Abstract
Longitudinal in vivo imaging studies characterizing subarachnoid hemorrhage (SAH)-induced large artery vasospasm (LAV) in mice are lacking. We developed a SAH-scoring system to assess SAH severity in mice using micro CT and longitudinally analysed LAV by intravenous digital subtraction angiography (i.v. DSA). Thirty female C57Bl/6J-mice (7 sham, 23 SAH) were implanted with central venous ports for repetitive contrast agent administration. SAH was induced by filament perforation. LAV was assessed up to 14 days after induction of SAH by i.v. DSA. SAH-score and neuroscore showed a highly significant positive correlation (rsp = 0.803, p < 0.001). SAH-score and survival showed a negative significant correlation (rsp = −0.71, p < 0.001). LAV peaked between days 3–5 and normalized on days 7–15. Most severe LAV was observed in the internal carotid (Δmax = 30.5%, p < 0.001), anterior cerebral (Δmax = 21.2%, p = 0.014), middle cerebral (Δmax = 28.16%, p < 0.001) and basilar artery (Δmax = 23.49%, p < 0.001). Cerebral perfusion on day 5 correlated negatively with survival time (rPe = −0.54, p = 0.04). Arterial diameter of the left MCA correlated negatively with cerebral perfusion on day 3 (rPe = −0.72, p = 0.005). In addition, pseudoaneurysms arising from the filament perforation site were visualized in three mice using i.v. DSA. Thus, micro-CT and DSA are valuable tools to assess SAH severity and to longitudinally monitor LAV in living mice.
Keywords: Subarachnoid hemorrhage, cerebral vasospasm, longitudinal digital subtraction angiography, micro-CT, in vivo
Introduction
Subarachnoid hemorrhage (SAH) related to aneurysm rupture is associated with high morbidity and mortality with almost 15% of patients dying immediately after hemorrhage and about 30% of patients within the first 24 h.1,2 Approximately 40–70% of the surviving patients develop cerebral vasospasm within the first two weeks after SAH,3 and up to 30% of these patients have been described to suffer from vasospasm-related cerebral ischemia leading to permanent neurological deficits.
Numerous animal studies have been carried out to better understand the pathophysiology of SAH-related neurological deficits and vasospasm. Different sources indicate that delayed cerebral vasospasms are multifactorial events,4,5 in which factors such as cytokines, chemokines, leukocytes and platelet adhesion proteins play a crucial role.6,7 To further evaluate the pathophysiology of SAH and to identify novel therapeutic approaches to effectively treat patients suffering from cerebral vasospasm, mouse models are being used with increasing frequency.
The most frequently used techniques to induce SAH in mice are the filament perforation model,8,9 single or repeated injection of blood into the cisterna magna10 or into the prechiasmatic cistern11 or intracisternal vessel transection.12 To determine the degree of vasospasm, most studies use predefined time points after induction of SAH to sacrifice their groups of animals concurrently10,13,14 and quantify cerebral vasospasm post mortem either micro- or macroscopically after perfusion of the animals with pigments, casting agents or radio-opaque substances.10,13,15 These methods and the animals’ death have been discussed to influence research results due to artificial changes of the blood vessel diameter.15
In vivo high-resolution digital subtraction angiography (DSA)16 and cerebral computed tomography angiography (CTA) of the murine cerebral circulation17,18 have been suggested to be useful to monitor cerebral vasospasm longitudinally. Nonetheless, micro-CT has been solely used in animal studies to establish vascular casts and to analyse cerebrovascular anatomy.19,20 To our best knowledge, no studies assessed SAH-associated LAV longitudinally in living mice, although longitudinal measurement of vasospasm offers several advantages, such as (i) the distinct reduction of the number of animals required, (ii) an improvement of the quality of results (by reducing variance through intra-individual comparisons) and (iii) the possibility to observe the clinical outcome and development of vasospasm over time in the same animal.
In this study, SAH was induced in mice by filament perforation and the feasibility to longitudinally monitor LAV in mice using intravenous (i.v.) DSA was explored. In addition, we developed a micro-CT-based novel scoring system for SAH in mice in vivo. Finally, we investigated the correlation between the degree of vasospasm, SAH- and neuroscore and described the longitudinal perfusion characteristics.
Materials and methods
Animals
All experiments were carried out after receiving approval by the local governmental committee (Regierungspräsidium Karlsruhe, Germany; approval number: 35-9185.81/G-42/16). Institutional guidelines of the Medical Faculty Mannheim for animal welfare and experimental conduct were followed according to the European Communities Council Directive (86/609/EEC) and the experiments have been reported in compliance with the ARRIVE guidelines. Thirty female 8–12-weeks-old C57Bl/6J mice weighing 18–22 g were used. In order to avoid social stress, animals were kept in small groups and only female animals were used. All animals were kept in individually ventilated cages with controlled temperature (24°C ± 1°C), humidity (50% ± 2%), a lighting circle of 12h light/dark. All animals had ad libitum access to autoclaved tap water and a standard research rodent diet (ssniff Spezialdiäten GmbH, Germany).
Surgical procedures
Implantation of a vascular access mini port
The vascular access mini port (VAMP) was custom-made and implanted (V.W.) as previously described.21 Briefly, the animals were anesthetized by subcutaneous injection of a mixture of medetomidine (0.5 mg/kg), midazolam (5 mg/kg) and fentanyl (0.05 mg/kg) (MMF). Animals were positioned supine on a warming plate (Medax GmbH; Neumünster, Germany) at 37°C. After depilating (Pilca; Glaxo Smith Kline, Bühl) and disinfecting (Softasept; Braun, Melsungen) the neck, the right jugular vein was cranially exposed. A first ligature was made at the cranial section of the vein, and a second filament was positioned cranially of the junction of the cephalic vein. