Abstract
Background
Diabetic nephropathy (DN) is the leading cause of impaired renal function. The purpose of this study was to investigate the effects of Mogroside IIIE (MG IIIE), a cucurbitane-type compound isolated from Siraitia grosvenorii, in high glucose (HG)-induced podocytes and the possible mechanisms.
Methods
MPC-5 cells were cultured under normal glucose or HG conditions. After treatment with MG IIIE, cell viability was examined using a cell counting kit-8 assay. The contents of inflammatory factors and oxidative stress-related markers were determined using the corresponding kits. Additionally, apoptosis of MPC-5 cells was determined using flow cytometry assay and the levels of apoptosis-associated proteins were evaluated by Western blot analysis. Moreover, the expression of proteins in AMPK/SIRT1 signaling was tested and the compound C, an AMPK inhibitor, was used to study whether the effects of MG IIIE on HG-induced MPC-5 cells were mediated by activation of the AMPK/SIRT1 signaling pathway.
Results
MG IIIE elevated the cell viability of HG-induced MPC-5 cells, reduced the concentrations of inflammatory cytokines and decreased the levels of oxidative stress-related markers. What’s more, the apoptosis of podocytes induced by HG was inhibited after MG IIIE intervention, accompanied by the upregulated expression of Bcl-2 and downregulated expression of Bax, cleaved caspase-3 and cleaved caspase-9. It was also found that MG IIIE could activate the AMPK/SIRT1 signaling, but compound C inhibited this pathway and reversed the inhibitory effects of MG IIIE on inflammation, oxidative stress and apoptosis in HG-stimulated podocytes.
Conclusion
MG IIIE can alleviate HG-induced inflammation and oxidative stress of podocytes by the activation of AMPK-SIRT1 signaling.
Keywords: podocytes, inflammation, oxidative stress, apoptosis, AMPK
Introduction
Diabetic nephropathy (DN) is named as a chronic complication of diabetes and it is characterized by proteinuria and progressive loss of renal function.1 DN is the leading cause of renal failure in adults and the elderly.2 Approximately one-third of patients with type I diabetes and half of the patients with type II diabetes are suffering from DN, which is clinically confirmed by the occurrence of renal dysfunction and increased excretion of proteinuria.3,4 To date, advances have been achieved in diabetes management, but the incidence of DN is still rising in our country. What’s more, molecular mechanism contributing to the occurrence of DN is not fully investigated yet. Mounting evidence supported that the function and structure podocyte injuries arise in the early stages of DN, which triggers the impairment of renal functions and results in the occurrence of proteinuria.5,6 Therefore, it is urgent to find an effective method to treat high glucose (HG)-induced podocytes injury.
Siraitia grosvenorii, produced in Guangxi province for over 200 years, is a perennial herb belonging to the cucurbitaceae family.7 Besides rich nutrients like vitamin C, it contains various constituents, such as flavonoids, triterpenoids and amino acids, and so on.8–10 Mogrosides, including Mogroside IV, IVE, V and IIIE, are the principal active extracts from S. grosvenorii saponins and have been approved by the US Food and Drug Administration as GRAS (generally recognized as safe) substances.7,11 Due to their sweet taste and nontoxic characteristic, they are used as beverages for patients who present with obesity and diabetes.12 Recently, their bioactivities including anti-bacterial, anti-allergic, anti-inflammatory and anti-diabetic effects have been reported.13 Mogroside IIIE (MG IIIE), the most widely distributed metabolite, can reduce blood glucose, and alleviate the clinical symptoms of pancreas at the early stage and pathological lesions.14,15 The treatment with MG IIIE can enhance the activation of AMP-activated protein kinase (AMPK), thereby decreased the levels of inflammatory cytokines to achieve the final goal of alleviating the symptoms of gestational diabetes.16 Furthermore, MG IIIE can alleviate the acute lung injury by inhibiting the activation of MAPKs and NF-ҡB signaling in lung tissues from lipopolysaccharide (LPS)-induced mice. Therefore, we speculated that MG IIIE might exert protective effects in HG-induced podocytes by a certain mechanism.
The aim of this study was to explore whether inflammation and oxidative stress of podocytes could be alleviated by MG IIIE in HG-induced podocytes and the underlying mechanism.
Materials and Methods
Cell Culture and Treatment
The mouse podocyte MPC-5 cell lines purchased from Cell Bank of the Chinese Academic of Sciences (Shanghai, China) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco; Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific) in a humidified incubator at 37°C containing 5% CO2.
