Abstract
Introduction:
Triptans, the most commonly prescribed acute treatments for migraine attacks are, per FDA labeling, contraindicated in cardiovascular (CV) disease patients and have warnings and precautions for those with CV risk factors.
Methods:
Headache specialists, cardiologists, and health economics and outcomes researchers convened to identify diagnostic codes for: (1) CV diseases contraindicating triptans based on FDA labeling; (2) conditions comprising “other significant underlying CV disease”; and (3) CV risk factors included as warnings and precautions for triptans. A retrospective, cross-sectional analysis of commercially insured adult US migraine patients in the 2017 Optum® Clinformatics® Data Mart (CDM) and the 2017 IBM® Watson Health MarketScan® Commercial Claims database was used to estimate the proportion of migraine patients with CV contraindications and warnings and precautions to triptans.
Results:
Of the 56,662 migraine patients analyzed from Optum CDM, 13.5% had ≥1 CV disease as specified in triptan labeling and an additional 8.5% had ≥1 “other CV disease” judged by the panel to constitute a “significant underlying CV disease” (total: 22.0% migraine patients). Of 176 724 migraine patients analyzed from MarketScan, 12.2% had ≥1 CV disease as specified in the labeling and an additional 8.0% had ≥1 “other significant underlying CV disease” (total: 20.2% of migraine patients). An additional 25.4% and 25.1% of migraine patients had ≥2 CV risk factors in Optum CDM and MarketScan. In total, 47.4% and 45.3% of migraine patients in both databases had a CV disease specified as a contraindication, an “other CV disease” endorsed as significant, or ≥2 CV risk factors identified as warnings and precautions to triptans.
Conclusions:
Analyses of more than 230,000 people with migraine showed that ≥20% of commercially insured US migraine patients have a CV condition that specifically contraindicates triptan treatment, and an additional 25% have ≥2 CV risk factors identified as warnings and precautions to triptans.
Keywords: migraine, acute treatment, triptan, cardiovascular disease, risk factors
Introduction
Migraine is a chronic disease that is characterized by recurrent attacks of headache pain and associated neurological symptoms.1 The incapacitating nature of migraine can lead to marked individual disability and can exert a substantial impact on many aspects of life, including social and emotional well-being, family, career, and finances.2-6 For these reasons, migraine was identified as one of the leading causes of years lived with disability in the 2017 Global Burden of Diseases, Injuries, and Risk Factors Study.7
Acute treatments for migraine are taken at the time of a migraine attack, with the goals of rapidly treating pain and associated symptoms, restoring functional ability, and minimizing the need for repeat dosing or rescue medications.8,9 Acute treatments for migraine include migraine-specific medications, such as triptans, ergots, gepants, and lasmiditan, and nonspecific medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and non-opioid analgesics.9,10 The American Academy of Neurology8 and the American Headache Society recommend triptans as first-line acute treatment for moderate or severe migraine attacks.9 Triptans are the most commonly prescribed acute treatments for migraine.11-13 A claims analysis of commercially insured patients in the United States from 2007 to 2013 revealed that 63% of patients who were prescribed any acute treatment for migraine had received a prescription for a triptan.11 Population-based studies in the US, including the American Migraine Prevalence and Prevention (AMPP) and Chronic Migraine Epidemiology and Outcomes (CaMEO) studies, have shown that approximately 22% of people with migraine reported current use of a triptan to treat their migraine attacks.14,15 Claims studies include only medically diagnosed migraine; lower rates of triptan use in population studies reflect the inclusion of people with migraine not being treated by health care professionals.
Triptans are selective serotonin (5-HT)1B/1D/1F receptor agonists.16 Designed specifically to constrict cerebral blood vessels, their mechanism of action was initially attributed to vasoconstriction mediated by agonism of the 5-HT1B receptors on intracranial blood vessels.16 However, triptan therapeutic efficacy is currently believed to be mediated, at least in part, by its agonism of 5-HT1F receptors located on sensory nerves of the trigeminal system, which innervate the face and oral and nasal cavities.16 Nevertheless, triptans are vasoconstrictors and these effects may increase the risk of serious ischemic events in patients who have underlying cardiovascular (CV) diseases or risk factors for CV disease.17 Indeed, migraine with aura is a significant and independent risk factor for ischemic stroke, and there is growing evidence of an association between migraine with aura and other CV disorders, including myocardial infarction, perioperative stroke, hypertension, venous thromboembolism, and atrial fibrillation.18-23
All triptans, as specified in the US Food and Drug Administration (FDA) labeling for the class, are contraindicated in patients with certain CV diseases (eg, coronary artery disease [CAD], coronary artery vasospasm, peripheral artery disease, stroke, uncontrolled hypertension, and ischemic bowel disease).24-30 However, there are ambiguities and differences in the labeling of various triptans regarding certain CV contraindications. Some triptan labels include nonspecific language stating that the triptan is contraindicated in patients with “other significant underlying CV disease.”24,26,29 The Warning and Precautions section of the labels advises an evaluation to identify underlying CV disease in patients with multiple risk factors, but does not provide clear insights into the specific CV risk factors that are deemed to be relevant in this context.24-30 Additionally, the lists of CV risk factors in the labels do not align with current clinical guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA).31 Given the ambiguities and inconsistencies, it is difficult to estimate the proportion of people with migraine who have contraindications and precautions for triptan use. There are no clear published guidelines for interpreting and operationalizing the triptan labeling.
The objectives of this study were to operationalize the Contraindications and the Warnings and Precautions regarding CV diseases and CV risk factors in the triptan labels, and apply these operations to real-world insurance claims data to estimate the proportions of people with migraine who have contraindications and precautions for triptan use.
