Abstract
Sleep problems are widely prevalent and associated with various comorbidities including anxiety. Valerian (Valeriana officinalis L.) is a popular herbal medicine used as a sleep aid, however the outcomes of previous clinical studies are inconsistent. This study was conducted to update and re-evaluate the available data in order to understand the reason behind the inconsistent outcomes and to provide a broader view of the use of valerian for associated disorders. PubMed, ScienceDirect, and Cochrane Library were searched to retrieve publications relevant to the effectiveness of valerian as a treatment of sleep problems and associated disorders. A total of 60 studies (n=6,894) were included in this review, and meta-analyses were performed to evaluate the effectiveness to improve subjective sleep quality (10 studies, n=1,065) and to reduce anxiety (8 studies, n=535). Results suggested that inconsistent outcomes were possibly due to the variable quality of herbal extracts and that more reliable effects could be expected from the whole root/rhizome. In addition, therapeutic benefits could be optimized when it was combined with appropriate herbal partners. There were no severe adverse events associated with valerian intake in subjects aged between 7 and 80 years. In conclusion, valerian could be a safe and effective herb to promote sleep and prevent associated disorders. However, due to the presence of multiple active constituents and relatively unstable nature of some of the active constituents, it may be necessary to revise the quality control processes, including standardization methods and shelf life.
Keywords: valeriana officinalis, herbal medicine, sleep, insomnia, anxiety, systematic review
Introduction
Sleep Problems
Sleep plays a crucial role in maintaining brain functions and systemic physiology, and chronic sleep problems could have a significant impact on our health.1,2 Insufficient sleep leads to reduced stress resilience, decreased quality of life, mood disorders, and cognitive, memory, and performance deficits.3 It can also contribute to metabolic disorders including hypertension, dyslipidemia, cardiovascular disease, and type 2 diabetes mellitus.2,4 In addition, it is associated with significantly increased dementia risk.5,6 Problems with sleep are widely prevalent,4 affecting 70 million (9-20%) of adults in the US and 45 million (7%) of adults in Europe.7,8 Typical manifestation of sleep disorders include sleep deprivation or fragmentation and events that occur during sleep.4 The major sleep disorders are insomnia, restless leg syndrome (RLS),9 obstructive sleep apnea syndrome, and narcolepsy,10,11 among which insomnia is the most common.12 Sedative-hypnotic medications commonly prescribed for insomnia are γ-aminobutyric acid type A (GABAA) receptor agonists, antidepressants, and antihistamines.13 However, the long term use of most sedative-hypnotic drugs is limited due to various side effects, such as cognitive and daytime performance impairments.14 In recent years, herbal supplements, such as valerian (Valeriana officinalis L.), sour jujube seeds (Ziziphus jujuba Miller var. spinosa Hu ex H. F. Chou), and kava (Piper methysticum G.Forst.), have gained popularity as alternatives to prescription drugs to improve sleep quality without side effects.15
Valerian for Sleep Problems and Associated Disorders
Recorded medicinal use of valerian dates back to the first century AD. In recent years, it is popular as a sedative and hypnotic. Historically, however, its metabolic stimulating features such as diuretic, carminative, and menstrual stimulant properties had been more valued.16 It was in the middle ages that the use of valerian in treating nervous disorders and insomnia was recorded.16,17 There are over 200 valerian species worldwide, including V. wallichii DC., V. edulis Nutt., and V. fauriei Briq., among which Valeriana officinalis L. is the most well known in Europe and North America as “valerian.” In the United States, valerian is regulated by the Food and Drug Administration (FDA) as a dietary supplement (https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional/). According to the European Medicine Agency (EMA), the well-established uses of V. officinalis root include the relief of mild nervous tension as well as sleep disorders. For relief of nervous tension, recommended oral dosages are 400-600 mg dry hydroalcoholic extract or comminuted herbal substance (root) 0.3-3 g up to 3 times daily.18 Valerian is considered relatively safe and well-tolerated, however EMA monograph notes gastrointestinal symptoms (e.g. nausea, abdominal cramps) as undesirable effects. Although hydroalcoholic extracts of valerian root in the recommended dosage improve sleep latency and quality, it is uncertain what constituents contribute to the efficacy.18
The effectiveness of valerian as a sleep aid has been the major research focus, and several systematic reviews were conducted previously. A systematic review published in 2000, which analyzed 9 randomized clinical trials, found contradictory results and significant inconsistency in terms of patients, experimental design and methodology among the trials.19 Another systematic review and meta-analysis, published in 2006, analyzed 16 studies and this study also found significant methodological problems.20 Taibi et al. (2007) conducted a systematic review on 37 studies, of which 29 were controlled trials and 8 were open-label trials. They concluded that, although it is a safe herb, evidence did not support the clinical efficacy of valerian as a sleep aid for insomnia.21 A meta-analysis of 18 randomized placebo-controlled trials, published in 2010, concluded that valerian’s effectiveness had not been demonstrated with quantitative or objective measures although valerian could improve subjective sleep quality.22 So far, these inconsistent outcomes have not been fully explained. In addition, it is not clear if valerian is effective in treating other disorders associated with, and possibly contributing to, sleep problems. The aim of the current study is to up-date the available data, to evaluate the effectiveness of valerian as a treatment of sleep problems and associated disorders, and to discuss possible reasons behind the inconsistent research outcomes, by particularly focusing on the herbal preparations used in the studies.
Summary
In summary, this study will focus on:
The herbal materials – extracts or whole root/rhizomes – that have been used in trials.
Potential applications of valerian, including but looking beyond its use for sleep problems.
Popular and effective herbal combinations.
Methods
In order to evaluate the therapeutic effects of valerian, systematic reviews and meta-analyses were conducted following PRISMA guidelines.23 Searches A-C were conducted using PubMed (https://www.ncbi.nlm.nih.gov/pubmed), ScienceDirect (https://www.sciencedirect.com/search/advanced), and Cochrane Library (https://www.cochranelibrary.com/search).
Search Strategy
Search A:
Clinical studies on valerian, published between January 1980 and November 2019, were retrieved using search term “Valeriana” in PubMed database under article type: Clinical Trial.
Search B:
ScienceDirect advanced search was conducted using search term “Valeriana clinical trial.” Articles published between January 2000 and November 2019 were sought.
Search C:
To identify clinical trials using valerian, the Cochrane Library was searched (up to December 2019) using the search term ‘Valeriana officinalis’.
Study Selection
Studies were screened initially for relevance based on the title and abstract. Reviews, unrelated studies, and works without available full text were excluded. Studies evaluated the effectiveness of valerian alone or in combination on sleep or related health problems were considered relevant. The full texts of potential studies were assessed following the exclusion and listed below.
Exclusion criteria
Articles published in any non-English language.
Studies using unknown substance.
Studies on non-human subjects.
The quality of randomized trials were assessed using Jadad scale.24 In order to extensively collect currently available information, both controlled trials and observational studies were included for reviewing, irrespective of potential risk of bias (Figure 1).
Figure 1.
PRISMA flowchart.
Meta-Analysis
The effectiveness of V. officinalis in promoting sleep and reducing anxiety were evaluated by meta-analyses. Due to the scarcity of data, all placebo-controlled studies reporting values that are convertible to effect sizes were included for statistical analysis, irrespective of trials’ quality (Jadad scale). The outcome variables were extracted from V. officinalis treatment group and placebo group by 1 author, which was checked by 2 authors. In order to evaluate the effectiveness as a sleep aid, data on subjective ‘sleep quality’ improvement by repeated administration (ranging between 5 days to 8 weeks) were included in the analysis. Due to the variable measures, both numerical scores and binary scores were converted to effect sizes for the analysis.25 To evaluate the anxiolytic effect, a study reporting the overall emotional symptoms including anxiety and a study reporting binary outcomes, in addition to the studies reporting standard anxiety test scores, were included. When several measures were reported over time in the same study, the one with the longest follow-up period was selected. When more than 1 dose were tested, the result of the most effective dose was selected. Adjusted effect sizes (Hedges’ g) were calculated from summary measures such as means and standard deviations or confidence intervals, odds ratio (for binary outcomes), and sample sizes, using reported formula.25–27 Meta-analyses were performed using Meta-Essentials.28 I2 statistic was used to estimate heterogeneity. Publication bias was evaluated using the funnel plots.29
Results
Based on the inclusion and exclusion criteria described above, a total of 60 articles were selected for reviewing (Table 1 and 2). Both quantitative and qualitative studies with human subjects were included. Of 40 articles using valerian as a single herb, 36 studies were randomized controlled trials (RCT)30–65 and 2 studies were observational.66,67 Two studies addressed the potential induction of liver enzyme cytochrome P450 (CYP) isoforms.68,69 In addition, 2 RCTs were conducted using valepotriates (80% didrovaltrate, 15% valtrate, and 5% acevaltrate)70,71 (Table 1). Nineteen articles assessed the effectiveness of herbal combinations, of which 13 studies were RCT65,72,73–80,81–83 and 6 were observational studies84–89 (Table 2).
Table 1.
Valeriana spp. as a Single Herb.
| First Author | Study design | Intervention and measures | Outcomes | Adverse events | * | |
|---|---|---|---|---|---|---|
| Hydroalcoholic extract | ||||||
| 1 | Roh D 201934 | Randomized, placebo-controlled, double-blind. Population: nonclinical volunteers suffering psychological stress (n=52) |
Intervention: V. officinalis 70%EtOH extract capsule 300 mg (0.8% valerenic acid) per day (100 mg t.i.d.) for 4 weeks. Measures: 1. Psychiatric variables (HAM-A, HAM-D, PSQI). 2. Functional brain connectivity changes (EEG) Summary measures for meta-analysis: difference in means |
1. Improvement in both treatment and control groups. No statistically significant difference between the groups. 2. Greater increases in frontal brain region alpha coherence in valerian group, which was correlated with anxiolysis |
No moderate or serious adverse reaction. Two adverse effects (one from each group): atypical chest pain (unrelated to the medication) in treatment group and gastrointestinal problems in the placebo group. |
(1) PSQI (2) HAM-A (3) 5 |
| 2 | Taibi DM 200954 | Randomized, placebo-controlled, single-blind, pilot study. Population: Arthritis patients with mild sleep disturbance (n=15) |
Intervention: V. officinalis 70%EtOH extract (0.8% valerenic acid) 600 mg for 5 nights Measures: Sleep outcomes (sleep diaries and wrist actigraphy) Summary measures for meta-analysis: difference in means |
No significant difference between the groups. | Dizziness and sleepiness | (1) Self-rated (2) n/a (3) 5 |
| 3 | Taibi DM 200953 | Randomized, placebo-controlled, double-blind, cross-over Population: Older women with insomnia (n=16) |
Intervention: V. officinalis 70% extract 300 mg (0.8% valerenic acid), 30 min before bedtime for 2 weeks Measures: (1) polysomnography (PSG); (2) self-rated sleep Summary measures for meta-analysis: difference in means |
No significant difference between the groups in sleep latency, wake after sleep onset, sleep efficiency, and self-rated sleep quality. | No severe adverse events. No difference between valerian and placebo | (1) Self-rated (2) n/a (3) 5 |
| 4 | Oxman AD 200752 | Randomized, placebo-controlled, double-blind (Web-based trial) Population: Volunteers with insomnia without treatment (n=405) |
Intervention: V. officinalis 60%EtOH extract 600 mg per day, 1 h before bedtime for 14 days. Measures: Sleep diary (sleep onset latency, number of night awakenings, sleep duration, sleep quality, energy level the following day). Summary measures for meta-analysis: odds ratio |
Global self-assessment of change was better for valerian group (p=0.04). Similar trends favoring the treatment group for sleep quality, night awakenings, and sleep duration. | No serious adverse events. No statistically significant differences in minor adverse events between the groups. | (1) Self-rated (2) n/a (3) 5 |
| 5 | Koetter U 200765 | Randomized, placebo-controlled, double-blind Population: Patients suffering from non-organic sleep disorders (n=27) |
Intervention: V. officinalis 45%MeOH extract 500 mg compared to placebo. For 4 weeks. Measures: (1) the reduction of the initially prolonged sleep latency; (2) WASO, sleep efficiency, relative proportion of the sleep stages, REM latency and CGI |
No significant difference between valerian extract and placebo. | No adverse events. | (1) n/a (2) n/a (3) 3 |
| 6 | Diaper A 200450 | Randomized, placebo-controlled, double-blind, cross-over Population: Sleep-disturbed, but otherwise normal subjects (n=16) |
Intervention: (1) V. officinalis 70%EtOH extract (LI 156) 300 mg; (2) V. officinalis 70%EtOH extract 600 mg; (3) placebo. Single dose, separated by 6 days washout. Measures: Sleep EEG, CFF, CRT, SMT, LARS, LSEQ, STDI, and STAI Summary measures for meta-analysis: difference in means |
No significant differences between the 3 groups. | Mild or moderate adverse events: Drowsiness was 4 under 600 mg valerian, 12 under 300 mg valerian, and 8 in placebo. |
(1) n/a (2) STAI (3) 3 |
| 7 | Donovan JL 200469 | Open-label, fixed treatment, cross-over Population: Healthy volunteers (n=12) |
Intervention: V. officinalis 70%EtOH extract 1000 mg (containing 11.02 mg valerenic acid) nightly for 14 days. Measures: CYP2D6 and CYP3A4 activities |
V. officinalis has a moderate effect on CYP3A4 (increase in maximum probe drug concentration), however there were no significant differences in other pharmacokinetic parameters. | No treatment-associated adverse events | (1) n/a (2) n/a (3) n/a |
| 8 | Hallam KT 200347 | Placebo-controlled, double-blind, cross-over Population: Healthy volunteers (n=9) |
Intervention: Single doses of V. officinalis 70%EtOH extract (0.25% valerenic acids) 500 mg or 1000 mg. Compared to triazolam 0.25 mg. Measures: Cognitive and psychomotor performance (CFF, CRT, DSST, SST, DST) and mood (VAS). Summary measures for meta-analysis: difference in means |
Single doses of valerian were without effect on either cognitive or psychomotor performance, while triazolam had detrimental effects on cognitive processes, in healthy volunteers. Although not statistically significant, valerian 500 mg may have tranquilizing / calming effect. |
(1) n/a (2) Self-rated calming effect (3) 3 |
|
| 9 | Ziegler G 200244 | Randomized, double-blind, comparative Population: Non-organic insomnia outpatients (n=202) |
Intervention: V. officinalis 70%EtOH extract (LI 156 sedonium) 600 mg for 6 weeks. Compared with oxazepam Measures: Sleep questionnaire |
Both Valerian and oxazepam improved sleep quality. | Mild or moderate adverse events in both oxazepam (36%) and valerian (28%) groups. | (1) n/a (2) n/a (3) 2 |
| 10 | Herrera-Arellano A 200142 | Randomized, controlled, double-blind, cross-over Population: Outpatients with clinical insomnia (n=20) |
Intervention: Single doses of (1) V. officinalis hydroalcoholic extract 450 mg or (2) V. edulis (hydroalcoholic extract) 450 mg. Prior to lights out. Compared to basal levels. Measures: Hypnotic effects (polysomnography), anterograde memory (measured by D. Wechsler Memory Scale) |
V. officinalis and V. edulis increased the REM sleep (better improved by V. officinalis), and morning sleepiness. V. edulis reduced the number of awaking episodes. |
Slight dyspepsia (2 for V. officinalis and one for V. edulis) | (1) n/a (2) n/a (3) 2 |
| 11 | Donath F 200036 | Randomized, placebo-controlled, double-blind, cross-over Population: Patients suffering from psychophysiological insomnia (n=16) |
Intervention: V. officinalis 70%EtOH extract (Sedonium) 600 mg, 1 h before bedtime. (1) Single dose, (2) 14 days. Measures: Objective sleep efficiency (SE) and sleep onset latency (SOL). NREM, REM, and slow wave sleep SWS). Subjective sleep quality (VAS) Summary measures for meta-analysis: difference in means |
(1) There was no significant effect after a single dose. (2) SE was significantly higher in both groups at the end of the trial periods. SWS latency was significantly shorter and SWS increased in valerian group. A tendency toward reduced subjective sleep latency was observed despite there was no significant difference in objective SOL between the groups. |
Fewer adverse events for Valerian group than placebo. | (1) Self-rated (2) n/a (3) 2 |
| 12 | Kuhlmann J 199937 | Randomized, placebo-controlled, double-blind Population: Healthy volunteers (n=102) |
Intervention: V. officinalis 70%EtOH extract (600 mg, LI 156). Single dose in the evening (compared to placebo and Flunitrazepam (FNZ)), and repeated evening administration for 2 weeks (compared to placebo). Measures: (1) Sleep quality and latency (self-assessment). (2) Reaction time (Vienna Determination Test), alertness, and concentration (2-handed co-ordination) at next morning. |
(1) Sleep quality and latency improved after single doses of valerian and FNZ. After 2 weeks with Valerian, sleep quality had a trend toward improvement, compared to placebo. (2) Neither single dose nor repeated administrations of valerian extract had a negative impact on reaction time, alertness, and concentration. |
No serious adverse events. Headache and weakness were most frequently reported, which had not inter-group difference (no causal relationship to valerian). Note: Single dose of FNZ caused hangover effect in 59.4%, while only 32.4% of placebo and 30.3% of valerian-treated groups reported this effect. |
(1) n/a (2) n/a (3) 3 |
