39100920).
Type of study
The study was not a randomised controlled clinical trial. Immune status detection and other relevant measurements were performed retrospectively.
Flow cytometry
Flow immunofluorocytometry (MACSQuant® Analyzer 16 flow cytometer, Miltenyi Biotec, Bergisch Gladbach, Germany) with necessary Miltenyi Biotec and Vector reagents and kits was used to perform immunological cell blood analyses. For isolation/detection of T killer cells, a MACSxpress® CD8 T Cell Isolation Kit was used and the presence/absence of specific markers (CD27–, CD28–, CD45RA+, PRF1++) was checked during flow cytometry. For Th1 cells a MACSxpress® CD4 T Cell Isolation Kit and an IFNγ Secretion Assay — Cell Enrichment and Detection Kit were used. For Th2 cells a Miltenyi® CD294 MicroBead Kit and an IL-4 Secretion Assay — Cell Enrichment and Detection Kit were used. For Th17 cells a Miltenyi® IL-17 Secretion Assay — Cell Enrichment and Detection Kit was used. For central memory CD4+ T cells a Miltenyi® CD4+ Central Memory T Cell Isolation Kit was used. For effector memory CD4+ T cells a Miltenyi® CD4+ Effector Memory T Cell Isolation Kit was used. For effector memory RA T cells a Miltenyi® CD4+CD45RA+ Effector T Cell Isolation Kit was used. For CD4+ naive lymphocytes a Miltenyi® Naive CD4+ T Cell Isolation Kit II and a CD4+ T Cell Isolation Kit were used. For exhausted T cells Vector® PD1 and TIM-3 isolation kits were used.
Cytokine amount measurements
IFNγ was detected by the ELISA technique with ELISA kits from PanEco (Moscow, Russia). For IFNγ production, phytohaemagglutinin was used as the mitogen. Protection of monolayer cell culture against vesicular stomatitis virus by interferon was investigated. IL-2, IL-10, TNFα and TGFβ levels were measured by the ELISA technique with ELISA kits from Protein Contour (St Petersburg, Russia).
Respiratory score
Usually, the PaO 2/FiO2 ratio is used as an indicator of respiratory distress. We found that use of an overall respiratory score (RS) instead may give a better result, since RS also concerns respiratory frequency (respiratory rate) and SpO2 besides the PaO 2/FiO2 ratio.
We define RS
where RR is the respiratory rate (per minute). Therefore, normal values of such an indicator will be approximately in the 0–4 range, and more rarely in the 0–5 range. Higher values will indicate respiratory distress caused by pneumonia, bronchitis, bronchiolitis, pulmonary embolism, etc. The respiratory score was calculated without reference to asymptomatic patients in all groups of patients. For group 1, RS up to 48 was observed (severe acute respiratory distress syndrome, severe respiratory failure of type I). Patients with high RS values, usually more than 25, required mechanical ventilator oxygenation or extracorporeal membrane oxygenation. For group 2, RS of 18.6 was observed as the highest value. Such patients required neither mechanical oxygenation nor extracorporeal membrane oxygenation. Instead, for some of them, non-invasive oxygenation was used to reduce RS and prevent severe respiratory distress.
Statistical treatment
OriginLab Origin 8.1 Pro was used for statistical calculations and visualisation.
