Abstract
Case summary
A 3-year-old neutered female domestic shorthair cat presented for a 2-week history of hyporexia, lethargy and weight loss. Aspartate aminotransferase, alanine aminotransferase and cholesterol were mildly elevated. Thoracic radiographs identified a lobulated soft tissue opacity in the caudal thorax to the right of midline, with the border effacing the caudal vena cava and broad-based towards the diaphragm. The broad base was suggestive of diaphragmatic hernia, with the other radiographic features and location suggestive of caval foramen hernia. Ultrasound confirmed diaphragmatic hernia with liver herniation. CT showed the herniation of multiple liver lobes and the gallbladder through a defect at the caval foramen. Herniorrhaphy was performed via ventral midline coeliotomy. Following this procedure, the cat’s clinical signs resolved and its weight has been regained.
Relevance and novel information
To our knowledge, this is the first report of successful caval foramen herniorrhaphy in a cat. Caval foramen hernia is a type of congenital diaphragmatic hernia. The authors suggest that its embryopathology involves defective septum transversum development. The case was detected during the standard diagnostic investigation of non-specific clinical signs. Its radiographic findings may easily be mistaken for a pulmonary mass. Although not seen in our case, caval foramen hernia is commonly associated with caudal vena cava obstruction, which can potentially result in Budd–Chiari-like syndrome.
Keywords: Congenital hernia, diaphragmatic defect, caval foramen, computed tomography
Case description
A 3-year-old neutered female domestic shorthair cat weighing 4.33 kg presented for a 2-week history of hyporexia and lethargy. It had also lost 130 g of body weight in the past 9 days. There was an incidental chronic history of intermittent feline acne. It had been housed indoors since being in the owner’s possession from 2 months of age. Other than scabs under the rostral chin and left ear base, clinical examination was unremarkable. Complete blood count, serum biochemistry, urinalysis, and feline leukaemia virus and feline immunodeficiency virus serology were performed. Aspartate aminotransferase (77 IU/l; reference interval [RI] 2–62 IU/l), alanine aminotransferase (214 IU/l; RI 19–100 IU/l) and cholesterol (7.5 mmol/l; RI 2.2–5.5 mmol/l) were mildly increased. Urinalysis showed proteinuria and bacteriuria, which was considered to be clinically insignificant. All other results were unremarkable.
Thoracic radiographs and abdominal ultrasound were requested for further investigation of the non-specific clinical findings.
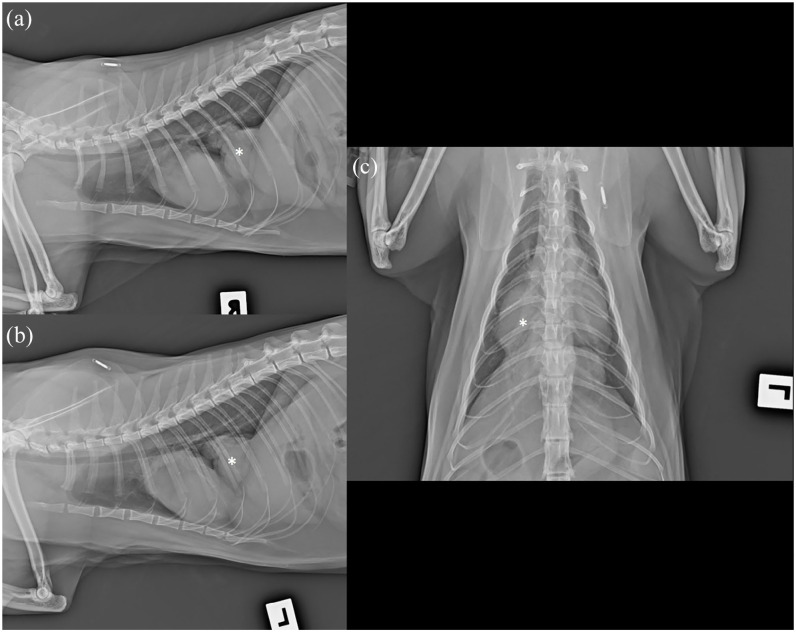
Thoracic radiographs (FDR D-EVO II G35 Flat Panel Detector; Fujifilm) included left- and right-lateral recumbency and dorsoventral projections. In the caudal thorax, to the right of the midline and at mid-height, there was a lobulated, well-defined smoothly margined soft tissue opacity with the border effacing the caudal vena cava; it was broad based and contiguous with the diaphragm (Figure 1). Differential diagnoses included a diaphragmatic hernia (caval foramen hernia, pleuroperitoneal hernia and, less likely, chronic traumatic diaphragmatic hernia), diaphragmatic eventration or (unlikely) pulmonary mass or caudal mediastinal mass (including granuloma, neoplasia).