| Aqueous extract | ||||||
| 13 | Pakseresht S 201155 | Randomized, placebo-controlled, double-blind Population: Obsessive-Compulsive Disorder (OCD) patients (n=31) |
Intervention: V. officinalis aqueous extract 750 mg per day (250 mg t.i.d.) for 8 weeks Measures: Yale-Brown scale for OCD (Y-BOCS) |
Significantly reduced Y-BOCS score in the treatment group compared to placebo. | No significant difference in observed side effects, however increased somnolence in the treatment group (53.3% in treatment group and 18.75% in placebo group) | (1) n/a (2) n/a (3) 5 |
| 14 | Schulz H 199438 | Randomized, placebo-controlled, double-blind Population: Elderly poor sleepers (n=14) |
Intervention: V. officinalis aqueous alkaline extract (Valdispert forte): (1) 405 mg single administration 1 h before sleep recording; (2) 405 mg t.i.d. for 7 days. Measures: Objective (polysomnography) |
(1) Acute administration had no effect. (2) V. officinalis increased SWS and K-complex density, and decreased sleep stage 1. (SWS increased in each single subject on valerian.) No effect on sleep onset time, time awake after sleep onset, or self-rated sleep quality. |
Not noted. | (1) n/a (2) n/a (3) 2 |
| 15 | Balderer G 198540 | Randomized, placebo-controlled, double-blind, cross-over Population: Healthy young volunteers (n=18) |
Intervention: Single doses of V. officinalis aqueous extract (450 or 900 mg) or placebo 30 min before bedtime. At home (n=10) and in the laboratory (n=8). Measures: Subjective: sleep questionnaires, self-rating scale, night-time motor activity (n=18). Objective: polysomnography (n=8). |
Home: V. officinalis dose-dependently reduced subjective sleep latency and wake time after sleep onset. Self-rating of subjective quality showed no difference (data not shown). Laboratory: No statistically significant difference between valerian and placebo. |
(1) n/a (2) n/a (3) 2 |
|
| 16 | Leathwood PD 198241 | Randomized, placebo-controlled, blinded Population: Volunteers. Self-reported habitually good sleeper, habitually poor or irregular sleepers (n=128) |
Intervention: Single doses of (1) V. officinalis aqueous extract 400 mg; (2) over-the-counter preparation containing valerian and hops (Hova, Zyma S.A.); (3) placebo. 1 h before bedtime. Each treatment 3 times, randomly on non-consecutive nights. Measures: Sleep latency and quality, dream recall, night awakenings, somnolence the next morning (sleep questionnaire). |
V. officinalis produced significant decrease in subjective sleep latency and improved sleep quality. Sleep quality improvement was most notable among poor or irregular sleepers and smokers. Proprietary preparation produced ‘hangover effect’ the next morning, which was absent after valerian intake. |
No notable side effects | (1) n/a (2) n/a (3) 5 |
| Unknown extract | ||||||
| 17 | Farah GJ 201933 | Randomized, split-mouth, controlled, double-blind, cross-over Population: anxious patients undergoing osteotomy and odontosection (n=20) |
Intervention: V. officinalis extract 100 mg. Control drug: midazolam 15 mg. Both in an identical-looking capsules). 60 min before the surgical procedures. Measures: Oxygen saturation, HR, systolic and diastolic BP, RR, sedation (Ramsay scale). Self-assessment of anterograde amnesia. |
More patients with valerian were rated as calm. No difference between valerian and midazolam (benzodiazepine) in oxygen saturation. HR and BP were lower with midazolam. Midazolam caused somnolence and anterograde amnesia, while valerian did not. |
The most commonly reported side effect was somnolence. No statistically significant difference between the 2 drugs. | (1) n/a (2) n/a (3) 5 |
| 18 | Mineo L 201731 | Randomized, placebo-controlled, double-blind, cross-over. Population: Healthy volunteers (n=15) |
Intervention: Single dose of V. officinalis extract capsule (300 mg with 0.8% valerenic acid) Measures: Cortical excitability (TMS) |
Significant reduction in intracortical facilitation (ICF) at 1 h after Valerian extract intake, without affecting other TMS parameters. ICF returned to normal at 6 h. | No adverse events | (1) n/a (2) n/a (3) 4 |
| 19 | Behboodi Moghadam Z 201663 | Randomized, placebo-controlled, double-blind Population: Female students having premenstrual syndrome (n=100) |
Intervention: V. officinalis extract 630 mg twice daily, in the last 7 days of menstrual period for 3 cycles. Measures: Severity of emotional, behavioral, and physical premenstrual symptoms (questionnaire) Summary measures for meta-analysis: difference in means |
Valerian improved emotional, behavioral, and physical premenstrual symptom severity, whole placebo had no effects. | Not noted | (1) n/a (2) self-rated emotional state (3) 5 |
| 20 | Jacobs BP 200551 | Randomized, placebo-controlled, double-blind (internet-based) Population: Volunteers with anxiety and insomnia (n=391). Recruited via e-mail and banner advertisements on websites |
Intervention: (1) V. officinalis extract (6.4 mg (1%) valerenic acid); (2) Piper methysticum extract (100 mg total (30%) kavalactones); (3) placebo. For 28 days Measures: Anxiety (STAI) and insomnia (ISI) Summary measures for meta-analysis: difference in means |
Similar decrease in anxiety and insomnia symptoms among the 3 groups. | Diarrhea (valerian 18%, Kava 11%, control 8%) | (1) ISI (2) STAI (3) 5 |
| 21 | Gurley BJ 200568 | Cross-over Population: Healthy volunteers (n=12) |
Intervention: (1) V. officinalis extract (125 mg 3 times daily); (2) Hydrastis canadensis (900 mg 3 times daily); (3) Piper methysticum (100 mg twice daily); (4) Cimicifuga racemosa (1090 mg twice daily). For 28 days separated by 30 days washout. Measures: CYP1A2, CYP2D6, CYP2E1 and CYP3A4/5 phenotypes |
No significant changes in phenotypic ratios were observed for V. officinalis. (H. canadensis inhibited CYO2D6 and CYP3A4/5, P. methysticum reduced CYP2E1, and C. racemosa inhibited CYP2D6.) |
No serious adverse events | (1) n/a (2) n/a (3) n/a |
| 22 | Gutierrez S 200449 | Randomized, placebo-controlled, double-blind, cross-over Population: Healthy volunteers (n=10) |
Intervention: Single doses of V. officinalis standardized extract 600, 1200, and 1800 mg Measures: Subjective mood (including sedation by ARCI) and psychomotor performance (DSST) Summary measures for meta-analysis: difference in means |
No significant difference from placebo. | (1) n/a (2) self-rated sedation (3) 4 |
|
| 23 | Coxeter PD 200348 | Randomized, placebo-controlled, double-blind, n-of-1 Population: Patients with chronic insomnia (n=24) |
Intervention: V. officinalis extract 450 mg equivalent to 2000 mg dry root / rhizome (5.88 mg valerenic acids, 0.96 mg valerenal, and 2.46 mg valtrates), 30 min before bed for 3 weeks. 5 days wash-out. Measures: Sleep diary |
Fair response for energy level in the previous day, but only modest response for other variables. | There was no significant difference in the number, distribution, or severity of side effects between valerian and placebo. | (1) n/a (2) n/a (3) 5 |
| 24 | Cropley M 200243 | Randomized, controlled Population: Young healthy volunteers (n=54) |
Intervention: (1) V. officinalis standardized extract 600 mg (Lichtwer-Pharma UK Ltd); (2) Piper methysticum (120 mg) For 7 days. Compared to non-placebo control. Measures: Blood pressure, heart rate, and subjective ratings of pressure in response to standardized mental stress task-induced psychological stress were assessed. |
V. officinalis (and Piper methysticum) reduced psychological pressure and systolic blood pressure responsivity during stress task. V. officinalis reduced heart rate reaction to mental stress. |
(1) n/a (2) n/a (3) 1 |
|
| 25 | Wheatley D 200166 | Observational, cross-over Population: Outpatients suffering from stress-induced insomnia (n=19) |
Intervention: (1) Piper methysticum 120 mg; (2) V. officinalis 600 mg (Lichtwer-Pharma UK Ltd). For 6 weeks. 2 weeks wash-out. Piper methysticum first. Measures: Severity of stress (VAS) and sleep disturbance (subjective). |
Severity of stress: Significant reduction during the first weeks on P. methysticum. There was no further change during the second 6 weeks on V. officinalis. Severity of insomnia: Score fell after he first 6 weeks on P. methysticum, and further fell after the final 6 weeks on V. officinalis. |
1. Vivid dreams, daytime drowsiness, heavy sleep and depression for V. officinalis. 2. Dry mouth, gastric disturbance, diarrhea, dizziness, craving for trial drug, and depression for P. methysticum. |
(1) n/a (2) n/a (3) n/a |
| 26 | Kohnen 198839 | Randomized, placebo-controlled, double-blind Population: Healthy volunteers (n=48) |
Intervention: Single doses of (1) Valerian extract 100 mg, (2) propranolol (20 mg), (3) placebo, and (4) valerian + propranolol Measures: Pulse frequency, test performance, psychological strain, and somatic arousal under stress conditions after intervention |
Valerian induced a slight improvement in the concentration task, while propranolol and valerian-propranolol combination reduced the performance values. Valerian reduced the feelings of somatic arousal. Valerian had no effect on psychological strain. | Not noted | (1) n/a (2) n/a (3) 2 |
| Whole root | ||||||
| 27 | Samaei A 201832 | Randomized, placebo-controlled, double-blind, cross over Population: haemodialysis patients (n=39) |
Intervention: V. officinalis root/rhizome 530 mg, 60 min before bedtime for 1 month. Measures: Cognitive brain functions (Mini-Mental State Examination: MMSE) and EEG |
Valerian significantly improved cognitive status (MMSE), although no significant changes were observed in EEG. | Not noted. | (1) n/a (2) n/a (3) 4 |
| 28 | Ahmadi M 201735 | Randomized, placebo-controlled, double-blind. Population: HIV-positive patients receiving efavirenz (n=51) |
Intervention: V. officinalis root/rhizome 530 mg (> 0.05% valerenic acid), nightly 1 h before sleep for 4 weeks Measures: Neuropsychiatric status (HAM-D, HAM-A, PANSS, PANSI, and PSQI) Summary measures for meta-analysis: difference in means |
Sleep and anxiety significantly improved in Valerian treatment group. | There was no significant difference between the groups. | (1) PSQI (2) HAM-A (3) 3 |
| 29 | Jenabi E 201764 | Randomized, placebo-controlled, triple-blind Population: postmenopausal women with hot flashes (n=60, all women) |
Intervention: V. officinalis root/rhizome 225 mg 3 times per day for 8 weeks Measures: Severity and frequency of hot flashes (Kupperman index) |
Significantly lower severity after 1 and 2 months and reduced frequency after 2 months in V. officinalis treatment group | No side effects | (1) n/a (2) n/a (3) 4 |
| 30 | Thomas K 201662 | Randomized, placebo-controlled, double-blind, cross-over Population: Healthy volunteers (n=40) |
Intervention: Single dose of V. officinalis root/rhizome 1600 mg (containing 0.8% valerenic acid) Measures: SVRT, subjective sleepiness scales (Karolinska Sleepiness Scale), SFST performance scores, driving simulator performance |
There were no significant differences in SVRT, sleepiness scales, SFST, and driving simulator performance | (1) n/a (2) n/a (3) 5 |
|
| 31 | Hassani S 201561 | Randomized, placebo-controlled, double-blind Population: Coronary artery bypass graft (CABG) surgery patients (n=61) |
Intervention: V. officinalis root/rhizome 1,060 mg per day (530 mg twice daily, every 12 h) for 8 weeks Measures: Cognitive brain function (MMSE test) |
MMSE score was restored on the 60th day in valerian treatment group, whereas reduced in placebo group. | (1) n/a (2) n/a (3)5 |
|
| 32 | Pinheiro ML 201460 | Randomized, placebo-controlled, double-blind, split-mouth Population: Dental surgery patients (n=20) |
Intervention: V. officinalis comminuted root/rhizome 100 mg, 1 h prior to each surgical procedure Measures: Anxiety (assessed by surgeons and researchers) and physical parameters Summary measures for meta-analysis: odds ratio |
Valerian significantly reduced anxiety. Valerian was effective in maintaining systolic blood pressure and heart rate after surgery. |
There was no significant difference between the groups. | (1) n/a (2) Surgeon-assessed anxiety (3) 5 |
| 33 | Mirabi P 201359 | Randomized, placebo-controlled, double-blind Population: Menopausal women with the chief complaint of hot flash (n=68) |
Intervention: V. officinalis root/rhizome 675 mg per day (225 mg t.i.d), for 8 weeks Measures: Severity and frequency of hot flashes |
Valerian reduced the severity and frequency of hot flashes | – | (1) n/a (2) n/a (3) 4 |
| 34 | Mirabi P 201158 | Randomized, placebo-controlled, double-blind Population: Female students experiencing moderate-severe dysmenorrhoea (n=100) |
Intervention: V. officinalis root/rhizome 675 mg per day (225 mg t.i.d) for 3 days from the first day of menstruation, continued for 2 menstrual cycles. Measures: Pain severity (VAS, 3 times per day), the severity of associated systemic symptoms (fatigue, diarrhea, syncope, nausea and vomiting, lack of energy, headache, and mood swings) |
The pain severity was reduced in both groups, but the extent of the reduction was larger in the treatment group. There was no difference between the groups in the total scores of associated systemic manifestations, except for syncope (improvement in V. officinalis treatment group) | No adverse effects | (1) n/a (2) n/a (3) 5 |
| 35 | Taavoni S 201157 | Randomized, placebo-controlled, triple-blind Population: Postmenopausal women with self-reported insomnia (n=100) |
Intervention: V. officinalis root/rhizome 530 mg (Sedamin) twice daily for 4 weeks Measures: PSQI Summary measures for meta-analysis: difference in means |
Significant improvement in PSQI score and improved percentage in the intervention group compared to placebo. | No reported adverse effects. | (1) PSQI (2) n/a (3) 4 |
| 36 | Barton DL 201156 | Randomized, placebo-controlled, double-blind Population: Cancer patients (n=227) |
Intervention: V. officinalis root/rhizome 450 mg (containing 0.8% valerenic acid) 1 h before bedtime for 8 weeks Measures: (1) PSQI. (2) Functional outcomes of sleep questionnaire, fatigue (BFI), and mood (POMS). (3) Toxicity Summary measures for meta-analysis: difference in means |
(1) No difference in PSQI score. (2) Significantly better fatigue end points (BFI and POMS), less trouble with sleep, and less drowsiness in the treatment group over control. |
No difference in toxicities, except for alkaline phosphatase increase (slightly more common in the placebo group). | (1) PSQI (2) n/a (3) 4 |
| 37 | Cuellar NG 200930 | Randomized, placebo-controlled, triple-blind Population: Restless legs syndrome (RLS) patients (n=37) |
Intervention: V. officinalis root/rhizome 800 mg (containing 1.16 mg valerenic acid) per day 60 min before bedtime for 8 weeks Measures: Sleep disturbances (PSQI and ESS) and severity of RLS Summary measures for meta-analysis: difference in means |
Although there was no statistically significant difference valerian group showed a greater improvement in the majority of sleep quality and RLS scores. The most obvious changes were seen in participants with ESS score of ≥10. |
GI disturbances, fatigue, vivid dreams, agitation/restlessness, headache, dizziness, rash | (1) PSQI (2) n/a (3) 5 |
| 38 | Glass JR 200345 | Randomized, placebo-controlled, double-blind, cross-over. Population: Healthy elderly volunteers (n=14) |
Intervention: Single doses of V. officinalis root/rhizome 400 or 800 mg (containing 0.683% valerenic acids). Compared to temazepam, diphenhydramine, and placebo. Measures: Psychomotor performance (Manual Tracking Test, DSST, manual tracking and digit symbol substitution test); sedation and mood (VAS) |
Valerian was not different from placebo on any measure of psychomotor performance, while temazepam and diphenhydramine caused psychomotor impairment. No sedation or any side effects as a result of valerian treatment. |
(1) n/a (2) n/a (3) 2 |
|
| 39 | Francis AJ 200246 | Randomized, placebo-controlled, double-blind, cross-over Population: Children with intellectual deficit (ID) and primary sleep problems (n=5) |
Intervention: Valeriana edulis (dried and crushed whole root, containing 1.1% of valtrate/isovaltrate) 20mg/kg body weight, nightly 1 h before bedtime for 2 weeks. Wash-out 7 days. Measures: Sleep latency, time spent awake during the night, and sleep quality (VAS). |
V. edulis treatment significantly reduced sleep latencies and nocturnal time awake, lengthened total sleep time, and improved sleep quality. The treatment was most effective in children with hyperactivity. | No significant adverse effects. | (1) n/a (2) n/a (3) 5 |
| 40 | Dominguez RA 200067 | Observational Population: Hispanic outpatients receiving mental health services and complaining of insufficient sleep (n=20) |
Intervention: V. officinalis root/rhizome (Nature’s Way) 470 mg each night 30-60 min prior to retiring (possibly increased up to max. 1410 mg after 1 week) for 4 weeks. Measures: Sleep questionnaire |
Week 1: 16 patients rated moderately improved. Week 2: 15 patients rated more than moderately improved. Global improvement at week 2 was significantly better at week 2 than week 1. |
(1) n/a (2) n/a (3) n/a |
|
| Isolates | ||||||
| 41 | Andreatini R 200271 | Randomized, placebo-controlled, double-blind Population: Outpatients with generalized anxiety disorders (n=36) |
Intervention: (1) Valepotriates (80% dihydrovaltrate, 15% valtrate, and 5% acevaltrate) (average dose: 81.3mg/day); (2) placebo; (3) diazepam (average dose:6.5mg/day). Flexible doses according to the patient’s therapeutic response. 4 weeks. Measures: Anxiety (HAM-A and STAI). |
Total HAM-A score: significant reduction in all 3 groups. (No significant difference among the 3 groups.) Psychological factor of HAM-A: Reduced in valepotriates and diazepam groups. |
No moderate or serious adverse reaction was observed. | (1) n/a (2) n/a (3) 5 |
| 42 | Poyares DR 200270 | Randomized, placebo-controlled, double-blind Population: (A) Primary insomnia patients with poor sleep despite chronic use of BZDs (n=19); (B) Healthy individuals (n=18) |
Intervention: valepotriates: 80% didrovaltrate, 15% valtrate, and 5% acevaltrate (from Valeriana wallichii root). Three times daily in 100 mg doses for 15 days. After BZD withdrawal (over 2 weeks + 48 h without BZD). Measures: Sleep diaries and sleep parameters (sleep latency, total sleep time, sleep efficiency, number of arousals, WASO, REM and NREM; sleep EGG |
Valerian group reported significantly better sleep quality than placebo during the second week of treatment. Sleep EEG data showed a significant increase in alpha count during Sleep stage 3 and 4 NREM in valerian group. Valerian group showed a significant decrease in WASO compared to placebo, while placebo group presented shorter sleep latency. |
Gastrointestinal symptoms (diarrhea, stomachache, and bitter taste in their mouth) in the first week of valerian treatment. | (1) n/a (2) n/a (3) 3 |
* (1) data included in meta-analysis for sleep quality; (2) data included in meta-analysis for anxiety; (3) Jadad scale.
