Abstract
Background:
Immune checkpoint inhibition has been shown to have limited efficacy in patients with metastatic prostate cancer. Prostate cancers that harbor certain homologous recombination (HR) DNA repair gene mutations, inactivating CDK12 mutations or have underlying mismatch repair deficiency may be effectively treated with immunotherapy. Combination therapy may improve clinical response rates to immune checkpoint blockade. We observed profound prostate-specific antigen (PSA) and/or objective responses to immune checkpoint blockade following prior treatment with bipolar androgen therapy (BAT) and enzalutamide.
Methods:
We report three cases of patients with metastatic castration resistant prostate cancer (mCRPC) undergoing therapy with anti-PD-1 inhibitors. All patients underwent both somatic molecular testing and germline genetic testing.
Results:
Two of the three patients with mCRPC harbored an inactivating mutation in an HR DNA repair gene (BRCA2, ATM). No patient demonstrated mismatch repair deficiency, nor were CDK12 alterations present. All three patients had been treated with BAT and enzalutamide before immune checkpoint blockade, a paradoxical approach for the treatment of mCRPC developed by our group.
Conclusions:
These cases of mCRPC suggest that immune checkpoint blockade may have therapeutic potential in patients with prostate cancer, especially following immune activation (“priming”) using BAT and enzalutamide.
Keywords: DNA repair, immunotherapy, prostate cancer, testosterone
1 |. BACKGROUND
The average tumor mutational burden (TMB) of prostate cancer is low, which may confer poor response rates to immune checkpoint blockade.1,2 Two phase III trials using anti-CTLA4 antibodies have failed to meet their primary endpoint in patients with metastatic castration resistant prostate cancer (mCRPC).3,4 However, a subset of patients with mCRPC do derive significant benefit from immune checkpoint inhibitors.5 These immunologically responsive tumors may harbor homologous recombination (HR), DNA repair gene mutations,6 inactivating CDK12 mutations,7,8 or mismatch repair deficiency.9,10
Recently, combination therapy has been studied as a means to improve the clinical response rate of patients with prostate cancer to immunotherapy. For instance, the addition of pembrolizumab at the time of progression on enzalutamide showed promising preliminary activity in one small study.11 Further exploration of this phenomenon is needed to better understand how to make an immunologically inert tumor respond to immune checkpoint inhibition.
Bipolar androgen therapy (BAT) is a novel treatment strategy for mCRPC. During BAT, patients are treated with intramuscular injections of a testosterone depot resulting in supraphysiologic levels of serum testosterone.12 Over a 28-day cycle, serum testosterone returns to castrate levels before the next injection. Here, we report three cases of patients with mCRPC who achieved profound prostate-specific antigen (PSA) and/or objective responses to anti-PD-1 therapy following prior treatment with BAT and enzalutamide.
2 |. MATERIALS AND METHODS
We identified three patients with mCRPC that achieved a PSA and/or objective response to anti-PD-1 therapy and treated at the Johns Hopkins Hospital. A retrospective review of the medical record was performed to identify prior therapies. All patients underwent commercially available, saliva-based germline genetic testing. Clinical grade molecular testing was performed on the primary tumor (patient 1), metastatic site (patient 2), or circulating tumor DNA (patient 3).
We conducted an audit of patients with mCRPC treated with immune checkpoint blockade at Johns Hopkins Hospital between 2014 and 2019. Forty-one patients met these criteria. Data were collected on PSA50 responses to immunotherapy, which is defined as a 50% or greater decline in PSA from baseline. The number of PSA50 responses was compared between two groups (prior BAT vs no prior BAT) using a Fisher’s exact test. Clinical significance was set at P ≤ .05.
3 |. RESULTS
Patient 1 was found to have Gleason 5 + 4 = 9 bone-predominant mCRPC with a germline frameshift BRCA2 mutation. In addition, clinical-grade molecular testing of his primary tumor revealed a somatic mutation in ROS1 (Table 1). His TMB was 5 mutations/Mb, and microsatellite testing was negative. His treatment history included a prolonged response to abiraterone acetate, followed by sequential BAT, and then enzalutamide treatment (Figure 1A). The patient then received treatment with a PARP inhibitor, followed by a second course of enzalutamide. Although retreatment with enzalutamide yielded only transient PSA stability, an anti-PD-1 inhibitor induced an immediate and complete PSA response after two cycles of immunotherapy. He has maintained this complete biochemical response for 5 months, which is ongoing. No additional imaging has been obtained since the start of immune checkpoint blockade.
