Abstract
Aim: Accumulating evidence reveals that sedentary behavior is associated with mortality and cardiometabolic disease; however, there are potential age and sex differences in sedentary behavior and health outcomes that have not been adequately addressed. This study aimed to determine the association of sedentary behavior with cardiometabolic diseases such as hypertension, dyslipidemia, diabetes mellitus, and its risk factors in a large Japanese population according to age and sex.
Methods: Using data from the Japan Multi-Institutional Collaborative Cohort Study obtained from baseline surveys, data of 62,754 participants (27,930 males, 34,824 females) were analyzed. This study uses a cross-sectional design and self-administered questionnaires to evaluate sedentary time and anamnesis. For the logistic regression analysis, sedentary time < 5 h/day was used as the reference and then adjusted for age, research areas, leisure-time metabolic equivalents, and alcohol and smoking status. From the analysis of anthropometric and blood examinations, 35,973 participants (17,109 males, 18,864 females) were analyzed.
Results: For hypertension and diabetes, sedentary time was associated with a significantly higher proportion of male participants. Both sexes were associated with a significantly higher proportion of participants with dyslipidemia. Participants who had longer sedentary time tended to have increased levels of blood pressure, triglycerides, and non-high-density lipoprotein cholesterol (HDL-C), and decreased levels of HDL-C, especially in the 60–69 years group.
Conclusions: Independent of leisure-time physical activity, sedentary time was associated with cardiometabolic diseases in a large Japanese population classified by age and sex. Our findings indicate that regularly interrupting and replacing sedentary time may contribute to better physical health-related quality of life.
Keywords: Sedentary time, Cardiometabolic diseases, Population approach
Introduction
Accumulating evidence reveals that sedentary (sitting or reclining posture) behavior is associated with negative health connotations1), including cardiovascular-specific and overall mortality2). Similarly, studies have demonstrated a relationship between sedentary behavior and the development of metabolic disease (e.g., obesity, metabolic syndrome, and type 2 diabetes mellitus)3). More specifically, these studies report an association between prolonged periods of sedentary behavior and all-cause morbidity and mortality, which cannot be simply explained by differences in engagement in low-, moderate-, or vigorous-intensity physical activity4). Sedentary time is associated with an increased risk of mortality and cardiometabolic disease, although there are potential age and sex differences in sedentary behavior and health outcomes that have not been adequately addressed5).
In Japan, some studies showed an association between sedentary time and erectile dysfunction among patients with type 2 diabetes mellitus6), kidney function decline7), chronic obstructive pulmonary disease8), coronary artery disease9), and all-cause mortality10). However, in Japan, few studies have examined the association between sedentary time and cardiovascular risk according to age and sex.
Aim
This study aimed to determine the association of sedentary behavior with cardiometabolic diseases such as hypertension, dyslipidemia, diabetes mellitus, and its risk factors in a large Japanese population according to age and sex.
Methods
Study Participants
In this study, we evaluated participant data collected during the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study11) from baseline surveys using cross-sectional data. The cohort study evaluated the general Japanese population using genetic and clinical data to detect and confirm gene–environment interactions related to lifestyle-associated diseases. The study participants were 35–69 years old and were enrolled after responding to study announcements in 13 research areas, attending health check-up examinations that were commissioned by their local governments, visiting local health check-up centers, or visiting a cancer hospital. Fig. 1 shows the flow chart of the study participants. Of the 13 research sites, two did not collect data on daily life activities, including sitting time from the participants. Excluding the participants in these two research sites, 72,714 participants were initially included in the current study (the dataset is ver. 20190729). Among the 72,714 participants, we excluded a total of 9,960 participants who lacked self-administered questionnaire data as follows: 478 without data on history of hypertension, dyslipidemia, or diabetes; 5,198 without data on smoking and drinking status or daily physical activity times including sitting time; 1,306 without data on medical history of ischemic heart disease and stroke; and 49 without data on drug treatment for hypertension, dyslipidemia, or diabetes. We also excluded 2,929 participants with a medical history of ischemic heart disease and stroke. Data for a total of 62,754 participants (27,930 males, 34,824 females) were analyzed for the association of sedentary time with hypertension, dyslipidemia, and diabetes.
Fig. 1.
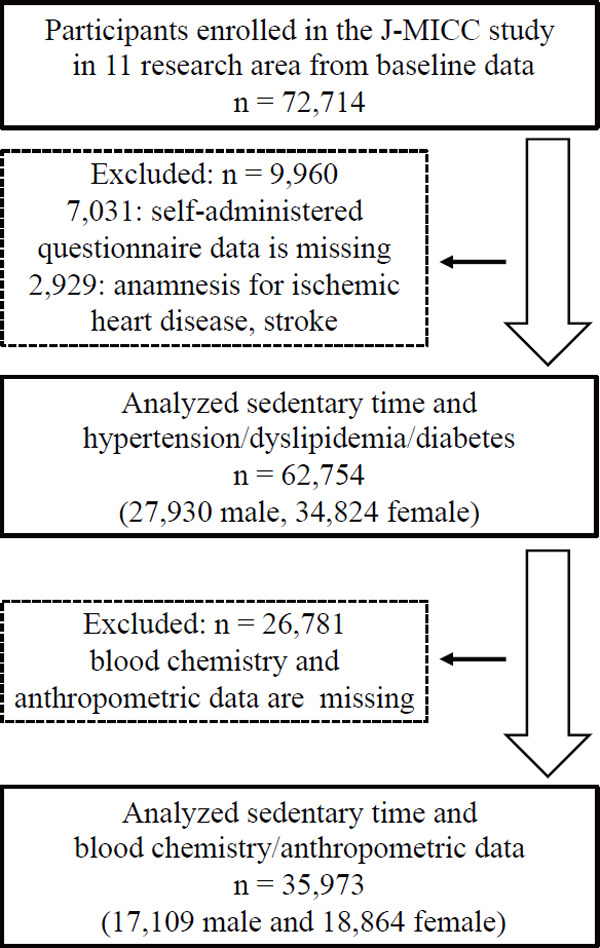
Flow chart of the study participants
Furthermore, we analyzed participants from seven research sites who underwent anthropometric and blood examinations. We excluded 26,781 participants with missing data for body mass index (BMI), blood pressure (systolic blood pressure [SBP] and diastolic blood pressure [DBP]), and biochemical measurements, including serum triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), non-HDL-C, and glycated hemoglobin (HbA1c). Finally, a total of 35,973 participants (17,109 males and 18,864 females) were included in the analysis of sedentary time.
