Abstract
The global burden of infections and the rapid spread of viral diseases show the need for new approaches in the prevention and development of effective therapies. To this end, we aimed to explore novel inhibitor compounds that can stop replication or decrease the viral load of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), for which there is currently no approved treatment. Besides using the angiotensin-converting enzyme (ACE2) receptor as a main gate, the CoV-2 can bind to the glucose-regulating protein 78 (GRP78) receptor to get into the cells to start an infection. Here, we report potential inhibitors comprising small molecules and peptides that could interfere with the interaction of SARS-CoV-2 and its target cells by blocking the recognition of the GRP78 cellular receptor by the viral Spike protein. These inhibitors were discovered through an approach of in silico screening of available databases of bioactive peptides and polyphenolic compounds and the analysis of their docking modes. This process led to the selection of 9 compounds with optimal binding affinities to the target sites. The peptides (satpdb18674, satpdb18446, satpdb12488, satpdb14438, and satpdb28899) act on regions III and IV of the viral Spike protein and on its binding sites in GRP78. However, 4 polyphenols such as epigallocatechin gallate (EGCG), homoeriodictyol, isorhamnetin, and curcumin interact, in addition to the Spike protein and its binding sites in GRP78, with the ATPase domain of GRP78. Our work demonstrates that there are at least 2 approaches to block the spread of SARS-CoV-2 by preventing its fusion with the host cells via GRP78.
Keywords: SARS-CoV-2, Spike, inhibitors, small molecule, peptide, GRP78
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new virus strain of the Coronaviridae family. Coronaviruses (CoVs) are enveloped viruses with a single-stranded positive-sense RNA containing approximately 30 000 base pairs (bps).1 CoVs are involved in many diseases affecting the upper respiratory tract, gastrointestinal system, and central nervous system in humans and animals.1 Two groups of proteins characterize CoVs: structural proteins, including Spike (S), envelope (E), membrane (M) and nucleocapsid (N),2-4 and nonstructural proteins, such as proteases (nsp3 and nsp5) and RdRp (nsp12).5,6 The CoV S glycoprotein is a precursor protein composed of 1300 amino acids, located on the outer envelope of the virion. It plays an essential role in the attachment, fusion, and entry of the virus into the host cells. Particularly, S protein mediates the fusion of the viral and the cellular membranes 7 by binding to different surface receptors of the host cells via its receptor-binding domain (RBD). The transmembrane (S) spike trimeric glycoprotein of the virus consists of 2 functional subunits: S1 and S2. The first, which made up of 4 domains (S1 A, S1 B, S1 C, and S1 D), contributes to the attachment of the virus to the host cell receptor. Then, S2 coordinates the fusion of the 2 membranes.8 This fusion activates proteases and allows the proteolytic cleavage of the S protein.9 The latter causes conformational changes to prime the S2 subunit for the fusion of viral and cell membranes.8 Thus, RBD is the key component by which the virus is translocated inside the cells through its interactions with the angiotensin-converting enzyme (ACE2),10,11 dipeptidyl peptidase-4 (DPP4) and or glucose-regulating protein 78 (GRP78).12
The SARS-CoV-2 RBD contains 13 disulfide bonds that correspond to 13 cyclic regions considered to be similar to the cyclic form of Pep42.13 These regions can interact with the GRP78 cell surface.
The GRP78 or binding immunoglobulin protein (BiP) is a member of the heat shock protein family.14 It is traditionally regarded as a major chaperone of the endoplasmic reticulum (ER) that facilitate protein folding and assembly, protein quality control, Ca2+ binding, and regulating ER stress signaling.15 When GRP78 moves to the surface of the cell, it can interact with many ligands or other proteins like a multifunctional receptor. It is therefore involved in inflammatory and autoimmune diseases16-18 and overexpressed in various human cancers, including prostate cancer, breast cancer, lung cancer, melanoma, and ovarian cancer.19-21 In addition to its role in the proliferation, invasion, and metastasis of many cancer cells, GRP78 also has a sensitivity to the recognition of viruses through its substrate-binding domain (SBD)6 and is involved in the assembly of their envelope proteins.22-25 Recently, GRP78 has been recognized as an attachment factor for the Middle East respiratory syndrome coronavirus (MERS-CoV), which improves the entry of the virus in the presence of DPP4,12 and as one of the SARS-CoV entry receptors in the human cell.1
Most research carried out, or measures taken, are reposed on the idea that ACE2 is the primary receptor of SARS-CoV-2.10,11 Providing GRP78 as a second receptor in the presence of special physiological conditions when the expression of GRP78 is high,16-21 this could potentially be more favorable to infection with SARS-CoV-2. Thus, the presence of ACE2 and GRP78 at a high quantity could classify these additional categories to very high-risk types. As there are no efficacy tools to fight against this disease, it is therefore essential to find out how to protect persons with chronic illnesses (cancer, diabetes, and high blood pressure). These people are at greater risk of developing an aggressive form of SARS-CoV-2 infection.
Like SARS-CoV and MERS-CoV, SARS-CoV-2 is a member of Betacoronavirus5,26 which also causes a severe respiratory tract infection with a higher mortality rate.12,27 Sequence and structure analysis of S proteins from different CoV strains showed that the specificity of interaction between S proteins and their receptors is the main determinant of the host tropism of these viruses.28 The main cellular receptors for viral S proteins include ACE2 and DPP4, as well as other molecules that may be involved in the interaction between the virus and the host cell.29,30 It has been suggested that GRP78 may also act as another receptor that assists SARS-CoV-2 to penetrate the host cells.13
This same proposition has confirmed by Aguiar et al,31 who concluded that the GRP78-binding site overlaps with the ACE2-binding site, although the residues involved in the interactions may be a little different. For this purpose, a targeted analysis of the expression of candidate genes involved in SARS-CoV-2 infection confirmed the presence of the GRP78 protein in vitro in epithelial cells of the human respiratory tract and lung tissue.31 This analysis suggests that for ACE2 to be an integral receptor for SARS-CoV-2, there are probably other mechanisms dynamically regulating the expression of ACE2 in the respiratory mucosa in the context of infection with SARS-CoV-232 and/or possibly other co-receptors. These can contribute to the functioning of ACE2 and TMPRSS2 in the binding and fusion of SARS-CoV-2 in this cell type.31 In the same context, Palmeira et al33 have verified that the level of expression of the GRP78 gene is high in the blood of patients with SARS-CoV-2 pneumonia.