Drug Treatment
Cells were maintained in medium containing normal glucose concentrations (NG, 5.5 mM glucose), 5.5 mM glucose+22.5 mM mannitol (MA) as the osmotic pressure control, or HG (25 mM glucose) at 37 °C for 24 hrs. MPC-5 cells were exposed to MG IIIE (Chengdu Biopurify Phytochemical Co., Ltd., Chengdu, China) for 24 h at the concentrations of 1, 10 or 50 μM after glucose challenge. Additionally, cells were pretreated with compound C (Selleck Chemicals, Houston, Texas), an AMPK inhibitor, for 2 h before exposure to MG IIIE.
Cell Counting Kit-8 (CCK-8) Assay
The cell viability was determined by CCK-8 kit (Dojindo Molecular Technologies, Gaithersburg, MD, USA). Briefly, the cells were seeded in a 96-well plate (5 × 104 cells per well) for 24 hrs with different glucose concentrations, followed by treatment with MG IIIE for another 24 hrs. Then, 10 μL of the CCK-8 reagent was added into each well and cultured at 37°C for 3 hrs. Subsequently, the optical density at a wavelength of 450 nm was detected using the microplate reader (Bio-Rad Laboratories, Richmond, CA, USA).
Test for the Concentrations of Inflammatory Factors
The levels of inflammatory factors including tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 in culture medium samples were determined with Enzyme-linked immunosorbent (ELISA) kits (Shanghai Xitang Biotechnology Co., Ltd., Shanghai, China) according to the manufacturer’s instructions.
Detection of the Levels of Oxidative Stress-Related Markers
The content of malondialdehyde (MDA) and the activities of superoxide dismutase (SOD) and catalase (CAT) were detected using the specific commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s recommendations. Subsequently, the absorbance was measured by a microplate reader (Bio-Rad Laboratories, Richmond, CA, USA).
Flow Cytometry Analysis
Cells were washed twice with PBS. Subsequently, 250 μL binding buffer was used to suspend cells to adjust cell concentration to 5×105/mL. 200 μL cell suspension was added with 10 μL Annexin-FITC and 10 μL of 20 μg/mL propidium iodide (PI) (BD Biosciences, Franklin Lakes, NJ, USA) and incubated at room temperature in the dark for 10 min. Then, the harvested cells were re-suspended in a 500 µL binding buffer. The cell apoptosis was analyzed by flow cytometry (BD Biosciences, NJ, USA). The percentage of apoptotic cells was analyzed using FlowJo software (Becton-Dickinson-San Jose CA, USA).
Western Blot Analysis
The protein was extracted from cells using a RIPA lysis buffer (Beyotime Institute of Biotechnology, Nanjing, China) and quantified using a BCA protein assay kit (Thermo Fisher Scientific, Inc.). The same amount of protein was subjected to 10% SDS-PAGE electrophoresis. Then, the separate protein was transferred onto PVDF membranes (EMD Millipore, Billerica, MA, USA) and blocked in 5% non-fat milk for 1hr. The transferred membranes were then incubated in the primary antibodies at 4°C overnight, followed by being washed with Tris-buffered saline with Tween (TBST) and incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling Technology, Inc.) at room temperature for 1.5 h. The protein bands were visualized using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Amersham, UK) and quantified using NIH ImageJ software (ImageJ, Bethesda, MD). The protein expression of the bands was normalized against the gray value of GAPDH.
Statistical Analysis
The experimental results were generated from three independent repeats (N=3). All values were presented as mean ± standard deviation. Statistical differences between groups were analyzed by Student’s t-tests. Statistical comparisons among multiple groups were conducted using analysis of variance (ANOVA) followed by Tukey’s post hoc test. P < 0.05 was considered to show a statistical significance.
Results
MG IIIE Elevates the Cell Viability of HG-Induced Podocytes
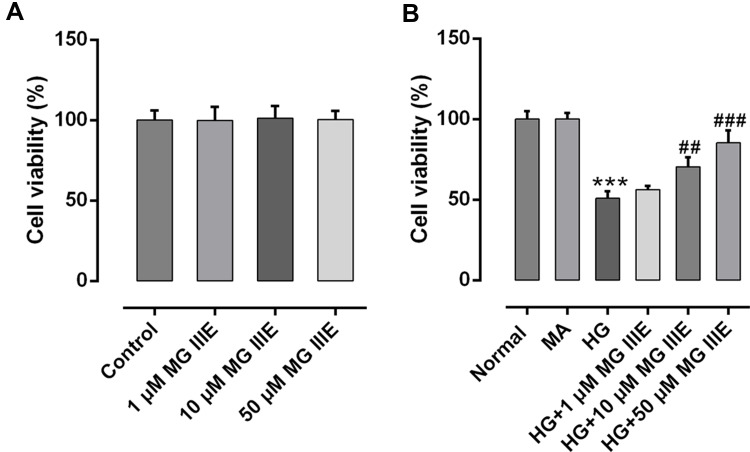
The cell viability was detected on normal podocytes and podocytes treated with different concentrations of MG IIIE. As shown in Figure 1A, the cell viability showed no significance between the groups. Then, HG (30 μM) was used to construct the podocyte model, while mannitol and normal glucose (5.5 μM) were seen as MA group and control group, respectively. Figure 1B exhibits no significance between the control group and MA group. What is more, HG challenge significantly decreased the cell viability of podocytes, while MG IIIE increased the cell viability in a dose-dependent manner, indicating that MG IIIE could recover the cell viability once significantly damaged by HG. Then, MG IIIE with the dose of 50 μM was chosen for the next experiments due to its relative high restorative effect.