All study participants gave written informed consent. The study protocol was approved by the Ethics Committees at Aichi Cancer Center, the Nagoya University Graduate School of Medicine (IRB No. 939-13), and other institutions participating in the J-MICC study. This study was conducted according to the principles expressed in the World Medical Association Declaration of Helsinki.
Lifestyle and Blood Biochemistry Data
In this study, we evaluated the lifestyle and medical information obtained through self-administered questionnaires (smoking and drinking status and physical activity, including sitting time). Physical activity was determined using a format similar to a short format of the International Physical Activity Questionnaire (IPAQ)12). Leisure-time physical activity was assessed in terms of metabolic equivalents (LT-METs), as previously reported13, 14). In brief, METs-hours per day of leisure-time activity was estimated by multiplying the reported daily time that was spent in each activity by the relevant MET intensity. The duration of sitting time was classified into one of the following eight categories: none, < 1 h/day, 1 to < 3 h/day, 3 to < 5 h/day, 5 to < 7 h/day, 7 to < 9 h/day, 9 to < 11 h/day, and ≥ 11 h/day. Sitting time was then categorized based on the quartile value: 5 h/day, 5-< 7 h/day, 7-< 9 h/day, or ≥ 9 h/day. BMI was calculated as weight (kg) divided by the square of height (m2). Anamnesis and medication history were assessed using self-administered questionnaires. Hypertension, dyslipidemia, and diabetes were defined as the presence or absence of anamnesis and/or current use of medication. In addition, blood chemistry data (serum levels of TG, total cholesterol, HDL-C, non-HDL-C, and HbA1c) and anthropometric data were obtained from health check-ups performed in the research areas. Laboratories, in each research area, analyzed the serum samples.
Statistical Analyses
Continuous variables are expressed as means, and categorical data are expressed as sums and percentages. We classified sedentary time into four groups according to sitting time (< 5 h, 5 to < 7 h, 7 to < 9 h, and ≥ 9 h) and analyzed each group for sex and age in years (35–49, 50–59, and 60–69 years). Odds ratios (OR) and 95% confidence intervals (CI) were calculated using logistic regression analyses to evaluate the associations of sedentary time with the prevalence of hypertension, dyslipidemia, and diabetes. Sedentary time < 5 h was used as a reference. Multiple regression analysis was performed to assess the sitting time (< 5 h, 5 to < 7 h, 7 to < 9 h, and ≥ 9 h) influence on variables of cardiometabolic risk factors. The following factors were considered as independent variables: age, research areas, LT-METs, and drinking and smoking status (never, former, and current). Tests for linear trends (e.g., P trend tests) were conducted by including four groups according to sitting time as ordinal variables. This provided a test of significance for the hypothesis that as the amount of sedentary time increases, the risk of cardiometabolic diseases tends to increase. All statistical tests were two-tailed, and differences with a p-value < 0.05 were considered statistically significant. JMP 13 software (SAS Institute Inc., Cary, NC) was used for all statistical analyses.
Results
Participant Characteristics
Table 1 presents the participants' characteristics, including drinking and smoking status, anamnesis of hypertension/dyslipidemia/diabetes, and distribution of age and sex differences according to sedentary time. Among the 27,930 participants included in the current analysis for male sex, 9,529 (34.1%), 5,458 (19.5%), 4,491 (16.1%), and 8,452 (30.3%) spent sedentary time < 5 h, 5 to < 7 h, 7 to < 9 h, and ≥ 9 h per day, respectively. Similarly, among the 34,824 participants included in the current analysis for female sex, 14,121 (40.5%), 8,727 (25.1%), 5,715 (16.4%), and 6,261 (18.0%) spent sedentary time < 5 h, 5 to < 7 h, 7 to < 9 h, and ≥ 9 h per day, respectively.
Table 1. Characteristics of participants according to sedentary time.
| Male |
||||||||
|---|---|---|---|---|---|---|---|---|
| < 5 h | 5 to < 7 h | 7 to < 9 h | ≥ 9 h | |||||
|
n = 9,529 |
n = 5,458 |
n = 4,491 |
n = 8,452 |
|||||
| n/mean | (%)/SD | n/mean | (%)/SD | n/mean | (%)/SD | n/mean | (%)/SD | |
| years | ||||||||
| 35–49 | 2,616 | 31.5% | 1,480 | 17.8% | 1,340 | 16.1% | 2,868 | 34.5% |
| 50–59 | 2,926 | 33.9% | 1,612 | 18.7% | 1,367 | 15.8% | 2,727 | 31.6% |
| 60–69 | 3,987 | 36.3% | 2,366 | 21.5% | 1,784 | 16.2% | 2,857 | 26.0% |
| 35–49 years | ||||||||
| Drinking status | ||||||||
| Current | 1,944 | 74.3% | 1,158 | 78.2% | 1,045 | 78.0% | 2,180 | 76.0% |
| Former | 46 | 1.8% | 25 | 1.7% | 16 | 1.2% | 48 | 1.7% |
| Never | 626 | 23.9% | 297 | 20.1% | 279 | 20.8% | 640 | 22.3% |
| Smoking status | ||||||||
| Current | 1,100 | 42.0% | 522 | 35.3% | 430 | 32.1% | 844 | 29.4% |
| Former | 751 | 28.7% | 465 | 31.4% | 456 | 34.0% | 934 | 32.6% |