The entry of the virus is a key step that can be targeted by a possible therapeutic strategy opting for the prediction of a molecule capable of causing simultaneous inhibition of the 2 proteins.34,35 Based on the knowledge gained about the SARS-CoV-2 virus, 2 axes of investigation were explored in this study to prevent the translocation of the virus inside host cells. The first was the inhibition of GRP78 recognition by SARS-CoV-2, by targeting both the nucleotide-binding domain (NBD) and SBD of GRP78 with ATP-competitive small molecules and/or peptides. The second consisted of the inhibition of the viral S protein of SARS-CoV-2 at the GRP78-binding site. Finally, we identified 9 compounds that could act as potential blockers of SARS-CoV-2 cell entry through GRP78 for further consideration as possible therapies against this virus.
Materials and Methods
Ligands retrieval
A library of 100 active phytochemicals (polyphenols)36-39 was generated from PubChem database (pubchem.ncbi.nlm.nih.gov). Biologically active peptides were obtained from the Structurally Annotated Therapeutic Peptides Database (SATPdb) (https://doi.org/10.1093/nar/gkv1114).
Targeted docking sites on the GRP78
Two binding domains characterize the structure of GRP78. The first is the NBD that houses ATP, whereas the second (SBD) receives the substrate (peptide or protein) in the form of an excluded segment or partially folded protein.40 The inhibition of the ATP-binding site interrupts the functional cycle of the protein by modifying its conformation, which may lead to the inhibition of viral penetration.
ATP docking was conducted on GRP78 (PDB ID:5F1X) structure to identify the key residues of its ATPase site.41 The residues (ASP-34, THR-38, TYR-39, ILE-61, GLU-201, ASP-224, PHE-258, GLY-228, GLY-249, ASP-250, GLY-364, SER-365, ILE-368, and ASP-391) were used as references for the search space determination. Similarly, it was considered advisable to prevent the Spike from being attached to the GRP78 by directly targeting their interaction site. The GRP78 residues involved in Spike binding are Ile-426, Thr-428, Val-429, Val-432, Thr-434, Phe-451, Ser-452, Val-457, and Ile-459 as was previously described.14
Target sites on SARS-CoV-2 Spike
According to the study by Ibrahim et al,13 GRP78 binds to the SARS-CoV-2 Spike in 4 regions, in which regions III (C391-C525) and IV (C480-C488) showed stronger affinities for the Spike. Consequently, it was proposed in our study to target these 2 regions to prevent any possibility of Spike binding to the GRP78 receptor.
Preparation of molecular docking files
Structures of the GRP78 (PDB ID: 5E84) and SARS-CoV-2 Spike (PDB ID: 6LZG) were downloaded from the RCSB PDB database (http://www.rcsb.org/pdb/home.do) in PDB format. The small-molecule SDF files (molecule.sdf) downloaded in the previous step were converted to PDB format using Open Babel.42 All inhibitors and receptors have been optimized and converted to PDBQT format using the standard protocol of the Autodock Tools.43
Molecular docking
Molecular docking of small molecules (ATP and inhibitors) was performed using AutoDock Vina 1.1.2. Docking of ATP on GRP78 was conducted to determine the key residues of its ATPase site. Docking search space was defined on each targeted site of GRP78 and Spike proteins as explained previously. The ClusPro server44,45 was used for peptide-protein docking of the selected peptides. The resulting peptide-protein complexes were ranked by cluster size and visual inspection. Small molecules and peptides were selected for each protein site based on their estimated binding energy and the protein residues with which they are to interact.
Postdocking analysis and visualization
Visual analysis of docking poses and image rendering was performed using PyMOL version 2.3 (Schrodinger, LLC).
Results and Discussion
The global burden of infections and the rapid spread of viral diseases point to the need for new preventive approaches and more effective therapeutic strategies. Therefore, particular priority is being given to the emerging SARS-CoV-2, which is considered a pandemic under current circumstances. In this direction, our study focused on the repositioning of approved drugs as well as the investigation of other bioactive compounds that may prevent the penetration of SARS-CoV-2 into host cells by targeting the region of GRP78 that is required for the interaction with the Spike protein of the virus. Two factors have guided our compound selection process. The first is the identification of peptides that can block the interaction of the SARS-CoV-2 Spike and GRP78, and the second is the screening of small molecules suitable for docking inside GRP78’s ATP-binding pocket to inhibit its activity and thus its interaction with the Spike.
The effect of the selected phytochemicals and peptides on SARS-CoV-2 Spike protein and GRP78 was elucidated by molecular docking analysis. The promising candidates were selected based on their binding mode and affinity against the 2 targets.
Peptide-protein docking
Peptide-protein docking performed between the peptides and their designated targets. The peptides docked against the Spike protein and its binding sites in GRP78. Results from their binding modes analysis were favorable, indicating that the selected compounds could potentially inhibit or prevent the entry of the virus. The sequences of the selected peptides are presented in Table 1.
Table 1.
Set of peptides selected to inhibit spike-SARS-CoV-2 and GRP78.