Results and discussion
T cell response
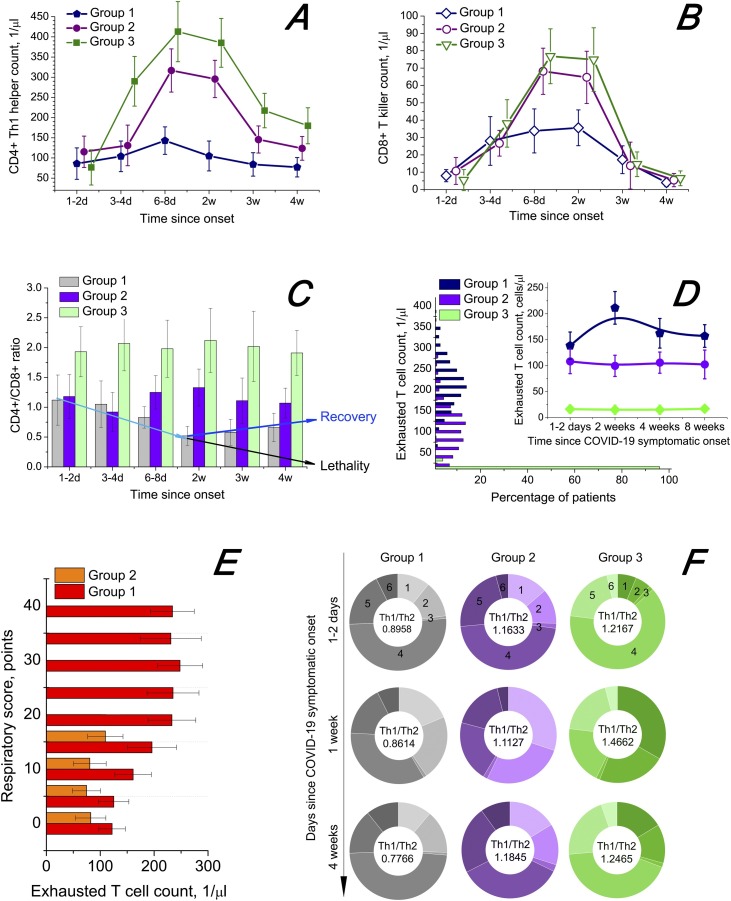
Figure 1 shows the T cell profiles in the case of HIV/SARS-Cov-2 co-infection. The dynamics of CD4+ Th1 cells (Figure 1A) and CD8+ T killer cells, or effector cytotoxic lymphocytes (Figure 1B), shows that SARS-CoV-2-positive patients without HIV infection exhibit a more effective and powerful T cell response to the pathogen. The weakest response was found in cases of HIV/SARS-CoV-2 co-infection without ART (dark blue lines). A clear reduction of the CD4+/CD8+ ratio was observed for group 1, with the minimum achieved in nearly 2 weeks from the symptomatic onset of COVID-19 (Figure 1C). From the third week of the COVID-19 course, a further decrease in the CD4+/CD8+ ratio (in some cases down to 0.015) with large probability led to significant aggravation of a patient’s condition and sometimes death. From that time, further negative dynamics was detected for lethal cases (7 of 171 participants, case fatality rate 4.09%) and positive dynamics of the CD4+/CD8+ ratio for the convalescent cohort of group 1. A rise in the CD4+/CD8+ ratio from the third week usually led to faster recovery from COVID-19.
Figure 1.
T lymphocyte response to HIV/SARS-CoV-2 co-infection. The onset of COVID-19 symptoms is taken as the starting point. The confidence interval is 95%. Whiskers show the standard error of mean. (A) Dynamics of Th1 cell [distinctive membrane phenotype CD3+CD4+CD94+CD183 (CXCR3)+CD195 (CCR5)+CCR3–CCR4–CXCR4–] count in serum. (B) Dynamics of T killer cells [CD3+CD8+CD45RA+CD27–CD28–CCR7–CD178 (FasL)+CD107a (LAMP-1)+IFNγ++GZMB++PRF1++] count in serum. (C) Dynamics of CD4+/CD8+ ratio. (D) Distribution of exhausted T cells (distinctive cytometry markers PD1 and TIM-3) and dynamics of exhausted T cell count in serum (count versus percentage of patients). (E) Relationship between exhausted T cell count in serum and respiratory score (see the “Methods” section for a detailed description). (F) Change of composition of CD4+ T cells in serum between the states at the beginning of COVID-19 (1–2 days), during the disease (1 week) and after it (4 weeks): 1, Th1 cells (distinctive membrane phenotype see above); 2, Th2 cells [CD3+CD4+CD193 (CCR3)+CD194 (CCR4)+CD184 (CXCR4)+]; 3, Th17 cells [CD3+CD4+CD161+CD194 (CCR4)+CD196 (CCR6)+]; 4, CD4+ naive lymphocytes [CD3+CD4+CD27+CD28+ CD45RA+ CD45RO–CD127+ CD197 (CCR7)+]; 5, CD4+ regulatory T cells [CD3+CD4+CD27+CD25+CD152 (CTLA-4)+CD127lowFOXP3+]; 6, CD4+ memory T cells [CD3+CD4+CD95+ T cells, detected as central memory CD27+CD28+CD57–CD62L+CD127+CD197 (CCR7)+ T cells, effector memory CD28–CD57+CD62L–CD197 (CCR7)– T cells and effector memory CD27–CD28–CD57+CD62L–CD127–CD197 (CCR7)–CD45RO–CD45RA+ lymphocytes]. The Th1/Th2 cell ratio is provided in the centres of the corresponding doughnuts.