| Never | 765 | 29.2% | 493 | 33.3% | 454 | 33.9% | 1,090 | 38.0% |
| Hypertension | 224 | 8.6% | 133 | 9.0% | 129 | 9.6% | 269 | 9.4% |
| Dyslipidemia | 279 | 10.7% | 178 | 12.0% | 182 | 13.6% | 409 | 14.3% |
| Diabetes | 78 | 3.0% | 41 | 2.8% | 48 | 3.6% | 85 | 3.0% |
| LT-METs (METs·hrs/day) | 1.90 | 3.43 | 1.83 | 3.00 | 1.78 | 2.73 | 1.49 | 2.20 |
| 50–59 years | ||||||||
| Drinking status | ||||||||
| Current | 2,285 | 78.1% | 1,255 | 77.9% | 1,078 | 78.9% | 2,154 | 79.0% |
| Former | 62 | 2.1% | 48 | 3.0% | 35 | 2.6% | 74 | 2.7% |
| Never | 579 | 19.8% | 309 | 19.2% | 254 | 18.6% | 499 | 18.3% |
| Smoking status | ||||||||
| Current | 1,041 | 35.6% | 547 | 33.9% | 413 | 30.2% | 818 | 30.0% |
| Former | 1,114 | 38.1% | 683 | 42.4% | 585 | 42.8% | 1,199 | 44.0% |
| Never | 771 | 26.3% | 382 | 23.7% | 369 | 27.0% | 710 | 26.0% |
| Hypertension | 640 | 21.9% | 373 | 23.1% | 343 | 25.1% | 631 | 23.1% |
| Dyslipidemia | 449 | 15.3% | 320 | 19.9% | 331 | 24.2% | 671 | 24.6% |
| Diabetes | 221 | 7.6% | 134 | 8.3% | 108 | 7.9% | 239 | 8.8% |
| LT-METs (METs·hrs/day) | 1.76 | 3.14 | 1.99 | 3.11 | 1.84 | 2.61 | 1.66 | 2.32 |
| 60–69 years | ||||||||
| Drinking status | ||||||||
| Current | 3,113 | 78.1% | 1,812 | 76.6% | 1,345 | 75.4% | 2,059 | 72.1% |
| Former | 128 | 3.2% | 111 | 4.7% | 84 | 4.7% | 176 | 6.2% |
| Never | 746 | 18.7% | 443 | 18.7% | 355 | 19.9% | 622 | 21.8% |
| Smoking status | ||||||||
| Current | 925 | 23.2% | 530 | 22.4% | 390 | 21.9% | 650 | 22.8% |
| Former | 1,826 | 45.8% | 1,142 | 48.3% | 922 | 51.7% | 1,504 | 52.6% |
| Never | 1,236 | 31.0% | 694 | 29.3% | 472 | 26.5% | 703 | 24.6% |
| Hypertension | 1,328 | 33.3% | 834 | 35.2% | 598 | 33.5% | 1,005 | 35.2% |
| Dyslipidemia | 712 | 17.9% | 519 | 21.9% | 407 | 22.8% | 678 | 23.7% |
| Diabetes | 486 | 12.2% | 301 | 12.7% | 245 | 13.7% | 383 | 13.4% |
| LT-METs (METs·hrs/day) | 3.25 | 4.67 | 3.31 | 3.92 | 3.02 | 3.53 | 2.47 | 3.25 |
| Female |
||||||||
|---|---|---|---|---|---|---|---|---|
| < 5 h | 5 to < 7 h | 7 to < 9 h | ≥ 9 h | |||||
|
n = 14,121 |
n = 8,727 |
n = 5,715 |
n = 6,261 |
|||||
| n/mean | (%)/SD | n/mean | (%)/SD | n/mean | (%)/SD | n/mean | (%)/SD | |
| years | ||||||||
| 35–49 | 4,952 | 39.9% | 2,750 | 22.1% | 2,007 | 16.2% | 2,709 | 21.8% |
| 50–59 | 4,605 | 42.3% | 2,745 | 25.2% | 1,723 | 15.8% | 1,823 | 16.7% |
| 60–69 | 4,564 | 39.7% | 3,232 | 28.1% | 1,985 | 17.2% | 1,729 | 15.0% |
| 35–49 years | ||||||||
| Drinking status | ||||||||
| Current | 2,358 | 47.6% | 1,379 | 50.1% | 995 | 49.6% | 1,391 | 51.3% |
| Former | 118 | 2.4% | 49 | 1.8% | 42 | 2.1% | 80 | 3.0% |
| Never | 2,476 | 50.0% | 1,322 | 48.1% | 970 | 48.3% | 1,238 | 45.7% |
| Smoking status | ||||||||
| Current | 516 | 10.4% | 232 | 8.4% | 165 | 8.2% | 292 | 10.8% |
| Former | 541 | 10.9% | 306 | 11.1% | 211 | 10.5% | 305 | 11.3% |
| Never | 3,895 | 78.7% | 2,212 | 80.4% | 1,631 | 81.3% | 2,112 | 78.0% |
| Hypertension | 188 | 3.8% | 93 | 3.4% | 87 | 4.3% | 96 | 3.5% |
| Dyslipidemia | 223 | 4.5% | 122 | 4.4% | 84 | 4.2% | 153 | 5.6% |
| Diabetes | 36 | 0.7% | 25 | 0.9% | 18 | 0.9% | 23 | 0.8% |
| LT-METs (METs·hrs/day) | 1.54 | 2.74 | 1.51 | 2.51 | 1.33 | 2.18 | 1.12 | 1.86 |
| 50–59 years | ||||||||
| Drinking status | ||||||||
| Current | 1,836 | 39.9% | 1,093 | 39.8% | 728 | 42.3% | 765 | 42.0% |
| Former | 64 | 1.4% | 44 | 1.6% | 27 | 1.6% | 33 | 1.8% |
| Never | 2,705 | 58.7% | 1,608 | 58.6% | 968 | 56.2% | 1,025 | 56.2% |
| Smoking status | ||||||||
| Current | 410 | 8.9% | 180 | 6.6% | 107 | 6.2% | 150 | 8.2% |
| Former | 331 | 7.2% | 211 | 7.7% | 129 | 7.5% | 162 | 8.9% |
| Never | 3,864 | 83.9% | 2,354 | 85.8% | 1,487 | 86.3% | 1,511 | 82.9% |
| Hypertension | 697 | 15.1% | 421 | 15.3% | 227 | 13.2% | 244 | 13.4% |
| Dyslipidemia | 753 | 16.4% | 496 | 18.1% | 305 | 17.7% | 352 | 19.3% |
| Diabetes | 151 | 3.3% | 86 | 3.1% | 45 | 2.6% | 62 | 3.4% |
| LT-METs (METs·hrs/day) | 1.85 | 2.88 | 2.00 | 2.86 | 1.65 | 2.24 | 1.38 | 2.11 |
| 60–69 years | ||||||||
| Drinking status | ||||||||
| Current | 1,372 | 30.1% | 1,071 | 33.1% | 669 | 33.7% | 577 | 33.4% |
| Former | 51 | 1.1% | 53 | 1.6% | 37 | 1.9% | 44 | 2.5% |
| Never | 3,141 | 68.8% | 2,108 | 65.2% | 1,279 | 64.4% | 1,108 | 64.1% |
| Smoking status | ||||||||
| Current | 166 | 3.6% | 99 | 3.1% | 79 | 4.0% | 79 | 4.6% |
| Former | 194 | 4.3% | 142 | 4.4% | 105 | 5.3% | 108 | 6.2% |
| Never | 4,204 | 92.1% | 2,991 | 92.5% | 1,801 | 90.7% | 1,542 | 89.2% |
| Hypertension | 1,155 | 25.3% | 846 | 26.2% | 535 | 27.0% | 470 | 27.2% |
| Dyslipidemia | 1,279 | 28.0% | 1,056 | 32.7% | 625 | 31.5% | 549 | 31.8% |
| Diabetes | 255 | 5.6% | 181 | 5.6% | 114 | 5.7% | 100 | 5.8% |
| LT-METs (METs·hrs/day) | 2.72 | 3.69 | 2.84 | 3.31 | 2.49 | 3.18 | 1.96 | 2.68 |
With increasing age, the proportion of sedentary time decreased from 34.5% to 26.0% for males in the 35–49 years old group vs. the 60–69 years old group and decreased from 21.8% to 15.0% for females.