Abbreviations: ARCI-Addiction Research Center Inventory, CFF-Critical Flicker Fusion, CRT-Choice Reaction Time, DSST-Digit Symbol Substitution Test, DST-Digit Span Test, EEG-Electroencephalogram, HAM-A-Hamilton Anxiety Rating Scale, HAM-D-Hamilton Depression Rating Scale, ISI-Insomnia Severity Index, LARS-Line Analogue Rating Scales, LSEQ-Leeds Sleep Evaluation Questionnaire, MMSE-Mini-Mental State Examination, PANSS-Positive and Negative Syndrome Scale, PANSI-The Positive and Negative Suicide Ideation, PSQI-Pittsburgh Sleep Quality Index, SFST-Standardized Field Sobriety Test, SMT-Short term Memory Test, SST-Symbol Search Test, STDI-State Trait Depression Inventory, SVRT-Simple Visual Reaction Test, WASO-Wake Time After Sleep Onset.
Table 2.
Herbal Combinations.
| First Author | Study design | Intervention and measures | Outcomes | Adverse events | Combined with | Jadad scale | |
|---|---|---|---|---|---|---|---|
| 1 | Abdellah SA 201988 | Open-label, observational Population: Adults with adjustment insomnia (n=22) |
Intervention: Combination of V. officinalis extract 32 mg and Eschscholtzia californica extract 80 mg per tablet, Max. 4 tablets per day. For 4 weeks Measures: Insomnia severity (ISI), quality of sleep and waking state (VAS), and anxiety (HAM-A). Sleep diary. |
ISI score decreased by approx. 30%, night sleep duration increased, the number of awakening decreased, and anxiety score significantly decreased by 50%. Sleep latency did not improve. | Mild nocturnal pollakiuria in 1 patient. No serious adverse events. | E. californica | n/a |
| 2 | Meier S 201877 | Randomized, placebo-controlled, double blind Population: Healthy adults (n=72, only male) |
Intervention: Combination of V. officinalis 45%MeOH extract 90 mg, Passiflora incarnata 50%EtOH extract 90 mg, Melissa officinalis 20%EtOH extract 60 mg (Ze 185) Measures: Biological (salivary cortisol) and self-reported psychological responses to a standardized psychosocial stress paradigm (TSST) |
Attenuated self-reported anxiety. There was no difference between the groups in salivary cortisol levels. |
No difference between the groups. |
P. incarnata
M. officinalis |
5 |
| 3 | Toolika E 201587 | Observational Population: Primary insomnia patients (n=30) |
Intervention: V. wallichii root/rhizome 4 g with milk after meal, 3 times daily (12 g per day) for 1 month. Measures: initiation of sleep, duration of sleep, disturbed sleep, and disturbance in daily functioning |
V. wallichii with milk improved the initiation and duration sleep, reduced sleep disturbance, and improved daily functioning. | Not mentioned | Milk | n/a |
| 4 | Gromball J 201484 | Observational study Population: Primary school children with hyperactivity and concentration difficulties (n=169) |
Intervention: Combination of V. officinalis 62%EtOH extract 640 mg and Melissa officinalis 30%EtOH 320 mg per day, for 7 weeks. Measures: (1) Concentration problems, hyperactivity, impulsiveness, impaired social behavior, difficulties to fall asleep, and morning fatigue (rated by paediatricians). (2) Questionnaire (filled out by parents). |
Doctors’ rating: The treatment improved concentration and reduced hyperactivity and impulsiveness. Parents’ rating: Social behavior, sleep and symptom burden improved. |
Two patients had transient moderate adverse reactions, however causal relation with the medication was judged unlikely. | M. officinalis | n/a |
| 5 | Taavoni S 201376 | Randomized, placebo-controlled, triple-blind Population: Menopausal women with sleep problems (n=100) |
Intervention: Valerian essence 160 mg and lemon balm 80 mg per day for 1 month Measures: PSQI |
Significantly reduced sleep disorder score after 1 month of Valerian/Lemon balm treatment | No adverse events | M. officinalis | 3 |
| 6 | Maroo N 201375 | Randomized, controlled, double-blind Population: Primary insomnia patients (n=78) |
Intervention: 1. NSF-3: Polyherbal extract of V. officinalis (300 mg with 0.8% valerenic acid), Passiflora incarnata (80 mg with 4% isovitexin), and Humulus lupulus (30 mg with 0.35% rutin) at bedtime for 2 weeks. 2. Control: zolpidem 10 mg Measures: Insomnia (Insomnia severity index and ESS score) |
Significant improvement in total sleep time, sleep latency, number of nightly awakenings and severity index score in both groups. No statistically significant difference between the groups. | Mild adverse events (e.g. drowsiness): 12 with herbal treatment (1), 16 with zolpidem (2) |
P. incarnata
H. lupulus |
4 |
| 7 | Trompetter I 201386 | Observational (multicentre) Population: Children (6-12 years) suffering from nervous agitation due to affective disorders (n=115) |
Intervention: Herbal triplet containing dried extracts of Valeriana officinalis (40%EtOH extract 28 mg), Hypericum perforatum (38%EtOH extract 60 mg), and Passiflora incarnata (60%EtOH extract 32 mg) per tablet. Average dose: 1-3 tablets per day. Duration: 2-4 weeks. Measures: A physician-completed questionnaire and a standardized parent-report questionnaire |
81.6-93.9% of affected children had no or just mild symptoms (depression, anxiety, sleeping problems, and different physical problems) at the end of observation. | A good tolerability for 97.4% of the children. Moderate to poor tolerability in 7 cases. Paradox reactions: restlessness, weepiness, increased irritability, or aggressiveness; redness in cheekbone region (1 case); stomach pain (1 case) |
H. perforatum
P. incarnata |
n/a |
| 8 | Chen JH 201274 | Randomized, controlled Population: Stable and less critical ICU patients (n=95) |
Intervention: Valerian acupressure (application of 2.5% V. officinals essential oil 2 drops per point before acupressure). Compared to regular treatment Measures: Sleep (observation, actigraphy measures (10pm-6 am), Stanford Sleepiness Scale (SSS) measures on the next morning |
Valerian acupressure increased sleeping hours, reduced wake frequency and SSS grades. Heart rate variability indicated immediate relaxation response. | Acupressure | n/a | |
| 9 | Melzer J 200973 | Randomized, controlled, double-blind Population: Somatoform disorder patients (n=167) |
Intervention: (1) Ze185 (4-combination) containing 90 mg of V. officinalis (45%MeOH extract), 90 mg of Petasites (90%EtOH extract), 90 mg of Passiflora incarnata (50%EtOH extract), and 60 mg of Melissa officinalis (20%EtOH extract); (2) 3-combination without Petasites; (3) placebo. 3 times daily for 2 weeks Measures: Anxiety (VAS) and depression (BDI) |
Significantly reduced anxiety in both treatment groups (3- and 4- combination), but not in placebo. Effectiveness: 4-combination > 3-combination > placebo |
Adverse events (1) 4-combination: vomiting and flatulence (2) 3-combination: nausea and constipation |
P. incarnata
M. officinalis Petasites |
4 |
| 10 | Dimpfel W 200872 | Randomized, placebo-controlled, double-blind Population: Otherwise healthy volunteers having poor sleep (n=42) |
Intervention: V. officinalis (tinct. 1:10) 460 mg and Humulus lupulus (tinct. 1:12) 460 mg. Single administration, 15 min before the lights were turned off at 22:00. Measures: (1) Depth of sleep (EEG); (2) Sleep quality (sleep inventory SF-A sub-score) |
Time spent in sleep and deeper sleep was significantly higher in the treatment group, which was correlated with the difference in sleep quality. |
H. lupulus
|
3 | |
| 11 | Koetter U 200765 | Randomized, placebo-controlled, double-blind Population: Patients suffering from non-organic sleep disorders (n=27) |
Intervention: (1) V. officinalis 45%MeOH extract 500 mg; (2) V. officinalis 45%MeOH extract 500 mg and Humulus lupulus 45%MeOH extract 120 mg (fixed combination Ze91019); (3) placebo. For 4 weeks. Measures: (1) the reduction of the initially prolonged sleep latency; (2) WASO, sleep efficiency, relative proportion of the sleep stages, REM latency and CGI |
The fixed combination was significantly superior to placebo in reducing the sleep latency, whereas the single valerian extract did not differ significantly from placebo. | No adverse events. | H. lupulus | 3 |
| 12 | Müller SF 200689 | Observational study Population: Children (average age: 8.3 years) with motor restlessness and sleep disturbance (n=918) |
Intervention: V. officinalis 62%EtOH extract 160 mg and Melissa officinalis 30%EtOH extract 80 mg per tablet (Euvegal forte, Schwabe Pharmaceuticals, Germany), Max. 2x2 tablets/day (average: 3.5 tablets/day) for 4±1 weeks (average: 31.9 days). Measures: Severity of symptoms and improvement efficacy. Scored by the investigators and the parents. |
Dyssomnia and restlessness were reduced from moderate/severe to mild or absent in most of the patients. (80.9% of dyssomnia patients and 70.4% of restlessness patients improved.) Both parents and investigators assessed efficacy to be very good or good (60.5% and 67.7%). Tolerability was good or very goof in 96.7% of the patients. | No adverse events. | M. officinalis | n/a |
| 13 | Kennedy DO 200678 | Randomized, placebo-controlled, double-blind, cross-over Population: Healthy volunteers (n=24) |
Intervention: 600 mg, 1200 mg, and 1800 mg of a standardized V. officinalis and Melissa officinalis extracts (V. officinalis: M. officinalis 3: 2). Three single doses, separated by a 7 day wash out period. Measures: (1) Modulation of mood and anxiety (Bond-Lader scales, STAI) after DISS battery exposure; (2) DISS performance |
The 600 mg dose ameliorated the negative effects of DISS on anxiety, however 1800 mg increased anxiety. All 3 doses led to decrements in performance on the Stroop task module, and the 2 lower doses led to decrements on the overall score generated on DISS. | M. officinalis | 3 | |
| 14 | Morin CM 200579 | Randomized, placebo-controlled Population: Mild insomnia patients (n=184) |
Intervention: (1) Combination of V. officinalis (45%MeOH extract) 187 mg and Humulus lupulus (45%MeOH extract) 41.9 mg, nightly for 28 days; (2) placebo for 28 days; (3) diphenhydramine 25 mg for 14 days followed by placebo for 14 days. Measures: Sleep parameters measured by daily diaries and polysomnography, clinical outcome ratings from patients and physicians, and quality of life. |
Modest improvements of subjective sleep parameters in valerian-hops (1) and diphenhydramine (3). Valerian-hops produced slightly greater reductions of sleep latency relative to placebo and diphenhydramine after 14 days, and greater reductions than placebo after 28 days. No group difference on polysomnography. Quality of life was significantly more improved in valerian-hops group than the placebo group after 28 days. | No serious adverse events | H. lupulus | 3 |
| 15 | Schellenberg R 200480 | Randomized, placebo-controlled Population: Healthy volunteers (n=32) |
Intervention: Single doses of V. officinalis / Humulus lupulus 45%MeOH extract combination (500 mg / 120 mg, or 1500 mg / 360 mg). Valerian extract contained 0.388% if valerenic acids. Measures: competition between caffeine and valerian/hop on the central nervous system (EEG) |
Valerian 500mg/hops 120 mg reduced, and valerian 1500mg/hops 360 mg inhibited caffeine-induced arousal, 60 min after oral administration. | Not mentioned | H. lupulus | 3 |
| 16 | Farag NH 200381 | Randomized, placebo-controlled, double-blind, cross-over Population: Sleep onset insomnia (n=25) |
Intervention: Combination of V. wallichi 320 mg, Rosa centifolia, Nardostachys jatamansi, Tinospora cordifolia, Withania somnifera, Piper negrum, Zingiber officinalis, Convolvulus pluricalis, and Glycyrrhiza glabra. Compared to placebo. 4 nights each with 10 days washout. Measures: Sleep latency (questionnaire) |
The supplement reduced sleep latency. The effect was greater in subjects with worse insomnia. | None | Ayurvedic herbs | 4 |
| 17 | Müller D 200385 | Observational Population: Patients suffering from mild to moderately severe depression (n=2,462) |
Intervention: (1) V. officinalis 70%EtOH extract (Euvegal Balance) 500 mg or 1000 mg and Hypericum perforatum 60%EtOH extract (Neuroplant) 600 mg per day. For 6 weeks. Measures: Rating of 16 symptoms during each doctor’s visit (ICD-10 and HAM-A). |
Core symptoms of depression and the main symptoms of anxiety disorder receded significantly. Efficacy was ‘very good’ or ‘good’ in 87.2%, and ‘poor’ in 1.6%. Tolerability was ‘very good’ or ‘good’ in 96.8%. Higher Valerian dosage could be more effective. | No significant side effects | H. perforatum | n/a |
| 18 | Vonderheid-Guth B 200082 | Randomized, placebo-controlled, single-blind, cross-over Population: Young healthy male volunteers (n=12) |
Intervention: Single dosages of V. officinalis and H. lupulus 45%MeOH extract combination (Ze91019). Low dosage: Valerian 500 mg and hops 120 mg. High dosage: valerian 1500 mg and hops 360 mg. Measures: Quantitative topographical EEG (qEEG). Performance Test (CPT) according to Dueker and Lienert (1965). Prior to, 1, 2, and 4 hours after drug intake. |
qEEG: High dosage led to power increases in delta, decreases in alpha and beta. CPT: Concentration and performance capability were hardly influenced. (A minimal increase of mean answer time and mean time for correct answer was observed after 4 h after high dosage intake and 1 h after low dosage intake. – More pronounced after the low dosage.) |
H. lupulus | 2 | |
| 19 | Lindahl O 198983 | Randomized, placebo-controlled, double-blind, cross-over Population: Volunteers with sleep difficulties and fatigue. (n=27) |
Intervention: Single doses of (1) test preparation: V. officinalis extract (equivalent to 400 mg of the root), H. lupulus extract (375 mg equivalent) and M. officinalis extract (160 mg equivalent) or (2) Control: same amounts of hops and lemon balm and V. officinalis 4 mg equivalent Measures: Subjective rating of sleep quality |
78% rated high valerian preparation better than the control. | No side effects. |
H. lupulus
M. officinalis |
2 |
Abbreviations: BDI-Beck Depression Inventory, DISS-Defined Intensity Stress Simulator, DSST-Digit Symbol Substitution Test, DST-Digit Span Test, EEG-Electroencephalogram, HAM-A-Hamilton Anxiety Rating Scale, ICD-10-International Classification of Diseases 10th Revision, ISI-Insomnia Severity Index, PSQI-Pittsburgh Sleep Quality Index, TSST -, WASO-Wake Time After Sleep Onset
V. Officinalis as a Single Herb
Sleep problems
To evaluate the effectiveness of valerian for sleep problems, 40 articles using V. officinalis as a single herb were assessed. As a result, it was found that 23 studies addressed the effectiveness of valerian for sleep problems. Among them, 13 studies found valerian effective as a sleep aid. Three studies were performed with healthy subjects,37,40,41 4 studies with insomnia patients,36,42,52,66 and 1 study in RLS patients.30 In addition, studies conducted with mental health patients,67 cancer patients,56 HIV-positive patients,35 postmenopausal women,57 and the elderly,38 also found that valerian could improve sleep quality.35,38,56,57 On the other hand, 10 studies found that valerian was not significantly effective compared to control36,38,40,48,50,51,53,54,56,65 at least for the outcome measures used in those studies. Study outcomes were assessed using various measures, such as sleep questionnaires and diaries, such as Pittsburgh Sleep Quality Inventory (PSQI),90 Insomnia Severity Index (ISI),91 and Epworth Sleepiness Scale (ESS), to polysomnography.92
Six studies measured immediate responses after single dose administration,36,38,40–42,50 while most studies evaluated the effectiveness of repeated doses.30,35,54,56,57,65,67,36–38,44,48,51–53 The intervention periods ranged from 5 days to 8 weeks.
In order to extract possible associations between herbal preparations and study outcomes, the reports were classified into 4 groups (Table 1): 8 studies using hydroalcoholic extracts,36,42,44,50,52–54,65 3 studies using aqueous extracts,38,40,41 3 studies using extracts using unspecified solvents,37,48,51 and 5 studies using herbal substance (the whole root / rhizome).30,35,56,57,67 Among the 3 studies using aqueous extracts, 2 studies with single dose found improved subjective sleep quality and latency in healthy volunteers40,41 (RCTs [Jadad scale 2 and 5]), while there was no significant difference in polysomnography40 (RCTs [Jadad scale 2]). Schulz et al. found that repeated administration of V. officinalis aqueous extract (for 1 week) increased deep sleep (slow-wave sleep: SWS) in elderly subjects, although single dose of the same preparation did not have any significant impact on polysomnography38 (RCT [Jadad scale 2]). As a single dose, V. officinalis hydroalcoholic extracts did not improve sleep quality36,50 (RCT, Jadad scale 2-3), however improved REM sleep was observed in insomnia patients42 (RCT [Jadad scale 2]). Repeated administration of hydroalcoholic extracts also led to inconsistent outcomes: sleep quality was improved in 4 studies using extract 600 mg (2 to 6 weeks)36,37,44,52 (RCTs [Jadad scale 2-5]), whereas 3 studies using extract 300-600 mg (5 days to 4 weeks) found no improvement53,54,65 (RCTs [Jadad scale 3-5]). Donath et al. found improved sleep latency and deep sleep after 2 weeks, while no improvement was observed after a single dose36 (RCTs [Jadad scale 2]), which suggests that repeated administration is needed to exert observable effects. There were 3 studies using extracts with unspecified procedures.48,51,66 The outcomes from those studies were also inconsistent: negative outcomes for 2 studies48,51 (RCTs [Jadad scale 5]) and positive for an observational study.66 On the other hand, all 5 studies using the dried root/rhizome showed that the interventions led to improved sleep at least in 1 subgroup30,35,56,57,67 (RCTs [Jadad 3-5] and 1 observational study), suggesting that maximum efficacy could be expected from the root / rhizome before extraction.
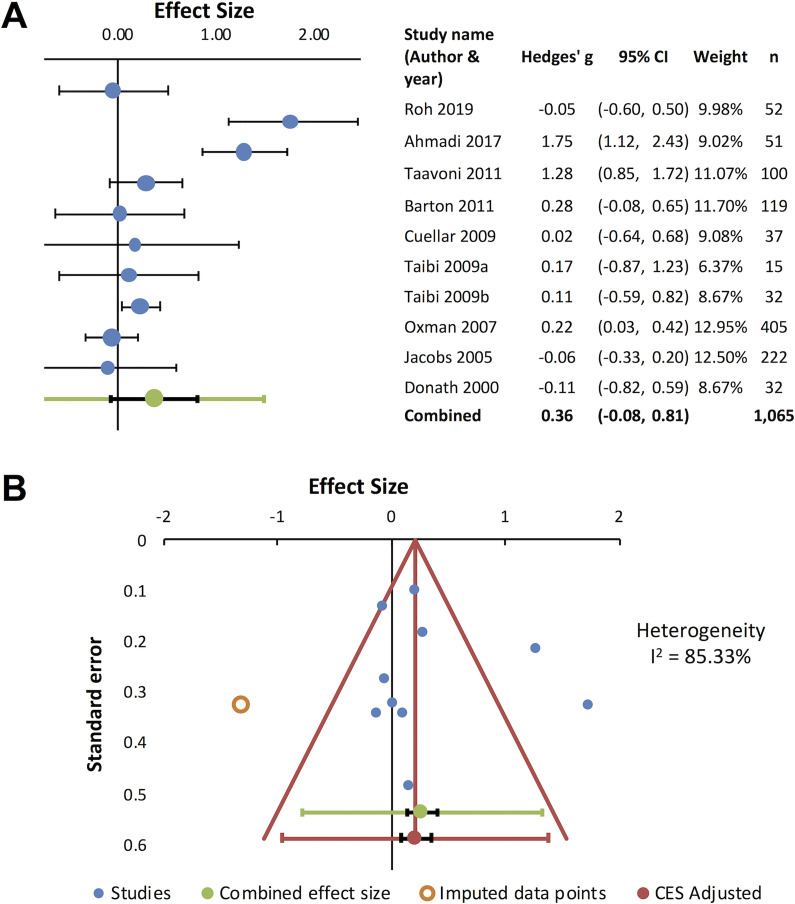
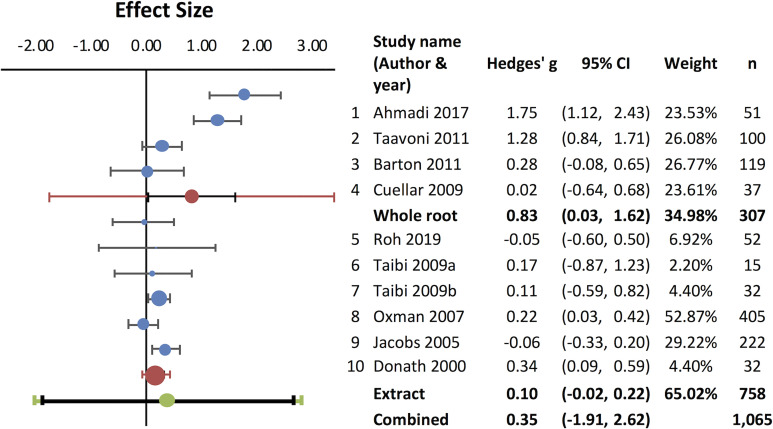
It has been suggested that valerian may be useful to improve subjective sleep quality22 and that repeated administration is required to obtain significant effects.36 In order to evaluate if repeated administrations could consistently improve sleep quality, a meta-analysis was carried out (Figure 2). Studies using repeated valerian administrations were assessed to extract numerical values for the meta-analysis, and 10 randomized placebo-controlled trials reporting quantifiable results30,34–36,51–54,56,57 were included in the analysis. The combined effect size was 0.36 (95% CI: -0.08 to 0.81) (Figure 2A). The funnel plot indicated missing data (Figure 2B) and high heterogeneity was observed (I2=85.33%). As described above, differences in herbal preparation could potentially contribute to the heterogenous outcomes. In fact, subgroup analysis for the whole root30,35,56,57 (RCTs [Jadad 3-5]) and the extract34,36,51–54 (RCTs [Jadad 2-5]) revealed that the combined effect size for the whole root was considerably higher 0.83 (95% CI: 0.03 to 1.62), compared to the extract (0.10; 95% CI: -0.02 to 0.22) (Figure 3), further supporting the use of the whole root rather than extract.
Figure 2.
Sleep quality improvement by repeated administration of V. officinalis. Ten studies were included in meta-analysis. Positive values indicate enhanced sleep quality. (A) Forest plot for sleep quality improvement. Effect sizes (Hedges’ g) for 10 studies (blue circles) and combined effect size (green circle) are shown. Black horizontal bars indicate 95% CI. (B) Funnel plot. Included data points are presented as filled blue circles and imputed data are shown as open orange circles.
Figure 3.
Subgroup analysis for V. officinalis whole root and extract as a sleep aid. Subgroup analysis was performed with 4 studies (1 ∼ 4) using the whole root/rhizome and 6 studies (5 ∼ 10) using extracts. Effect sizes for each group are shown as red circles. Black horizontal bars indicate 95% CI.
Anxiety
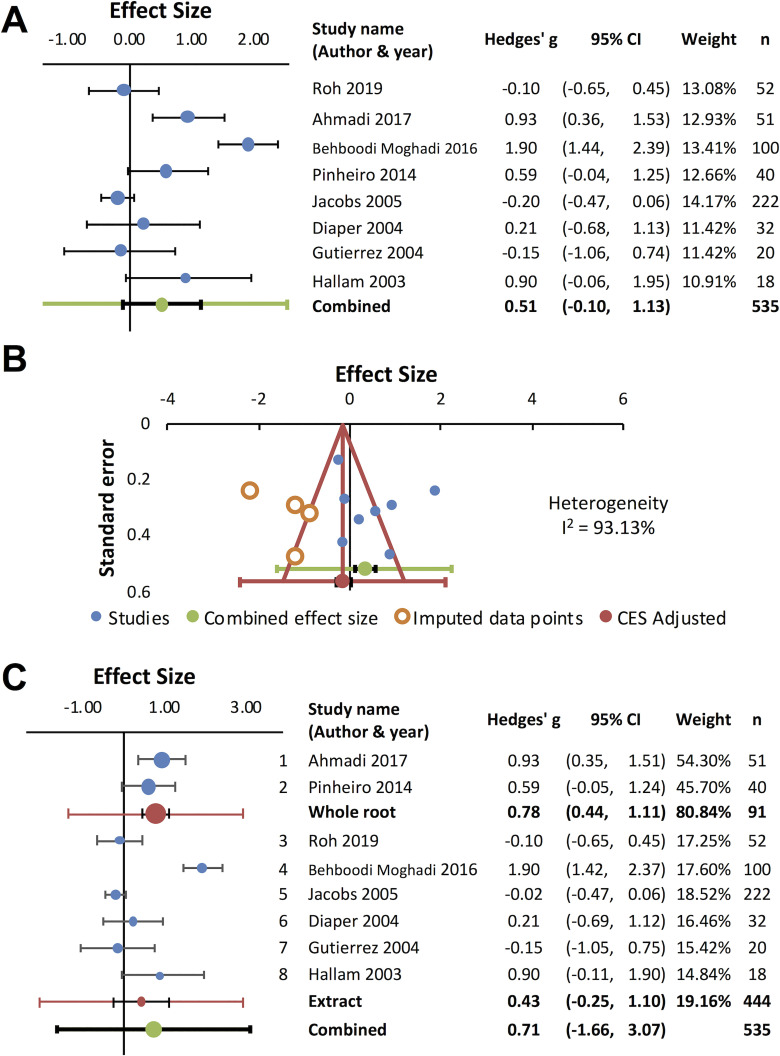
Anxiety was assessed by Hamilton Anxiety Rating Scale (HAM-A): a clinician-rated scale that considers psychological and somatic factors,93,94 State-Trait Anxiety Inventory (STAI): a patient-rated scale that measures state and trait anxiety,95,96 mental stress test using the color/word interference task,97 or other questionnaires. Seven studies examined valerian’s anxiolytic potential, and positive outcomes were observed in 6 studies.33,35,43,60,63,71 Valerian standardized extract 600 mg per day for 1 week reduced psychological and physiological stress reactivity under stress in healthy adult subjects43 (RCT [Jadad scale 1]). Average 81.3 mg per day of valepotriates for 4 weeks reduced the score of HAM-A in generalized anxiety disorder patients71 (RCT [Jadad scale 5]), suggesting that valepotriates are the active anxiolytic constituents. Valerian root / rhizome 530 mg per day for 4 weeks reduced anxiety in HIV-positive patients receiving efavirenz35 (RCT [Jadad scale 3]), suggesting valerian could prevent neuropsychiatric adverse effects caused by the antiretroviral medication. Valerian extract 100 mg33 or root / rhizome 100 mg,60 single dose 60 min before the initiation of surgical operation, reduced anxiety in anxious patients undergoing dental operation33,60 (RCTs [Jadad scale 5]). In addition, valerian extract 1,260 mg per day for 7 days for 3 menstrual cycles effectively reduced premenstrual anxiety63 (RCT [Jadad scale 5]). However, an internet-based study with volunteers with non-clinical anxiety and insomnia found no significant difference in STAI compared to placebo51 (RCT [Jadad scale 5]). Figure 4 shows the result of meta-analysis for valerian’s anxiolytic effects based on 7 randomized placebo-controlled trials that reported quantitative outcomes34,35,47,49,51,60,63 (Figure 4A). The funnel plot indicated significant publication bias, missing data in the area of negative outcome, and high heterogeneity (I2=93.13%) (Figure 4B). Subgroup analysis revealed high variability for valerian extracts34,47,49,51,63 (RCTs [Jadad scale 3-5]), whereas positive outcomes were observed for the whole root35,60 (RCTs [Jadad scale 3 and 5]). However, due to the scarcity of data (2 studies for the whole root), it is difficult to draw a conclusion.