Methods
Data Sources
We conducted parallel analyses in 2 large administrative claims data sets to assess the consistency of results across 2 commercially insured populations. Data were extracted from the Optum® Clinformatics® Data Mart [CDM] (Eden Prairie, MN) and the IBM® Watson Health MarketScan® Commercial Claims and Encounters database (Somers, NY). Optum CDM contains administrative health claims data from members of a large national insurer in the United States. Administrative claims submitted for payment by providers and pharmacies are verified, adjudicated, adjusted, and de-identified prior to inclusion in the database. For this study, data on commercially insured patients only were included. The MarketScan database contains cost, utilization, and clinical information associated with inpatient, outpatient, and prescription drug claims for employees and dependents covered by employer-sponsored private health insurance in the United States. All analyses were conducted in both databases.
Study Sample
This was a retrospective cross-sectional analysis of administrative claims among commercially insured US patients with at least 1 inpatient or at least 2 outpatient medical claims with a migraine diagnosis (International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM] code G43.XX) between January 1, 2017, and December 31, 2017. Patients were required to be aged 18 years or older on January 1, 2017, and have continuous 12-month medical and prescription benefits between January 1, 2017, and December 31, 2017.
Identification and Operationalization of CV Disease
Contraindications Specifically Listed in the Triptan Labels
To address ambiguity in triptan labeling, we convened an expert panel composed of 5 neurologist-headache specialists, 1 family practitioner-headache specialist, 2 cardiologists, and 4 health economics and outcomes researchers to review the CV disease contraindications listed in triptan labels. The labels reviewed were those for almotriptan malate tablets,29 naratriptan hydrochloride tablets,30 frovatriptan succinate tablets,28 sumatriptan succinate tablets,27 rizatriptan benzoate tablets,26 eletriptan hydrobromide tablets,25 and zolmitriptan orally disintegrating tablets.24 Upon careful review, the panel agreed on 5 broad categories of CV disease listed as contraindications in the triptan labels, which included ischemic heart disease, cerebrovascular disease, peripheral artery disease, uncontrolled hypertension, and gastrointestinal ischemia. Diseases included within each of these 5 broad CV disease groups were identified by ICD-10-CM codes on medical claims (Supplemental Table S1).
“Other Significant Underlying CV Disease.”
Some of the triptan labels include nonspecific language stating that the triptan is contraindicated in patients with “other significant underlying CV disease.”24,26,29 The cardiologist members of the expert panel generated a list of conditions that were not expressly listed in the triptan labels but were considered to be diseases that met the clinical definition of “other significant underlying CV disease.” This resulted in the identification of 4 additional conditions that potentially contraindicate triptans, including potentially life-threatening arrhythmias, structural heart disease, the presence of cardiac implants (eg, coronary grafts), and other serious cardiac conditions (eg, cardiac arrest). The panel reviewed ICD-10-CM codes by disease condition to identify relevant codes for the purpose of appropriately identifying these conditions. The conditions and their corresponding ICD-10-CM codes and criteria for claims identification are listed in Supplemental Table S2 in the supplementary data.
Identification and Operationalization of CV Risk Factors
In addition to contraindications, the Warnings and Precautions in the triptan labels advise that “triptan-naive patients who have multiple CV risk factors (eg, increased age, diabetes, hypertension, smoking, obesity, and strong family history of CAD)” should have a CV evaluation performed prior to receiving the triptan.24-26,28-30 One of the triptan labels (almotriptan malate) “strongly recommends that [almotriptan] not be given to patients in whom unrecognized CAD is predicted by the presence of risk factors.”29 The panel identified CV risk factors for evaluation in the claims databases based on the following criteria: (1) generally accepted definition of CV risk per the ACC/AHA guidelines31; (2) generally available in claims data; and (3) consistent with the label caution regarding unrecognized CAD. Laboratory data (eg, fasting lipid profiles, fasting blood sugar, and blood pressure) and historical data on family history were unavailable in the claims databases. Accordingly, the identification of CV risk factors was limited to a non-laboratory–based set of criteria available in administrative claims data that could be specifically identified by diagnosis codes and medication fills. Based on these criteria, the panel identified the following 6 CV risk factors: hypertension, hyperlipidemia, diabetes mellitus, obesity, increased age, and smoking. The criteria and corresponding ICD-10-CM and medication codes used to identify these risk factors are provided in Supplemental Table S3 in the supplementary data.
Elixhauser comorbidity counts, the total of the numbers of comorbidity categories from an established set of 30 comorbidities,32 were determined from ICD-10-CM codes.
Statistical Analyses
The proportion of patients with migraine and CV disease and/or risk factors was estimated for 2017 in the Optum CDM and MarketScan commercial claims databases. Data were summarized using descriptive statistics. Sensitivity analysis was conducted for the definition of CV disease by including and excluding the criteria for essential hypertension, and for the definition of CV risk factors by varying the specific risk factors that were included as well as the number of risk factors required to identify a patient with CV risk (data not shown).