Figure 1.
MG IIIE increased the cell viability of HG-induced podocytes. (A) Cell viability was evaluated by a cell counting kit-8 assay after MG IIIE treatment. (B) A cell counting kit-8 assay was employed to determine viability of HG-induced podocytes in the presence or absence of MG IIIE. ***P<0.001 vs. MA; ##P<0.01 and ###P<0.001 vs. HG.
Abbreviations: MG IIIE, Mogroside IIIE; NG, normal glucose; HG, high glucose; MA, mannitol.
MG IIIE Alleviates Inflammation and Oxidative Stress of HG-Induced Podocytes
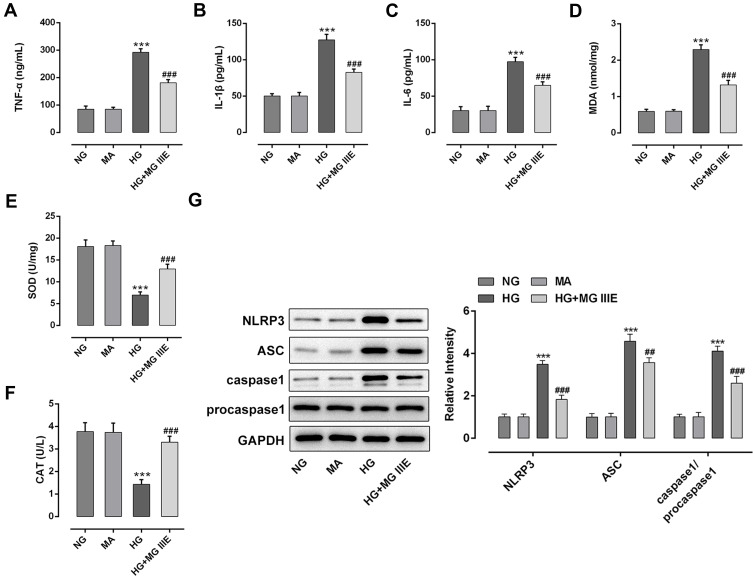
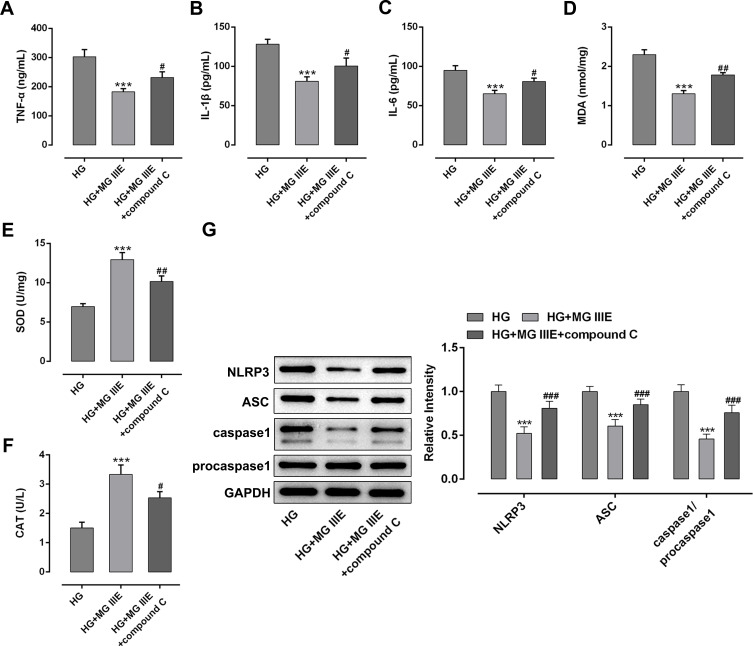
To explore the anti-inflammatory effect of MG IIIE in HG-induced podocytes, the contents of inflammation-related cytokines were detected by ELISA assay. As exhibited in Figure 2A–C, the levels of TNF-α, IL-1β and IL-6 were notably elevated after HG exposure, but MG IIIE dramatically reversed these effects. Similarly, significantly increased MDA content and decreased SOD and CAT activities were noticed in the HG group compared with the MA group (Figure 2D–F). However, the levels of the above-mentioned oxidative stress-related markers were reversed by MG IIIE. Additionally, HG-stimulation remarkably upregulated the expression of NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and caspase1 while MG IIIE intervention dramatically restored these changes (Figure 2G). These results suggested that MG IIIE could alleviate inflammation and oxidative stress of HG-induced podocytes.