AIDS-defining events
A CD4+ count below 200 1/μl (which is the usual cut-off for commencing Pneumocystis pneumonia prophylaxis and talking of increased risk of AIDS-defining events) became apparent in 16 patients from group 1 during the COVID-19 course. This potentially could open wide prospects for the occurrence of AIDS-defining events in such HIV/COVID-19 patients (recurrent bacterial, viral, fungal or protozoan pneumonia, or more specifically the fungal infections Pneumocystis carinii-associated pneumonia or Histoplasma capsulatum-related disease). In group 1, before diagnosis of COVID-19, Pneumocystis pneumonia was diagnosed in four patients (2.34%) and histoplasmosis was diagnosed in one patient (0.58%). COVID-19 did not add to these numbers, but led to many secondary bacterial pneumonia co-infections (42 cases of 70 pneumonia), with Haemophilus influenzae, Moraxella catarrhalis and Legionella pneumophila being the most common (12, 9 and 7 of 42 cases, respectively). Of seven deaths registered in group 1, all patients had bacterial pneumonias and most patients had an absolute CD4+ count less than 200 1/μl. Therefore, one can assume that the deaths were connected mainly to AIDS-defining events. The AIDS-defining events in patients from group 1 were not caused by SARS-CoV-2. SARS-CoV-2 induced impairment of the condition of untreated HIV patients.
T cell exhaustion
A close relationship between HIV infection progress and the process of T lymphocyte exhaustion has been repeatedly described in the literature (Blank et al., 2019, Wherry, 2011, Yi et al., 2010). We detected exhausted T cells by their most common specific markers PD1 and TIM-3, although sometimes different markers are used. The surge in exhausted T cell count detected by us for group 1, at 2 weeks from COVID-19 onset (Figure 1D), is probably related to SARS-CoV-2 synergic action with HIV, not just HIV infection progress, as one may observe a drop in exhausted T cells for a longer time (possibly, due to their gradual apoptosis in PLWH after COVID-19). A negligibly small level of T cell exhaustion was detected for group 3. In Figure 1E, we may observe that severe COVID-19 cases with pronounced respiratory distress (high values of RS) are also associated with high level of T lymphocyte exhaustion.
Th1/Th2 cell ratio
In Figure 1F, one can see that the Th1/Th2 cell ratio decreases in group 1 (left column) during the COVID-19 course along with a diminishing CD4+/CD8+ ratio. It remains lower after COVID-19 than before the disease, while for groups 2 and 3 there is no such tendency. Besides, we can also discern from Figure 1F, that T helper cell activation from CD4+ naive lymphocytes and proliferation are much more effective for PLWH with ART, to say nothing of people without HIV infection, in comparison with patients in group 1.