The multivariate-adjusted OR for anamnesis of hypertension, dyslipidemia, and diabetes, according to sedentary time, is shown in Table 2. For the logistic regression analysis, sedentary time < 5 h/day was used as the reference and then adjusted for age, research areas, LT-METs, and alcohol and smoking status. For hypertension, a sedentary time of 7 to < 9 h/day in the 50–59 years group (OR: 1.096, CI: 1.016–1.182) and in the 60–69 years group (OR: 1.041, CI: 1.006–1.077) were associated with a significantly higher proportion of male participants. In female participants, sedentary time was not associated with a higher proportion of hypertension. For dyslipidemia, both male (excluding 5 to < 7 h/day in the 35–49 years group) and female (excluding 5 to < 7 h/day and 7 to < 9 h/day in the 35–49 years group and the 50–59 years group) sexes were associated with a significantly higher proportion of participants with dyslipidemia. For diabetes, sedentary time ≥ 9 h/day in the 50–59 years group (OR: 1.067, CI: 1.000–1.137) was associated with a significantly higher proportion of male participants. In female participants, sedentary time was not associated with diabetes.
Table 2. Associations between anamnesis and sedentary time.
| Male |
||||||||
|---|---|---|---|---|---|---|---|---|
| years | < 5h | 5 to < 7h |
7 to < 9h |
≥ 9 h |
||||
| OR | 95%CI | OR | 95%CI | OR | 95%CI | |||
| Hypertension | 35–49 | ref | 1.035 | 0.824–1.301 | 1.049 | 0.935–1.178 | 1.036 | 0.973–1.104 |
| 50–59 | ref | 1.071 | 0.925–1.240 | 1.096 | 1.016–1.182 | 1.024 | 0.982–1.068 | |
| 60–69 | ref | 1.097 | 0.984–1.222 | 1.011 | 0.952–1.073 | 1.041 | 1.006–1.077 | |
| Dyslipidemia | 35–49 | ref | 1.135 | 0.927–1.389 | 1.031 | 1.031–1.262 | 1.122 | 1.061–1.185 |
| 50–59 | ref | 1.377 | 1.175–1.614 | 1.333 | 1.231–1.444 | 1.216 | 1.163–1.272 | |
| 60–69 | ref | 1.291 | 1.137–1.465 | 1.166 | 1.088–1.249 | 1.131 | 1.086–1.177 | |
| Diabetes | 35–49 | ref | 0.950 | 0.646–1.397 | 1.121 | 0.932–1.349 | 1.011 | 0.910–1.124 |
| 50–59 | ref | 1.103 | 0.881–1.380 | 1.037 | 0.919–1.169 | 1.067 | 1.000–1.137 | |
| 60–69 | ref | 1.047 | 0.898–1.221 | 1.071 | 0.986–1.163 | 1.042 | 0.993–1.093 | |
| Female |
||||||||
|---|---|---|---|---|---|---|---|---|
| years | < 5h | 5 to < 7h |
7 to < 9h |
≥ 9 h |
||||
| OR | 95%CI | OR | 95%CI | OR | 95%CI | |||
| Hypertension | 35–49 | ref | 0.864 | 0.670–1.114 | 1.069 | 0.938–1.218 | 0.980 | 0.901–1.067 |
| 50–59 | ref | 0.999 | 0.875–1.141 | 0.914 | 0.843–0.992 | 0.951 | 0.902–1.003 | |
| 60–69 | ref | 1.041 | 0.938–1.154 | 1.037 | 0.977–1.102 | 1.028 | 0.986–1.073 | |
| Dyslipidemia | 35–49 | ref | 0.968 | 0.771–1.215 | 0.965 | 0.848–1.098 | 1.103 | 1.027–1.184 |
| 50–59 | ref | 1.121 | 0.988–1.272 | 1.049 | 0.974–1.130 | 1.083 | 1.033–1.136 | |
| 60–69 | ref | 1.252 | 1.134–1.381 | 1.097 | 1.035–1.162 | 1.075 | 1.032–1.119 | |
| Diabetes | 35–49 | ref | 1.244 | 0.745–2.078 | 1.120 | 0.842–1.489 | 1.058 | 0.887–1.263 |
| 50–59 | ref | 0.938 | 0.716–1.230 | 0.893 | 0.754–1.059 | 1.024 | 0.926–1.134 | |
| 60–69 | ref | 1.007 | 0.827–1.226 | 1.015 | 0.906–1.138 | 1.019 | 0.940–1.104 | |
Adjusted for age, research areas, LT-METs, drinking and smoking status
Multiple regression analysis was then performed to identify the variables strongly associated with sedentary time (Table 3). The lipid cardiometabolic risk factors were significantly associated with sedentary time. As shown in Table 3, sedentary time was significantly associated with TG, HDL-C, and non-HDL-C in males and females. Furthermore, in the 60–69 years group, sedentary time was significantly associated with several variables except HDL-C (β = < 0.016, p = 0.171) as follows: BMI (β = 0.058, p < 0.001), SBP (β = 0.042, p < 0.001), DBP (β = 0.047, p < 0.001), TG (β = 0.054, p < 0.001), non-HDL-C (β = 0.039, p = 0.001), and HbA1c (β = 0.024, p = 0.044). Similarly, sedentary time was significantly associated with BMI (β = 0.034, p = 0.004), SBP (β = 0.056, p < 0.001), DBP (β = 0.057, p < 0.001), TG (β = 0.045, p < 0.001), and HDL-C (β = 0.042, p = 0.001) in obese women. Supplemental Table 1 shows the mean values of BMI, SBP, DBP, TG, HDL-C, non-HDL-C, and HbA1c levels in each group. Compared with participants who spent < 5 h/day of sedentary time, those who had longer sedentary time tended to have increased levels of BMI, SBP, DBP, TG, and non-HDL-C and decreased levels of HDL-C, especially in the 60–69 years group. Although sedentary time was not associated with a higher proportion of hypertension among female participants, SBP and DBP tended to increase, resulting from increased sedentary time.