Results
Patient Characteristics
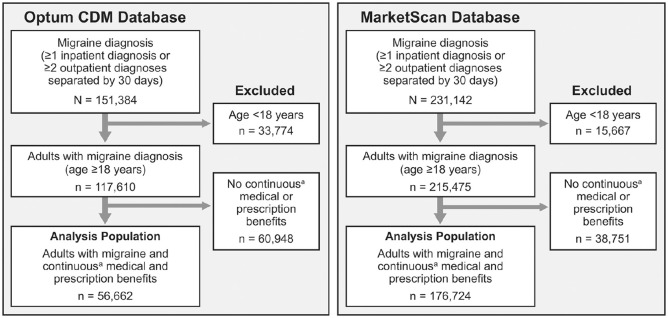
In the Optum CDM database, 151 384 migraine patients were identified in 2017. Of these, 56 662 were adults with continuous medical and prescription coverage for 12 months and were included in the analyses (Figure 1). In the MarketScan database, 231 142 migraine patients were identified in 2017, of whom 176 724 were adults with continuous medical and prescription coverage and were included in the analyses (Figure 1). Demographics and clinical characteristics are summarized by database in Table 1. Both populations were predominantly female, aged 35 to 64 years, and in both populations, approximately one fourth had received a diagnosis of chronic migraine.
Figure 1.
Sample selection.
Abbreviation: CDM, Clinformatics Data Mart.
aContinuous enrollment was defined as 12 months.
Table 1.
Demographics and Clinical Characteristics of the Migraine Population.
| Optum CDM N = 56 662 | MarketScan N = 176,724 | |
|---|---|---|
| Age group, years | ||
| 18–24 | 4843 (8.5) | 16 806 (9.5) |
| 25–34 | 10 004 (17.7) | 28 474 (16.1) |
| 35–44 | 15 500 (27.4) | 46 320 (26.2) |
| 45–64 | 26 315 (46.4) | 85 124 (48.2) |
| Sex | ||
| Female | 47 731 (84.2) | 150 256 (85.0) |
| Male | 8927 (15.8) | 26 468 (15.0) |
| Insurance plan type | ||
| Preferred provider organization | 722 (1.3) | 97 435 (55.1) |
| Point of service | 42 845 (75.6) | 14 287 (8.1) |
| Exclusive provider organization | 6445 (11.4) | 34 886 (19.7) |
| Health maintenance organization | 6334 (11.2) | 18 710 (10.6) |
| Other or unknown | 316 (0.6) | 11 406 (6.5) |
| Presence of chronic migraine diagnosis | 14 203 (25.1) | 47 669 (27.0) |
| Elixhauser comorbidity count | ||
| 0 | 12 974 (22.9) | 43 100 (24.4) |
| 1 | 14,359 (25.3) | 47 288 (26.8) |
| 2–3 | 17 968 (31.7) | 57 004 (32.3) |
| 4–5 | 7273 (12.8) | 20 328 (11.5) |
| ≥6 | 4088 (7.2) | 9004 (5.1) |
| Migraine-related comorbidities | ||
| Mood disorder | 16 974 (30.0) | 46 609 (26.4) |
| Sleep disturbances | 13 838 (24.4) | 39 163 (22.2) |
| Rhinitis | 10 744 (19.0) | 31 614 (17.9) |
| Pain | 6640 (11.7) | 17 173 (9.7) |
| Inflammatory bowel syndrome | 3443 (6.1) | 8853 (5.0) |
| Epilepsy | 2135 (3.8) | 6267 (3.5) |
Abbreviations: CDM, Clinformatics Data Mart; CV, cardiovascular.
All data are n (%).
Prevalence of CV Disease
Optum CDM Dataset
Of the 56 662 migraine patients identified in the Optum CDM database, 13.5% had at least 1 CV disease specified as a contraindication in the triptan label (Table 2). The most common of these individual CV disease groups were cerebrovascular disease (6.8%), uncontrolled hypertension (4.1%), and ischemic heart disease (3.8%). An additional 8.5% of patients had at least 1 “other CV disease” endorsed as significant by the panel of experts (eg, structural heart disease [5.5%] and arrhythmias [4.6%]). Overall, 22.0% of migraine patients had at least 1 of these CV diseases listed as a contraindication or referenced as “other significant CV disease” in the triptan label. Rates of the specific CV diseases within each disease group are in Table 3.
Table 2.
One-Year Rates of Cardiovascular Diseases Contraindicating Use of Triptans in Migraine Patients (2017 Data).
| Optum CDM N = 56 662 | MarketScan N = 176 724 | |
|---|---|---|
| ≥1 contraindication listed in triptan label | 7653 (13.5) | 21 495 (12.2) |
| Cerebrovascular/cardiovascular disease | 3830 (6.8) | 10 495 (5.9) |
| Ischemic cerebrovascular disease | 3085 (5.4) | 8482 (4.8) |
| Other cerebrovascular disease | 1369 (2.4) | 3683 (2.1) |
| Uncontrolled hypertension | 2324 (4.1) | 6154 (3.5) |
| Ischemic heart disease | 2136 (3.8) | 5751 (3.3) |
| Peripheral artery disease | 1409 (2.5) | 3885 (2.2) |
| Gastrointestinal ischemia | 51 (0.1) | 128 (0.1) |
| ≥1 contraindication considered as “other significant cardiovascular disease” in triptan label | 4833 (8.5) | 14 180 (8.0%) |
| Structural heart disease | 3113 (5.5) | 8737 (4.9) |
| Arrhythmias | 2582 (4.6) | 7369 (4.2) |
| Cardiac surgery | 484 (0.9) | 1031 (0.6) |
| Other cardiac condition | 4244 (7.5) | 11 037 (6.2) |
| ≥1 contraindication listed in triptan label or considered as “other significant cardiovascular disease” in triptan label | 12 486 (22.0) | 35 675 (20.2) |
Abbreviations: CDM: Clinformatics Data Mart.
All data are n (%). Diagnosis codes included within each disease category are listed in Supplemenatal Tables S1 and S2 in the supplementary material.
Table 3.