TABLE 1.
Molecular profile of patients with mCRPC with extreme response to immune checkpoint blockade
| Gene | Mutation | ||
|---|---|---|---|
| Patient 1 | BRCA2 | N319fs*8 | Germline |
| ROS1 | D725N | Somatic | |
| MS-S | |||
| TMB 5 mut/Mb | |||
| Patient 2 | ATM | R2244Kfs*5 | Somatic |
| DNMT3A | D564_L566del | Somatic | |
| ERBB2 | E930D | Somatic | |
| KMT2A | M1923I | Somatic | |
| POLD1 | R218H | Somatic | |
| RB1 | P777Lfs*33 | Somatic | |
| TSC1 | D1002V | Somatic | |
| TSC1 | E990K | Somatic | |
| AR | Amplification | ||
| FGFR1 | Amplification | ||
| MYC | Amplification | ||
| MS-S | |||
| TMB NR | |||
| Patient 3 | BRCA2 | D1754Na | Somatic |
| BRCA2 | R2991Ca | Somatic | |
| DNMT3A | R882H | Somatic | |
| JAK2 | V617F | Somatic | |
| NTRK2 | R598C | Somatic | |
| TP53 | Y205D | Somatic | |
| TP53 | H193L | Somatic | |
| MS-S | |||
| TMB NR |
Abbreviations: MS-S , microsatellite stable; NR , not reported; TMB, tumor mutational burden.
Note: The bold values used to discern between MSI/TMB status and the mutated genes listed.
VUS, variant of unknown significance.
FIGURE 1.
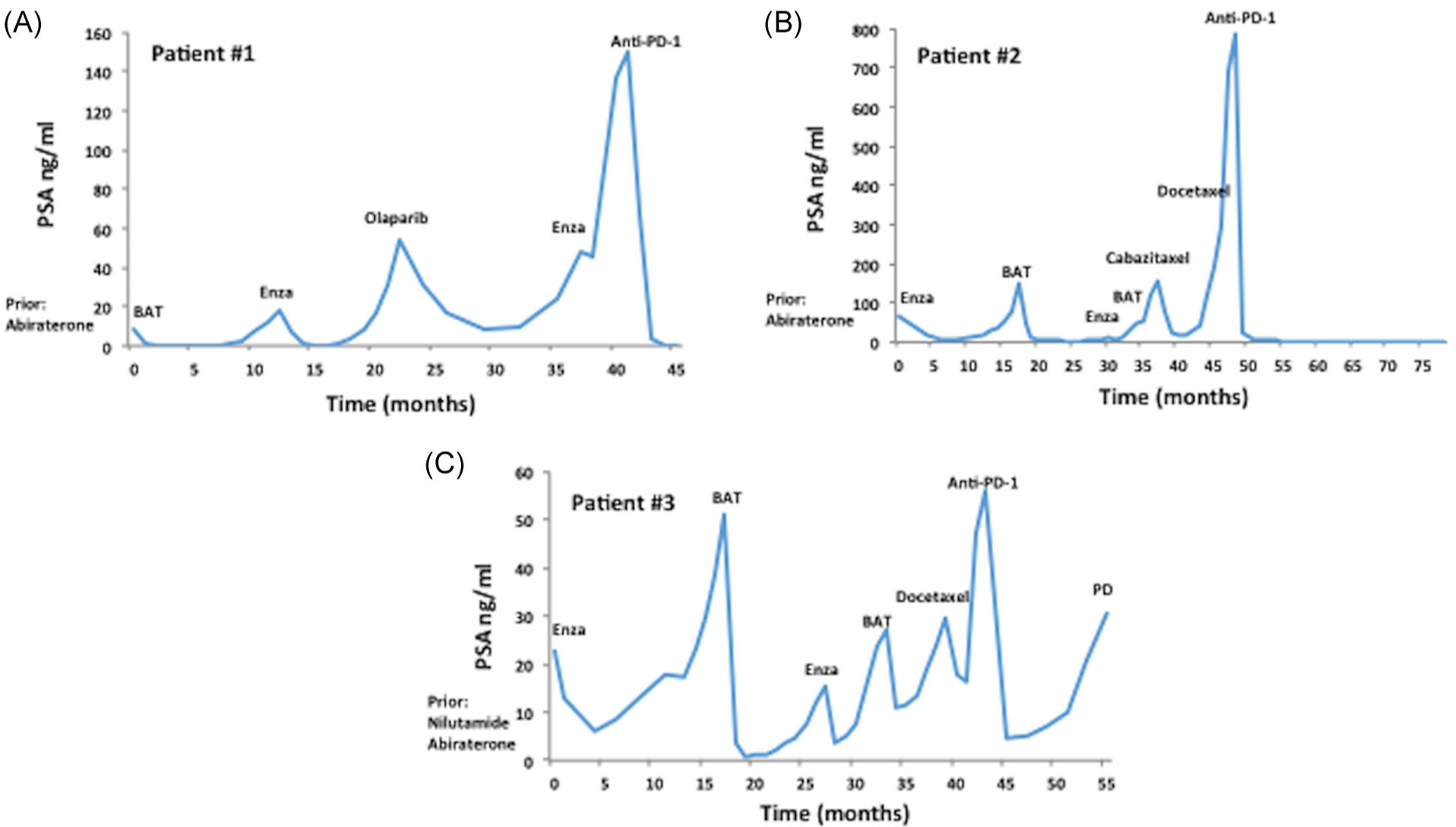
Clinical course of patients with mCRPC treated with immune checkpoint blockade. A, Patient 1 with mCRPC harboring a germline pathogenic BRCA2 mutation. BAT and enzalutamide were administered before immune checkpoint blockade. B, Patient 2 had mCRPC with an underlying inactivating somatic ATM mutation, who achieve a durable response on immunotherapy. He was treated with two cycles of enzalutamide followed by BAT. C, Patient 3 with mCRPC and two variants of unknown significance in BRCA2. This patient also was treated with two cycles of enzalutamide followed by BAT before anti-PD-1 therapy. BAT, bipolar androgen therapy; Enza, enzalutamide; mCRPC, metastatic castration resistant prostate cancer
Patient 2 had a long-standing history of Gleason 3 + 3 = 6 mCRPC. Clinical-grade germline genetic testing was negative for any pathogenic mutations. Commercially available molecular testing was performed on a paraspinal metastasis. Numerous somatic aberrations were identified, which included a pathogenic, frameshift mutation in ATM, an inactivating RB1 mutation, as well as AR and MYC amplification (Table 1). The tumor was microsatellite stable, and the TMB was not reported. No mutations were observed in DNA mismatch repair genes. The patient had an extensive treatment history for mCRPC (Figure 1B). Following abiraterone acetate, he was treated with BAT following progression on enzalutamide. He achieved a near-complete biochemical response as well as an objective partial response (by RECIST1.1 criteria) upon receiving BAT over a 12-month period. He was subsequently treated again with the enzalutamide followed by BAT with no clinical benefit. After progressing through two lines of different taxane chemotherapies, the patient was treated with an anti-PD-1 immune checkpoint inhibitor. His PSA nadired at 0.1 ng/mL, and he also achieved a partial radiographic response by (RECIST1.1 criteria) after 21 months of therapy (Figure 2). He self-discontinued the therapy and remains clinically stable without further treatment (except for medical castration) 12 months after stopping the immunotherapy.
FIGURE 2.

CT Imaging in patients with mCRPC before and during treatment with anti-PD-1 checkpoint inhibition. Patients 2 and 3 achieved a partial radiographic response to immune checkpoint blockade. Pretreatment and on-treatment representative CT images are shown. Red arrows indicate key target lesions. CT, computed tomography; mCRPC, metastatic castration resistant prostate cancer
Patient 3 had Gleason 4+3=7 mCRPC with multiple prior therapies. Cell-free tumor DNA sequencing from plasma showed several missense mutations including two mutations in BRCA2 that are considered variants of unknown significance and two inactivating TP53 mutations. His tumor was reported as microsatellite stable, and no TMB was provided. Interestingly, this patient had an inactivating mutation in a DNA methyltransferase, DNMT3A, and as well as an activating JAK2 (V617F) mutation, which may be indicative of an underlying myelodysplastic syndrome or clonal hematopoiesis. Similar to patient 2, enzalutamide and BAT were used in sequence (Figure 1C). Both agents induced a PSA response. This treatment paradigm (enzalutamide followed by BAT) was implemented on a second occasion, and again yielded a PSA response. After a short course of docetaxel for three cycles, which was discontinued due to toxicity, the patient was treated with an anti-PD-1 agent. Within 6 weeks, his PSA decreased by more than 90% and he achieved a partial objective response (via RECIST1.1 criteria) on his initial restaging (Figure 2). He maintained a partial response for 13 months, before discontinuing therapy due to eventual radiographic progression.