Table 3. Comparison of the association of cardiometabolic parameter and sedentary time.
| Male | 35–49 years | 50–59 years | 60–69 years | |||
|---|---|---|---|---|---|---|
|
n = 4,800 |
n = 5,388 |
n = 6,921 |
||||
| beta | p-value | beta | p-value | beta | p-value | |
| BMI | 0.072 | 0.072 | 0.015 | 0.278 | 0.058 | < 0.001 |
| SBP | 0.006 | 0.690 | 0.005 | 0.693 | 0.042 | < 0.001 |
| DBP | 0.004 | 0.808 | 0.021 | 0.122 | 0.047 | < 0.001 |
| TG | 0.029 | 0.049 | 0.043 | 0.002 | 0.054 | < 0.001 |
| HDL-C | −0.049 | 0.001 | −0.054 | < 0.001 | −0.016 | 0.171 |
| non-HDL-C | 0.031 | 0.034 | 0.038 | 0.006 | 0.039 | 0.001 |
| HbA1c | −0.015 | 0.309 | −0.008 | 0.572 | 0.024 | 0.044 |
| Female | 35–49 years | 50–59 years | 60–69 years | |||
|---|---|---|---|---|---|---|
|
n = 5,929 |
n = 5,986 |
n = 6,949 |
||||
| beta | p-value | beta | p-value | beta | p-value | |
| BMI | −0.008 | 0.518 | −0.006 | 0.654 | 0.034 | 0.004 |
| SBP | 0.034 | 0.009 | 0.019 | 0.139 | 0.056 | < 0.001 |
| DBP | 0.045 | 0.001 | 0.030 | 0.018 | 0.057 | < 0.001 |
| TG | 0.038 | 0.004 | 0.031 | 0.017 | 0.045 | < 0.001 |
| HDL-C | 0.020 | 0.127 | 0.007 | 0.610 | 0.042 | 0.001 |
| non-HDL-C | 0.046 | 0.001 | 0.029 | 0.027 | 0.021 | 0.080 |
| HbA1c | −0.036 | 0.006 | −0.040 | 0.002 | −0.003 | 0.815 |
Adjusted for age, research area, LT-METs, drinking and smoking status
Supplemental Table 1. The mean values of BMI, SBP, DBP, TG, HDL-C, nonHDL-C and HbA1c levels in each sedentary time group.
| Male | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| years | < 5 h | 5 to < 7 h | 7 to < 9 h | ≥ 9 h | p trend | |||||
| n = 6,077 | n = 3,440 | n = 2,755 | n = 4,837 | |||||||
| mean | SD | mean | SD | mean | SD | mean | SD | |||
| 35–49 | BMI (kg/m2) | 23.76 | 3.34 | 23.66 | 3.18 | 23.90 | 3.38 | 23.89 | 3.33 | 0.176 |
| SBP (mmHg) | 122.8 | 16.02 | 122.7 | 15.89 | 122.6 | 15.77 | 123.9 | 15.84 | 0.017 | |
| DBP (mmHg) | 77.72 | 11.81 | 77.85 | 11.47 | 77.68 | 11.27 | 78.24 | 11.18 | 0.162 | |
| TG (mg/dl) | 143.1 | 124.0 | 154.7 | 179.1 | 150.5 | 109.1 | 153.2 | 113.5 | < 0.001 | |
| HDL-C (mg/dl) | 57.94 | 15.26 | 57.14 | 14.24 | 56.31 | 15.41 | 56.25 | 14.24 | 0.002 | |
| non-HDL-C (mg/dl) | 145.3 | 35.35 | 145.6 | 37.74 | 144.7 | 33.53 | 148.5 | 35.18 | 0.005 | |
| HbA1c (%) | 5.47 | 0.63 | 5.41 | 0.55 | 5.48 | 0.71 | 5.41 | 0.60 | < 0.001 | |
| 50–59 | BMI (kg/m2) | 23.68 | 2.98 | 23.87 | 3.11 | 23.84 | 2.92 | 23.82 | 2.98 | 0.151 |
| SBP (mmHg) | 129.3 | 18.21 | 128.5 | 18.20 | 130.1 | 18.83 | 130.0 | 17.98 | 0.114 | |
| DBP (mmHg) | 81.34 | 11.60 | 81.26 | 11.20 | 82.43 | 11.60 | 82.38 | 11.56 | 0.004 | |
| TG (mg/dl) | 141.6 | 98.71 | 159.1 | 122.9 | 160.4 | 165.6 | 158.2 | 111.3 | < 0.001 | |
| HDL-C (mg/dl) | 58.82 | 15.57 | 56.76 | 15.16 | 56.83 | 14.91 | 56.46 | 14.65 | < 0.001 | |
| non-HDL-C (mg/dl) | 146.1 | 33.98 | 150.7 | 33.73 | 150.4 | 35.55 | 150.0 | 33.78 | 0.001 | |
| HbA1c (%) | 5.66 | 0.75 | 5.71 | 0.90 | 5.67 | 0.75 | 5.61 | 0.72 | < 0.001 | |
| 60–69 | BMI (kg/m2) | 23.22 | 2.71 | 23.29 | 2.73 | 23.42 | 2.85 | 23.66 | 2.93 | < 0.001 |
| SBP (mmHg) | 133.6 | 18.71 | 135.3 | 19.37 | 135.6 | 19.43 | 135.6 | 19.40 | < 0.001 | |
| DBP (mmHg) | 80.94 | 10.74 | 81.87 | 10.93 | 82.22 | 10.76 | 82.16 | 11.04 | < 0.001 | |
| TG (mg/dl) | 127.8 | 88.90 | 136.3 | 97.06 | 139.5 | 92.21 | 142.1 | 95.58 | < 0.001 | |
| HDL-C (mg/dl) | 59.49 | 15.98 | 58.96 | 15.52 | 58.13 | 15.74 | 58.16 | 15.66 | 0.002 | |
| non-HDL-C (mg/dl) | 142.8 | 32.27 | 144.4 | 32.49 | 146.1 | 33.83 | 146.1 | 34.62 | 0.001 | |
| HbA1c (%) | 5.71 | 0.74 | 5.71 | 0.72 | 5.73 | 0.69 | 5.74 | 0.76 | 0.370 | |
| Female | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| years | < 5 h | 5 to < 7 h | 7 to < 9 h | ≥ 9 h | p trend | |||||
|
n = 7,768 |
n = 4,485 |
n = 3,067 |
n = 3,344 |
|||||||
| mean | SD | mean | SD | mean | SD | mean | SD | |||
| 35–49 | BMI (kg/m2) | 21.71 | 3.21 | 21.64 | 3.13 | 21.64 | 3.13 | 21.60 | 3.57 | 0.020 |
| SBP (mmHg) | 115.6 | 16.51 | 117.3 | 16.61 | 116.2 | 16.24 | 117.4 | 17.08 | 0.003 | |