Overall cytokine dynamics
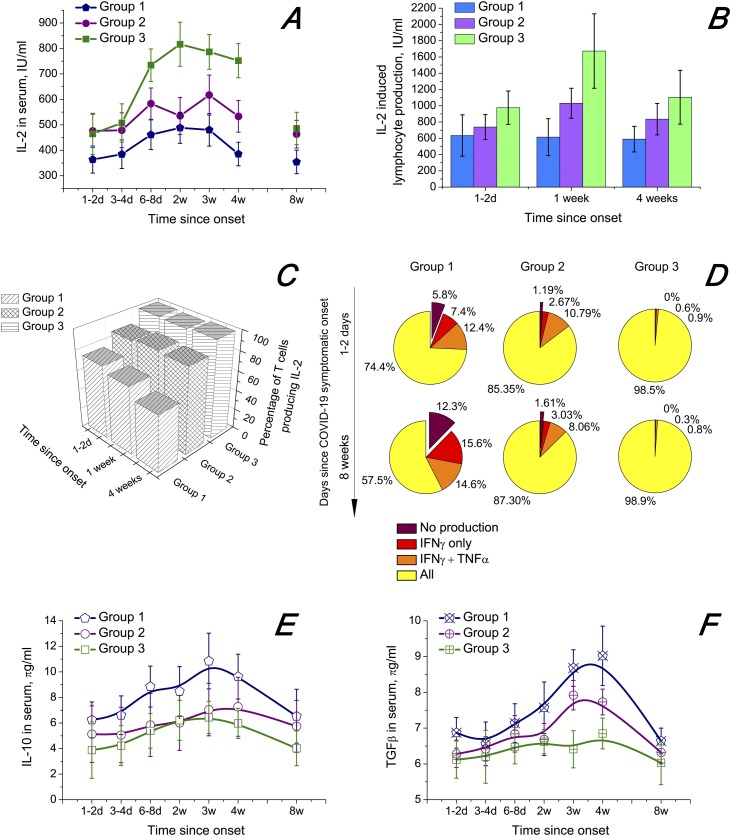
The results of the cytokine dynamics study are presented in Figure 2 . The cytokines IL-2, TNFα, IFNγ, IL-10 and TGFβ1, which are deemed to be closely connected with PLWH immunity degradation (Pett et al., 2010, Rich et al., 2019, Yarilin, 2010), were investigated.
Figure 2.
Dynamics of serum concentrations and induced lymphocytic production of certain cytokines. COVID-19 symptom onset is taken as the starting point. The confidence interval is 95%. Whiskers show the standard error of mean. (A) IL-2 serum amounts. (B) IL-2 production by lymphocytes. Mitogen: phytohaemagglutinin (10 μg/ml); 2.5 × 106 cells/ml; 24 h of exposure. (C) Percentage of T lymphocytes capable of producing IL-2. (D) Production ability of T cells with regard to IL-2, TNFα and IFNγ. (E) IL-10 serum amounts. (F) TGFβ1 serum amounts.
Inflammatory cytokines
We found that SARS-CoV-2 might exacerbate the process of T cell exhaustion by decreasing the capability of T cells to produce sufficient amounts of IL-2, TNFα and IFNγ (Figure 2A–D). These inflammatory cytokines are involved in regulation of T cell activation and sustaining many T cell subpopulations. They may be regarded as very important factors of T cell response in people with HIV/SARS-CoV-2 co-infection. The temporal changes in IL-2 serum amounts are presented in Figure 2. TNFα and IFNγ serum amount dynamics were strongly correlated with IL-2 (Table 1 ). Free serum concentrations of all three cytokines were substantially lower in PLWH without ART (group 1, blue line for IL-2). SARS-COV-2 infection led to an increase in the serum amounts of the three cytokines, with the return to pre-COVID values approximately in the eighth week from COVID-19 onset. IL-2, TNFα and IFNγ induced lymphocytic production was also suppressed in patients from group 1 in comparison with groups 2 and 3 (Figure 2B). The correlation coefficients for patients in group 1 are provided in Table 2 .
Table 1.
Correlation between dynamics of free serum amounts of IL-2, TNFα and IFNγ of group 1 patients (Pearson correlation coefficients and corresponding levels of significance).
| IL-2 | TNFα | IFNγ | |
|---|---|---|---|
| IL-2 | 0.8447 | 0.7215 | |
| TNFα | p = 0.0821 | 0.7862 | |
| IFNγ | p = 0.1638 | p = 0.1207 |
Table 2.