| Peptides ID | Sequence | Function |
|---|---|---|
| satpdb12488 | PTTFMLKYDENGTITDAVDC | Antiviral, antimicrobial |
| satpdb14438 | SNNTIAIPTNFSISITTEVM | Antiviral, antimicrobial |
| satpdb28899 | RDVSDFTDSVRDPKTSEILD | Antiviral, antimicrobial |
| satpdb18674 | QYGSFCTQLNRALSGIAAEQ | Antiviral, antimicrobial |
| satpdb18446 | VLYNSTFFSTFKCYGVSATK | Antiviral, antimicrobial |
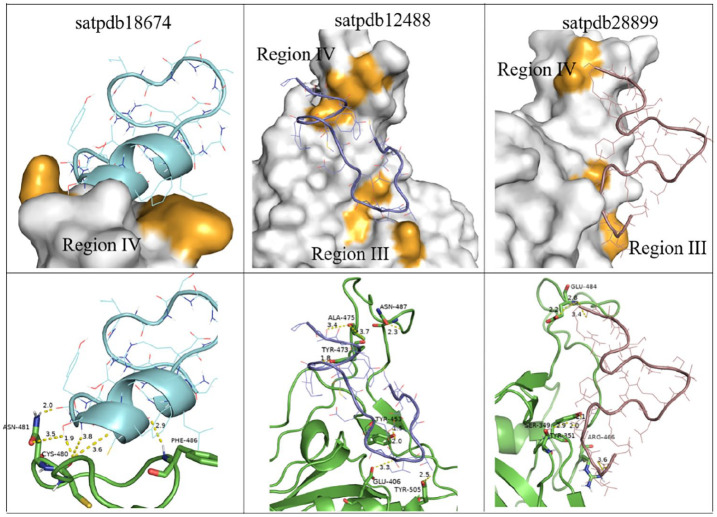
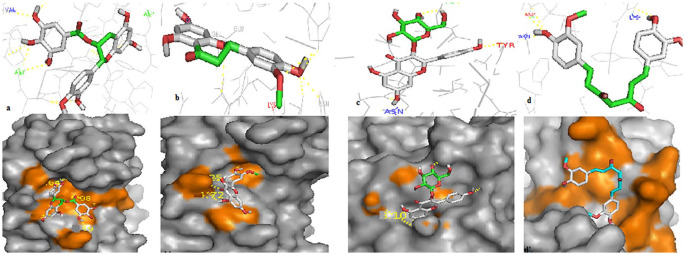
The peptide satpdb18674 selected to target the Spike protein was mainly bound to region IV (C480-C488), whereas satpdb12488 and satpdb28899 showed more affinity to region III (C391-C525) (Figure 1). Thus, this could directly interfere with the Spike/GRP78 interaction as both regions are necessary for the Spike to recognize the GRP78.
Figure 1.
Binding modes of 3 candidate peptides housed at the GRP78-binding regions on SARS-CoV-2 Spike (PDB ID: 6LZG). The spike is represented here by white surface (top) and green cartoon (bottom). The interacting residues of the Spike protein are colored in orange in the surface representation. The peptide satpdb18674 residues SER-4, TYR-2, and PHE-5 interacted with the CYS-480, ASN-481, and PHE-486 of region IV of the Spike. Satpdb12488 residues MET-5, PHE-4, THR-13, ASP-16, THR-2, THR-3, and TYR-15 interacted with TYR-473, TYR-453, ALA-475, and ARG-403 from region III and satpdb28899 (ARG-1, ASP-20, LEU-19, GLU-17, THR-15, and SER-16) interacted with GLU-484, ARG-466, TYR-351, SER-349, and ARG-346 from the same region. GRP78 indicates glucose-regulating protein 78; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
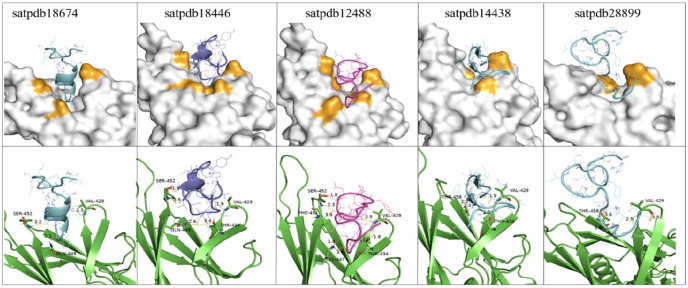
Peptides reported as inhibitors of GRP78 (satpdb18674, satpdb18446, satpdb12488, satpdb14438, and satpdb28899) were mainly bound to the region I426-I459 (Figure 2). This region of GRP78 appears to be critical for the Spike to attach to the host cell.
Figure 2.
Binding modes of the 5 candidate peptides housed in the substrate-binding region of GRP78 (PDB: 5E84). They are represented here by white surface (top) and green cartoon (bottom). The interacting residues of GRP78 are colored in orange in the surface representation. The interaction of the satpdb18674 peptide residues (GLN-1, SER, and THR-7) with the residues (VAL-429, GLN-449, and SER-452) of the GRP78-binding site. The peptide satpdb18446 residues TYR-3, ASN-4, VAL-1, and THR-10 interacted with THR-434, VAL-429, GLN-449, and SER-452 of the same target. For satpdb12488 residues, ASP-19, THR-2, ASP-16, and MET-5 interacted with the THR-434, VAL-429, LYS-447, PHE-451, and SER-452 of the GRP78. The residues (ILE-13, SER-12, and THR-9) of satpdb14438 interacted with GRP78 residues THR-434, VAL-429, and THR-458. Finally, the peptide satpdb28899 residues ASP-20, LEU-19, SER-16, and THR-15 interacted with VAL-429 and THR-458 from the same region. The 5 compounds established multiple hydrogen bonds with the residues of the GRP78 substrate-binding pocket. Interactions with V429 and S452 were observed in the 5 peptides, except for satpdb28899 (V429 only). satpdb12488, satpdb14438, and satpdb18446 interacted with THR-434; satpdb14438 and satpdb28899 interacted with GLN-449; satpdb14438 and satpdb28899 interacted with THR-458; and satpdb12488 interacted with LYS-447 and PHE-451. GRP indicates glucose-regulating protein.
These results revealed that the peptides satpdb18674, satpdb12488, and satpdb28899 had strong affinities simultaneously with the Spike and with both regions of GRP78 (regions III and IV). We therefore hypothesize that these peptides can prevent the entry of the virus by binding either to regions III and IV of the Spike or to their binding site in GRP78.
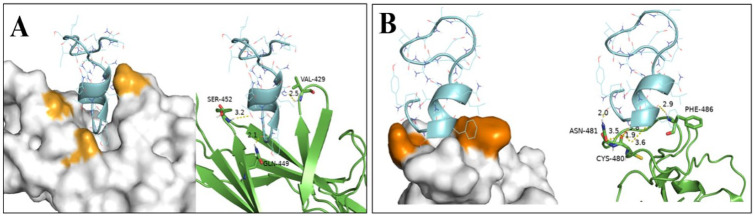
The peptide satpdb18674 was particularly bound to the key residues (Figure 3) at the GRP78 target site (VAL-429, GLN-449, and SER-452) and to those in the region IV of the Spike (CYS-480, ASN-481, and PHE-486). This region showed a strong affinity for GRP78, reason why we consider satpdb18674 to be the most appropriate peptide to block any potential binding of the Spike protein to the host cell via GRP78.