Figure 2.
MG IIIE alleviated inflammation and oxidative stress of HG-induced podocytes. The contents of inflammatory factors including (A) TNF-α, (B) IL-1β and (C) IL-6 were examined using ELISA kits, respectively. The level of (D) MDA and the activities of (E) SOD and (F) CAT were assessed using commercially available kits, respectively. (G) Western blot analysis was employed to measure the expression of MCP-1, NLRP3, ASC and caspase1 proteins. ***P<0.001 vs. MA; ##P<0.01, ###P<0.001 vs. HG.
Abbreviations: MG IIIE, Mogroside IIIE; NG, normal glucose; HG, high glucose; MA, mannitol; TNF-α, tumor necrosis factor-α; IL, interleukin; ELISA, enzyme-linked immunosorbent assay; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase.
MG IIIE Reduces the Cell Apoptosis of HG-Induced Podocytes
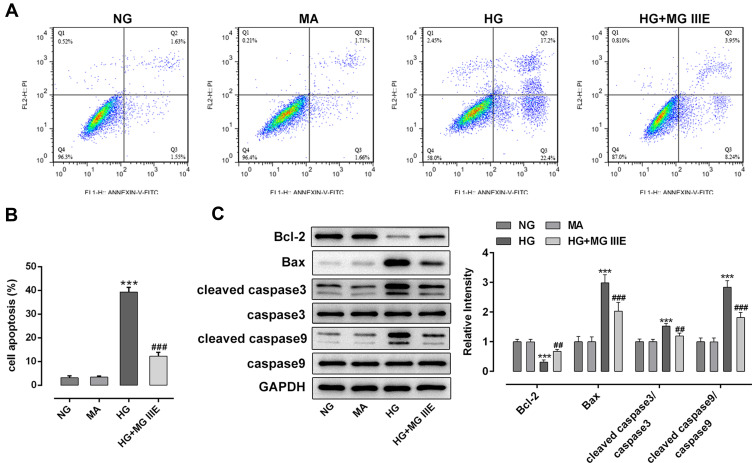
Next, the cell apoptosis of podocytes and apoptosis-related proteins were respectively detected by flow cytometry and Western blot analysis. Results of Figure 3A and B exhibited that the cell apoptosis was significantly increased after the induction of HG into podocytes, when compared with the control group. However, the addition of MG IIIE in HG-induced podocytes showed a marked decrease in cell apoptosis relative to the HG group. Consistently, the expression of Bcl-2 was notably downregulated, accompanied by upregulated expression of Bax, cleaved caspase-3 and cleaved caspase-9 in the HG condition, while MG IIIE treatment attenuated the effects of HG on the levels of fore-mentioned apoptosis-associated proteins (Figure 3C).
Figure 3.
MG IIIE attenuated the cell apoptosis of HG-induced podocytes. (A) Apoptotic cells were detected via flow cytometry. (B) The apoptotic rate of podocytes was quantified. (C) The expression of apoptosis-related proteins were evaluated using Western blot analysis. ***P<0.001 vs. MA; ##P<0.01, ###P<0.001 vs. HG.
Abbreviations: MG IIIE, Mogroside IIIE; NG, normal glucose; HG, high glucose; MA, mannitol.
Therefore, it could be confirmed that MGIIIE could reduce the cell apoptosis of HG-induced podocytes.