We identified 41 patients with mCRPC treated with immune checkpoint blockade at Johns Hopkins between 2014 and 2019. Patients were stratified by prior BAT treatment vs no prior BAT (Table 2). Six patients were identified as having prior BAT exposure. In addition to the three patients reported above, a fourth patient with mCRPC received BAT and achieved a PSA50 response to immune checkpoint blockade (N = 4/6 = 66.7%; P = 0.008 vs 11.4% in patients without prior BAT). This patient received enzalutamide after BAT, but before immunotherapy. Germline genetic testing was negative for pathogenic mutations. Somatic testing was not conducted. Thirty-five mCRPC patients treated with immune checkpoint inhibition did not receive BAT. Four of these 35 patients (N = 4/35 = 11.4%) achieved a PSA50 response to immunotherapy. One patient had a germline, pathogenic BRCA2 mutation and a second patient harbored an inactivating CDK12 mutation. Molecular testing was not performed in the remaining two patients.
TABLE 2.
PSA50 response following immune checkpoint blockade in mcrpc patients stratified by prior bipolar androgen therapy
| Prior BAT | No prior BAT | |
|---|---|---|
| (N = 6) | (N = 35) | |
| PSA50 response | 4 | 4 |
| No PSA50 response | 2 | 31 |
Abbreviations: BAT, bipolar androgen therapy; N, number of patients. P = .008
4 |. DISCUSSION
Identifying patients that will respond to immune checkpoint blockade with tumor types that are largely immunologically inert is an ongoing clinical challenge. The Food and Drug Administration’s approval of pembrolizumab based on a molecular phenotype (ie, mismatch repair deficient/microsatellite instability [MSI]-high status) rather than a specific tumor type was revolutionary. However, in each of the three cases described above, these tumors did not contain a mismatch repair gene mutation and were reported as microsatellite stable. However, there are a few caveats to this observation. First, it is possible that exon-only sequencing may have missed a structural rearrangement not present within the coding region of a mismatch repair gene. For instance, structural aberrations have been frequently identified in MSH2 and MSH6 in advanced prostate cancers.13 Since two of the three cases did not report TMB, it is also possible that a hypermutated tumor may have been missed, although this is unlikely given the microsatellite stability. Second, although each tumor was reported as microsatellite stable using clinical grade testing, this may have been a false negative. In a small case series of patients with mismatch repair-deficient prostate cancer, more than 25% of the patients did not have a single MSI marker shifted using the traditional five-marker NIH panel.14 An expanded MSI panel validated specifically in prostate cancer may be more sensitive for identifying MSI-high patients with mCRPC.15
Patients with mCRPC and an HR DNA repair gene mutation have responded favorably to the combination of nivolumab (anti-PD-1) plus ipilimumab (anti-CTLA4) in a small prospective trial.6 Patients 1 (germline BRCA2 alteration) and 2 (somatic ATM alteration) both had pathogenic HR DNA repair mutations. It is unclear from the commercially available molecular testing whether biallelic inactivation had occurred in each case. However, patient 1 did receive and respond favorably to a PARP inhibitor, suggesting that he had biallelic inactivation of BRCA2 and this fact may have predisposed this patient to clinical benefit from immunotherapy. Patient 2 has not received a PARP inhibitor at the time of this report. However, in Boudadi et al,6 two patients with mCRPC who harbored a somatic inactivating ATM mutation did not achieve a PSA response to combination immune checkpoint blockade. Finally, although patient 3 had two missense alterations in BRCA2 on cell-free DNA analysis, these were considered variants of unknown significance. Patient 3 received a PARP inhibitor following immune checkpoint blockade, but did not achieve any PSA or objective response, perhaps as anticipated. Therefore, whereas an HR mutation may have contributed to the immunotherapy response observed in patient 1, the inactivating ATM (patient 2), and nonpathogenic BRCA2 (patient 3) alterations would likely have had little impact on immunotherapy sensitivity.