Figure 4.
V. officinalis for anxiety. Eight studies were included in meta-analysis. Positive values indicate either reduced anxiety or enhanced calmness. (A) Forest plot for anxiety reduction. Effect sizes (Hedges’ g) for each study (blue circles) and combined effect size (green circle) are shown. (B) Funnel plot. Included data points are presented as filled blue circles. (C) Subgroup analysis for V. officinalis whole root (1 and 2) and extracts (3 ∼ 8).
Two studies investigated the effects of V. officinalis extracts on anxiety-associated brain activities.31,34 Single dose of 300 mg extract reduced intracortical facilitation, possibly via GABAA enhancement31 (RCT [Jadad scale 4]), and hydroalcoholic extract 300 mg per day for 4 weeks increased frontal brain region alpha coherence, which was correlated with anxiolysis34 (RCT [Jadad scale 5]). These findings suggest that V. officinalis could induce immediate as well as long-lasting effects on anxiety-associated brain activities.
Other therapeutic effects
In addition to insomnia and anxiety, several other therapeutic benefits were suggested (Figure 5). Valerian aqueous extract 750 mg per day for 8 weeks was effective in reducing the symptoms of obsessive-compulsive disorder (OCD)55 (RCT [Jadad scale 2]). Valerian root / rhizome 530 mg per day for 1 month improved cognition in haemodialysis patients32 (RCT [Jadad scale 2]), and valerian root / rhizome 1,060 mg per day for 8 weeks prevented cognitive dysfunction after coronary bypass surgery61 (RCT [Jadad scale 5]). Valerian root / rhizome 675 mg or extract 1,060 mg per day for 8 weeks reduced severity and frequency of hot flashes in menopausal and postmenopausal women59,64 (RCTs [Jadad scale 4]). In addition, valerian root / rhizome 765 mg per day for 3 days for 2 cycles reduced pain severity and syncope in young females with dysmenorrhea58 (RCT [Jadad scale 5]), and valerian extract 1,260 mg for 7 days for 3 cycles reduced premenstrual symptoms63, supporting the traditional use of valerian as a menstrual stimulant.16
Figure 5.
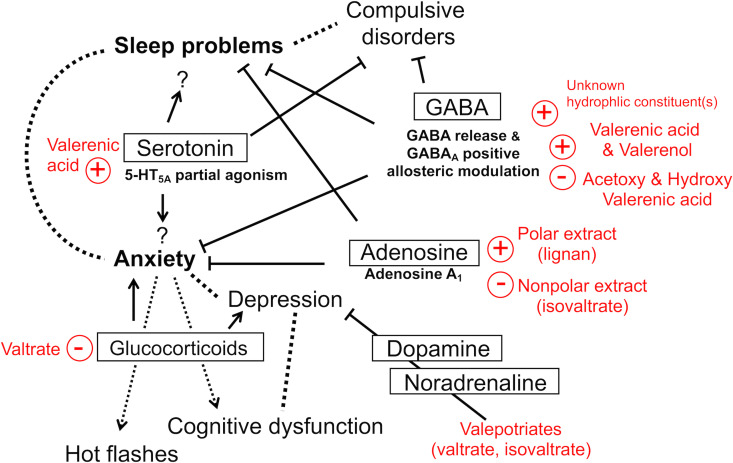
Valerian’s active constituents and potential therapeutic benefits.
Other Valerian Species
Three studies investigated the effectiveness of other Valeriana spp. as a sleep aid: 2 studies on V. edulis 42,46 and 1 study on V. wallichii.70 V. edulis 20 mg/kg for 2 weeks reduced sleep latencies and improved sleep quality in children with intellectual deficit and primary sleep problems46 (RCT [Jadad scale 5]), and V. edulis hydroalcoholic extract 450 mg single dose increased REM sleep and reduced the number of awaking in insomnia patients42 (RCT [Jadad scale 5]). A concoction of valepotriates from V. wallichii 300 mg per day for 15 days reduced Wake Time After Sleep Onset (WASO) and improved sleep quality in insomnia patients70 (RCT [Jadad scale 3]). Of note, chemical characteristics of the 3 species (V. officinalis, V. edulis, and V. wallichi) significantly differ; valerenic acids (valerenic acid, acetoxyvalerenic acid, hydroxyvalerenic acid) are specific to V. officinalis, whereas valepotriates (valtrate and isovaltrate) are common among the 3 species,98 suggesting that valepotriates at least in part contribute to valerian’s sleep promoting activity. Due to the scarcity of data, it was not possible to perform meta-analysis for V. edulis and V. wallichi.
Valerian in Herbal Combinations
In addition to the effectiveness of valerian alone, 19 studies evaluated the effectiveness of valerian in combination with other herbs or other agents, among which 16 studies used V. officinalis,65,72,82–86,88,89,73–80 2 studies used V. wallichii,81,87 and 1 study used an unknown valerian species.76 One study treated menopausal women with valerian essence in combination with lemon balm and observed sleep improvement, however the species and extraction methods were unidentified.76
Among 16 reports using herbal combinations with V. officinals, 8 studies examined the effects on sleep quality and/or latency and all those interventions led to positive outcomes.65,72,75,79,83,86,88,89 Six studies evaluated anxiolytic potential, and all those studies found positive outcomes at least for one of the measures at one of the tested dosages73,77,78,85,86,88 (RCTs [Jadad scale 3-5] and 3 observational studies). However, a study using the combination of V. officinals, Passiflora incarnata (syn. P. edulis), and Melissa officinalis extracts (single dose) found that the intervention attenuated anxiety under stress, although it did not alter the level of salivary cortisol, a physiological parameter of stress reactions77 (RCT [Jadad scale 5]). In another study, V. officinalis and M. officinalis combination (extract weight ratio 3:2) at 1,800 mg (single dose) increased anxiety while the same formulation at 600 mg reduced anxiety78 (RCT [Jadad scale 3]). Both of these negative outcomes were observed for single doses in healthy subjects. On the other hand, the other 4 studies, conducted with subjects with adjustment insomnia,88 somatoform disorder patients73 (RCT [Jadad scale 4]), depression patients85 (observational), and children with affective disorders86 (observational), found that the herbal combinations with V. officinalis were effective in reducing anxiety. Of note, when depression patients were treated with V. officinalis (500 or 1,000 mg extract) and Hypericum perforatum (600 mg extract) for 6 weeks, higher valerian dose (1,000 mg) reduced anxiety more effectively85 (observational). In addition, a study found that a combination of V. officinalis and M. officinalis extracts was effective in reducing the symptoms in children with hyperactivity and concentration difficulties84 (observational). In a study with Intensive Care Unit (ICU) patients, V. officinalis essential oil combined with acupressure effectively induced relaxation and improved sleep74 (observational). V. wallichii with 8 ayurvedic herbs improved sleep in sleep onset insomnia patients81 (RCT [Jadad scale 4]), and V. wallichii with milk improved sleep in primary insomnia patients87 (observational).
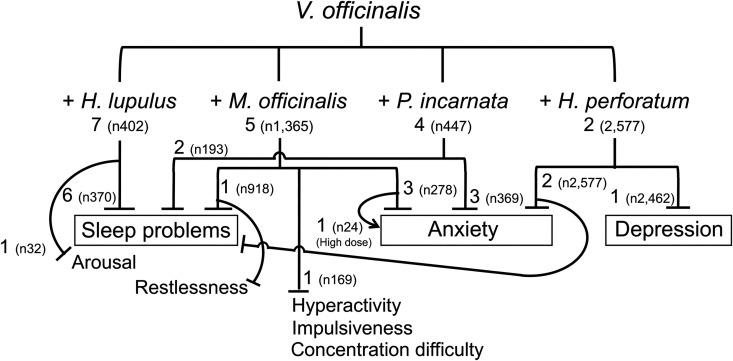
With respect to the potential herbal partners of V. officinalis, Humulus lupulus was the most frequently used in the clinical trials. All 7 reports that examined the effects on sleep or associated parameters observed sleep improvement65,72,79,83 (RCTs [Jadad scale 2-3]), EEG power alteration toward sleep82 (RCT [Jadad scale 2]), and reduced arousal80 (RCT [Jadad scale 3]) were observed. M. officinalis used in 5 trials,73,77,78,84,89 was the second most frequently combined herb, followed by P. incarnata used in 4 trials.73,75,77,86 The 2 herbs were used in combination with V. officinalis for sleep problems and anxiety, resulting in positive outcomes.73,75,77,78,84,86,89 Two studies were conducted on the combination of V. officinalis and H. perforatum with depression patients85 (observational) and children with affective disorders86 (observational), and the intervention reduced anxiety,85,86 improved sleep,86 and reduced depression symptoms.85 Two studies used M. officinalis and P. incarnata 73,77 (RCTs [Jadad scale 4-5]), and 1 study used H. lupulus and P. incarnata 75 (RCT [Jadad scale 4]) in combination with V. officinalis. These 4 frequently used herbal partners and the study outcomes are summarized in Figure 6. It was not possible to perform meta-analysis for herbal combinations due to the highly variable outcome measures.
Figure 6.
V. officinalis and herbal partners. Frequently used herbal partners and potential uses supported by evidence are shown with number of studies and total number of subjects (n).
Safety
There were no serious adverse events reported in the studies included in this review (60 studies, total n=6,894). Paradoxical stimulation,16,99 such as agitation and restlessness, was experienced only in minority.30,78,86 In older women with insomnia, valerian extract increased WASO,53 suggesting it could have stimulatory effects. However, a study found that the same formulation at a higher dose increased anxiety while a lower dose was anxiolytic,78 suggesting that paradoxical stimulation could be avoided by cautious dosing. Six studies investigated possible adverse effects on cognitive performance after V. officinalis extract intake (single dose) in healthy adults37,39,47,49,62 and elderly volunteers.45 V. officinalis standardized extracts at 100-1,600 mg did not impair cognitive or psychomotor performance37,39,45,47,49,62 and were proven safer compared to triazolam, temazepam (benzodiazepines), and diphenhydramine (antihistamine).45,47 Valerian did not cause any adverse events in postmenopausal women with insomnia,57 older women with insomnia,53 children with intellectual deficits and primary sleep problems,46 and psychophysiological insomnia patients.36 Mild adverse events were reported in RLS patients (vivid dreams and fatigue),30 arthritis patients with sleep disturbance (dizziness and sleepiness),54 sleep-disturbed subjects (drowsiness),50 insomnia patients (gastrointestinal symptoms),42,70 and outpatients with stress-induced insomnia (vivid dreams, drowsiness, heavy dream and depression),66 however there was no clear association with the treatments. Potential herb-drug interaction was addressed by examining the effects on cytochrome p450 (CYP) expression levels, and no significant impact was detected at least for CYP1A2, CYP2D6, CYP2E1 and CYP3A4/5.69,68 As a whole, valerian is safe in all ages.
Discussion
Concurring sleep problems and anxiety are frequently observed and their onset and course are interrelated.100,101 In addition, these disorders are associated with a number of comorbid conditions including depression,101–103 dementia,5,6 OCD,104 and hot flashes105–107 (Figure 6). Our results demonstrated that sleep promotion and anxiolytic effects were the major therapeutic benefits expected of valerian (V. officinalis), and this herb could be also useful in treating OCD, cognitive dysfunction, menopausal hot flashes, as well as menstrual problems. However, the study outcomes considerably differed particularly when herbal extracts were used. We address this issue by discussing potential mechanisms underlying the therapeutic actions of valerian.
Key Constituents and Mechanisms of Actions
Although the use of valerian extracts as a sedative and sleep aid dates back to the 18th century and a number of constituents have been identified in the last 120 years,108 the active constituents and underlying mechanisms for the reported activities remain obscure.109 The identified constituents include iridoids known as valepotriates (valtrate, isovaltrate, didrovaltrate and acevaltrate),110 essential oil constituents including monoterpenes (e.g. borneol, bornyl acetate), sesquiterpenes (e.g. valerenal, valerenic acid), and carboxylic compounds (valeric / isovaleric acid),111 lignans,112 flavonoids,108,113 and low levels of γ-aminobutyric acid (GABA).114 Valeriana edulis (Mexican valerian) Valeriana wallichii (Indian valerian) contain higher levels of iridoids, while valerenic acid and acetoxyvalerenic acid are unique to V. officinalis. 98
Valerian and GABAergic Signaling
GABA is an inhibitory neurotransmitter within the central nervous system and is a key target of pharmacotherapies in the treatment of anxiety and sleep disorders.115,116 GABA is present in the central nervous system (CNS), where it plays a role in the maintenance of balance between excitatory and inhibitory neurotransmissions. GABA acts via 3 subclasses of receptors termed GABAA, GABAB, and GABAC, each of which has distinct characteristics. GABAA and GABAC receptors are ligand-gated ion channels, while GABAB members are G-protein coupled receptors. GABAA receptors are heteropentameric transmembrane protein complexes made up of α1-6, β1-3, γ1-3, δ, ε, θ, π subunits, and have numerous allosteric binding sites.116 Although it is not very likely that GABA in valerian, taken orally, directly exerts any therapeutic action, valerenic acid and valerenol can allosterically modulate GABAA receptors to enhance the response of GABAA to GABA in vitro,117–119 and both valerenic acid and valerenol exerted anxiolytic activity in a GABAA β3 subunit-dependent manner in mice.117 On the other hand, valerenic acid derivatives (e.g. acetoxy valerenic acid and hydroxy valerenic acid) bind the identical sites without inducing allosteric modulation, suggesting that these constituents can potentially compete with valerenic acid.120 In fact, in an anxiety model in rodents, a valerian extract containing valerenic acid at a high level and acetoxy valerenic acid at a low level exhibited higher anxiolytic potency compared to an extract with acetoxy valerenic at a high level, indicating that standardization with respect to the total amount of valerenic acids (both valerenic acid and acetoxy valerenic acid) is inappropriate.120 In addition, an aqueous extract of valerian induced GABA release in rat brain synaptosomes in vitro,121 suggesting the presence of hydrophilic constituents potentially promotes GABAergic signaling. Expected mechanisms of actions are summarized in Figure 5. Of note, the expression patterns of GABA receptors in humans are sex- and age-dependent, and GABAA β3 subunit levels in the superior temporal gyrus were lower in older women than old men,122 suggesting that valerian’s actions via GABAergic signaling could depend on the sex and age.
Valerian and Serotonergic Signaling
Serotonin (5-HT) plays essential roles in the regulation of sleep and mood, and serotonergic system is a promising therapeutic target for psychiatric disorders including anxiety, depression, and sleep disorders.123–126 Both valerian extract and valerenic acid exhibit partial agonist activity at 5-HT receptor 5A (5-HT5A) in vitro,127 suggesting the involvement of serotonergic system in the actions of valerian. There are 7 families of its receptors (5-HT1-7). Although the functional significance of 5-HT5A remains obscure, 5-HT5A is present at high levels in the regions that regulate circadian rhythms (the suprachiasmatic nucleus, the intergeniculate leaflet,128,129 the median raphe nuclei, and dorsal raphe nucleus) and colocalized with serotonin, suggesting the auto-receptor role of 5-HT5A in circadian regulation.128 In addition, 5-HT5A is expressed in the brain areas such as the cerebral cortex, hippocampus, amygdala and hypothalamus,129 suggesting that it is potentially involved in stress reactivity and resilience. Indeed, a study demonstrated that 5-HT5A antagonists could have different sedative, anxiolytic, and anti-depressant properties130 (Figure 6). These data suggest that valerian, as a 5-HT5A partial agonist, may act differently depending on what circumstances the subjects were placed and this could possibly explain the inconsistent outcomes among the trials.
Valerian and Adenosine Signaling
Adenosine receptors play important roles in mood and anxiety disorders.131 Adenosine A1 receptor activation reduces anxiety-like behavior in mice132 and positive allosteric modulators of adenosine A1 receptor could be potential anxiolytic therapy.133,134 In addition, adenosine helps maintain brain homeostasis by preventing over excitation and regulating the sleep-wake rhythm.135–137 Sleep stages are divided into 1 stage of rapid eye movement (REM) sleep and 4 stages of non-rapid eye movement (NREM) sleep (Stages 1-4) that are characterized by increasing sleep depth.4,138 The deeper stages (Stages 3 and 4) are believed to be the most restorative and collectively called SWS.4,138 Adenosine acting through A1 receptors facilitates sleep and increases slow wave activity (SWA) in SWS, whereas A1 receptor antagonism suppresses SWA.139 Importantly, 2 studies found that V. officinalis could increase SWS,36,38 possibly contributing to the improved subjective sleep quality while objective sleep latency remained unchanged.36 These observations suggest that valerian may promote the sleep quality by inducing deep sleep via adenosine A1 receptor signaling. In fact, a polar extract of valerian exhibited partial agonistic activity at A1 receptors140 and valerian contains hydrophilic constituents that interact with adenosine signaling, such as lignans of a olivil derivatives that is a partial agonist of adenosine A1 receptors.112,141 Thus, valerian’s hydrophilic constituents could exert the anxiolytic and sleep-inducing effects via adenosine signaling. Importantly, while the hydrophilic extract was agonistic, isovaltrate in the hydrophobic extract acted as an inverse agonist at adenosine A1 receptor.140 These findings indicate the hydrophilic and hydrophobic components of valerian act in opposite directions at adenosine receptors,140 supporting the traditional use of water-based extract as a sleep aid,16,140 and possibly explaining the contradictory findings regarding the efficacy of valerian hydroalcoholic extracts.
Valepotriates
Valepotriates are unstable thermolabile compounds that rapidly decompose. The characteristic odor of dried valerian is due to isovaleric acid released from decomposing valepotriates.142 Although the mechanistic roles of valepotriates in the therapeutic effects are yet to be clarified, pre-clinical evidence suggests that valepotriates exert anxiolytic143 and antidepressant-like effects,144,145 possibly via the dopaminergic and noradrenergic neurotransmission but not via the serotonergic system.145 The monoaminergic neurotransmitters (dopamine, noradrenaline, and 5-HT) play overlapping roles in the etiology of anxiety and depression, and medications targeting these systems are proved to be effective in treating both anxiety and depression.146 Moreover, those monoamines play a role in sleep-wake regulation.147 Thus, the presence of valepotriates is likely to be essential in treating these disorders. In addition, valtrate reduced the serum glucocorticoid level in a rat anxiety model,148 suggesting hypothalamus-pituitary-adrenal axis modulatory potential of valepotriates. Considering the significant association between neuroendocrine-immune axis imbalance and stress vulnerability and resilience,149 the potential impact on the hypothalamus-pituitary-adrenal axis is particularly important. Stress can disturb the sleep, and stress reactivity is implicated in vulnerability to insomnia and other stress-related disorders such as anxiety and depression.8,150 Furthermore, glucocorticoid imbalance could lead to amyloid formation and tau accumulation, hallmarks of Alzheimer’s disease,151 and elevated glucocorticoid levels are associated with neurodegenerative disorders.152 Thus, valepotriates may prevent stress-induced neuropsychiatric disorders including anxiety and depression, as well as cognitive dysfunction, by restoring endocrine-immune balance. Expected mechanisms of action are summarized in Figure 6.
Quality and Shelf Life
As described above, multiple constituents, including valerenic acids, lignan, and valepotriates, differentially contribute to the therapeutic effectiveness of valerian. However, most of the commercially available valerian extracts are standardized to the levels of valerenic acids, while the contents of valepotriates are often unknown. Importantly, Bos et al. compared freshly prepared tincture (70% EtOH extract) and the same extract stored at room temperature for 2 months, and found that the levels of valepotriates (valtrate and isovaltrate) after 2 months were less than 5% of the original, whereas the levels of valerenic acids (valerenic acid and acetoxyvalerenic acid) were not affected.98 This finding suggests that the shelf life of valerian extracts may be significantly shorter if the levels of valepotriates are considered. It is possible that the negative outcomes observed in the trials using valerian extracts, rather than the raw materials, could have been caused by the loss of valepotriates in those extracts.
In the United States, valerian-containing products are widely available as dietary supplements, in various forms under various trade names. Examples are Valerian (NOW FOODS, IL, US; Nature’s Way, WI, USA), Standardized Valerian (root extract standardized to 0.8% valerenic acids; Nature’s Way), and Amantilla (hydroalcoholic extract; NutraMedix, FL, USA), to name a few. In Europe, the Committee on Herbal Medicine Products (HMPC) concluded that valerian-containing medicinal products have to be assessed by the responsible national authorities. Available products include Baldrian (85% ethanol extract; Kneipp, Würzburg, Germany), and Baldrianwurzel (70% ethanol extract; Hofapotheke St. Afra, Augsburg, Germany). Herbal combinations are also available (e.g. Valeirana-Melissa extract [standardized to 0.8% valerenic acids; Bonusan, Rotterdam, Netherlands]). Of note, the recommended storage temperatures for most of these products are at or below room temperature (around 25°C).
Limitation
The current study has reviewed the effectiveness of valerian without specifying target populations. This raises a limitation as the etiology underlying sleep problems and associated health problems could be diverse depending on age, sex, and general health conditions. Consequently, there could be potential differences in the outcomes depending on the target populations, however such possibilities were not addressed in this review. In addition, heterogeneity in the outcome measures limited the number of data sources for meta-analysis, which in turn limits the generalizability of the results. Further research is needed to assess the effectiveness for specific populations and outcomes.
Conclusion
According to the 1905 edition of King’s American dispensatory,99 ‘valerian excites the cerebro-spinal system.’ and ‘in medicinal doses it acts as a stimulant-tonic,…’, which suggests that valerian is not a simple hypnotic or anxiolytic agent. In addition, the text continues that ‘The extract of valerian is worthless, but the fluid extract has been found to possess all the medicinal virtues of the root’,99 suggesting that extraction methods could play a crucial role in preserving the therapeutic potency. Valerian has diverse chemical properties. As summarized in Table 3, the differences in polarity can lead to variability in the quality of valerian extracts depending on the extraction solvents. Our study has found that the hydroalcoholic extracts used in the clinical trials are variable with respect to the solvents (Table 4), and their phytochemical properties are not characterized in most cases. Furthermore, there is no information regarding the storage conditions such as temperature and storage period. Considering the low stability of some of the important active constituents,98,142 it is unknown to what extent the active constituents had been preserved at the end of those trials. The absence of such information limits the discussion as to why some extracts were ineffective while others exhibited effectiveness in those clinical trials. Due to the multiple active constituents and complex mechanisms of actions (Figure 5), it is imperative to use well characterized plant materials and extracts in the future clinical trials, in order for the evaluation of valerian’s true effectiveness to be possible.
Table 3.
Polarity Indices of Major Constituents.
| Constituent | Molecular formula | molecular weight | Polarity index | |||
|---|---|---|---|---|---|---|
| Partition coefficient XLogP3-AA | Topological Polar Surface Area (Å2) | Hydrogen bond donor number / acceptor number | ||||
| Essential oil | ||||||
| 1 | borneol | C10H18O | 154.25 | 2.7 | 20.2 | 1/1 |
| 2 | bornyl acetate | C12H20O2 | 196.29 | 3.3 | 26.3 | 0/2 |
| 3 | valerenal | C15H22O | 218.33 | 3.2 | 17.1 | 0/1 |
| 4 | valerenic acid | C15H22O2 | 234.33 | 3.2 | 37.3 | 1/2 |
| 5 | valeric acid | C5H10O2 | 102.13 | 1.4 | 37.3 | 1/2 |
| 6 | isovaleric acid | C5H10O2 | 102.13 | 1.2 | 37.3 | 1/2 |
| Valepotriates | ||||||
| 7 | valtrate | C22H30O8 | 422.5 | 1.8 | 101 | 0/8 |
| 8 | isovaltrate | C22H30O8 | 422.5 | 1.8 | 101 | 0/8 |
| 9 | didrovaltrate | C22H32O8 | 424.5 | 2.3 | 101 | 0/8 |
| 10 | acevaltrate | C24H32O10 | 480.5 | 0.9 | 127 | 0/10 |
| Flavonoids | ||||||
| 11 | 6-methylapigenin | C16H12O5 | 284.26 | 2.1 | 87 | 3/5 |
| 12 | linarin | C28H32O14 | 592.5 | -0.5 | 214 | 7/14 |
| 13 | hesperidin | C28H34O15 | 610.6 | -1.1 | 234 | 8/15 |
| Amino acid | ||||||
| 14 | γ-aminobutyric acid | C4H9NO2 | 103.12 | -3.2 | 63.3 | 2/3 |
| Lignan | ||||||
| 15 | 4’-O-β-D-glucosyl-9-O-(6”-deoxysaccharosyl)olivil | C38H54O22 | 862.8 | -3.9 | 346 | 13/22 |
Polarity index values were obtained from PubChem database (https://pubchem.ncbi.nlm.nih.gov).
XLogP3-AA (computed by XLogP3 3.0) indicates hydrophobicity, whereas Topological Polar Surface Area (computed by Cactvs 3.4.6.11) and Hydrogen Bond Donor / Acceptor (computed by Cactvs 3.4.6.11) indicate hydrophilicity.
Table 4.
Solvents for Valerian Extract Preparation.
| Extraction solvent | References | |
|---|---|---|
| 1 | 70% Ethanol | Roh D, 201934; Hallam KT, 200347; Taibi DM, 200954; Taibi DM, 200953; Diaper A, 200450; Donovan JL, 200469; Ziegler G, 200244 |
| 2 | 45% Methanol | Meier S, 201877; Melzer J, 200973; Koetter U, 200765; Morin CM, 200579; Schellenberg R, 200480; Vonderheid-Guth B, 200082 |
| 3 | 62% Ethanol | Gromball J, 201484; Müller SF, 200689 |
| 4 | 60% Ethanol | Oxman AD, 200746 |
| 5 | 40% Ethanol | Trompetter I, 201386 |
This study demonstrated that valerian could be a safe and useful herb alone and also in combination in treating sleep problems, anxiety, and associated comorbidities. One possibility for exploration that arises is whether valerian is particularly useful in treating insomnia where there are higher levels of anxiety. In fact, the observation that V. edulis reduced sleep problems most effectively in children with hyperactivity46 may suggest that valerian is more useful in treating insomnia associated with particular psychiatric conditions such as anxiety. The safety has been proven in a wide range from childhood46,84,86 to old age.38,45,53 Sleep problems are associated with a number of comorbidities including anxiety, depression, and dementia in both adulthood123,153 and childhood.154 As we age, the quantity and quality of sleep decrease,155,156 and insomnia and daytime drowsiness are frequently reported in the older population.157 Thus, valerian may help improve the quality of life in all ages by improving sleep quality, thereby preventing a number of psychiatric and cognitive dysfunctions. However, due to the presence of multiple active constituents and the unstable nature of some components, it may be useful to revise the standardization methods and shelf life of the extracts. Repeated treatments with the whole root / rhizome consistently promoted sleep quality at 450-1410 mg per day for 4-8 weeks, whereas valerian extracts 300-600 mg per day for 5 days-4 weeks resulted in inconsistent outcomes. Evidence is poor with respect to the effectiveness of single dose intervention as a sleep aid. Developing effective standardization methods would be desirable. Meanwhile, the usage of whole herbal substances (root / rhizome), rather than extracts, may be the way to obtain optimal efficacy for the time being.
Lastly, due to the subjective nature of sleep problems and associated mood disorders, there remains a difficulty in determining the best measure for the efficacy of interventions. The ultimate goal of any therapies, whether it be traditional, conventional or integrative, beyond disease ‘cure’ or modulation, is to improve the quality of life for patients. While objective measures in a laboratory setting provide valuable information and mechanistic understanding, it may be useful to consider additional and more important factors that need to be addressed specifically in each individual. Such measures and tailor-made approaches would necessarily involve sufficient interaction and communication between patients and practitioners. Developing effective trial strategies to address this issue is a future challenge.
Supplemental Material
Supplemental Material, PRISMA_checklist_200107 for Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis by Noriko Shinjyo, Guy Waddell and Julia Green in Journal of Evidence-Based Integrative Medicine
Acknowledgments
We thank the collaborative environment at University of Westminster, Heartwood Education, and Chiba University, which enabled this study.
Authors Note: All research done by the authors.
Author Contributions: NS wrote this systematic review with input and contributions of GW and JG. NS, GW and JG planned the search in databases and defined criteria for the selection of articles. NS carried out the search, screen the materials and proposed a selection. All authors contributed to the evaluation of the studies. All authors contributed to the interpretation of data and discussion. All authors reviewed the whole manuscript, read and approved the submitted version.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Noriko Shinjyo  https://orcid.org/0000-0003-4501-4513
https://orcid.org/0000-0003-4501-4513
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Olesen J, Gustavsson A, Svensson M, et al. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19(1):155–162. [DOI] [PubMed] [Google Scholar]
- 2. Watson N, Badr M, Belenky G, et al. Joint consensus statement of the American academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015;38(8):1161–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou E, Gardiner P, Bertisch S. Integrative medicine for insomnia. Med Clin North Am. 2017;101(5):865–879. doi:10.1016/j.mcna.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 4. Medic G, Wille M, Hemels M. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics. 2015;15(1):65–74. doi:10.1111/psyg.12069 [DOI] [PubMed] [Google Scholar]
- 6. de Almondes K, Costa M, Malloy-Diniz L, Diniz B. Insomnia and risk of dementia in older adults: systematic review and meta-analysis. J Psychiatr Res. 2016;77:109–115. doi:10.1016/j.jpsychires.2016.02.021 [DOI] [PubMed] [Google Scholar]
- 7. Chaiard J, Weaver T. Update on research and practices in major sleep disorders: part I. Obstructive sleep apnea syndrome. J Nurs Sch. 2019;51(5):500–508. doi:10.1111/jnu.12489 [DOI] [PubMed] [Google Scholar]
- 8. Kalmbach D, Anderson J, Drake C. The impact of stress on sleep: pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J Sleep Res. 2018;27(6):e12710 doi:10.1111/jsr.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koo B. Restless leg syndrome across the globe: epidemiology of the restless legs syndrome/Willis-Ekbom disease. Sleep Med Clin. 2015;10(3):189–205. [DOI] [PubMed] [Google Scholar]
- 10. Chaiard J, Weaver T. Update on research and practices in major sleep disorders: Part II-insomnia, Willis-Ekbom disease (Restless Leg Syndrome), and narcolepsy. J Nurs Sch. 2019;51(6):624–633. [DOI] [PubMed] [Google Scholar]
- 11. Ohayon M, Priest R, Zulley J, Smirne S, Paiva T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology. 2002;58(12):1826–1833. [DOI] [PubMed] [Google Scholar]
- 12. Burman D. Sleep disorders: insomnia. FP Essent. 2017;460:22–28. [PubMed] [Google Scholar]
- 13. Borja N, Daniel K. Ramelteon for the treatment of insomnia. Clin Ther. 2006;28(10):1540–1555. [DOI] [PubMed] [Google Scholar]
- 14. Roth T, Drake C. Evolution of insomnia: current status and future direction. Sleep Med. 2004;5(Suppl 1):S23–30. [DOI] [PubMed] [Google Scholar]
- 15. Brandt J, Leong C. Benzodiazepines and Z-Drugs: an updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D. 2017;17(4):493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bone K, Mills S. Principles and Practice of Phytotherapy: Modern Herbal Medicine. 2nd ed Churchill Livingstone; 2013. [Google Scholar]
- 17. Klein S, Rister R. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicine (English Translation). (Blumenthal M, ed.). American Botanical Council; 1998. [Google Scholar]
- 18. EMA. European Union Herbal Monograph on Valeriana Officinalis L. Radix; 2016. [Google Scholar]
- 19. Stevinson C, Ernst E. Valerian for insomnia: a systematic review of randomized clinical trials. Sleep Med. 2000;1(2):91–99. [DOI] [PubMed] [Google Scholar]
- 20. Bent S, Padula A, Moore D, Patterson M, Mehling W. Valerian for sleep: a systematic review and meta-analysis. Am J Med. 2006;119(12):1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taibi D, Landis C, Petry H, Vitiello M. A systematic review of valerian as a sleep aid: safe but not effective. Sleep Med Rev. 2007;11(3):209–230. [DOI] [PubMed] [Google Scholar]
- 22. Fernández-San-Martín MI, Masa-Font R, Palacios-Soler L, Sancho-Gómez P, Calbó-Caldentey C, Flores-Mateo G. Effectiveness of Valerian on insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2010;11(6):505–511. doi:10.1016/j.sleep.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med [Internet]. 2009;6(7):e1000097 doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jadad A, Moore R, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 25. Borenstein M, Hedges L, Higgins J, Rothstein H. Converting among effect sizes In: Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, eds. Introduction to Meta-Analysis. John Wiley & Sons; 2009. [Google Scholar]
- 26. Hedges L, Olkin I. Statistical Methods for Meta-Analysis. Academic Press; 1985. [Google Scholar]
- 27. Durlak J. How to select, calculate, and interpret effect sizes. J Pediatr Psychol. 2009;34(9):917–928. [DOI] [PubMed] [Google Scholar]
- 28. Suurmond R, van Rhee H, Hak T. Introduction, comparison and validation of Meta-Essentials: a free and simple tool for meta-analysis. Res Synth Methods. 2017;8(4):537–553. doi:10.1002/jrsm.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cuellar N, Ratcliffe S. Does valerian improve sleepiness and symptom severity in people with restless legs syndrome? Altern Ther Heal Med. 2009;15(2):22–28. [PubMed] [Google Scholar]
- 31. Mineo L, Concerto C, Patel D, et al. Valeriana officinalis root extract modulates cortical excitatory circuits in humans. Neuropsychobiology. 2017;75(1):46–51. doi:10.1159/000480053 [DOI] [PubMed] [Google Scholar]
- 32. Samaei A, Nobahar M, Hydarinia-Naieni Z, et al. Effect of valerian on cognitive disorders and electroencephalography in hemodialysis patients: a randomized, cross over, double-blind clinical trial. BMC Nephrol. 2018;19(1):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farah G, Ferreira G, Danieletto-Zanna C, Luppi C, Jacomacci W. Assessment of Valeriana officinalis l. (Valerian) for conscious sedation of patients during the extraction of impacted mandibular third molars: a randomized, split-mouth, double-blind, crossover study. J Oral Maxillofac Surg. 2019;77(9):1796.e1–1796.e8. doi:10.1016/j.joms.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 34. Roh D, Jung J, Yoon K, et al. Valerian extract alters functional brain connectivity: a randomized double-blind placebo-controlled trial. Phytother Res. 2019;33(4):939–948. [DOI] [PubMed] [Google Scholar]
- 35. Ahmadi M, Khalili H, Abbasian L, Ghaeli P. Effect of Valerian in preventing neuropsychiatric adverse effects of efavirenz in HIV-positive patients: a pilot randomized, placebo-controlled clinical trial. Ann Pharmacother. 2017;51(6):457–464. [DOI] [PubMed] [Google Scholar]
- 36. Donath F, Quispe S, Diefenbach K, Maurer A, Fietze I, Roots I. Critical evaluation of the effect of valerian extract on sleep structure and sleep quality. Pharmacopsychiatry. 2000;33(2):47–53. [DOI] [PubMed] [Google Scholar]
- 37. Kuhlmann J, Berger W, Podzuweit H, Schmidt U. The influence of valerian treatment on “reaction time, alertness and concentration” in volunteers. Pharmacopsychiatry. 1999;32(6):235–241. [DOI] [PubMed] [Google Scholar]
- 38. Schulz H, Stolz C, Müller J. The effect of valerian extract on sleep polygraphy in poor sleepers: a pilot study. Pharmacopsychiatry. 1994;27(4):147–151. [DOI] [PubMed] [Google Scholar]
- 39. Kohnen R, Oswald W. The effects of valerian, propranolol, and their combination on activation, performance, and mood of healthy volunteers under social stress conditions. Pharmacopsychiatry. 1988;21(6):447–448. [DOI] [PubMed] [Google Scholar]
- 40. Balderer G, Borbély A. Effect of valerian on human sleep. Psychopharmacol. 1985;87(4):406–409. [DOI] [PubMed] [Google Scholar]
- 41. Leathwood P, Chauffard F, Heck E, Munoz-Box R. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacol Biochem Behav. 1982;17(1):65–71. [DOI] [PubMed] [Google Scholar]
- 42. Herrera-Arellano A, Luna-Villegas G, Cuevas-Uriostegui M, et al. Polysomnographic evaluation of the hypnotic effect of Valeriana edulis standardized extract in patients suffering from insomnia. Planta Med. 2001;67(8):695–699. [DOI] [PubMed] [Google Scholar]
- 43. Cropley M, Cave Z, Ellis J, Middleton R. Effect of kava and valerian on human physiological and psychological responses to mental stress assessed under laboratory conditions. Phytother Res. 2002;16(1):23–27. [DOI] [PubMed] [Google Scholar]
- 44. Ziegler G, Ploch M, Miettinen-Baumann A, Collet W. Efficacy and tolerability of valerian extract LI 156 compared with oxazepam in the treatment of non-organic insomnia—a randomized, double-blind, comparative clinical study. Eur J Med Res. 2002;7(11):480–486. [PubMed] [Google Scholar]
- 45. Glass J, Sproule B, Herrmann N, Streiner D, Busto U. Acute pharmacological effects of temazepam, diphenhydramine, and valerian in healthy elderly subjects. J Clin Psychopharmacol. 2003;23(3):260–268. [DOI] [PubMed] [Google Scholar]
- 46. Francis A, Dempster R. Effect of valerian, Valeriana edulis, on sleep difficulties in children with intellectual deficits: randomised trial. Phytomedicine. 2002;9(4):273–279. [DOI] [PubMed] [Google Scholar]
- 47. Hallam K, Olver J, McGrath C, Norman T. Comparative cognitive and psychomotor effects of single doses of Valeriana officianalis and triazolam in healthy volunteers. Hum Psychopharmacol. 2003;18(8):619–625. [DOI] [PubMed] [Google Scholar]
- 48. Coxeter P, Schluter P, Eastwood H, Nikles C, Glasziou P. Valerian does not appear to reduce symptoms for patients with chronic insomnia in general practice using a series of randomised n-of-1 trials. Complement Ther Med. 2003;11(4):215–222. [DOI] [PubMed] [Google Scholar]
- 49. Gutierrez S, Ang-Lee M, Walker D, Zacny J. Assessing subjective and psychomotor effects of the herbal medication valerian in healthy volunteers. Pharmacol Biochem Behav. 2004;78(1):57–64. [DOI] [PubMed] [Google Scholar]
- 50. Diaper A, Hindmarch I. A double-blind, placebo-controlled investigation of the effects of two doses of a valerian preparation on the sleep, cognitive and psychomotor function of sleep-disturbed older adults. Phytother Res. 2004;18(10):831–836. [DOI] [PubMed] [Google Scholar]
- 51. Jacobs B, Bent S, Tice J, Blackwell T, Cummings S. An internet-based randomized, placebo-controlled trial of kava and valerian for anxiety and insomnia. Med. 2005;84(4):197–207. [DOI] [PubMed] [Google Scholar]
- 52. Oxman A, Flottorp S, Håvelsrud K, et al. A televised, web-based randomised trial of an herbal remedy (valerian) for insomnia. PLoS One. 2007;2(10):e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Taibi D, Vitiello M, Barsness S, Elmer G, Anderson G, Landis C. A randomized clinical trial of valerian fails to improve self-reported, polysomnographic, and actigraphic sleep in older women with insomnia. Sleep Med. 2009;10(3):319–328. doi:10.1016/j.sleep.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Taibi D, Bourguignon C, Gill Taylor A. A feasibility study of valerian extract for sleep disturbance in person with arthritis. Biol Res Nurs. 2009;10(4):409–417. doi:10.1177/1099800408324252 [DOI] [PubMed] [Google Scholar]
- 55. Pakseresht S, Boostani H, Sayyah M. Extract of valerian root (Valeriana officinalis L.) vs. placebo in treatment of obsessive-compulsive disorder: a randomized double-blind study. J Complement Integr Med. 2011;8:pii: /j/jcim.2011.8.issue-1/1553-3840.1465/1553-38 doi:10.2202/1553-3840.1465 [DOI] [PubMed] [Google Scholar]
- 56. Barton DL, Atherton PJ, Bauer BA, et al. The use of Valeriana officinalis (Valerian) in improving sleep in patients who are undergoing treatment for cancer: a phase III randomized, placebo-controlled, double-blind study (NCCTG Trial, N01C5). J Support Oncol. 2011;9(1):24–31. doi:10.1016/j.suponc.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taavoni S, Ekbatani N, Kashaniyan M, Haghani H. Effect of valerian on sleep quality in postmenopausal women: a randomized placebo-controlled clinical trial. Menopause. 2011;18(9):951–955. doi:10.1097/gme.0b013e31820e9acf [DOI] [PubMed] [Google Scholar]
- 58. Mirabi P, Dolatian M, Mojab F, Majd H. Effects of valerian on the severity and systemic manifestations of dysmenorrhea. Int J Gynaecol Obs. 2011;115(3):285–288. doi:10.1016/j.ijgo.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 59. Mirabi P, Mojab F. The effects of valerian root on hot flashes in menopausal women. Iran J Pharm Res. 2013;12(1):217–222. [PMC free article] [PubMed] [Google Scholar]
- 60. Pinheiro M, Alcântara C, de Moraes M, de Andrade E. Valeriana officinalis L. for conscious sedation of patients submitted to impacted lower third molar surgery: a randomized, double-blind, placebo-controlled split-mouth study. J Pharm Bioallied Sci. 2014;6(2):109–114. doi:10.4103/0975-7406.129176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hassani S, Alipour A, Darvishi Khezri H, et al. Can Valeriana officinalis root extract prevent early postoperative cognitive dysfunction after CABG surgery? A randomized, double-blind, placebo-controlled trial. Psychopharmacol. 2015;232(5):843–850. doi:10.1007/s00213-014-3716-x [DOI] [PubMed] [Google Scholar]
- 62. Thomas K, Canedo J, Perry P, et al. Effects of valerian on subjective sedation, field sobriety testing and driving simulator performance. Accid Anal Prev. 2016;92:240–244. doi:10.1016/j.aap.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 63. Behboodi Moghadam Z, Rezaei E, Shirood Gholami R, Kheirkhah M, Haghani H. The effect of Valerian root extract on the severity of pre menstrual syndrome symptoms. J Tradit Complement Med. 2016;6(3):309–315. doi:10.1016/j.jtcme.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jenabi E, Shobeiri F, Hazavehei S, Roshanaei G. The effect of Valerian on the severity and frequency of hot flashes: a triple-blind randomized clinical trial. Women Heal. 2017;58(3):297–304. doi:10.1080/03630242.2017 [DOI] [PubMed] [Google Scholar]
- 65. Koetter U, Schrader E, Käufeler R, Brattström A. A randomized, double blind, placebo-controlled, prospective clinical study to demonstrate clinical efficacy of a fixed valerian hops extract combination (Ze 91019) in patients suffering from non-organic sleep disorder. Phytother Res. 2007;21(9):847–851. [DOI] [PubMed] [Google Scholar]
- 66. Wheatley D. Kava and valerian in the treatment of stress-induced insomnia. Phytother Res. 2001;15(6):549–551. [DOI] [PubMed] [Google Scholar]
- 67. Dominguez R, Bravo-Valverde R, Kaplowitz B, Cott JM. Valerian as a hypnotic for Hispanic patients. Cult Divers Ethn Minor Psychol. 2000;6(1):84–92. [DOI] [PubMed] [Google Scholar]
- 68. Gurley B, Gardner S, Hubbard M, et al. In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clin Pharmacol Ther. 2005;77(5):415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Donovan J, DeVane C, Chavin K, et al. Multiple night-time doses of valerian (Valeriana officinalis) had minimal effects on CYP3A4 activity and no effect on CYP2D6 activity in healthy volunteers. Drug Metab Dispos. 2004;32(12):1333–1336. [DOI] [PubMed] [Google Scholar]
- 70. Poyares D, Guilleminault C, Ohayon M, Tufik S. Can valerian improve the sleep of insomniacs after benzodiazepine withdrawal? Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):539–545. [DOI] [PubMed] [Google Scholar]
- 71. Andreatini R, Sartori V, Seabra M, Leite J. Effect of valepotriates (valerian extract) in generalized anxiety disorder: a randomized placebo-controlled pilot study. Phytother Res. 2002;16(7):650–654. [DOI] [PubMed] [Google Scholar]
- 72. Dimpfel W, Suter A. Sleep improving effects of a single dose administration of a valerian/hops fluid extract—a double blind, randomized, placebo-controlled sleep-EEG study in a parallel design using electrohypnograms. Eur J Med Res. 2008;13(5):200–204. [PubMed] [Google Scholar]
- 73. Melzer J, Schrader E, Brattström A, Schellenberg R, Saller R. Fixed herbal drug combination with and without butterbur (Ze 185) for the treatment of patients with somatoform disorders: randomized, placebo-controlled pharmaco-clinical trial. Phytother Res. 2009;23(9):1303–1308. doi:10.1002/ptr.2771 [DOI] [PubMed] [Google Scholar]
- 74. Chen J, Chao Y, Lu S, Shiung T, Chao Y. The effectiveness of valerian acupressure on the sleep of ICU patients: a randomized clinical trial. Int J Nurs Stud. 2012;49(8):913–920. doi:10.1016/j.ijnurstu.2012.02.012 [DOI] [PubMed] [Google Scholar]
- 75. Maroo N, Hazra A, Das T. Efficacy and safety of a polyherbal sedative-hypnotic formulation NSF-3 in primary insomnia in comparison to zolpidem: a randomized controlled trial. Indian J Pharmacol. 2013;45(1):34–39. doi:10.4103/0253-7613.106432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Taavoni S, Nazem Ekbatani N, Haghani H. Valerian/lemon balm use for sleep disorders during menopause. Complement Ther Clin Pr. 2013;19(4):193–196. doi:10.1016/j.ctcp.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 77. Meier S, Haschke M, Zahner C, et al. Effects of a fixed herbal drug combination (Ze 185) to an experimental acute stress setting in healthy men—an explorative randomized placebo-controlled double-blind study. Phytomedicine. 2018;39:85–92. doi:10.1016/j.phymed.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 78. Kennedy D, Little W, Haskell C, Scholey A. Anxiolytic effects of a combination of Melissa officinalis and Valeriana officinalis during laboratory induced stress. Phytother Res. 2006;20(2):96–102. [DOI] [PubMed] [Google Scholar]