MG IIIE Exerts Protective Effects on the HG-Induced Podocytes by Activating the AMPK/SIRT1 Signaling Pathway
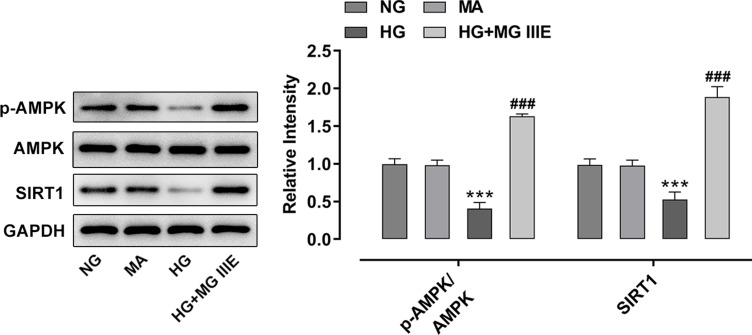
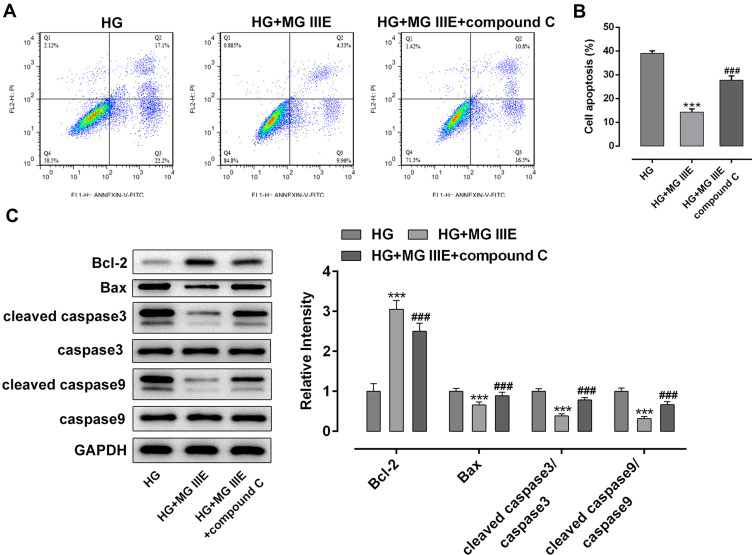
To explore the mechanism by which MG IIIE exerts its protective effects on HG-induced podocytes, the expressions of AMPK and silent information regulator T1 (SIRT1) were examined using Western blot analysis. It was observed that HG induction significantly decreased the expression of p-AMPK and SIRT1 while MG IIIE reversed this effect, suggesting that the AMPK/SIRT1 signaling pathway that was once inhibited by HG could be reactivated by MG IIIE (Figure 4). Then, after pretreatment with Compound C, an AMPK inhibitor, for 1 h, the HG-stimulated podocytes with 50 μM MG IIIE administration exhibited enhanced contents of inflammatory factors when compared with the HG+50 μM MG IIIE group (Figure 5A–C). Similar results could also be found in Figure 5D–G, which demonstrated that the levels of oxidative stress were reduced after HG-induced podocytes were administrated with 50 μM MG IIIE. Nevertheless, when cells were pretreated with Compound C for 1 h, they exhibited higher levels of oxidative stress than those without Compound C pretreatment. Moreover, as it is observable from Figure 6, Compound C intervention remarkably elevated the number of apoptotic cells, coupled with downregulated Bcl-2 expression and upregulated Bax, cleaved caspase-3 and cleaved caspase-9 expression in HG-stimulated podocytes. Summing up, these data provide evidence that MG IIIE could exert protective effects on the HG-induced podocytes by activating the AMPK/SIRT1 signaling pathway.
Figure 4.
MG IIIE activated AMPK/SIRT1 signaling pathway in HG-induced podocytes. Western blot analysis was applied for detecting the expression of p-AMPK and AMPK. ***P<0.001 vs. MA; ###P<0.001 vs. HG.
Abbreviations: MG IIIE, Mogroside IIIE; NG, normal glucose; HG, high glucose; MA, mannitol; AMPK, AMP-activated protein kinase; SIRT1, silent information regulator T1.
Figure 5.
MG IIIE mitigated inflammation and oxidative stress by activating AMPK/SIRT1 signaling pathway in HG-induced podocytes. The levels of (A) TNF-α, (B) IL-1β and (C) IL-6 were determined using ELISA kits, respectively. The content of (D) MDA and the activities of (E) SOD and (F) CAT were evaluated using commercially available kits, respectively. (G) The expression of MCP-1, NLRP3, ASC and caspase1 proteins was examined by Western blot analysis. ***P<0.001 vs. HG; #P<0.05, ##P<0.01, ###P<0.001 vs. HG+ MG IIIE.
Abbreviations: MG IIIE, Mogroside IIIE; HG, high glucose; TNF-α, tumor necrosis factor-α; IL, interleukin; ELISA, enzyme-linked immunosorbent assay; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase.
Figure 6.
MG IIIE relieved apoptosis of HG-induced podocytes by activating AMPK/SIRT1 signaling pathway. (A) Apoptotic cells were evaluated via flow cytometry. (B) The apoptotic rate of podocytes was quantified. (C) The expression of apoptosis-related proteins were evaluated using Western blot analysis. ***P<0.001 vs. MA; ###P<0.001 vs. HG.