Correlation between dynamics of IL-2, TNFα and IFNγ lymphocytic producing ability of group 1 patients (Pearson correlation coefficients and corresponding levels of significance).
| IL-2 | TNFα | IFNγ | |
|---|---|---|---|
| IL-2 | 0.9023 | 0.8735 | |
| TNFα | p = 0.0150 | 0.8947 | |
| IFNγ | p = 0.0726 | p = 0.0533 |
Connection of T cell exhaustion with inflammatory cytokine levels
Exhausted T cells are known to lose their ability to produce IL-2, TNFα and IFNγ during the process of their exhaustion. At first, T cells stop synthesising IL-2, then TNFα and finally IFNγ (Blank et al., 2019, Wherry, 2011). Our results indicate that HIV/SARS-CoV-2 co-infection augments the process of T cell exhaustion. Figure 2C shows both a comparison between the groups of patients and the time dynamics within the groups, with the quantity of IL-2-producing T cells being the marker of exhaustion. It may be observed that the percentage of T cells incapable of producing IL-2 in sufficient amounts grows with the COVID-19 progress for group 1 only, while groups 2 and 3 do not exhibit such a trend. Figure 2D further explicates the composition and time dynamics of exhausted T cells during SARS-CoV-2 infection. Both the initial count of exhausted T cells and the dynamics of exhaustion were much greater in group 1.
Immunosuppressive cytokines
Elevated serum concentrations of the immunosuppressive cytokines IL-10 and TGFβ in groups 1 and 2 with maximums at around 4 weeks after COVID-19 onset (Figure 2E and F) may have increased T cell exhaustion further. IL-10 and TGFβ serum amounts have been reported to be higher in PLWH than in the general population (D’Ettorre et al., 2020, Ho et al., 2020, Yarilin, 2010). In our study, we observed higher pre-COVID-19 amounts of IL-10 and TGFβ and their more pronounced growth during the COVID-19 course in group 1 than in group 2 or especially group 3 (Figure 2E and F). That may indicate a weaker immune response to acute SARS-CoV-2 infection in patients in group 1 than in group 2 or group 3. IL-10 and TGFβ time dynamics were well correlated, with the Pearson correlation coefficient being 0.8835 at p = 0.008349.
Conclusions
Our study proves that HIV infection without ART can be a dangerous comorbidity of COVID-19, while PLWH with ART do not generally suffer from additional immunity deterioration that may be associated with SARS-CoV-2 infection.
Studying 30 patients with HIV/SARS-CoV-2 co-infection, a research team from Bichat-Claude Bernard University Hospital (Paris, France) deduced recently that HIV infection is probably not an independent risk factor for COVID-19 (Isernia et al., 2020). We support their view strongly, with the proviso that HIV is virologically suppressed with proper ART, HIV viral load in serum is less than 50 copies per millilitre and the CD4+ lymphocyte count is higher than 500 1/μl. Indeed, in such a patient, as we observed in our investigation, HIV infection does not represent a serious COVID-19 comorbidity statistically, whereas SARS-CoV-2 infection does not influence the immunity of such a patient more than the immunity of the general population. However, if ART interruption lasts for 2–3 months and especially 6 months or more, HIV infection may become a very dangerous risk factor for poor COVID-19 clinical course and outcome. Therefore, providing proper ART to PLWH during the COVID-19 pandemic is crucial for healthcare systems around the world (Nakamoto et al., 2020, Patel et al., 2020, Sharov, 2020a, Sharov, 2020b). The healthcare systems must be sustained by governmental actions aimed at prevention of pharmaceutical supply chain disruptions, as in a truly global society such disruptions may cause tremendous harm to the world population (Donskikh, 2019, Etienne et al., 2020, Guo et al., 2020, Lysenko, 2019, Moskovkin, 2020).HIV-1 and SARS-CoV-2 are likely to exhibit a synergic effect. The levels and time dynamics of (1) exhausted T lymphocyte count in serum, (2) IL-2, TNFα and IFNγ serum amounts, (3) IL-2, TNFα and IFNγ lymphocytic producing capability, and (4) IL-10 and TGFβ serum amounts during the COVID-19 course may be an effective complex prognostic marker of this synergy.
Funding
This work was funded by the government programme of basic research in Koltzov Institute of Developmental Biology, Russian Academy of Sciences, in 2020 (no.0108-2019-0002).
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethics approval
The Ethical Committee of Koltzov Institute of Developmental Biology of the Russian Academy of Sciences regarded the current study ethically appropriate and exempt from human participant review, as no clinical trials were performed and the authors were not personally involved in collecting the clinical data and hence do not possess any information that might identify the patients.
Consent to participate and for publication
In hospitals and clinics involved in treating the patients, written informed consent for use of their data for scientific research and publications was obtained from every patient. All written informed consent forms duly signed are kept in the hospitals, clinics, ambulance and medical centres.