One-Year Rates of Specific Cardiovascular Diseases in Patients With Migraine (2017 Data).
| Optum CDM N = 56 662 | MarketScan N = 176 724 | |
|---|---|---|
| Contraindications specifically listed in triptan labela | ||
| All cerebrovascular/cardiovascular diseases | 3830 (6.8) | 10 495 (5.9) |
| Ischemic cerebrovascular disease | 3085 (5.4) | 8482 (4.8) |
| Transient ischemic attack | 2000 (3.5) | 4800 (2.7) |
| Cerebral infarction | 1287 (2.3) | 3736 (2.1) |
| Cerebral occlusion | 874 (1.5) | 2634 (1.5) |
| Cerebral ischemia | 178 (0.3) | 519 (0.3) |
| Cerebrovascular syndrome | 43 (0.1) | 164 (0.1) |
| Other cerebrovascular disease | 1369 (2.4) | 3683 (2.1) |
| Sequelae of cerebrovascular disease | 203 (0.4) | 445 (0.3) |
| Intracerebral hemorrhage | 50 (0.1) | 102 (0.1) |
| Intracranial hemorrhage | 50 (0.1) | 106 (0.1) |
| Subarachnoid hemorrhage | 30 (0.1) | 71 (<0.1) |
| Other cerebrovascular disease | 979 (1.7) | 2908 (1.6) |
| Familial hypercholesterolemia | 187 (0.3) | 249 (0.1) |
| Wolff-Parkinson-White syndrome | 46 (0.1) | 128 (0.1) |
| Uncontrolled hypertension code | 2324 (4.1) | 6154 (3.5) |
| End organ hypertension | 1317 (2.3) | 3298 (1.9) |
| Uncontrolled essential hypertension | 873 (1.5) | 2517 (1.4) |
| Emergent hypertension | 345 (0.6) | 359 (0.2) |
| Secondary hypertension | 218 (0.4) | 711 (0.4) |
| Ischemic heart disease | 2136 (3.8) | 5751 (3.3) |
| Chronic ischemic heart disease | 1408 (2.5) | 3899 (2.2) |
| Angina pectoris | 733 (1.3) | 1933 (1.1) |
| Myocardial infarction | 576 (1.0) | 1171 (0.7) |
| Angioplasty or grafts for ischemic heart disease | 395 (0.7) | 560 (0.3) |
| Prinzmetal’s angina | 183 (0.3) | 500 (0.3) |
| Acute ischemic heart disease | 122 (0.2) | 231 (0.1) |
| ≥1 Peripheral artery disease | 1409 (2.5) | 3885 (2.2) |
| Peripheral atherosclerosis native vessels | 429 (0.8) | 1072 (0.6) |
| Atherosclerosis | 102 (0.2) | 250 (0.1) |
| Diabetic peripheral artery disease | 82 (0.1) | 185 (0.1) |
| Angioplasty or grafts for peripheral artery disease | 26 (<0.1) | 24 (<0.1) |
| Peripheral atherosclerosis graft | 9 (<0.1) | 10 (<0.1) |
| Other peripheral artery disease | 973 (1.7) | 2681 (1.5) |
| Other arterial disease | 4 (<0.1) | 8 (<0.1) |
| Gastrointestinal ischemia | 51 (0.1) | 128 (0.1) |
| Contraindications considered as “other significant cardiovascular disease” in triptan labela | ||
| Structural heart disease | 3113 (5.5) | 8737 (4.9) |
| Valvular heart disease | 2349 (4.1) | 6687 (3.8) |
| Heart failure | 609 (1.1) | 1532 (0.9) |
| Congenital heart disease | 581 (1.0) | 1538 (0.9) |
| Hypertrophic cardiomyopathy | 36 (0.1) | 111 (0.1) |
| Arrhythmias | 2582 (4.6) | 7369 (4.2) |
| Other arrhythmia | 1240 (2.2) | 3572 (2.0) |
| Ventricular arrhythmia | 805 (1.4) | 2453 (1.4) |
| Atrial arrhythmia | 709 (1.3) | 1957 (1.1) |
| Channelopathy | 202 (0.4) | 418 (0.2) |
| Heart block | 145 (0.3) | 333 (0.2) |
| Cardiac surgery/implant | 484 (0.9) | 1031 (0.6) |
| Other cardiac condition | 4244 (7.5) | 11 037 (6.2) |
| Syncope | 2939 (5.2) | 8408 (4.8) |
| Personal history of embolism or sudden cardiac arrest | 1001 (1.8) | 1503 (0.9) |
| Abnormal result cardiac testing | 272 (0.5) | 814 (0.5) |
| Aneurysm | 182 (0.3) | 514 (0.3) |
| Embolic disease | 103 (0.2) | 215 (0.1) |
| Cardiac arrest | 45 (0.1) | 118 (0.1) |
| Family history of sudden cardiac death | 28 (<0.1) | 48 (<0.1) |
| Aortic dissection | 26 (<0.1) | 57 (<0.1) |
Abbreviation: CDM, Clinformatics Data Mart.
Category totals represent the proportion of people with at least 1 condition in the category and are not the sum of all subcategories. Some people may have more than 1 condition (ie, transient ischemic attack and cerebral infarction).
MarketScan Dataset
Of the 176 724 patients with migraine identified in the MarketScan database, 12.2% had at least 1 CV disease specified as a contraindication in the triptan labels (eg, cerebrovascular disease [5.9%], uncontrolled hypertension [3.5%], and ischemic heart disease [3.3%]; Table 2). An additional 8.0% of patients had at least 1 “other CV disease” endorsed as significant by cardiologists (eg, structural heart disease [4.9%] and arrhythmias [4.2%]). Overall, 20.2% of migraine patients had at least 1 of these CV diseases listed as a contraindication or referenced as “other significant CV disease” in the triptan label. Rates of the specific CV diseases within each disease group are listed in Table 3.