Abbreviations: MG IIIE, Mogroside IIIE; HG, high glucose.
Discussion
DN is a common complication of diabetes with high mortality.17 Mounting evidence supported that the function and structure podocyte injuries arise in the early stages of DN, which triggers the impairment of renal functions and results in the occurrence of proteinuria.5,6 Traditional medicine plays excellent effects on the treatment of DN. The present study aimed to identify the effects of MG IIIE, which has been reported to play protective effects on various metabolic disorders, on HG-induced podocytes in order to find a new strategy to ameliorate podocyte injury during DN.
Both inflammation and oxidative stress are reported to be closely associated with HG-induced podocyte injury in the development of DN.18,19 The amplified and continued production of inflammatory cytokines including TNF-α, IL-1β and IL-6 can be induced by exposure of podocytes to HG.20 Additionally, NLRP3 inflammasome is known to act as a sensor and is shown to be involved in inflammatory responses.21 Recently, the NLRP3 inflammasome pathway, which is recognized as key pathophysiology of DN, has greatly caught the public’s attention.22,23 The NLRP3 inflammasome serves as an intracellular inflammatory machinery, which can be activated by HG condition.24 Due to the lack of caspase recruitment domain, NLRP3 needs to be combined with ASC to recruit pro caspase1 and cut into mature caspase1 to participate in the release of inflammatory factors.25,26 In the present study, the protective effect and the underlying mechanism of MG IIIE on podocytes treated with HG were studied in vivo to investigate the pathogenesis of DN and the possible novel therapies for it. It was found that MG IIIE could attenuate inflammation and oxidative stress caused by HG, which was correspondent with the findings of previous studies,15,16 by downregulation of NLRP3, ASC and caspase1 expression. Moreover, accumulating evidence has strongly suggested that HG stimulation markedly enhanced the apoptotic rate of podocytes via induction of inflammation and oxidative stress.27,28 Remarkably reduced apoptotic podocytes were observed after MG IIIE treatment, accompanied by increased expression of Bcl-2 and decreased expression of Bax, cleaved caspase-3 and cleaved caspase-9. These findings provided a clue that MG IIIE protects against HG-induced podocytes injury.
To further explore the mechanism underlying the crucial role of MG IIIE on DN, we investigated whether MG IIIE exerted its effect on the AMPK/SIRT1 signaling pathway. A previous study reported that glycyrrhizic acid, a triterpenoid saponin glycoside that is recognized as the most efficacious component of the licorice plant, exerts a protective effect from DN in db/db mice by activating the AMPK/SIRT1/PGC-1α signaling pathway.29 Moreover, some researchers identified that by activating the renal AMPK/SIRT1 signaling pathway, salidroside could alleviate the symptoms of diabetic nephropathy in rats.30 AMPK is a highly conserved serine/threonine kinase that serves as an energy-sensing enzyme.31 It is responsible for the energy metabolism either by promoting the production of energy or by inhibiting energy-consuming pathways. Damaged AMPK activities can trigger insulin resistance.32 Therefore, AMPK has always been associated with diseases regarding metabolic syndrome, such as obesity and diabetes.33 SIRT1, the downstream effector of AMPK, shares commonalities with AMPK in metabolism and cellular survival.34 It is involved in the modulation of the inflammatory response through deacetylating target molecules, thus provoking the suppressed activities of transcription factors, altered chromatin conformation, and finally transcriptional suppression of genes related to inflammation.35 AMPK can suppress inflammatory responses via SIRT1, and SIRT1 can prepare the cells to survive under stress conditions.36 Existing study has shown that MG IIIE can enhance the activation of AMPK to achieve the final goal of alleviating the symptoms of gestational diabetes.16 In this study, the elevated expressions of AMPK and SIRT1 by MG IIIE treatment indicated that the AMPK/SIRT1 signaling pathway was activated, resulting in the alleviation of inflammation, oxidative stress and cell apoptosis. Previous studies confirmed the role of MG IIIE in activating this signaling pathway, thereby inhibiting the production of pro-inflammatory cytokines.37 Compound C, an AMPK inhibitor, significantly reversed the inhibitory effects of MG IIIE on inflammation, oxidative stress and apoptosis of HG-stimulated podocytes, which further verified that MG IIIE mediated the AMPK/SIRT1 signaling pathway to protect against HG-induced podocytes injury.