Acknowledgements
We thank Wolfgang Sassin (International Institute for Applied System Analysis, Laxenburg, Austria) for constant think-tank discussions of the main ideas of this article, Alexandre Gnes (in-house translator at the Institute of Archaeology and Ethnography of the Siberian Branch of the Russian Academy of Sciences) for fruitful discussions and improving the language quality and Maria Baranovskaya, Anna Gorenintseva, Dmitri Kochetkov, Daria Miloslavskaya, Anton Panin, Yulia Smirnova and Maxim Tsarkov for their provision of clinical data.
References
- Blank C.U., Haining W.N., Held W., Hogan P.G., Kallies A., Lugli E. Defining ‘T cell exhaustion’. Nat Rev Immunol. 2019;19(11):665–674. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza L., Bon I., Tadolini M., Borderi M., Colangeli V., Badia L. COVID-19 in patients with HIV-1 infection: a single-centre experience in northern Italy. Infection. 2020 doi: 10.1007/s15010-020-01492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ettorre G., Recchia G., Ridolfi M., Siccardi G., Pinacchio C., Innocenti G. Analysis of type I IFN response and T cell activation in severe COVID-19/HIV-1 coinfection: a case report. Medicine (Baltimore) 2020;99(36):e21803. doi: 10.1097/MD.0000000000021803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donskikh O.A. Horror Zivilisationis, oder Horror der Subjektivität. Beacon J Stud Ideol Ment Dimens. 2019;2(2) doi: 10.5281/zenodo.3733791. [DOI] [Google Scholar]
- Etienne N., Karmochkine M., Slama L., Pavie J., Batisse D., Usubillaga R. HIV infection and COVID-19: risk factors for severe disease. AIDS. 2020;34(12):1771–1774. doi: 10.1097/QAD.0000000000002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A., Chen Z., Segal F.P., Carrington M., Streeck H., Chakraborty A.K. Predicting the immunogenicity of T cell epitopes: from HIV to SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.05.14.095885. [DOI] [Google Scholar]
- Guo W., Ming F., Dong Y., Zhang Q., Liu L., Gao M. Driving force of Covid-19 among people living with HIV/AIDS in Wuhan, China. AIDS Res Ther. 2020 doi: 10.21203/rs.3.rs-53351/v1. [DOI] [PubMed] [Google Scholar]
- Härter G., Spinner C.D., Roider J., Bickel M., Krznaric I., Grunwald S. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection. 2020;48:681–686. doi: 10.1007/s15010-020-01438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H.E., Peluso M.J., Margus C., Lopes J.P.M., He C., Gaisa M.M. Clinical outcomes and immunologic characteristics of Covid-19 in people with HIV. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isernia V., Julia Z., Le Gac S., Bachelard A., Landman R., Lariven S. SARS-COV2 infection in 30 HIV-infected patients followed-up in a French University Hospital. Int J Infect Dis. 2020;101:49–51. doi: 10.1016/j.ijid.2020.09.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysenko L.A. Back to anthropology: what does it mean to development studies? Beacon J Stud Ideol Ment Dimens. 2019;2(2) doi: 10.5281/zenodo.3946695. [DOI] [Google Scholar]
- Madge S., Barber T.J., Hunter A., Bhagani S., Lipman M., Burns F. Descriptive account of 18 adults with known HIV infection hospitalised with SARS-CoV-2 infection. Sex Transm Infect. 2020 doi: 10.1136/sextrans-2020-054660. [DOI] [PubMed] [Google Scholar]
- Mascolo S., Romanelli A., Carleo M.A., Esposito V. Could HIV infection alter the clinical course of SARS-CoV-2 infection? When less is better. J Med Virol. 2020;92:1777–1778. doi: 10.1002/jmv.25881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondi A., Cimini E., Colavita F., Cicalini S., Pinnetti C., Matusali G. COVID-19 in people living with HIV: clinical implications of dynamics of the immune response to SARS-CoV-2. J Med Virol. 2020 doi: 10.1002/jmv.26556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovkin V.M. Do we need a great reset? COVID-19, black revolution, inequality and common good. Beacon J Stud Ideol Ment Dimens. 2020;3(1) doi: 10.5281/zenodo.3934708. [DOI] [Google Scholar]