| DBP (mmHg) | 70.64 | 11.16 | 71.60 | 10.97 | 71.26 | 11.06 | 71.99 | 11.15 | 0.001 | |
| TG (mg/dl) | 85.18 | 64.51 | 88.34 | 58.70 | 90.77 | 67.06 | 91.08 | 63.35 | < 0.001 | |
| HDL-C (mg/dl) | 71.15 | 15.93 | 69.84 | 16.11 | 70.02 | 16.23 | 70.90 | 17.09 | 0.121 | |
| non-HDL-C (mg/dl) | 126.4 | 32.59 | 127.8 | 31.96 | 129.4 | 30.46 | 130.3 | 32.56 | < 0.001 | |
| HbA1c (%) | 5.34 | 0.41 | 5.33 | 0.41 | 5.32 | 0.44 | 5.28 | 0.34 | < 0.001 | |
| 50–59 | BMI (kg/m2) | 22.30 | 3.17 | 22.35 | 3.20 | 22.24 | 3.15 | 22.30 | 3.36 | 0.735 |
| SBP (mmHg) | 125.2 | 19.07 | 127.2 | 19.91 | 126.1 | 20.17 | 126.6 | 19.15 | 0.022 | |
| DBP (mmHg) | 75.91 | 11.49 | 76.84 | 11.83 | 76.25 | 12.00 | 77.19 | 11.58 | 0.013 | |
| TG (mg/dl) | 108.5 | 66.27 | 117.2 | 78.15 | 113.0 | 68.94 | 115.9 | 75.11 | < 0.001 | |
| HDL-C (mg/dl) | 69.90 | 17.09 | 68.05 | 16.79 | 69.11 | 16.00 | 69.35 | 17.18 | 0.170 | |
| non-HDL-C (mg/dl) | 151.3 | 34.76 | 155.0 | 34.34 | 154.0 | 35.24 | 154.6 | 37.01 | 0.004 | |
| HbA1c (%) | 5.58 | 0.59 | 5.55 | 0.63 | 5.53 | 0.53 | 5.50 | 0.48 | < 0.001 | |
| 60–69 | BMI (kg/m2) | 22.59 | 3.05 | 22.61 | 3.12 | 22.74 | 3.27 | 23.00 | 3.54 | 0.023 |
| SBP (mmHg) | 131.6 | 19.65 | 133.1 | 20.19 | 134.1 | 20.41 | 135.0 | 20.91 | < 0.001 | |
| DBP (mmHg) | 76.88 | 10.97 | 77.60 | 11.16 | 78.26 | 11.16 | 78.63 | 11.57 | < 0.001 | |
| TG (mg/dl) | 112.3 | 61.32 | 122.0 | 76.42 | 121.5 | 77.91 | 121.8 | 64.79 | < 0.001 | |
| HDL-C (mg/dl) | 65.97 | 15.79 | 65.95 | 16.91 | 66.13 | 16.66 | 66.94 | 17.01 | 0.469 | |
| non-HDL-C (mg/dl) | 154.0 | 33.82 | 155.9 | 34.46 | 155.2 | 33.35 | 156.7 | 33.11 | 0.017 | |
| HbA1c (%) | 5.65 | 0.57 | 5.65 | 0.64 | 5.62 | 0.59 | 5.64 | 0.67 | 0.001 | |
Discussion
Considerable evidence suggests that sedentary time affects health outcomes regardless of physical activity4. Especially, many previous studies showed that cardiovascular disease and its risk factors are associated with sedentary time3, 15–17). This study was conducted to determine the associations of sedentary behavior with cardiometabolic diseases such as hypertension, dyslipidemia, and diabetes mellitus in a large Japanese population according to age and sex.
Among male participants, sedentary time was associated with cardiometabolic diseases. Although sedentary time was not associated with hypertension in female participants, Sedentary time increased; thus, SBP and DBP tended to increase. These results suggest the association between sedentary time and cardiometabolic diseases in Japanese males and females. In agreement with many previous studies, our study confirmed that sedentary time was associated with cardiometabolic diseases2, 3, 16, 18–21). Notably, our results further revealed that sedentary time was strongly associated with dyslipidemia and lipid metabolism, as indicated by the levels of TG, HDL-C, and non-HDL-C. A recent study showed the mechanism of adverse effects of sedentary time; that is, the sedentary time has a potential role in the increased production of reactive oxygen species, low-grade inflammation, and metabolic impairment, which contribute to sitting-induced impaired vascular function1). Furthermore, inactivity, such as sitting, quickly engages signals for specific molecular responses contributing to poor lipid metabolism by suppression of skeletal muscle lipoprotein lipase (LPL22); a protein important for controlling plasma TG catabolism, HDL-C, and other metabolic risk factors) activity23). LPL activity was associated with reduced TG uptake and decreased HDL-C levels24). In contrast, the response of lipids and lipoproteins was improved by physical activity, including regular aquatic endurance25), cardiorespiratory exercise26), and fitness aerobic exercise27). These studies show that sedentary time and physical activity has a significant effect on lipid metabolism, which is consistent with our results. The findings of the present study partly support these previous results. To the best of our knowledge, this is the first study to show that sedentary time was strongly associated with lipid metabolism in a large Japanese population.