Conclusion
In conclusion, these findings demonstrated that MG IIIE can alleviate HG-induced inflammation, oxidative stress and apoptosis of podocytes by the activation of AMPK-SIRT1 signaling pathway. Further investigations about the effects of MG IIIE on DN in vivo and other kinds of cells in kidney tissue, such as renal mesangial cells, macrophages, etc., will be performed in the future investigations, which are limitations of the current study.
Funding Statement
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Lu ZY, Liu N, Wang F. Epigenetic regulations in diabetic nephropathy. J Diabetes Res. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ioannou K. Diabetic nephropathy: is it always there? Assumptions, weaknesses and pitfalls in the diagnosis. Hormones. 2017;16(4):351–361. [DOI] [PubMed] [Google Scholar]
- 3.Thomas MC, Weekes AJ, Broadley OJ, Cooper ME, Mathew TH. The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study). Med J Aust. 2006;185(3):140–144. doi: 10.5694/j.1326-5377.2006.tb00499.x [DOI] [PubMed] [Google Scholar]
- 4.Dwyer JP, Parving HH, Hunsicker LG, Ravid M, Remuzzi G, Lewis JB. Renal dysfunction in the presence of normoalbuminuria in type 2 diabetes: results from the DEMAND Study. Cardiorenal Med. 2012;2(1):1–10. doi: 10.1159/000333249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99(2):342–348. doi: 10.1172/JCI119163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai RY, Jiang JJ. LncRNA ANRIL silencing alleviates high glucose-induced inflammation, oxidative stress, and apoptosis via upregulation of MME in podocytes. Inflammation. 2020;9. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Dai L, Liu Y, Dou D, Sun Y, Ma L. Pharmacological activities of mogrosides. Future Med Chem. 2018;10(8):845–850. doi: 10.4155/fmc-2017-0255 [DOI] [PubMed] [Google Scholar]
- 8.Li C, Lin LM, Sui F, et al. Chemistry and pharmacology of Siraitia grosvenorii: a review. Chin J Nat Med. 2014;12(2):89–102. [DOI] [PubMed] [Google Scholar]
- 9.Qing ZX, Zhao H, Tang Q, et al. Systematic identification of flavonols, flavonol glycosides, triterpene and siraitic acid glycosides from Siraitia grosvenorii using high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a screening strategy. J Pharm Biomed Anal. 2017;138:240–248. doi: 10.1016/j.jpba.2017.01.059 [DOI] [PubMed] [Google Scholar]
- 10.Zhou G, Wang M, Li Y, Peng Y, Li X. Rapid and sensitive analysis of 27 underivatized free amino acids, dipeptides, and tripeptides in fruits of Siraitia grosvenorii swingle using HILIC-UHPLC-QTRAP((R))/MS (2) combined with chemometrics methods. Amino Acids. 2015;47(8):1589–1603. doi: 10.1007/s00726-015-2002-5 [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Wang C, Qi X, Zou J, Sun Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J Food Sci Technol. 2018;55(5):1880–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Song Y, Ding Y, et al. Effects of mogrosides on high-fat-diet-induced obesity and nonalcoholic fatty liver disease in mice. Molecules. 2018;23(8):1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di R, Huang MT, Ho CT. Anti-inflammatory activities of mogrosides from Momordica grosvenori in murine macrophages and a murine ear edema model. J Agric Food Chem. 2011;59(13):7474–7481. doi: 10.1021/jf201207m [DOI] [PubMed] [Google Scholar]
- 14.Xu F, Li DP, Huang ZC, et al. Exploring in vitro, in vivo metabolism of mogroside V and distribution of its metabolites in rats by HPLC-ESI-IT-TOF-MS(n). J Pharm Biomed Anal. 2015;115:418–430. doi: 10.1016/j.jpba.2015.07.024 [DOI] [PubMed] [Google Scholar]
- 15.Tao L, Cao F, Xu G, Xie H, Zhang M, Zhang C. Mogroside IIIE attenuates LPS-induced acute lung injury in mice partly through regulation of the TLR4/MAPK/NF-kappaB axis via AMPK activation. Phytother Res. 2017;31(7):1097–1106. doi: 10.1002/ptr.5833 [DOI] [PubMed] [Google Scholar]