Figure 3.
Modes of satpdb18674 binding to Spike protein and GRP78. (A) The interacting satpdb18674 residues GLN-1, SER-4, and THR-7 with the residues of the GRP78 site (VAL-429, GLN-449, and SER-452). (B) Interacting satpdb18674 residues SER-4, TYR-2, and PHE-5 with the residues from region IV of the Spike (CYS-480, ASN-481, and PHE-486). The interacting residues of GRP78 are colored in light orange, and those of the Spike are colored in dark orange (surface representation). The satpdb18674 peptide interacted, respectively, with the residues of the GRP78 site and those of the region IV of Spike. GRP78 indicates glucose-regulating protein 78.
Analysis of the molecular interactions of the peptides stabilizes the target protein by interacting with the residues of their different binding pockets. Several hydrogen bonds have been observed, ensuring strong bonds with the spike binding site residues (regions III and IV) and with those of GRP78. Table 2 illustrates the details of the interactions between the proteins and peptides selected.
Table 2.
Recapitulating table details the size and bonds types of different residues between the targets (SARS-CoV-2 peak, GRP78) and the peptides (satpdb18674, satpdb18446, and satpdb12488, satpdb14438, and satpdb28899).
| Peptide | Target |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Interaction residues Peptides-GRP78 |
Interaction residues Peptides-Spike |
||||||||
| Peptide | GRP78 | Interaction size (A) | interaction Types | Peptide | Spike |
Interaction size (A) | interaction Types | ||
| Region III | Region IV | ||||||||
| Satpdb12488 | ASP-19 | THR-434 | 2; 1.8; 2.6 | O-NH; OH-O; O-O | MET-5 | TYR-473 | – | 1.8; | OH-O |
| THR-2 | VAL-429 | 2.5 | NH-O | PHE-4 | TYR-473 | 3.2 | OH-O | ||
| ASP-16 | LYS-447 | 1.8; 2.7 | NH-O. N-O | THR-13 | TYR-453 | 2.8 | OH-O | ||
| ALA-17 | LY-447 | 3.4 | NH-NH | ASP-16 | TYR-453 | 3.8 | OH-O | ||
| MET-5 | SER-452 | 1.8, 2.6 | OH-O; O-O | THR-13 | ARG-403 | 2.2 | NH-O | ||
| 2.3, 3.9 | NH-O; O-O | THR-2 | ALA-475 | 3.4 | O-O | ||||
| PHE-4 | SER-452 | 2.3 | NH-O | THR-3 | ALA-475 | 3.7 | O-O | ||
| TYR-15 | TYR-505 | 3.6 | OH-O | ||||||
| GLU-10 | LYS-417 | 3.0 | O-NH | ||||||
| Satpdb14438 | ILE-13 | THR-434 | 1.9 | OH-O | – | – | – | – | – |
| 2.9 | O-O | ||||||||
| SER-12 | VAL-429 | 1.9; 2.9 | NH-O; O-O | ||||||
| THR-9 | THR-458 | 2.2; 3.1; 3.3 | NH-O; N-N;O-O | ||||||
| Satpdb18446 | TYR-3 | THR-434 | 3.4 | O-OH | – | – | – | – | – |
| ASN-4 | VAL-429 | 1.9; 2.8 | NH-O; O-N | ||||||
| TYR-3 | GLN-449 | 2.6; 1.9; 3.6 | NH-OH: NH-O | ||||||
| N-OH | |||||||||
| VAL-1 | SER-452 | 3.9 | NH-NH | ||||||
| THR-10 | SER-452 | 1.9; 2.8 | O-OH; O-O | ||||||
| Satpdb18674 | GLN-1 | VAL-429 | 2.5; 2.5; 2.9 | NH-NH; 2H-N | SER-4 | – | PRO-479 | 1.9; 2.8 | OH-O; O-O |
| SER-4 | GLN-449 | 2.1; 2.8 | NH-O; N-O | PHE-5 | PRO-479 | 3.6 | NH-O | ||
| THR-7 | SER-452 | 3.2 | NH-O | SER-4 | CYS-480 | 3.8 | O-NH | ||
| SER-4 | ASN-481 | 3.5 | OH-O | ||||||
| TYR-2 | ASN-481 | 2.0; 3 | NH-O; O-N | ||||||
| PHE-5 | PHE-486 | 2.9 | NH-O | ||||||
| Satpdb28899 | ASP-20 | VAL-429 | 3.9 | O-O | ARG-1 | GLU-484 | – | 2.2; 2.8; 3.4 | NH-N; 2NH-O |
| LEU-19 | VAL-429 | 2.9; 3.4 | NH-O; O-N | ASP-20 | ARG-466 | 1.7; 2-6; | NH-O; N-O | ||
| SER-16 | THR-458 | 1.8; 2.6 | OH-O; O-O | LEU-19 | ARG-466 | 3.6 | NH-O | ||
| THR-15 | THR-458 | 3.6 | NH-O | GLU-17 | TYR-351 | 2.1; 3 | OH-O; O-O | ||
| THR-15 | TYR-351 | 3.7 | OH-O | ||||||
| GLU-17 | SER-349 | 2.9 | OH-O | ||||||
| SER-16 | ARG-346 | 2.0; 2.6 | NH-O; N-O | ||||||
Abbreviations: GRP78, glucose-regulating protein 78; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Small molecules and protein docking
The screening of polyphenols was performed against the Spike protein, its binding site in GRP78, and the ATPase domain of GRP78. Postdocking analysis showed favorable binding modes, indicating that the compounds epigallocatechin gallate (EGCG), homoeriodictyol, isorhamnetin, and curcumin could bind simultaneously to the 3 sites.