From the analysis of continuous variables, compared with female participants who had < 5 h per day of sedentary time, those who had ≥ 9 h of sedentary time per day in the 60–69 years group had 3.4 mmHg higher SBP. In The Japanese Society of Hypertension Guidelines for the Management of Hypertension, the cardiovascular disease-reducing effects of lowering blood pressure were estimated using the EPOCH-JAPAN database28). Briefly, a decrease of only 4 mmHg in the average SBP in the Japanese population is estimated to reduce the age-adjusted mortality from stroke in males and females by 8.9% and 5.8%, respectively (the total number of deaths from stroke will decrease by 10,000 per year) and that for coronary heart disease by 5.4% and 7.2%, respectively (the total number of deaths from coronary heart disease will decrease by 5,000 per year)28). This may indicate that reducing sedentary time was an effective strategy to lower the blood pressure of the population. Furthermore, one study indicated that replacing sedentary time with the same amount of moderate- to vigorous-intensity physical activity may contribute to better physical health-related quality of life of Japanese older adults29). In summary, regularly interrupting and replacing sedentary time may contribute to better health-related quality of life because of population approach for the prevention of cardiometabolic diseases.
In contrast, HbA1c tended to decrease, in the 35–49 years females group and the 50–59 years both sexes group. The impact of sedentary time on glycaemic biomarkers was limited in a systematic review30). It seems difficult to evaluate the relationship between sitting time and HbA1c in the general population31) or to use HbA1c as an indicator of glucose metabolism32). To clarify the relationship between glycemic biomarkers and sedentary time, further study and/or more sensitive measures of insulin resistance are necessary.
Despite our novel findings, this study has some limitations. This study uses a cross-sectional design and self-administered questionnaires to evaluate sedentary time and anamnesis. Although questionnaire evaluation of sitting time is controversial, IPAQ was an acceptable international physical activity surveillance instrument33). Most of the studies about sedentary time made use of self-reported sedentary behaviors19). Furthermore, a previous study reported that both accelerometer and self-report measurements are similarly associated with cardiometabolic risk factors in the Japanese population34). Similarly, a previous study reported that stroke and myocardial infarction appears sensitive enough to be used for baseline evaluation of patient characteristics in Japanese cohort studies35). In a follow-up survey, we plan to assess the participants using their actual medical records; therefore, we expect that these additional data will help with further detailed analysis of the direct effect of sedentary time. The strength of the study is that a large number of participants were included, and we implemented a population-based cohort design.
Conclusion
Independent of leisure-time physical activity, sedentary time was associated with cardiometabolic diseases in a large Japanese population classified by age and sex. Our findings indicate that regularly interrupting and replacing sedentary time may contribute to better health-related quality of life because of population approach for the prevention of cardiometabolic diseases.
Financial Support
This study was funded by Grants-in-Aid for Scientific Research on Priority Areas of Cancer (No. 17015018) and on Innovative Areas (No. 221S0001), Platform of Supporting Cohort Study and Biospecimen Analysis (JSPS KAKENHI Grant Number JP16H06277) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and by Grants-in-Aid for Scientific Research (C) (JSPS KAKENHI Grant Number JP15K08778 and JP18K10086) from the Japan Society for the Promotion of Science.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1). Carter S, Hartman Y, Holder S, Thijssen DH, Hopkins ND: Sedentary Behavior and Cardiovascular Disease Risk: Mediating Mechanisms. Exerc Sport Sci Rev, 2017; 45: 80-86 [DOI] [PubMed] [Google Scholar]
- 2). Young DR, Hivert MF, Alhassan S, Camhi SM, Ferguson JF, Katzmarzyk PT, Lewis CE, Owen N, Perry CK, Siddique J, Yong CM: Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation, 2016; 134: e262-279 [DOI] [PubMed] [Google Scholar]
- 3). Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T, Biddle SJ: Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia, 2012; 55: 2895-2905 [DOI] [PubMed] [Google Scholar]
- 4). Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA: Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med, 2015; 162: 123-132 [DOI] [PubMed] [Google Scholar]
- 5). Copeland JL, Ashe MC, Biddle SJ, Brown WJ, Buman MP, Chastin S, Gardiner PA, Inoue S, Jefferis BJ, Oka K, Owen N, Sardinha LB, Skelton DA, Sugiyama T, Dogra S: Sedentary time in older adults: a critical review of measurement, associations with health, and interventions. Br J Sports Med, 2017; 51: 1539. [DOI] [PubMed] [Google Scholar]
- 6). Furukawa S, Sakai T, Niiya T, Miyaoka H, Miyake T, Yamamoto S, Kanzaki S, Maruyama K, Tanaka K, Ueda T, Senba H, Torisu M, Minami H, Tanigawa T, Matsuura B, Hiasa Y, Miyake Y: Self-reported sitting time and prevalence of erectile dysfunction in Japanese patients with type 2 diabetes mellitus: The Dogo Study. J Diabetes Complications, 2017; 31: 53-57 [DOI] [PubMed] [Google Scholar]
- 7). Lee S, Shimada H, Lee S, Makizako H, Doi T, Harada K, Bae S, Harada K, Hotta R, Tsutsumimoto K, Yoshida D, Nakakubo S, Anan Y, Park H, Suzuki T: Association between sedentary time and kidney function in community- dwelling elderly Japanese people. Geriatr Gerontol Int, 2017; 17: 730-736 [DOI] [PubMed] [Google Scholar]
- 8). Ukawa S, Tamakoshi A, Yatsuya H, Yamagishi K, Ando M, Iso H: Association Between Average Daily Television Viewing Time and Chronic Obstructive Pulmonary Disease-Related Mortality: Findings From the Japan Collaborative Cohort Study. J Epidemiol, 2015; 25: 431-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Ikehara S, Iso H, Wada Y, Tanabe N, Watanabe Y, Kikuchi S, Tamakoshi A: Television viewing time and mortality from stroke and coronary artery disease among Japanese men and women -- the Japan Collaborative Cohort Study. Circ J, 2015; 79: 2389-2395 [DOI] [PubMed] [Google Scholar]