Prevalence of CV Risk Factors
Optum CDM dataset
Estimates of the prevalence of CV risk factors in the 2017 Optum CDM dataset are presented in Table 4. More than 20% of all migraine patients had individual CV risk factors of hyperlipidemia (30.1%), hypertension (29.5%), and obesity (24.8%). Overall, 31.6% of migraine patients had at least 2 CV risk factors, 14.6% had at least 3 CV risk factors, and 5.3% had at least 4 CV risk factors. Among migraine patients without any claims-based evidence of a CV disease (n = 44,176), the rates of individual CV risk factors ranged from 6.5% for diabetes mellitus to 25.1% for hyperlipidemia (Table 4). In total, 25.4% of the patients without CV disease had at least 2 CV risk factors, 10.0% had at least 3 CV risk factors, and 3.1% had at least 4 CV risk factors.
Table 4.
One-Year Rates of Cardiovascular Risk Factors in Migraine Patients (2017 Data).
| Optum CDM |
MarketScan |
All N = 176 724 | Without CV diseasea n = 141 049 | |
|---|---|---|---|---|
| All N = 56,662 | Without CV Diseasea n = 44,176 | |||
| Cardiovascular risk factors | ||||
| Hypertension | 16 742 (29.5) | 10 222 (23.1) | 52 231 (29.6) | 33 943 (24.1) |
| Hyperlipidemia | 17 076 (30.1) | 11 106 (25.1) | 51 544 (29.2) | 34 953 (24.8) |
| Diabetes mellitus | 4926 (8.7) | 2866 (6.5) | 14 959 (8.5) | 9351 (6.6) |
| Obesity | 14 025 (24.8) | 9966 (22.6) | 41 496 (23.5) | 30 788 (21.8) |
| Increased ageb | 5107 (9.0) | 3131 (7.1) | 17 978 (10.2) | 11 830 (8.4) |
| Smoking | 5236 (9.2) | 3453 (7.8) | 11 722 (6.6) | 8064 (5.7) |
| No. of risk factors | ||||
| ≥1 risk factor | 33 253 (58.7) | 23 536 (53.3) | 103 385 (58.5) | 75 787 (53.7) |
| ≥2 risk factors | 17 923 (31.6) | 11 214 (25.4) | 53 978 (30.5) | 35 366 (25.1) |
| ≥3 risk factors | 8263 (14.6) | 4422 (10.0) | 23 580 (13.3) | 13 476 (9.6) |
| ≥4 risk factors | 3006 (5.3) | 1353 (3.1) | 7605 (4.3) | 3753 (2.7) |
Abbreviations: CDM, Clinformatics Data Mart; CV, cardiovascular.
All data are n (%).
Patients without a CV disease specified as a contraindication in the triptan labels or referenced as “other significant CV disease” in the triptan labels.
Men aged 55 years or older and women aged 60 years or older.
MarketScan dataset
More than 20% of migraine patients in the 2017 MarketScan dataset had risk factors of hypertension (29.6%), hyperlipidemia (29.2%), and obesity (23.5%) (Table 4). Overall, 30.5% had at least 2 CV risk factors, 13.3% had at least 3 CV risk factors, and 4.3% of patients had at least 4 CV risk factors. Among patients without claims-based evidence of CV disease (n = 141 049), the rates of individual CV risk factors ranged from 6.6% for diabetes mellitus to 24.8% for hyperlipidemia (Table 4). In total, 25.1% of patients without CV disease had at least 2 CV risk factors, 9.6% had at least 3 CV risk factors, and 2.7% had at least 4 CV risk factors.
Discussion
This retrospective analysis of 2 insurance claims databases estimated the prevalence of contraindications and warnings and precautions for the use of triptans. In the 2 independent samples, at least 12% had a contraindication to triptans specifically mentioned in the labeling, and an additional 8% had an “other CV disease” classified as significant by an expert panel. In total, at least 20% of migraine patients had at least 1 of these CV contraindications. Rates of CV diseases in these commercially insured populations generally align with those reported in US population-based surveys of people with migraine.33,34 An analysis of the 2009 AMPP Study data in respondents with episodic migraine (headache frequency of less than 15 days per month) found that 11.8% of respondents in the 40 to 59 year age group and 25.2% in the 60 years and older age group had a history of CV events, conditions, or procedures.
This study also shows that even among migraine patients without any claims-based evidence of CV disease as defined in triptan labels, approximately 25% had at least 2 CV risk factors. Our rates in all migraine patients are lower than those found in a 2009 population survey (the AMPP Study), which showed that 38.9% of women with episodic migraine and 41.6% of men with episodic migraine had at least 2 CV risk factors (among high cholesterol, hypertension, diabetes mellitus, obesity, and smoking).35 The higher rate of CV risk factors in the AMPP Study may be partially attributable to the older age of the AMPP population (78% at least 40 years of age)33 compared with the claims populations (46%–48% at least 45 years of age). Additionally, participants in the AMPP Study self-reported CV risk factors (eg, smoking, obesity),35 which may be underreported in claims data. Nevertheless, our study still found that a substantial proportion of people with migraine have multiple CV risk factors.