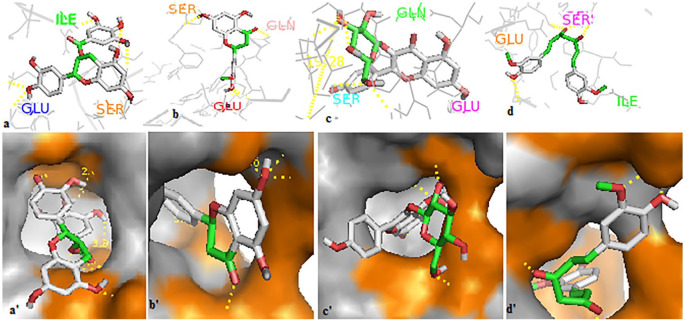
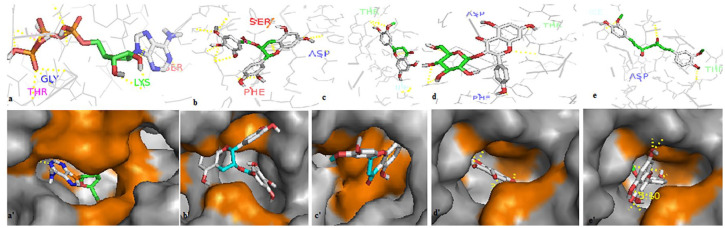
The 4 polyphenols have established multiple hydrogen bonds with the residues of the GRP78 pocket (Figure 4). Also, these compounds showed high affinity toward the regions III and IV of the Spike protein (Figure 5). The docking results of the compounds EGCG, homoeriodictyol, isorhamnetin, and curcumin in the ATP-binding site of GRP78 showed their favorable positioning inside the receptor’s cavity, mimicking ATP in its mode of interaction with the ATPase domain of GRP78 (Figure 6) with binding affinities higher than or close to that of ATP. Therefore, these compounds could potentially interfere with the virus entry to the host cells. Finally, as CoVs acquire the ability to bind to multiple receptors, and cross the interspecies barrier, this work could provide new insights in the development of effective therapies against SARS-CoV-2 infections.
Figure 4.
Modes of polyphenols binding on the GRP78 site. The active site in GRP78 is colored orange in (a′, b′, c′, and d′). The 4 compounds established multiple hydrogen bonds with the residues at the active site of GRP78. All the compounds interacted with the Glu-427 residue, and other interactions were observed with Ser-455, Ser-452, Ile-450, Ala-454, Gln-458 for EGCG (a, a′). Homoeriodictyol (b, b′) interacted with Gly-430, Ile-450, and Val-429; isorhamnetin (c, c′) interacted with Gly-430, Ser-448, and Gln-458; and curcumin (d, d′) interacted with Ser-425, Ile-450, and Thr-441. The best affinity was attributed to EGCG (–10.5 kcal/mol). EGCG interacts through the H bonds with Glu-427, Gln-458, Ser-455, Ala-454 and a hydrophobic bond with Ile-450; homoeriodictyol and isorhamnetin establish the same H bonds with Glu-427, Glu-430, Ser-448, and Gln-485; curcumine interact with 2 residues Glu-427 and Ile-450 through the H bonds and 2 hydrophobic bonds through Ser-425 and Thr-441. EGCG indicates epigallocatechin gallate; GRP78, glucose-regulating protein 78.
Figure 5.
Modes of polyphenols binding in the GRP78/SARS-CoV-2 Spike interaction site (Spike side). The binding region of the Spike is colored in orange. The 4 compounds established multiple hydrogen bonds with the residues at the binding region of the Spike. Interactions with Lys-417, Asp-420, Asn-422, and Tyr-421 were observed for all compounds: EGCG (a, a′), homoeriodictyol (b, b′), curcumin (d, d′), except isorhamnetin (c, c′) that established H bonds with Pro-463 and Tyr-505 instead the Asn-420 and Lys-417; EGCG also interacted with Asn-460 and Lys-464. EGCG indicates epigallocatechin gallate; GRP78, glucose-regulating protein 78; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 6.
Binding modes of the 4 selected candidates in the substrate-binding site of GRP78 ATPase domain (PDB: 5F1X). (a, a′) ATP; (b, b′) EGCG; (c, c′) homoeriodictyol; (d, d′) isorhamnetin; and (e, e′) curcumin. The ATP docking was used to define the key residues in the catalytic site (Asp-34, Thr-38, Thr-39, Ile-61, Glu-201, Asp-224, Phe-258, Gly-228, Gly-249, Asp-249, Gly-364, Ser-365, Ile-368, and Asp-39). The active site is colored in orange. The 4 molecules have established at least 6 hydrogen bonds with these residues in GRP78. The best affinity was attributed to EGCG (–10.2 kcal/mol), higher than that of ATP (–9.6 kcal/mol). All molecules interact through hydrogen bonds with Gly-228, Phe-258, Asp-224 residues; EGCG established 3 other H bonds with Gly-364, Ser-365, and Ile-368. Homoeriodictyol, isorhamnetin, and curcumine interact through hydrophobic bonds with Thr-38 and Thr-39. EGCG indicates epigallocatechin gallate; GRP78, glucose-regulating protein 78.
The set of interactions between the small molecules and the active sites of the 2 targets (ATPase domain, GRP78/spike interaction sites) is summarized in Table 3. The best affinity was attributed to EGCG at all the interaction sites. Binding to EGCG regularly folds the protein while ATP binding unfolds the protein. A slightly unfolded form is the native functional form of GRP78. Thus, due to the link of the EGCG and the movements with SBD, GRP78 will not be able to perform its appropriate functions given the changes in conformation. This may be one of the likely mechanisms by which the EGCG inhibitor acts on the full-length GRP78 protein. The several researches show that polyphenols as an antiviral and anti-inflammatory agent that can be helpful for both prevention and treatment of new emerging CoV.46 However, well-designed clinical trials are needed to demonstrate the potential efficacy of curcumin against SARS-CoV-2 infection and its ensuing complications.47
Table 3.