Figure 1.
Thoracic radiographs of a 3-year-old neutered female domestic shorthair cat with hyporexia, lethargy and weight loss. (a) Right lateral, (b) left lateral recumbency and (c) dorsoventral projections. There is a lobulated soft tissue opacity in the caudal thorax just right of the midline at mid-height, with a broad base to the diaphragm (asterisk). The caudal vena cava is not seen
Abdominal ultrasound (EPIQ 5G [Philips Medical Systems] using microconvex 8–5 MHz and linear 12–5 MHz transducers) showed a loss of continuity in the mid-diaphragmatic interface with herniation of hepatic parenchyma (Figure 2). Ultrasound was otherwise unremarkable. CT was then performed under general anaesthesia for surgical planning.
Figure 2.
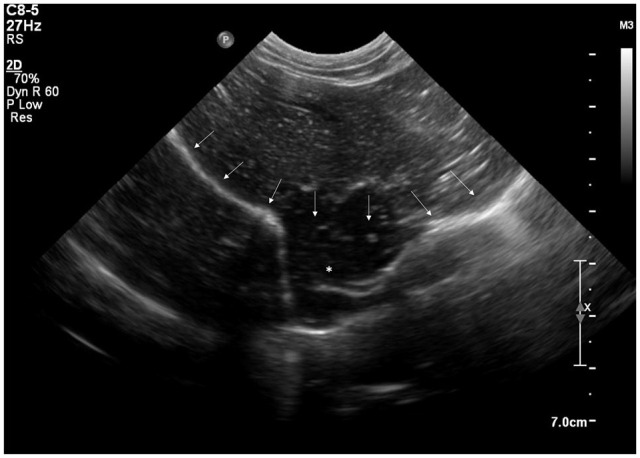
Ultrasound image of the thoracoabdominal region of a 3-year-old neutered female domestic shorthair cat with hyporexia, lethargy and weight loss via a subcostal window. There is an interruption in the diaphragmatic interface (arrows), with herniation of hepatic parenchyma (asterisk)
The cat was premedicated with 0.9 mg butorphanol and 21.5 µg medetomidine intravenously (IV), induced with 4 mg alfaxalone IV, intubated with a 4.5 mm endotracheal tube and maintained on isoflurane and oxygen. Multiphase contrast-enhanced CT images (Brilliance 16; Philips Medical Systems) of the thorax and abdomen were acquired in the transverse plane in sternal recumbency. Intravenous contrast (2 ml/kg Omnipaque 350 mg/ml [General Electric Healthcare]) was administered via the left cephalic vein using a power injector (EmpowerCTA; E-Z-EM). CT images were viewed using smoothing and edge enhancement algorithms on a dedicated workstation (RadiForce GS220; Eizo) using Osirix (v10.0.5; Pixmeo SARL).