- 16.Zou C, Zhang Q, Zhang S. Mogroside IIIE attenuates gestational diabetes mellitus through activating of AMPK signaling pathway in mice. J Pharmacol Sci. 2018;138(3):161–166. doi: 10.1016/j.jphs.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 17.Morigi M, Perico L, Corna D, et al. C3a receptor blockade protects podocytes from injury in diabetic nephropathy. JCI Insight. 2020;5(5):5. doi: 10.1172/jci.insight.131849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang DZ, Jin MY, Zhao XY, et al. FGF1(Delta HBS) ameliorates chronic kidney disease via PI3K/AKT mediated suppression of oxidative stress and inflammation. Cell Death Dis. 2019;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou HH, Yang PP, Huang TL, Zheng XX, Xu GS. PLK2 plays an essential role in high D-glucose-induced apoptosis, ROS generation and inflammation in podocytes. Sci Rep. 2017;7:14. doi: 10.1038/s41598-017-00686-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Ding XQ, Gu TT, et al. Pterostilbene and allopurinol reduce fructose-induced podocyte oxidative stress and inflammation via microRNA-377. Free Radic Biol Med. 2015;83:214–226. doi: 10.1016/j.freeradbiomed.2015.02.029 [DOI] [PubMed] [Google Scholar]
- 21.Jiang T, Jiang D, Zhang L, Ding M, Zhou H. Anagliptin ameliorates high glucose- induced endothelial dysfunction via suppression of NLRP3 inflammasome activation mediated by SIRT1. Mol Immunol. 2019;107:54–60. doi: 10.1016/j.molimm.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 22.Xiong W, Meng XF, Zhang C. Inflammasome activation in podocytes: a new mechanism of glomerular diseases. Inflamm Res. 2020;69(8):731–743. doi: 10.1007/s00011-020-01354-w [DOI] [PubMed] [Google Scholar]
- 23.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Shen XF, Liu J, et al. High glucose mediates NLRP3 inflammasome activation via upregulation of ELF3 expression. Cell Death Dis. 2020;11(5):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakker PJ, Butter LM, Kors L, et al. Nlrp3 is a key modulator of diet-induced nephropathy and renal cholesterol accumulation. Kidney Int. 2014;85(5):1112–1122. doi: 10.1038/ki.2013.503 [DOI] [PubMed] [Google Scholar]
- 26.Hong JN, Li GB, Zhang QH, Ritter J, Li WW, Li PL. D-ribose induces podocyte NLRP3 inflammasome activation and glomerular injury via AGEs/RAGE pathway. Front Cell Dev Biol. 2019;7:13. doi: 10.3389/fcell.2019.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu BC, Song X, Lu XY, et al. High glucose induces podocyte apoptosis by stimulating TRPC6 via elevation of reactive oxygen species. Biochim Biophys Acta. 2013;1833(6):1434–1442. doi: 10.1016/j.bbamcr.2013.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhan X, Yan C, Chen Y, et al. Celastrol antagonizes high glucose-evoked podocyte injury, inflammation and insulin resistance by restoring the HO-1-mediated autophagy pathway. Mol Immunol. 2018;104:61–68. doi: 10.1016/j.molimm.2018.10.021 [DOI] [PubMed] [Google Scholar]
- 29.Yao Y, Wang L, Jin P, et al. Methane alleviates carbon tetrachloride induced liver injury in mice: anti-inflammatory action demonstrated by increased PI3K/Akt/GSK-3beta-mediated IL-10 expression. J Mol Histol. 2017;48(4):301–310. [DOI] [PubMed] [Google Scholar]
- 30.Shati AA. Salidroside ameliorates diabetic nephropathy in rats by activating renal AMPK/SIRT1 signaling pathway. J Food Biochem. 2020;44(4):e13158. doi: 10.1111/jfbc.13158 [DOI] [PubMed] [Google Scholar]
- 31.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144(12):5179–5183. doi: 10.1210/en.2003-0982 [DOI] [PubMed] [Google Scholar]
- 32.Lage R, Dieguez C, Vidal-Puig A, Lopez M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14(12):539–549. doi: 10.1016/j.molmed.2008.09.007 [DOI] [PubMed] [Google Scholar]
- 33.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89(3):1025–1078. doi: 10.1152/physrev.00011.2008 [DOI] [PubMed] [Google Scholar]
- 34.Ruderman NB, Xu XJ, Nelson L, et al. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298(4):E751–E760. doi: 10.1152/ajpendo.00745.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teng L, Fan L, Peng Y, et al. Carnosic acid mitigates early brain injury after subarachnoid hemorrhage: possible involvement of the SIRT1/p66shc signaling pathway. Front Neurosci. 2019;13:26. doi: 10.3389/fnins.2019.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32(5):1183–1194. doi: 10.1002/stem.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao L, Cao F, Xu G, Xie H, Zhang M, Zhang C. Mogroside IIIE Attenuates LPS-Induced Acute Lung Injury in Mice Partly Through Regulation of the TLR4/MAPK/NF-kappaB Axis via AMPK Activation. Phytother Res. 2017;31(7):1097–1106 [DOI] [PubMed]