Interaction residues between targets (SARS-CoV-2 Spike, GRP78) and small molecules (EGCG, homoeriodictyol, isorhamnetin, and curcumin).
| Interaction sites | Small molecule |
||||
|---|---|---|---|---|---|
| EGCG | Homoeriodictyol | isorhamnetin | Curcumin | ||
| ATPase domain of GRP78 | Docking score | −10.2 kcal/mol | −9.0 kcal/mol | −8.8 kcal/mol | −8.2 kcal/mol |
| Residues | Gly-228, Phe-258, Gly-364, Ser-365, Ile-368, Asp-390 | Thr-38, Thr-39; Ile-61, Glu-201, Asp-224, Phe-258, Gly-228, Glu-249, Asp-249 | Thr-38, Thr-39, Ile-61, Glu-201, Asp-224, Phe-258 | Thr-39, Ile-61, Glu-201, Asp-224, Phe-258, Glu-228 | |
| GRP78 (Spike Binding Site) |
Docking score | −10.5 kcal/mol | −8.1 kcal/mol | −7.2 kcal/mol | −7.7 kcal/mol |
| Residues | Ser-455, Ser-452, Glu-427, Ile-450, Ala-454, Gln-458 | Glu-427, Glu-430, Ser-448, Gln-458 | Glu-427, Gly-430, Ser-448, Gln-458 | Glu-427, Ser-425, Ile-450, Thr-441 | |
| Spike (GRP78 Binding Site) |
Docking score | −10.0 kcal/mol | −7.5 kcal/mol | −7.9 kcal/mol | −7.9 kcal/mol |
| Residues | Asn-422, ASP-420, Tyr-421, Lys-417, Asn-460, Lys-464, Val-427 | Asn-420, Asp-420, Tyr-421, Lys-417 | Tyr-421, Lys-417, Pro-463, Tyr-505 | Asn-420, Asp-420, Tyr-421, Lys-417 | |
Abbreviations: EGCG, epigallocatechin gallate; GRP78, glucose-regulating protein 78; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The table details the hydrogen and hydrophobic links between the 2 targets and the small molecules.
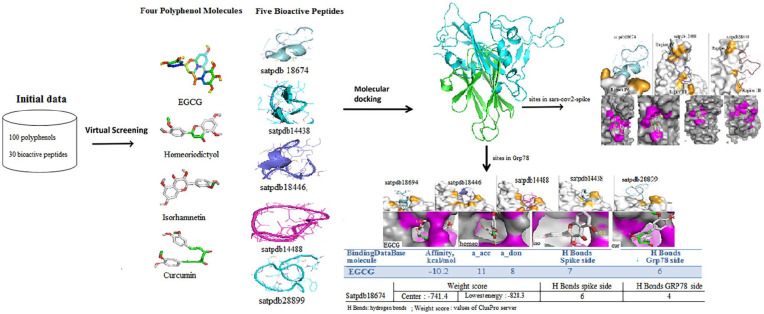
Current drugs have limited efficacy in the treatment of SARS-CoV-2, given the high number of deaths caused by this virus. Designing new drugs that target specific activities of this virus or stop the stages of its infection cycle is a crucial step. Due to the diverse behavior of SARS-CoV-2 depending on the population type, the production of an appropriate vaccine against this disease is difficult, hence the need for new therapeutic strategies,48 proposing compounds affecting different stages of the virus’s life cycle, and strategies that target the entry of the virus on the ACE2 side and others on the SARS protease side.49 Our study will complement these strategies by targeting the different sites involved in the SARS/GRP78 interaction (Figure 7). The neglect of the latter causes a high risk of infections. The set of molecules (4 polyphenols and 5 bioactive peptide) was selected in this study to inhibit the SARS-CoV-2 Spike/GRP78 interaction. Satpdb18674 and EGCG gave the best results for all the targeted sites for the 2 proteins. Our results, therefore, meet an urgent need, given the absence of an effective drug available. As demonstrated in this combined approach, screening and reassigning bioactive molecules to prevent the entry of this virus on the Spike/GRP78 side can provide an accelerated approach to identify and develop new treatments for SARS-CoV-2 infection.
Figure 7.
The strategy description targeting the different sites involved in the SARS/GRP78 interaction. Chemical molecules have been extracted from compound libraries (PubChem and SATPdb). Screening of these molecules (100 polyphenols and 40 peptides) was carried out by the docking method by AutoDock Vina and ClusPro server. This method offers a precise and efficient anchoring tool, which is based on empirical notation functions. It is based on the average binding energy scores in AutoDock Vina,43 and the root-mean-square deviation (RMSD) in ClusPro to generate clusters with the most probable models of the complex. Selection of structures based on energy minimization. GRP78 indicates glucose-regulating protein 78.
Inhibition of the interaction between the spike protein SARS-CoV-2 and the receptor by blocking the GRP78 is a strategy interesting to identify drugs that decrease the rate of viral infection.13 Several studies have given confirmatory evidence to the presence of the GRP78 protein in patients infected with SARS-CoV-2.31-33 In addition, GRP78 is part of receptors that allow the virus to enter and facilitate the initial infection of host cells,31 the reason why it has become a molecular target to treat COVID-19. These results, as well as those of the aforementioned studies, allow us to suppose that the GRP78 protein could be a receptor for SARS-CoV-2 and to propose molecules capable of hindering this interaction. Reducing the level of expression or inhibition of GRP78 by small interfering RNAs or by siRNAs, respectively, blocked the entry of Japanese encephalitis virus50 and Ebola virus replication.51 A study recently showed by virtual screening that there are molecules that can in silico inhibit the GRP78 protein and prevent virus binding.33 Interestingly, our results also suggest a compound capable to simultaneously inhibit the 3 binding sites that allow entry of SARS-CoV-2 via GRP78.
The peptides proposed in this work targets 2 regions of the S protein (III and IV) and 1 of GRP78. This interaction could interfere with or competitively bind to the S protein S sites of SARS-CoV and GRP78. These results suggest a novel inhibitor mechanism distinct from the anti-SARS-CoV peptide that could disrupt the virus-host cell interaction. We propose, therefore, to combine 2 or 3 of these peptides before or after infection with the virus.
Inhibition of ATPase activity of the GRP78 NBD52 is another potential inhibitory mechanism of SARS-CoV-2 infection. Studies have previously reported that after competitive inhibition at the ATP-binding site of GRP78, the SBD of the latter adopts a conformation having a low affinity for the substrates, thus blocking their transport.53 Binding of various inhibitors to the ATP-binding site established by crystallographic studies54 allows altering the function of GRP78 and, therefore, theoretically avoids infection with SARS-CoV-2.
Pretreatment of GRP78 with the small molecule EGCG inhibited the Ebola virus infection,51 what supports our results obtained where EGCG is suggested to have an inhibitory effect on GRP78 with high affinity (–10.5 kcal/mol).