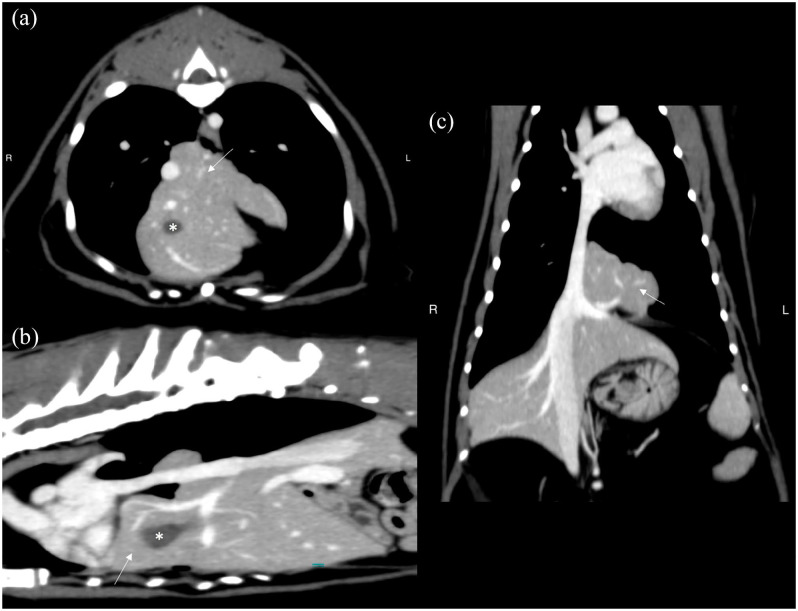
Just ventral to and including the caval foramen, there was a loss of continuity in the diaphragm through which multiple liver lobes (identified vascularly as likely the quadrate lobe and part of the right medial and left medial liver lobes) and most of the gallbladder were herniated, contacting and displacing the heart to the left side (Figure 3). Some hepatic parenchyma was in apposition with, but not compressing, the intrathoracic caudal vena cava. From these findings, the cat was diagnosed with caval foramen hernia.
Figure 3.
CT scan of a 3-year-old neutered female domestic shorthair cat with hyporexia, lethargy and weight loss. (a) Transverse, (b) sagittal and (c) dorsal image planes post-contrast portal phase after reconstruction with a smoothing algorithm and viewed in a soft tissue window. There is herniation of multiple liver lobes as a hepatic mass formation (arrow) and the gallbladder (asterisk) through the caval foramen
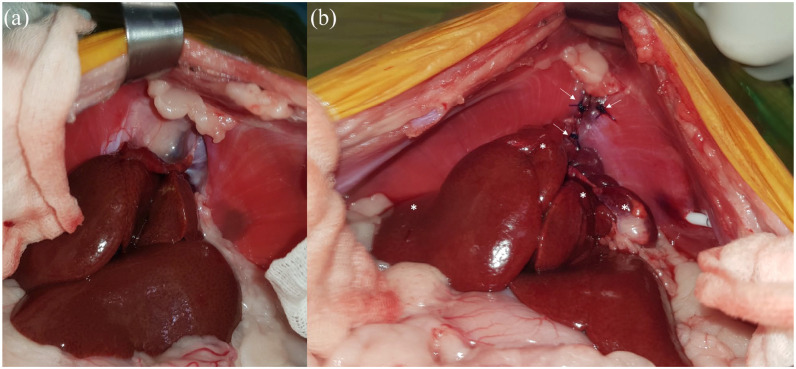
Forty-eight hours prior to midline exploratory coeliotomy, the cat began receiving 90 mg amoxicillin–clavulanate IV q8h and Hartmann’s IV fluid therapy at a maintenance rate. It was premedicated with 10 µg dexmedetomidine and 1 mg methadone IV, induced with 7.5 mg alfaxalone IV, intubated with a cuffed 4 mm endotracheal tube, and maintained on isoflurane and oxygen. Perioperative analgesia included a 0.5 mg morphine epidural injection and fentanyl (5–7 µg/kg/h) constant rate infusion (CRI). The cat was mechanically ventilated intraoperatively. Anaesthetic complications included mild hypotension, tachycardia and hypothermia. Intraoperatively, the cat received dopamine (5–10 µg/kg/h) CRI, 90 µg atropine IV, 100 mg amoxicillin–clavulanate IV q2h for two doses and 2.5 mg alfaxalone IV. A 3 cm defect was identified in the central tendon of the diaphragm at the caval foramen (Figure 4a). The hernial ring was extended ventrally to the sternum with sharp and blunt dissection using Steven’s tenotomy scissors and cotton tip applicators, respectively. The cranial aspect of the right lateral, right medial, quadrate along with the entire left medial liver lobe, gallbladder and omentum were herniated. The left and right lateral hepatic ligaments were transected to increase mobility of the liver lobes. The left medial liver lobe appeared lobulated and adhered to the pericardium. This adhesion was bluntly dissected off the pericardium to enable its reduction into the peritoneal cavity. The edges of the caval foramen defect were apposed with full-thickness simple interrupted sutures using 2/0 polydioxanone (PDS II; Ethicon) (Figure 4b). Laxity in the right component of the central tendon was addressed with horizontal mattress sutures using 2/0 polydioxanone following scalpel scarification on either side. Prior to its closure, a thoracostomy tube (MILA International) was placed through the left muscular component of the diaphragm and exited through the ventral abdomen. A 12 F oesophagostomy tube (MILA International) was also placed.