- 79. Morin C, Koetter U, Bastien C, Ware J, Wooten V. Valerian-hops combination and diphenhydramine for treating insomnia: a randomized placebo-controlled clinical trial. Sleep. 2005;28(11):1465–1471. [DOI] [PubMed] [Google Scholar]
- 80. Schellenberg R, Sauer S, Abourashed E, Koetter U, Brattström A. The fixed combination of valerian and hops (Ze91019) acts via a central adenosine mechanism. Planta Med. 2004;70(7):594–597. [DOI] [PubMed] [Google Scholar]
- 81. Farag N, Mills P. A randomized-controlled trial of the effects of a traditional herbal supplement on sleep onset insomnia. Complement Ther Med. 2003;11(4):223–225. [DOI] [PubMed] [Google Scholar]
- 82. Vonderheid-Guth B, Todorova A, Brattström A, Dimpfel W. Pharmacodynamic effects of valerian and hops extract combination (Ze 91019) on the quantitative-topographical EEG in healthy volunteers. Eur J Med Res. 2000;5(4):139–144. [PubMed] [Google Scholar]
- 83. Lindahl O, Lindwall L. Double blind study of a valerian preparation. Pharmacol Biochem Behav. 1989;32(4):1065–1066. [DOI] [PubMed] [Google Scholar]
- 84. Gromball J, Beschorner F, Wantzen C, Paulsen U, Burkart M. Hyperactivity, concentration difficulties and impulsiveness improve during seven weeks’ treatment with valerian root and lemon balm extracts in primary school children. Phytomedicine. 2014;21(8-9):1098–1103. doi:10.1016/j.phymed.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 85. Müller D, Pfeil T, von den Driesch V. Treating depression comorbid with anxiety—results of an open, practice-oriented study with St John’s wort WS 5572 and valerian extract in high doses. Phytomedicine. 2003;10(Suppl 4):25–30. [DOI] [PubMed] [Google Scholar]
- 86. Trompetter I, Krick B, Weiss G. Herbal triplet in treatment of nervous agitation in children. Wien Med Wochenschr. 2013;163(3-4):52–57. doi:s10.1007/s10354-012-0165 -1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Toolika E, Bhat N, Shetty S. A comparative clinical study on the effect of Tagara (Valeriana wallichii DC.) and Jatamansi (Nardostachys jatamansi DC.) in the management of Anidra (primary insomnia). Ayu. 2015;36(1):46–49. doi:10.4103/0974-8520.169008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Abdellah S, Berlin A, Blondeau C, et al. A combination of Eschscholtzia californica Cham. and Valeriana officinalis L. extracts for adjustment insomnia: a prospective observational study. J Tradit Complement Med. 2019;10(2):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Müller S, Klement S. A combination of valerian and lemon balm is effective in the treatment of restlessness and dyssomnia in children. Phytomedicine. 2006;13(6):383–387. [DOI] [PubMed] [Google Scholar]
- 90. Buysse D, Reynolds C, Monk T, Berman S, Kupfer D. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 91. Morin C, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ibáñez V, Silva J, Cauli O. A survey on sleep assessment methods. PeerJ. 2018;6:e4849 doi:10.7717/peerj.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. [DOI] [PubMed] [Google Scholar]
- 94. Bech P, Kastrup M, Rafaelsen O. Mini-compendium of rating scales for states of anxiety depression mania schizophrenia with corresponding DSM-III syndromes. Acta Psychiatr Scand Suppl. 1986;326:1–37. [PubMed] [Google Scholar]
- 95. Spielberger C. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; 1970. [Google Scholar]
- 96. Julian L. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res. 2011;63(suppl 11):S467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Steptoe A, Cropley M, Joekes K. Job strain, blood pressure and response to uncontrollable stress. J Hypertens. 1999;17(2):193–200. [DOI] [PubMed] [Google Scholar]
- 98. Bos R, Hendriks H, Scheffer J, Woerdenbag H. Cytotoxic potential of valerian constituents and valerian tinctures. Phytomedicine. 1998;5(3):219–225. doi:10.1016/S0944-7113(98)80032-9 [DOI] [PubMed] [Google Scholar]
- 99. Felter H, Lloyd J. King’s American Dispensatory. 19th ed Franklin Classics; 1905. [Google Scholar]
- 100. Glidewell R, McPherson Botts E, Orr W. Insomnia and anxiety: diagnostic and management implications of complex interactions. Sleep Med Clin. 2015;10(1):93–99. doi:10.1016/j.jsmc.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 101. Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A. Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol. 2011;21(12):841–860. doi:10.1016/j.euroneuro.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 102. Gebara M, Siripong N, DiNapoli E, et al. Effect of insomnia treatments on depression: A systematic review and meta-analysis. Depress Anxiety. 2018;35(8): 717–731. doi:10.1002/da.22776 [DOI] [PubMed] [Google Scholar]
- 103. Liu L, Liu C, Wang Y, Wang P, Li Y, Li B. Herbal medicine for anxiety, depression and insomnia. Curr Neuropharmacol. 2015;13(4):481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cox R, Parmar A, Olatunji B. Sleep in obsessive-compulsive and related disorders: a selective review and synthesis. Curr Opin Psychol. 2019;34:23–26. doi:10.1016/j.copsyc.2019.08.018 [DOI] [PubMed] [Google Scholar]
- 105. Freeman E, Sammel M. Anxiety as a risk factor for menopausal hot flashes: evidence from the Penn Ovarian Aging cohort. Menopause. 2016;23(9):942–949. doi:10.1097/GME.0000000000000662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ohayon M. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166(12):1262–1268. [DOI] [PubMed] [Google Scholar]
- 107. Bonanni E, Schirru A, Di Perri M, Bonuccelli U, Maestri M. Insomnia and hot flashes. Maturitas. 2019;126:51–54. doi:10.1016/j.maturitas.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 108. Fernández S, Wasowski C, Paladini AC, Marder M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol Biochem Behav. 2004;77(2):399–404. [DOI] [PubMed] [Google Scholar]
- 109. Houghton P. The scientific basis for the reputed activity of Valerian. J Pharm Pharmacol. 1999;51(5):505–512. [DOI] [PubMed] [Google Scholar]
- 110. Wang P, Hu J, Ran X, et al. Iridoids and sesquiterpenoids from the roots of Valeriana officinalis. J Nat Prod. 2009;72(9):1682–1685. doi:10.1021/np9003382 [DOI] [PubMed] [Google Scholar]
- 111. Samaneh E, Tayebeh R, Hassan E, Vahid N. Composition of essential oils in subterranean organs of three species of Valeriana L. Nat Prod Res. 2010;24(19):1834–1842. doi:10.1080/14786419.2010.482051 [DOI] [PubMed] [Google Scholar]
- 112. Schumacher B, Scholle S, Hölzl J, Khudeir N, Hess S, Müller C. Lignans isolated from valerian: identification and characterization of a new olivil derivative with partial agonistic activity at A1 adenosine receptors. J Nat Prod. 2002;65(10):1479–1485. [DOI] [PubMed] [Google Scholar]
- 113. Marder M, Viola H, Wasowski C, Fernández S, Medina JH, Paladini AC. 6-methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNS. Pharmacol Biochem Behav. 2003;75(3):537–545. [DOI] [PubMed] [Google Scholar]
- 114. Murphy K, Kubin Z, Shepherd J, Ettinger R. Valeriana officinalis root extracts have potent anxiolytic effects in laboratory rats. Phytomedicine. 2010;17(8-9):674–678. doi:10.1016/j.phymed.2009.10.020 [DOI] [PubMed] [Google Scholar]
- 115. Savage K, Firth J, Stough C, Sarris J. GABA-modulating phytomedicines for anxiety: a systematic review of preclinical and clinical evidence. Phytother Res. 2018;32(1):3–18. doi:10.1002/ptr.5940 [DOI] [PubMed] [Google Scholar]
- 116. Solomon V, Tallapragada V, Chebib M, Johnston G, Hanrahan J. GABA allosteric modulators: an overview of recent developments in non-benzodiazepine modulators. Eur J Med Chem. 2019;171:434–461. doi:10.1016/j.ejmech.2019.03.043 [DOI] [PubMed] [Google Scholar]
- 117. Benke D, Barberis A, Kopp S, et al. GABA A receptors as in vivo substrate for the anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology. 2009;56(1):174–181. doi:10.1016/j.neuropharm.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 118. Khom S, Baburin I, Timin E, et al. Valerenic acid potentiates and inhibits GABA(A) receptors: molecular mechanism and subunit specificity. Neuropharmacology. 2007;53(1):178–187. [DOI] [PubMed] [Google Scholar]
- 119. Trauner G, Khom S, Baburin I, Benedek B, Hering S, Kopp B. Modulation of GABAA receptors by valerian extracts is related to the content of valerenic acid. Planta Med. 2008;74(1):19–24. [DOI] [PubMed] [Google Scholar]
- 120. Felgentreff F, Becker A, Meier B, Brattström A. Valerian extract characterized by high valerenic acid and low acetoxy valerenic acid contents demonstrates anxiolytic activity. Phytomedicine. 2012;19(13):1216–1222. doi:10.1016/j.phymed.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 121. Santos M, Ferreira F, Cunha A, Carvalho A, Macedo T. An aqueous extract of valerian influences the transport of GABA in synaptosomes. Planta Med. 1994;60(3):278–279. [DOI] [PubMed] [Google Scholar]
- 122. Pandya M, Palpagama T, Turner C, Waldvogel H, Faull R, Kwakowsky A. Sex- and age-related changes in GABA signaling components in the human cortex. Biol Sex Differ. 2019;10(1):5 doi:10.1186/s13293-018-0214-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Blake M, Trinder J, Allen N. Mechanisms underlying the association between insomnia, anxiety, and depression in adolescence: implications for behavioral sleep interventions. Clin Psychol Rev. 2018;63:25–40. doi:10.1016/j.cpr.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 124. Żmudzka E, Sałaciak K, Sapa J, Pytka K. Serotonin receptors in depression and anxiety: insights from animal studies. Life Sci. 2018;210:106–124. doi:10.1016/j.lfs.2018.08.050 [DOI] [PubMed] [Google Scholar]
- 125. Venner A, Broadhurst R, Sohn L, Todd W, Fuller P. Selective activation of serotoninergic dorsal raphe neurons facilitates sleep through anxiolysis. Sleep. 2019. doi:10.1093/sleep/zsz231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Volk B, Nagy B, Vas S, Kostyalik D, Simig G, Bagdy G. Medicinal chemistry of 5-HT5A receptor ligands: a receptor subtype with unique therapeutical potential. Curr Top Med Chem. 2010;10(5):554–578. [DOI] [PubMed] [Google Scholar]
- 127. Dietz B, Mahady G, Pauli G, Farnsworth N. Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitro. Brain Res Mol Brain Res. 2005;138(2):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Duncan M, Jennes L, Jefferson J, Brownfield M. Localization of serotonin(5A) receptors in discrete regions of the circadian timing system in the Syrian hamster. Brain Res. 2000;869(1-2):178–185. [DOI] [PubMed] [Google Scholar]
- 129. Oliver K, Kinsey A, Wainwright A, Sirinathsinghji D. Localization of 5-ht(5A) receptor-like immunoreactivity in the rat brain. Brain Res. 2000;867(1-2):131–142. [DOI] [PubMed] [Google Scholar]
- 130. Kassai F, Schlumberger C, Kedves R, et al. Effect of 5-HT5A antagonists in animal models of schizophrenia, anxiety and depression. Behav Pharmacol. 2012;23(4):397–406. doi:10.1097/FBP.0b013e3283565248 [DOI] [PubMed] [Google Scholar]
- 131. van Calker D, Biber K, Domschke K, Serchov T. The role of adenosine receptors in mood and anxiety disorders. J Neurochem. 2019;151(1):11–27. [DOI] [PubMed] [Google Scholar]
- 132. Prediger R, da Silva G, Batista L, Bittencourt A, Takahashi R. Activation of adenosine A1 receptors reduces anxiety-like behavior during acute ethanol withdrawal (hangover) in mice. Neuropsychopharmacology. 2006;31(10):2210–2220. [DOI] [PubMed] [Google Scholar]
- 133. Vincenzi F, Borea P, Varani K. Anxiolytic properties of A1 adenosine receptor PAMs. Oncotarget. 2017;8(5):7216–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Romagnoli R, Baraldi P, Tabrizi M, Gessi S, Borea P, Merighi S. Allosteric enhancers of A1 adenosine receptors: state of the art and new horizons for drug development. Curr Med Chem. 2010;17(30):3488–3502. [DOI] [PubMed] [Google Scholar]
- 135. Basheer R, Halldner L, Alanko L, McCarley R, Fredholm B, Porkka-Heiskanen T. Opposite changes in adenosine A1 and A2A receptor mRNA in the rat following sleep deprivation. Neuroreport. 2001;12(8):1577–1580. [DOI] [PubMed] [Google Scholar]
- 136. Strecker R, Morairty S, Thakkar M, et al. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res. 2000;115(2):183–204. [DOI] [PubMed] [Google Scholar]
- 137. Basheer R, Porkka-Heiskanen T, Strecker R, Thakkar M, McCarley R. Adenosine as a biological signal mediating sleepiness following prolonged wakefulness. Biol Sig Recept. 2000;9(6):319–327. [DOI] [PubMed] [Google Scholar]
- 138. Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. US: National Academies Press; 2006. [PubMed] [Google Scholar]
- 139. Lazarus M, Oishi Y, Bjorness T, Greene R. Gating and the need for sleep: dissociable effects of adenosine A1 and A2A receptors. Front Neurosci. 2019;13:740 doi:10.3389/fnins.2019.00740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lacher S, Mayer R, Sichardt K, Nieber K, Müller C. Interaction of valerian extracts of different polarity with adenosine receptors: identification of isovaltrate as an inverse agonist at A1 receptors. Biochem Pharmacol. 2007;73(2):248–258. doi:10.1016/j.bcp.2006.09.029 [DOI] [PubMed] [Google Scholar]
- 141. Müller C, Schumacher B, Brattström A, Abourashed E, Koetter U. Interactions of valerian extracts and a fixed valerian–hop extract combination with adenosine receptors. Life Sci. 2002;71(16):1939–1949. doi:10.1016/s0024-3205(02)01964 -1 [DOI] [PubMed] [Google Scholar]
- 142. Müller L, de Andrade Salles L, Sakamoto S, et al. Effect of storage time and conditions on the diene valepotriates content of the extract of Valeriana glechomifolia obtained by supercritical carbon dioxide. Phytochem Anal. 2012;23(3):222–227. doi:10.1002/pca.1346 [DOI] [PubMed] [Google Scholar]
- 143. Maurmann N, Reolon G, Rech S, Fett-Neto A, Roesler R. A Valepotriate fraction of valeriana glechomifolia shows sedative and anxiolytic properties and impairs recognition but not aversive memory in mice. Evid Based Complement Altern Med. 2011;2011:720853 doi:10.1093/ecam/nep232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sah S, Mathela C, Chopra K. Antidepressant effect of Valeriana wallichii patchouli alcohol chemotype in mice: behavioural and biochemical evidence. J Ethnopharmacol. 2011;135(1):197–200. [DOI] [PubMed] [Google Scholar]
- 145. Müller L, Salles L, Stein A, et al. Antidepressant-like effect of Valeriana glechomifolia Meyer (Valerianaceae) in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36(1):101–109. doi:10.1016/j.pnpbp.2011.08.015 [DOI] [PubMed] [Google Scholar]
- 146. Liu Y, Zhao J, Guo W. Emotional roles of mono-aminergic neurotransmitters in major depressive disorder and anxiety disorders. Front Psychol. 2018;9:2201 doi:10.3389/fpsyg.2018.02201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Holst S, Landolt H. Sleep-wake neurochemistry. Sleep Med Clin. 2018;13(2):137–146. doi:10.1016/j.jsmc.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 148. Shi S, Shi J, Liu Y, et al. The anxiolytic effects of valtrate in rats involves changes of corticosterone levels. Evid Based Complement Altern Med. 2014;2014:325948 doi:10.1155/2014/325948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ménard C, Pfau M, Hodes G, Russo S. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology. 2017;42(1):62–80. doi:10.1038/npp.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Drake C, Pillai V, Roth T. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. Sleep. 2013;37:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Green K, Billings L, Roozendaal B, McGaugh J, LaFerla F. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26(35):9047–9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Vyas S, Rodrigues A, Silva J, et al. Chronic stress and glucocorticoids: from neuronal plasticity to neurodegeneration. Neural Plast. 2016;2016:6391686 doi:10.1155/2016/6391686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hertenstein E, Feige B, Gmeiner T, et al. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev. 2019;43:96–105. doi:10.1016/j.smrv.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 154. Gregory A, Sadeh A. Annual research review: sleep problems in childhood psychiatric disorders—a review of the latest science. J Child Psychol Psychiatry. 2016;57(3):296–317. doi:10.1111/jcpp.12469 [DOI] [PubMed] [Google Scholar]
- 155. Vitiello M. Sleep disorders and aging: understanding the causes. J Gerontol A Biol Sci Med Sci. 1997;52(4):M189–191. [DOI] [PubMed] [Google Scholar]
- 156. Ohayon M, Carskadon M, Guilleminault C, Vitiello M. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespa. Sleep. 2004;27(7):1255–1273. doi:10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- 157. Foley D, Monjan A, Brown S, Simonsick E, Wallace R, Blazer D. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, PRISMA_checklist_200107 for Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis by Noriko Shinjyo, Guy Waddell and Julia Green in Journal of Evidence-Based Integrative Medicine