- Nakamoto T., Kutsuna S., Yanagawa Y., Kanda K., Okuhama A., Akiyama Y. A case of SARS-CoV-2 infection in an untreated HIV patient in Tokyo, Japan. J Med Virol. 2020 doi: 10.1002/jmv.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidleman J., Luo X., Frouard J., Xie G., Gill G., Stein E.S. SARS-CoV-2-specific T cells exhibit phenotypic features of helper function, lack of terminal differentiation, and high proliferation potential. Cell Rep Med. 2020;1(6) doi: 10.1016/j.xcrm.2020.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoh A.K., Bishburg E., Grinberg S., Nagarakanti S. COVID-19 pneumonia in patients with HIV: a case series. J Acquir Immune Defic Syndr. 2020;85(1):e4–e5. doi: 10.1097/QAI.0000000000002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R.H., Acharya A., Mohan M., Byrareddy S.N. COVID-19 and AIDS: Outcomes from the coexistence of two global pandemics and the importance of chronic antiretroviral therapy. J Med Virol. 2020 doi: 10.1002/jmv.26416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pett S.L., Kelleher A.D., Emery S. Role of interleukin-2 in patients with HIV infection. Drugs. 2010;70(9):1115–1130. doi: 10.2165/10898620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Pinnetti C., Vergori A., Agrati C., Castilletti C., Campioni P., Gagliardini R. SARS-CoV-2 infection does not induce HIV viral escape in central nervous system: a case series. Int J Infect Dis. 2020;101:38–41. doi: 10.1016/j.ijid.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich R.R., Fleisher T.A., Shearer W.T., Schroeder H., Frew A., Weyand C. Elsevier; London: 2019. Clinical immunology. [Google Scholar]
- Sassin W. Deja vue? Beacon J Stud Ideol Ment Dimens. 2019;2(2) doi: 10.5281/zenodo.3733442. [DOI] [Google Scholar]
- Sassin W. Die Grenzen der Ökonomie: Globalisierung — Vom Füllhorn zum Giftbecher? Eur Crossrd. 2020;1(1) doi: 10.5281/zenodo.3978673. [DOI] [Google Scholar]
- Sassin W. Er-Schöpfung der Schöpfung, oder Eine neue Kulturstufe in der Entwicklung des homo. Beacon J Stud Ideol Ment Dimens. 2019;2(2) doi: 10.5281/zenodo.3732508. [DOI] [Google Scholar]
- Schmidt F., Weisblum Y., Muecksch F., Hoffmann H.H., Michailidis E., Lorenzi J.C.C. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med. 2020;217(11) doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V.K., Firmal P., Alam A., Ganguly D., Chattopadhyay S. Overview of immune response during SARS-CoV-2 infection: lessons from the past. Front Immunol. 2020;11:1949. doi: 10.3389/fimmu.2020.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov K.S. COVID-19 pandemic: a survival challenge to humanity unseen thus far or a déjà vu experience? Beacon J Stud Ideol Ment Dimens. 2020;3(1) doi: 10.5281/zenodo.3895809. [DOI] [Google Scholar]
- Sharov K.S. Creating and applying SIR modified compartmental model for calculation of COVID-19 lockdown efficiency. Chaos Soliton Fract. 2020;141 doi: 10.1016/j.chaos.2020.110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Arrieta S., Goulder P.J.R., Brander C. In silico veritas? Potential limitations for SARS-CoV-2 vaccine development based on T-cell epitope prediction. PLoS Pathog. 2020;16(6) doi: 10.1371/journal.ppat.1008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Luo L., Bu H., Xia H. One case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a low CD4+ T-cell count. Int J Infect Dis. 2020;96:148–150. doi: 10.1016/j.ijid.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry E.J. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Yarilin A.A. GEOTAR Media; Moscow: 2010. Immunology. [Google Scholar]
- Yi J.S., Cox M.A., Zajac A.J. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129(4):474–481. doi: 10.1111/j.1365-2567.2010.03255.x-. [DOI] [PMC free article] [PubMed] [Google Scholar]