Figure 4.
Hernial appearance during exploratory coeliotomy of a 3-year-old neutered female domestic shorthair cat with hyporexia, lethargy and weight loss. (a) Prior to hernia reduction: there is a defect in the central tendon of the diaphragm with reduced liver mass in the abdominal cavity. (b) Following herniorrhaphy: liver lobes have been reduced into the abdominal cavity (asterisks). Simple interrupted sutures appose the edges of the surgically extended hernial ring defect (arrows)
The cat recovered uneventfully. Postoperatively, it received Hartmann’s IV fluid therapy, fentanyl (2–7 µg/kg/h) CRI and 4 mg meloxicam PO for 2 days. It received 90 mg amoxicillin–clavulanate q12h IV for 2 days and PO for 3 days. The thoracostomy tube was removed 24 h postoperatively. Four days postoperatively, the cat began eating voluntarily with a good appetite and the oesophageal tube was removed. It remained under boarding care for 3 weeks, during which time it was bright and had a good appetite. At the referring veterinarian 4 months later, the cat’s body weight had increased to 4.5 kg. The cat is currently being managed with prednisolone and a hypoallergenic diet for a skin condition.
Discussion
In the present case, the caval foramen hernia was considered congenital owing to the absence of any history of trauma and the cat being housed indoors. Caval foramen hernia has been rarely reported in dogs and humans.1–5
Embryologically, the septum transversum is closely involved in the development of the diaphragm, liver and caudal vena cava. The septum transversum forms the central tendon of the diaphragm.6,7 It also supports the growth and proliferation of the liver and formation of the hepatic connective tissue, hepatic capsule and its peritoneal covering.8 The mature liver remains attached to the central tendon of the diaphragm via the coronary ligament.8 The caudal vena cava develops from the cranial segment of the right vitelline vein, which initially traverses through the septum transversum into the sinus venosus of the developing embryo.9 When fully developed, the adventitial layer of the caudal vena cava fuses with the central tendon of the diaphragm.10
The authors of a human case report suggested that the embryopathology of caval foramen hernia may involve overgrowth of hepatic cords through the septum transversum or late digression of hepatocyte precursors during the formation of the intrahepatic inferior vena cava.4 However, this does not explain the occurrence of omental fat herniation, nor in the present case, herniation of the gallbladder.3
We propose that defective septum transversum development can result in caval foramen hernia. This is supported by the diaphragmatic defect involving the central tendon in the present case.
The cat’s non-specific clinical signs were attributed to the hernia owing to their resolution following herniorrhaphy. It is unclear what may have caused the cat to develop these signs. Its presentation is similar to two canine cases, which developed anorexia and vomiting.1 However, another canine case reported lethargy since infancy and most cases were found incidentally in older patients.1–5 These presentations are similar to another congenital diaphragmatic hernia, peritoneopericardial diaphragmatic hernia (PPDH). Cats with PPDH were a median of 1–2 years of age at diagnosis, with diagnosis occurring as late as 12.3 years.11,12 Animals undergoing herniorrhaphy for PPDH were more likely to be younger and have clinical signs than those not undergoing surgery.11,12 Diagnosis was therefore more likely incidental in older animals.11,12
In other cases of caval foramen hernia, only a small portion of omental fat or a single liver lobe were herniated.1–5 In dogs, the right lateral liver lobe is most commonly affected, with ventral herniation with respect to the caudal vena cava.1 In the present case, the diaphragmatic defect was relatively large, allowing herniation of multiple organs. There was a mild elevation in liver enzymes and left medial liver lobe adhesions. These are not uncommon in other diaphragmatic hernias involving liver herniation.11–14 Perhaps in our case, because more structures were herniated, there was an increased risk of organ entrapment causing reversible disease. The herniation may also have resulted in relatively recent adhesion formation, causing secondary lobulation, irritation and hepatocellular damage, resulting in clinical signs.11 Another cause for the lobulated liver lobe could be defective septum transversum development given its role in the formation of the hepatic connective tissue and capsule.8
In the present case, thoracic radiographs and abdominal ultrasound were the initial imaging modalities used for the investigation of non-specific clinical signs. These modalities are inexpensive and relatively widely available. Radiographically, caval foramen hernias most often appear as a caudal dome-shaped soft tissue opacity broad-based to the diaphragm.1 These can be confused for pulmonary masses.1,5 In this case, the broad base helped prioritise diaphragmatic hernia over other origins. Caval foramen hernia was specifically considered because of the location of the soft tissue opacity and border effacement of the caudal vena cava. Ultrasound may be used if diaphragmatic hernia cannot be confirmed on survey radiographs.15 Typical ultrasonographic signs include identification of abdominal structures within the thorax and an interruption in the diaphragmatic outline.15 In this case, ultrasound confirmed diaphragmatic hernia involving the liver and ruled out other morphological features of disease in case the hernia was an incidental finding. Clinicians should be aware that caval foramen hernias have been mistaken for a right atrial mass during echocardiography.3,4 In the current case, radiography and ultrasound did not determine the extent of liver involvement, gallbladder herniation, size of the diaphragmatic defect or association with the caudal vena cava.