We hypothesize that prior therapies may have predisposed these patients to a dramatic response to immune checkpoint blockade. Notably, we observed that all of these patients had prior therapy with both BAT and enzalutamide. Prior treatment with enzalutamide may predispose patients with mCRPC to respond to immune checkpoint blockade, as suggested by one small study.11 However, we postulate that the use of BAT may produce a similar immunological “priming” effect. Supraphysiologic androgens have been showed to induce double-strand DNA damage and genomic rearrangements, and also inhibit expression of DNA repair genes in prostate cancer cell lines.16,17 Moreover, patients with mCRPC that harbor DNA repair gene mutations were more likely to respond to BAT.17 We speculate that BAT, when used in sequence with enzalutamide, may promote dsDNA damage and STING activation by cytoplasmic DNA fragments, facilitating immune system activation, T cell infiltration, and durable clinical response to immune checkpoint inhibition.
A major weakness of our findings is the temporal relationship between BAT, enzalutamide, and the subsequent response to immune checkpoint inhibition. In each patient case, other therapies were administered between BAT and immunotherapy such as PARP inhibition (patient 1) or chemotherapy (patient 2). This makes it difficult to associate BAT (or enzalutamide) with response to anti-PD-1 therapy. However, we did observe a significantly higher PSA50 response rate to immunotherapy in patients with mCRPC that received prior BAT compared with those patients not treated with BAT. This finding suggests that BAT may be important as a priming agent before immunotherapy. We are formally testing the relationship between BAT and anti-PD-1 inhibition to validate the findings in this report. We are conducting an ongoing phase II study investigating the effect of BAT administered in sequence with nivolumab (NCT03554317) in patients with mCRPC who have developed progressive disease following at least one novel hormonal therapy and one taxane chemotherapy. We are collecting serial biopsies and blood samples to explore the mechanism of how BAT may induce more robust clinical responses to immunotherapy.
5 |. CONCLUSION
These cases of mCRPC presented here suggest that immune checkpoint blockade may have therapeutic potential in patients with prostate cancer, especially following immune activation (“priming”) using BAT. In this analogy, the immune checkpoint agent serves as the immunological “boost” required to incite an antitumor and clinical immune response. Further prospective investigation is needed to identify additional mechanisms of action explaining how immune checkpoint blockade can induce meaningful clinical responses in traditionally immunologically inert tumors.
ACKNOWLEDGMENTS
This study was supported by the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins NIH grants P30 CA006973 and PCF Young Investigator and Challenge Awards. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Funding information
Prostate Cancer Foundation, Grant/Award Numbers: Challenge Award, Young Investigator Award; NIH, Grant/Award Number: P30 CA006973
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1.Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nature Genet. 2019;51(2):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014; 505(7484):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40–47. [DOI] [PubMed] [Google Scholar]
- 4.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonarakis ES. A new molecular taxonomy to predict immune checkpoint inhibitor sensitivity in prostate cancer. Oncologist. 2019; 24(4):430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boudadi K, Suzman DL, Anagnostou V, et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget. 2018;9(47):28561–28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu YM, Cieslik M, Lonigro RJ, et al. Team PSCIPCD, Robinson DR, Chinnaiyan AM. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate. Cancer Cell. 2018;173(7): 1770–1782 e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonarakis ES. Cyclin-dependent kinase 12, immunity, and prostate cancer. N Engl J Med. 2018;379(11):1087–1089. [DOI] [PubMed] [Google Scholar]
- 9.Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017; 357(6349):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graff JN, Alumkal JJ, Drake CG, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016; 7(33):52810–52817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denmeade SR, Isaacs JT. Bipolar androgen therapy: the rationale for rapid cycling of supraphysiologic androgen/ablation in men with castration resistant prostate cancer. Prostate. 2010;70(14):1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pritchard CC, Morrissey C, Kumar A, et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonarakis ES, Shaukat F, Isaacsson Velho P, et al. Clinical features and therapeutic outcomes in men with advanced prostate cancer and dna mismatch repair gene mutations. Eur Urol. 2019;75(3):378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hempelmann JA, Lockwood CM, Konnick EQ, et al. Microsatellite instability in prostate cancer by PCR or next-generation sequencing. J Immunother Cancer. 2018;6(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haffner MC, Aryee MJ, Toubaji A, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nature Genet. 2010;42(8):668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee P, Schweizer MT, Lucas JM, et al. Supraphysiological androgens suppress prostate cancer growth through androgen receptor-mediated DNA damage. J Clin Invest. 2019;129:130–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]