Interestingly, a recent study showed by molecular docking that certain natural products (estrogen and phytoestrogens) could interfere with the attachment of SARS-CoV-2 to stressed cells.55 As for the natural products (EGCG, homoeriodictyol, isorhamnetin, and curcumin) selected in our study, they could also interfere with this attachment.
There are several proofs on the antiviral potential of herbal compounds.56 Pharmacological investigations have revealed many pharmacological activities for certain phytochemicals as the EGCG, and Curcumin. Reducing the charge and inhibiting the expression of viral core proteins are the main effects of polyphenols.57,58 These effects are of great interest for the development of synthetic drugs against SARS-CoV-2-specific polyphenols. The inhibitory effect of curcumin on SARS-CoV replication has been demonstrated by Wen et al59. Likewise, the impact of curcumin on cells before or after a viral infection reach the infectivity of certain viruses.60 Recently, mooring studies revealed that curcumin could potentially inhibit ACE2 to remove COVID-19 entry in the cell (potential effect). These results and that shown in our work are proof that confirms the potential role of curcumin as a promising antiviral agent.
GRP78 has a strong affinity for hydrophilic or hydrophobic regions, such as those of the SARS-CoV-2 spike.13 As GRP78 can act as a co-receptor for SARS-CoV-2,61,62 compounds with a high affinity could hinder this interaction, by competing with the viral spike55; this specificity increases the probability of the compounds selected in our study to rival with the Spike and GRP78, thus preventing the entry of SARS-CoV-2 and the resulting infection.
Conclusions
Drug repositioning is an effective and rapid strategy for providing therapeutic solutions to COVID-19. In silico approaches can be very useful in identifying new indications for approved drugs whose pharmacokinetic data are already known, allowing them to move rapidly to the final phases of clinical trials.
In this work, we identified 4 phytochemicals (polyphenols) that could potentially inhibit the interaction of SARS-CoV-2 Spike protein with GRP78, in addition to 5 peptides (satpdb18674, satpdb18446, satpdb12488, satpdb14438, and satpdb28899) that target simultaneously the Spike protein and its binding region in GRP78. The satpdb18674 and EGCG gave the best results for all of the targeted sites for the 2 proteins. As these results appear very promising, further bioassays are needed to confirm the inhibitory activity of these compounds against SARS-CoV-2 infection.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was carried out under National Funding from the Moroccan Ministry of Higher Education and Scientific Research (PPR program) to AI. This work was also supported by a grant to AI from Institute of Cancer Research of the foundation Lalla Salma.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: LA: Conceptualization, Methodology, Writing original draft, Writing-review and Editing.
FG: Conceptualization, Methodology, Writing original draft, Writing-review and Editing.
MK: Methodolgy, Writing-review and editing.
N el H: Writing-review and editing.
R el J: Writing-review and editing.
J el H: Writing-review and editing.
BI: Writing-review and editing.
LB: Writing-review and editing.
AI: Supervisor.
ORCID iDs: Hakmi Mohammed  https://orcid.org/0000-0002-1548-3792
https://orcid.org/0000-0002-1548-3792
Badreddine Lmimouni  https://orcid.org/0000-0001-9163-4911
https://orcid.org/0000-0001-9163-4911
References
- 1. Gallagher TM, Buchmeier MJ. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu X, Wang Y, Liu Y, Chen H, Hu Y. Response of bacterial and fungal soil communities to Chinese fir (Cunninghamia lanceolate) long- monoculture plantations term. Front Microbiol. 2020;11:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Du L, Tai W, Zhou Y, Jiang S. Vaccines for the prevention against the threat of MERS-CoV. Expert Rev Vaccines. 2016;15:1123-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou Y, Jiang S, Du L. Prospects for a MERS-CoV spike vaccine. Expert Rev Vaccines. 2018;17:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elfiky AA, Mahdy SM, Elshemey WM. Quantitative structure-activity relationship and molecular docking revealed a potency of anti-hepatitis C virus drugs against human corona viruses. J Med Virol. 2017;89:1040-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ibrahim IM, Abdelmalek DH, Elfiky AA. GRP78: a cell’s response to stress. Life Sci. 2019;226:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saghazadeh A, Rezaei N. Towards treatment planning of COVID-19: rationale and hypothesis for the use of multiple immunosuppressive agents: anti-antibodies, immunoglobulins, and corticosteroids. Int Immunopharmacol. 2020;84:106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763-1767. [DOI] [PubMed] [Google Scholar]
- 11. Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu H, Chan C-M, Zhang X, et al. Middle East respiratory syndrome coronavirus and bat coronavirus HKU9 both can utilize GRP78 for attachment onto host cells. J Biol Chem. 2018;293:11709-11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020;80:554-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang J, Nune M, Zong Y, Zhou L, Liu Q. Close and allosteric opening of the polypeptide-binding site in a human Hsp70 chaperone BiP. Structure. 2015;23:2191-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373-381. [DOI] [PubMed] [Google Scholar]
- 16. Quinones QJ, de Ridder GG, Pizzo SV. GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum. Histol Histopathol. 2008;23:1409-1416. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009;11:2299-2306. [DOI] [PubMed] [Google Scholar]
- 18. Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434:181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45-54. [DOI] [PubMed] [Google Scholar]
- 20. Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14:263-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roller C, Maddalo D. The molecular chaperone GRP78/BiP in the development of chemoresistance: mechanism and possible treatment. Front Pharmacol. 2013;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181-191. [DOI] [PubMed] [Google Scholar]
- 23. Singh I, Doms RW, Wagner KR, Helenius A. Intracellular transport of soluble and membrane-bound glycoproteins: folding, assembly and secretion of anchor-free influenza hemagglutinin. EMBO J. 1990;9:631-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mulvey M, Brown DT. Involvement of the molecular chaperone BiP in maturation of Sindbis virus envelope glycoproteins. J Virol. 1995;69:1621-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choukhi A, Ung S, Wychowski C, Dubuisson J. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J Virol. 1998;72:3851-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28:465-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peck KM, Burch CL, Heise MT, Baric RS. Coronavirus host range expansion and Middle East respiratory syndrome coronavirus emergence: biochemical mechanisms and evolutionary perspectives. Annu Rev Virol. 2015;2:95-117. [DOI] [PubMed] [Google Scholar]