CT enables assessment of organs in multiple planes without superimposition.16 CT is commonly used to confirm caval foramen hernia.1,5 In this case, it also enabled assessment of vasculature and identification of herniated structures, aiding anaesthetic and surgical planning. If there is no access to CT or if the client is financially constrained, the combined clinical, radiographic and ultrasonographic findings would provide indication for surgical intervention.
Caval foramen hernias are commonly associated with caudal vena cava obstruction, and may be associated with hepatic vein dilation and pulmonary embolism.1,3 Interestingly, the majority of these patients remained asymptomatic.1,3 In the present case, multiple organs were herniated without caudal vena cava obstruction. This is likely attributable to the relatively large diaphragmatic defect allowing space for structures to herniate around the caudal vena cava. Nevertheless, caudal vena cava obstruction must be considered in cases of caval foramen hernia, and clinicians should be aware of the potential clinical signs. Caudal vena cava obstruction can result in Budd–Chiari-like syndrome. There is hepatic venous outflow obstruction and portal hypertension, leading to hepatomegaly and ascites.1,17,18 Additionally, a dog with intrathoracic caudal vena cava obstruction also had impaired venous return to the heart, reduced cardiac output and renal blood flow, leading to secondary azotaemia.19 This dog presented with more clinical findings including ascites, diarrhoea, lethargy, tacky mucous membranes, prolonged capillary refill time, a non-pulsatile left jugular vein and pitting oedema in the hindlimbs.19
Conclusions
To our knowledge, this is the first report of successful caval foramen herniorrhaphy in a cat. The hernia was detected on routine investigation of non-specific clinical signs. Radiographic features suggestive of caval foramen hernia were its positioning at the mid-height of the caudal thorax to the right of midline, border effacement of the caudal vena cava and broad based towards the diaphragm. These hernias have been radiographically mistaken for pulmonary masses. Therefore, recognition of these features can potentially significantly alter case management. CT confirmed caval foramen hernia and enabled assessment of the caudal vena cava to aid anaesthetic and surgical planning. Caudal vena cava obstruction is commonly associated with caval foramen hernia. Therefore, it may be considered as a differential diagnosis in cats presenting with clinical signs of Budd–Chiari-like syndrome.