The triptan labels24-30 advise that a CV evaluation should be performed in triptan-naive patients who have multiple CV risk factors (eg, increased age, diabetes mellitus, hypertension, smoking, obesity, strong family history of CAD). The nature of the recommended CV evaluation is not specified. If the evaluation reveals evidence of CV disease or coronary artery vasospasm, the patient should not be treated with a triptan. For patients who have multiple CV risk factors but a negative CV evaluation, the triptan labels recommend that the health care provider should consider administering the first dose of the triptan in a medically supervised setting and performing an electrocardiogram (ECG) immediately following triptan administration. Depending upon the CV evaluation and the clinician’s confidence that the evaluation was adequate, an alternative is to refer the patient to a cardiologist for CV evaluation.
Acute treatment options are limited by FDA warnings for migraine patients with underlying CV disease or multiple CV risk factors. Historically, these patients have been limited to migraine nonspecific treatments, such as NSAIDs, non-opioid analgesics, acetaminophen, and caffeine analgesic combinations. However, all NSAIDs (eg, ibuprofen, diclofenac, and naproxen) carry an FDA boxed warning because of their association with increased risks of heart attack and stroke, if taken chronically.36 An analysis of data from the CaMEO Study showed that respondents with both migraine and at least 1 CV comorbidity were 56% more likely than those without a CV comorbidity to be using an opioid to treat headache (odds ratio [OR], 1.56; 95% CI, 1.28-1.90).37 These findings suggest that in the past, persons unable to take triptans were more likely to be treated with opioids, which authoritative guidelines and headache specialists recommend against for patients with migraine because of a lack of evidence and the potential for medication overuse headache, as well as the potential for habituation, dependency, and addiction.38,39 The recent availability of novel migraine-specific agents approved for the acute treatment of migraine, including gepants (ie, ubrogepant and rimegepant) and lasmiditan, could help to address the unmet needs of some of these patients.9 Unlike triptans, gepants and lasmiditan are not vasoconstrictors and do not have CV contraindications and precautions in labeling.9,40-42 Therefore, gepants and lasmiditan may fill a treatment gap in the substantial proportion of patients identified in the current study who should not take triptans.
Our data showed that approximately 84% to 85% of migraine patients were women, a result that aligns with epidemiological reports that the prevalence of migraine is two- to three-fold higher in women than in men.3,43
Strengths and Limitations
This retrospective analysis of 2017 data from 2 comprehensive insurance claims databases allowed for the estimation of CV disease rates and CV risk factors in a large population of more than 230 000 patients with migraine overall. A sensitivity analysis of 2016 data in both databases yielded similar results, lending support to the consistency of the results over multiple years (data not shown). An additional strength of this study is that the criteria for clinically significant CV disease and CV risk factors were operationalized based on the consensus of an expert panel of headache specialists, a family practitioner, cardiologists, and health economics and outcomes researchers.
There are several limitations of our analysis. First, as with all retrospective claims analyses, administrative claims data are subject to coding errors, which may have resulted in misclassification of CV disease, CV risk factors, and/or study outcomes. Laboratory data (eg, fasting blood sugar, fasting lipid profile, or blood pressure values) were not available to confirm the presence of risk factors, although it is likely that the clinicians who assigned the diagnostic codes had access to this information. In addition, the prevalence of CV risk factors may be underestimated because of under-coding of some conditions in claims data, particularly smoking. Another limitation is that CV disease and CV risk factors were evaluated only over a 1-year time frame; as such, patients with CV disease or CV risk factors who had not consulted with their health care provider within the 1-year period were not included, potentially underestimating the true prevalence of CV disease and/or CV risk factors. However, results were similar in a separate analysis of MarketScan and Optum CDM claims from 2016 and a 2-year analysis of data from 2016 and 2017 (data not shown). Last, the sample was limited to patients with migraine enrolled in commercial insurance in the US; therefore, the results may not be generalizable to patients with other types of insurance (eg, Medicare or Medicaid) or those without health insurance. Migraine is likely under-ascertained in these commercial databases. On the other hand, the sample is likely to be representative of migraine patients who carry a diagnosis and are eligible for prescription treatment.
Conclusion
Real-world claims data indicate that at least 20% of commercially insured US patients with migraine have a CV condition that specifically contraindicates treatment with triptans, and that 25% of those without claims-based evidence of a CV condition have multiple risk factors identified as warnings and precautions for triptans. Clinicians should exercise caution in continuing to prescribe triptans to these patients. Although acute treatment options are limited for migraine patients with underlying CV disease or multiple CV risk factors, the recent approval of novel migraine-specific agents that are not contraindicated in patients with CV disease may help address the unmet acute treatment needs for these patients.