- 28. Li F. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J Virol. 2015;89:1954-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan C-M, Chu H, Wang Y, et al. Carcinoembryonic antigen-related cell adhesion molecule 5 is an important surface attachment factor that facilitates entry of Middle East respiratory syndrome coronavirus. J Virol. 2016;90:9114-9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Earnest JT, Hantak MP, Li K, McCray PB, Jr, Perlman S, Gallagher T. The tetraspanin CD9 facilitates MERS-coronavirus entry by scaffolding host cell receptors and proteases. PLoS Pathog. 2017;13:e1006546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aguiar JA, Tremblay BJ-M, Mansfield MJ, et al. Gene expression and in situ protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue. bioRxiv. https://www.biorxiv.org/content/10.1101/2020.04.07.030742v2.abstract. [DOI] [PMC free article] [PubMed]
- 32. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016-1035.e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmeira A, Sousa E, Köseler A, et al. Preliminary virtual screening studies to identify GRP78 inhibitors which may interfere with SARS-CoV-2 infection. Pharmaceuticals (Basel). 2020;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bupp K, Roth MJ. Alteration and analyses of viral entry with library-derived peptides. Adv Virus Res. 2005;65:147-172. [DOI] [PubMed] [Google Scholar]
- 35. Allam L, Fatima G, Wiame L, Hamid EA, Azeddine I. Molecular screening and docking analysis of LMTK3and AKT1 combined inhibitors. Bioinformation. 2018;14:499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fioravanti R, Celestino I, Costi R, et al. Effects of polyphenol compounds on influenza A virus replication and definition of their mechanism of action. Bioorg Med Chem. 2012;20:5046-5052. [DOI] [PubMed] [Google Scholar]
- 37. Musarra-Pizzo M, Ginestra G, Smeriglio A, Pennisi R, Sciortino MT, Mandalari G. The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) skin. Nutrients. 2019;11:2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fatima G, Loubna A, Wiame L, Azeddine I. In silico inhibition studies of AXL kinase by curcumin and its natural derivatives. J Appl Bioinforma Comput Biol. 2017;6:2. [Google Scholar]
- 39. Ghrifi F, Allam L, Wiame L, Ibrahimi A. Curcumin-synthetic analogs library screening by docking and quantitative structure-activity relationship studies for AXL tyrosine kinase inhibition in cancers. J Comput Biol. 2019;26:1156-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hughes SJ, Antoshchenko T, Chen Y, Lu H, Pizarro JC, Park HW. Probing the ATP site of GRP78 with nucleotide triphosphate analogs. PLoS ONE. 2016;11:e0154862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Allam L, Lakhlili W, Tarhda Z, et al. Three-dimensional structure prediction of the human LMTK3 catalytic domain in DYG-in conformation. J Biomol Res Ther. 2017;6:2. [Google Scholar]
- 42. O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. J Cheminform. 2011;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kozakov D, Hall DR, Xia B, et al. The ClusPro web server for protein–protein docking. Nat Protoc. 2017;12:255-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Porter KA, Xia B, Beglov D, et al. ClusPro PeptiDock: efficient global docking of peptide recognition motifs using FFT. Bioinformatics (Oxford, England). 2017;33:3299-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mani JS, Johnson JB, Steel JC, et al. Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res. 2020;284:197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zahedipour F, Hosseini SA, Sathyapalan T, et al. Potential effects of curcumin in the treatment of COVID-19 infection [published online ahead of print May 19, 2020]. Phytother Res. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zu ZY, Jiang MD, Xu PP, et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020;296:E15-E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hakmi M, Bouricha EM, Kandoussi I, Harti JE, Ibrahimi A. Repurposing of known anti-virals as potential inhibitors for SARS-CoV-2 main protease using molecular docking analysis. Bioinformation. 2020;16:301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nain M, Mukherjee S, Karmakar SP, et al. GRP78 is an important host factor for Japanese encephalitis virus entry and replication in mammalian cells. J Virol. 2017;91:e02274-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reid SP, Shurtleff AC, Costantino JA, et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res. 2014;109:171-174. [DOI] [PubMed] [Google Scholar]
- 52. Ermakova SP, Kang BS, Choi BY, et al. (−)−Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260-9269. [DOI] [PubMed] [Google Scholar]
- 53. Gurusinghe KSN, Mishra A, Mishra S. Glucose-regulated protein 78 substrate-binding domain alters its conformation upon EGCG inhibitor binding to nucleotide-binding domain: molecular dynamics studies. Sci Rep. 2018;8:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Macias AT, Williamson DS, Allen N, et al. Adenosine-derived inhibitors of 78 kDa glucose regulated protein (Grp78) ATPase: insights into isoform selectivity. J Med Chem. 2011;54:4034-4041. [DOI] [PubMed] [Google Scholar]
- 55. Elfiky AA. Natural products may interfere with SARS-CoV-2 attachment to the host cell [published online ahead of print May 5, 2020]. J Biomol Struct Dyn. doi: 10.1080/07391102.2020.1761881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Praditya D, Kirchhoff L, Brüning J, Rachmawati H, Steinmann J, Steinmann E. Anti-infective properties of the golden spice curcumin. Front Microbiol. 2019;10:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Adem S, Eyupoglu V, Sarfraz I, Rasul A, Ali M. Identification of potent COVID-19 main protease (Mpro) inhibitors from natural polyphenols: an in silico strategy unveils a hope against CORONA [published online ahead of print March 23, 2020]. Preprints. doi: 10.20944/preprints202003.0333.v1. [DOI] [Google Scholar]
- 58. Clark K, Grant P, Sarr A, et al. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Vet Microbiol. 1998;63:147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wen CC, Kuo YH, Jan JT, et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50:4087-4095. [DOI] [PubMed] [Google Scholar]
- 60. Chen TY, Chen DY, Wen HW, et al. Inhibition of enveloped viruses infectivity by curcumin. PLoS ONE. 2013;8:e62482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Triantafilou K, Fradelizi D, Wilson K, Triantafilou M. GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J Virol. 2002;76:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu Y-P, Chang C-M, Hung C-Y, Tsai M-C, Schuyler SC, Wang RY-L. Japanese encephalitis virus co-opts the ER-stress response protein GRP78 for viral infectivity. Virol J. 2011;8:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]