Footnotes
Accepted: 6 September 2020
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: QiCai Jason Hoon  https://orcid.org/0000-0002-1636-8622
https://orcid.org/0000-0002-1636-8622
References
- 1. Kim J, Kim S, Jo J, et al. Radiographic and computed tomographic features of caval foramen hernias of the liver in 7 dogs: mimicking lung nodules. J Vet Med Sci 2016; 78: 1693–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nonaka Y, Takashima K, Yamane T, et al. Caval foramen hernia in a dog. J Anim Clin Med 2008; 17: 81–85. [Google Scholar]
- 3. Benitez Lazzarotto A, O'Rourke NA, Fitzgerald BT, et al. Hernia of the diaphragmatic caval foramen causing right atrial ‘mass’, caval obstruction and pulmonary embolism. Int J Cardiol 2016; 207: 215–216. [DOI] [PubMed] [Google Scholar]
- 4. Kapoor H, Ramy Elashery A, El Khouli R, et al. Accessory hepatic caval foraminal herniation mimicking a right atrial mass. Circ Cardiovasc Imag 2019; 12. DOI: 10.1161/CIRCIMAGING.118.008765. [DOI] [PubMed] [Google Scholar]
- 5. Ng CS, Lee TW, Wan S, et al. Caval foramen hernia masquerading as a thoracic mass. Can J Surg 2006; 49: 64–65. [PMC free article] [PubMed] [Google Scholar]
- 6. McGeady T, Quinn P, Fitzpatrick E, et al. Coelomic cavities. In: McGeady T, Quinn P, Fitzpatrick E, et al. (eds). Veterinary embryology. Ames, IA: Blackwell Publishing, 2006, pp 59–65. [Google Scholar]
- 7. Noden D, DeLahunta A. Respiratory system and partitioning of body cavities. In: Noden D, DeLahunta A. (eds). The embryology of domestic animals: developmental mechanisms and malformations. Baltimore, MD: Williams & Wilkins, 1985, pp 287–291. [Google Scholar]
- 8. McGeady TA, Quinn PJ, FitzPatrick ES, et al. Digestive system. In: McGeady TA, Quinn PJ, FitzPatrick ES, et al. (eds). Veterinary embryology. Ames, IA: Blackwell Publishing, 2006, pp 205–224. [Google Scholar]
- 9. McGeady TA, Quinn PJ, FitzPatrick ES, et al. Cardiovascular system. In: McGeady TA, Quinn PJ, FitzPatrick ES, et al. (eds). Veterinary embryology. Ames, IA: Blackwell Publishing, 2006, pp 105–135. [Google Scholar]
- 10. Dyce K, Sack W, Wensing C. The locomotor apparatus. In: Dyce K, Sack W, Wensing C. (eds). Textbook of veterinary anatomy. St Louis, MO: Saunders Elsevier, 2010, pp 32–99. [Google Scholar]
- 11. Banz AC, Gottfried SD. Peritoneopericardial diaphragmatic hernia: a retrospective study of 31 cats and eight dogs. J Am Anim Hosp Assoc 2010; 46: 398–404. [DOI] [PubMed] [Google Scholar]
- 12. Burns CG, Bergh MS, McLoughlin MA. Surgical and nonsurgical treatment of peritoneopericardial diaphragmatic hernia in dogs and cats: 58 cases (1999–2008). J Am Vet Med Assoc 2013; 242: 643–650. [DOI] [PubMed] [Google Scholar]
- 13. Minihan AC, Berg J, Evans KL. Chronic diaphragmatic hernia in 34 dogs and 16 cats. J Am Anim Hosp Assoc 2004; 40: 51–63. [DOI] [PubMed] [Google Scholar]
- 14. Schmiedt CW, Tobias KM, Stevenson MA. Traumatic diaphragmatic hernia in cats: 34 cases (1991–2001). J Am Vet Med Assoc 2003; 222: 1237–1240. [DOI] [PubMed] [Google Scholar]
- 15. Randall E. Canine and feline diaphragm. In: Thrall D. (ed). Textbook of veterinary diagnostic radiology. 7th ed. St Louis, MO: Elsevier, 2018, pp 633–648. [Google Scholar]
- 16. d’Anjou M-A. Principles of computed tomography and magnetic resonance imaging. In: Thrall D. (ed). Textbook of veterinary diagnostic radiology. 7th ed. St Louis, MO: Elsevier, 2018, pp 71–95. [Google Scholar]
- 17. Hoehne SN, Milovancev M, Hyde AJ, et al. Placement of a caudal vena cava stent for treatment of Budd-Chiari-like syndrome in a 4-month-old Ragdoll cat. J Am Vet Med Assoc 2014; 245: 414–418. [DOI] [PubMed] [Google Scholar]
- 18. Holt D, Saunders HM, Aronson L, et al. Caudal vena cava obstruction and ascites in a cat treated by balloon dilation and endovascular stent placement. Vet Surg 1999; 28: 489–495. [DOI] [PubMed] [Google Scholar]
- 19. Stauthammer C, Tobias A, France M, et al. Caudal vena cava obstruction caused by redundant pacemaker lead in a dog. J Vet Cardiol 2009; 11: 141–145. [DOI] [PubMed] [Google Scholar]