Supplemental Material
Supplemental material, CV_Prop_Ms_7.16.20_Online_Supplementary_Data for Migraine Patients With Cardiovascular Disease and Contraindications: An Analysis of Real-World Claims Data by David W. Dodick, Anand S. Shewale, Richard B. Lipton, Seth J. Baum, Steven C. Marcus, Stephen D. Silberstein, Jelena M. Pavlovic, Nathan L. Bennett, William B. Young, Hema N. Viswanathan, Jalpa A. Doshi and Howard Weintraub in Journal of Primary Care & Community Health
Acknowledgments
Writing and editorial assistance was provided to the authors by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and was funded by AbbVie.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: David W. Dodick, MD, reports the following conflicts: Personal fees: AbbVie, AEON, Alder BioPharmaceuticals, Amgen, Amzak Health, Association of Translational Medicine, Autonomic Technologies, Axsome, Biohaven, Cerecin, Charleston Laboratories, Clexio, Daniel Edelman Inc., Dr Reddy’s Laboratories/Promius, electroCore LLC, Eli Lilly, eNeura, Equinox, Foresite Capital, Impel, Ipsen, Neurolief, Nocira, Novartis, Oppenheimer, Pieris, PSL Group Services, Revance, Salvia, Satsuma, Sun Pharma (India), Supernus, Teva, Theranica, University Health Network, Upjohn (Division of Pfizer), Vedanta, WL Gore, XoC, Zosano, and ZP Opco; Speaking fees: Amgen, Eli Lilly, Lundbeck, and Novartis Canada; CME fees or royalty payments: Academy for Continued Healthcare Learning, Cambridge University Press, Catamount, Chameleon, Global Access Meetings, Global Life Sciences, Global Scientific Communications, Haymarket, HealthLogix, Medicom Worldwide, MedLogix Communications, Mednet, Miller Medical, Oxford University Press, PeerView, Universal Meeting Management, UpToDate (Elsevier), WebMD Health/Medscape, and Wolters Kluwer Health; Stock options: Aural Analytics, Epien, Healint, King-Devick Technologies, Matterhorn, Nocira, Ontologics, Precon Health, Second Opinion/Mobile Health, and Theranica; Consulting without fee: Aural Analytics, Epien, Healint, Second Opinion/Mobile Health; Board of Directors: Epien, King-Devick Technologies, Matterhorn, Ontologics, Paranet North America, and Precon Health; Patent: 17189376.1-1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis, without fee; Research funding: American Migraine Foundation, Henry Jackson Foundation, PCORI, and US Department of Defense; Professional society fees or reimbursement for travel: American Academy of Neurology, American Brain Foundation, American Headache Society, American Migraine Foundation, Canadian Headache Society, and International Headache Society.
Anand S. Shewale, MS, PhD, is an employee of AbbVie, and may hold AbbVie stock.
Richard B. Lipton, MD, serves on the editorial boards of Neurology and Cephalalgia and as senior advisor to Headache. He has received research support from the NIH, the Migraine Research Foundation, and the National Headache Foundation. He has reviewed for the NIA and NINDS; serves as consultant, advisory board member, or has received honoraria from or research support from AbbVie, AEON, Amgen, Axsome, Biohaven, Dr. Reddy’s, electroCore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Impel, Merck, Novartis, Satsuma, Teva, Vector, and Vedanta. He receives royalties from Wolff’s Headache, 8th Edition (Oxford University Press, 2009) and Informa. He holds stock options in Biohaven and eNeura Therapeutics.
Seth J. Baum, MD, has nothing to disclose.
Steven C. Marcus, PhD, is a consultant for AbbVie.
Stephen D. Silberstein, MD, is a consultant and/or advisory panel member for and has received honoraria from AbbVie, Alder Biopharmaceuticals, Amgen, Avanir, electroCore Medical, eNeura, Labrys Biologics, Medscape, Medtronic, Neuralieve, NINDS, Pfizer, and Teva. His employer receives research support from AbbVie, Amgen, Cumberland Pharmaceuticals, electroCore Medical, Eli Lilly, Labrys Biologics, Mars, and Troy Healthcare.
Jelena Pavlovic, MD, PhD, is a consultant and/or advisory panel member, receives honoraria from AbbVie, Alder Biopharmaceuticals, Amgen, Biohaven, and Promius. She is funded by NIH/NIA K23 AG049466-01A1.
Nathan L. Bennett, MD, has performed clinical research for AbbVie, Amgen, Avanir, electroCore, Impax, Lilly, and Teva, and has served as a consultant/on an advisory board for AbbVie, Amgen, Lilly, Pernix, Promius, and Supernus, and as a speaker for AbbVie, Amgen, Avanir, Promius, Supernus, and Teva.
William B. Young, MD, is a consultant or acts on an advisory board for Biohaven, Lilly, Teva, and Theranica; is a speaker for Lilly and Teva; and has conducted research for Amgen, Cumberland, Eli Lilly, Novartis, PCORI, Satsuma, Scion, Teva, and Zosano.
Hema N. Viswanathan, BPharm, PhD, was an employee of AbbVie at the time of this study, and holds AbbVie stock.
Jalpa A. Doshi, PhD, is a consultant for AbbVie.
Howard Weintraub, MD, has conducted clinical research for Akcea, Amarin, Amgen, NovoNordisk, and Sanofi/Regeneron, and has served as a consultant and/or advisory panel member for AbbVie, Amarin, Amgen, AstraZeneca, and Sanofi/Regeneron. He has received speaking honoraria from Amarin and Amgen.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Allergan (prior to its acquisition by AbbVie).
ORCID iDs: Anand S. Shewale  https://orcid.org/0000-0002-3138-5623
https://orcid.org/0000-0002-3138-5623
Stephen D. Silberstein  https://orcid.org/0000-0001-9467-5567
https://orcid.org/0000-0001-9467-5567
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition Cephalalgia. 2018;38(1):1–211. [DOI] [PubMed] [Google Scholar]
- 2. Buse DC, Fanning KM, Reed ML, et al. Life with migraine: effects on relationships, career, and finances from the chronic migraine epidemiology and outcomes (CaMEO) study. Headache. 2019;59(8):1286–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–349. [DOI] [PubMed] [Google Scholar]
- 4. Lipton RB, Manack Adams A, Buse DC, Fanning KM, Reed ML. A comparison of the Chronic Migraine Epidemiology and Outcomes (CaMEO) study and American Migraine Prevalence and Prevention (AMPP) study: demographics and headache-related disability. Headache. 2016;56(8):1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buse DC, Scher AI, Dodick DW, et al. Impact of migraine on the family: perspectives of people with migraine and their spouse/domestic partner in the CaMEO study. Mayo Clin Proc. 2016;91(5):596-611. [DOI] [PubMed] [Google Scholar]
- 6. Martelletti P, Schwedt TJ, Lanteri-Minet M, et al. My migraine voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 2018;19(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55(6):754-762. [DOI] [PubMed] [Google Scholar]
- 9. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59(1):1-18. [DOI] [PubMed] [Google Scholar]
- 10. How gepants and ditans complement existing therapies. 2020. Available at: https://americanmigrainefoundation.org/resource-library/gepants-ditans-therapies/#:~:text=When%20a%20patient%20is%20dissatisfied,risk%20of%20medication%20overuse%20headache. Accessed: June 11, 2020.