- 10). Kikuchi H, Inoue S, Odagiri Y, Inoue M, Sawada N, Tsugane S: Occupational sitting time and risk of all-cause mortality among Japanese workers. Scand J Work Environ Health, 2015; 41: 519-528 [DOI] [PubMed] [Google Scholar]
- 11). Hamajima N: The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) to detect gene-environment interactions for cancer. Asian Pac J Cancer Prev, 2007; 8: 317-323 [PubMed] [Google Scholar]
- 12). Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P: International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc, 2003; 35: 1381-1395 [DOI] [PubMed] [Google Scholar]
- 13). Hara M, Higaki Y, Taguchi N, Shinchi K, Morita E, Naito M, Hamajima N, Takashima N, Suzuki S, Nakamura A, Ohnaka K, Uemura H, Nishida H, Hosono S, Mikami H, Kubo M, Tanaka H, Japan Multi-Institutional Collaborative Cohort Study G : Effect of the PPARG2 Pro12Ala polymorphism and clinical risk factors for diabetes mellitus on HbA1c in the Japanese general population. J Epidemiol, 2012; 22: 523-531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Hara M, Hachiya T, Sutoh Y, Matsuo K, Nishida Y, Shimanoe C, Tanaka K, Shimizu A, Ohnaka K, Kawaguchi T, Oze I, Matsuda F, Ito H, Kawai S, Hishida A, Okada R, Sasakabe T, Hirata A, Ibusuki R, Nindita Y, Furusyo N, Ikezaki H, Kuriyama N, Ozaki E, Mikami H, Nakamura Y, Suzuki S, Hosono A, Katsuura-Kamano S, Arisawa K, Kuriki K, Endoh K, Takashima N, Kadota A, Nakatochi M, Momozawa Y, Kubo M, Naito M, Wakai K: Genomewide Association Study of Leisure-Time Exercise Behavior in Japanese Adults. Med Sci Sports Exerc, 2018; 50: 2433-2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A, Lee IM: Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet, 2016; 388: 1302-1310 [DOI] [PubMed] [Google Scholar]
- 16). Patterson R, McNamara E, Tainio M, de Sa TH, Smith AD, Sharp SJ, Edwards P, Woodcock J, Brage S, Wijndaele K: Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol, 2018; 33: 811-829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Powell C, Herring MP, Dowd KP, Donnelly AE, Carson BP: The cross-sectional associations between objectively measured sedentary time and cardiometabolic health markers in adults - a systematic review with metaanalysis component. Obes Rev, 2018; 19: 381-395 [DOI] [PubMed] [Google Scholar]
- 18). Lim MS, Park B, Kong IG, Sim S, Kim SY, Kim JH, Choi HG: Leisure sedentary time is differentially associated with hypertension, diabetes mellitus, and hyperlipidemia depending on occupation. BMC Public Health, 2017; 17: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Lee PH, Wong FK: The association between time spent in sedentary behaviors and blood pressure: a systematic review and meta-analysis. Sports Med, 2015; 45: 867-880 [DOI] [PubMed] [Google Scholar]
- 20). Ford ES, Caspersen CJ: Sedentary behaviour and cardiovascular disease: a review of prospective studies. Int J Epidemiol, 2012; 41: 1338-1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Same RV, Feldman DI, Shah N, Martin SS, Al Rifai M, Blaha MJ, Graham G, Ahmed HM: Relationship Between Sedentary Behavior and Cardiovascular Risk. Curr Cardiol Rep, 2016; 18: 6. [DOI] [PubMed] [Google Scholar]
- 22). Olivecrona G: Role of lipoprotein lipase in lipid metabolism. Curr Opin Lipidol, 2016; 27: 233-241 [DOI] [PubMed] [Google Scholar]
- 23). Hamilton MT, Hamilton DG, Zderic TW: Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes, 2007; 56: 2655-2667 [DOI] [PubMed] [Google Scholar]
- 24). Bey L, Hamilton MT: Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol, 2003; 551: 673-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Igarashi Y, Nogami Y: Response of Lipids and Lipoproteins to Regular Aquatic Endurance Exercise: A Meta- Analysis of Randomized Controlled Trials. J Atheroscler Thromb, 2019; 26: 14-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Watanabe N, S SS, Shimada K, Lee IM, Gando Y, Momma H, Kawakami R, Miyachi M, Hagi Y, Kinugawa C, Okamoto T, Tsukamoto K, S NB: Relationship between Cardiorespiratory Fitness and Non-High-Density Lipoprotein Cholesterol: A Cohort Study. J Atheroscler Thromb, 2018; 25: 1196-1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Igarashi Y, Akazawa N, Maeda S: Effects of Aerobic Exercise Alone on Lipids in Healthy East Asians: A Systematic Review and Meta-Analysis. J Atheroscler Thromb, 2019; 26: 488-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S: The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res, 2014; 37: 253-390 [DOI] [PubMed] [Google Scholar]
- 29). Yasunaga A, Shibata A, Ishii K, Inoue S, Sugiyama T, Owen N, Oka K: Replacing sedentary time with physical activity: effects on health-related quality of life in older Japanese adults. Health Qual Life Outcomes, 2018; 16: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Wirth K, Klenk J, Brefka S, Dallmeier D, Faehling K, Roque IFM, Tully MA, Gine-Garriga M, Caserotti P, Salva A, Rothenbacher D, Denkinger M, Stubbs B: Biomarkers associated with sedentary behaviour in older adults: A systematic review. Ageing Res Rev, 2017; 35: 87-111 [DOI] [PubMed] [Google Scholar]
- 31). Bellettiere J, Winkler EAH, Chastin SFM, Kerr J, Owen N, Dunstan DW, Healy GN: Associations of sitting accumulation patterns with cardio-metabolic risk biomarkers in Australian adults. PLoS One, 2017; 12: e0180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Henson J, Yates T, Biddle SJ, Edwardson CL, Khunti K, Wilmot EG, Gray LJ, Gorely T, Nimmo MA, Davies MJ: Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia, 2013; 56: 1012-1020 [DOI] [PubMed] [Google Scholar]
- 33). Bauman A, Bull F, Chey T, Craig CL, Ainsworth BE, Sallis JF, Bowles HR, Hagstromer M, Sjostrom M, Pratt M: The International Prevalence Study on Physical Activity: results from 20 countries. Int J Behav Nutr Phys Act, 2009; 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Honda T, Chen S, Kishimoto H, Narazaki K, Kumagai S: Identifying associations between sedentary time and cardio-metabolic risk factors in working adults using objective and subjective measures: a cross-sectional analysis. BMC Public Health, 2014; 14: 1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Yamagishi K, Ikeda A, Iso H, Inoue M, Tsugane S: Self-reported stroke and myocardial infarction had adequate sensitivity in a population-based prospective study JPHC (Japan Public Health Center)-based Prospective Study. Journal of clinical epidemiology, 2009; 62: 667-673 [DOI] [PubMed] [Google Scholar]