- 11. Bonafede M, Sapra S, Shah N, Tepper S, Cappell K, Desai P. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache. 2018;58(5):700-714. [DOI] [PubMed] [Google Scholar]
- 12. Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache. 2014;54(2):278-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alam A, Munjal S, Reed ML, et al. Triptan use and discontinuation in a representative sample of persons with migraine: results from Migraine in America Symptoms and Treatment (MAST) study [abstract OR11]. Headache. 2018;58(suppl 2):68-69. [Google Scholar]
- 14. Holland S, Fanning KM, Serrano D, Buse DC, Reed ML, Lipton RB. Rates and reasons for discontinuation of triptans and opioids in episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) study. J Neurol Sci. 2013;326(1-2):10-17. [DOI] [PubMed] [Google Scholar]
- 15. Hutchinson S, Lipton RB, Ailani J, et al. Characterization of acute prescription migraine medication use: results from the CaMEO study. Mayo Clin Proc. 2020;95(4):709-718. [DOI] [PubMed] [Google Scholar]
- 16. Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bigal ME, Kurth T, Hu H, Santanello N, Lipton RB. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72(21):1864-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adelborg K, Szepligeti SK, Holland-Bill L, et al. Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ. 2018;360:k96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Androulakis XM, Kodumuri N, Giamberardino LD, et al. Ischemic stroke subtypes and migraine with visual aura in the ARIC study. Neurology. 2016;87(24):2527-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spector JT, Kahn SR, Jones MR, Jayakumar M, Dalal D, Nazarian S. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med. 2010;123(7):612-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Timm FP, Houle TT, Grabitz SD, et al. Migraine and risk of perioperative ischemic stroke and hospital readmission: hospital based registry study. BMJ. 2017;356:i6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurth T, Rist PM, Ridker PM, Kotler G, Bubes V, Buring JE. Association of migraine with aura and other risk factors with incident cardiovascular disease in women. JAMA. 2020;323(22):2281-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Øie LR, Kurth T, Gulati S, Dodick DW. Migraine and risk of stroke. J Neurol Neurosurg Psychiatry. 2020;91(6):593-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zomig ZMT [package insert]. Bridgewater, NJ: Amneal Specialty; 2019. [Google Scholar]
- 25. Relpax [package insert]. New York, NY: Roerig, Division of Pfizer Inc.; 2013. [Google Scholar]
- 26. Maxalt [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2019. [Google Scholar]
- 27. Imitrex [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017. [Google Scholar]
- 28. Frova [package insert]. Malvern, PA: Endo Pharmaceuticals, Inc; 2018. [Google Scholar]
- 29. Axert [package inset]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2017. [Google Scholar]
- 30. Amerge [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2016. [Google Scholar]
- 31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. [DOI] [PubMed] [Google Scholar]
- 33. Buse DC, Reed ML, Fanning KM, Kurth T, Lipton RB. Cardiovascular events, conditions, and procedures among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2017;57(1):31-44. [DOI] [PubMed] [Google Scholar]
- 34. Bigal ME, Kurth T, Santanello N, et al. Migraine and cardiovascular disease: a population-based study. Neurology. 2010;74(8):628-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lipton RB, Reed ML, Kurth T, Fanning KM, Buse DC. Framingham-based cardiovascular risk estimates among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2017;57(10):1507-1521. [DOI] [PubMed] [Google Scholar]
- 36. Drug Safety Communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. 2015. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-warning-non-aspirin-nonsteroidal-anti-inflammatory. Accessed: February 18, 2020.
- 37. Lipton RB, Buse DC, Friedman BW, et al. Characterizing opioid use in a US population with migraine: results from the CaMEO study. Neurology 2020; 95(5):e457-e468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3-20. [DOI] [PubMed] [Google Scholar]
- 39. Pringsheim T, Davenport WJ, Marmura MJ, Schwedt TJ, Silberstein S. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56(7):1194-1200. [DOI] [PubMed] [Google Scholar]
- 40. Ubrelvy [package insert]. Madison, NJ: Allergan USA, Inc.; 2020. [Google Scholar]
- 41. Reyvow [package insert]. Indianapolis, IN: Lilly USA; 2020. [Google Scholar]
- 42. Nurtec ODT [package insert]. New Haven, CT: Biohaven Pharmaceuticals, Inc.; 2020. [Google Scholar]
- 43. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CV_Prop_Ms_7.16.20_Online_Supplementary_Data for Migraine Patients With Cardiovascular Disease and Contraindications: An Analysis of Real-World Claims Data by David W. Dodick, Anand S. Shewale, Richard B. Lipton, Seth J. Baum, Steven C. Marcus, Stephen D. Silberstein, Jelena M. Pavlovic, Nathan L. Bennett, William B. Young, Hema N. Viswanathan, Jalpa A. Doshi and Howard Weintraub in Journal of Primary Care & Community Health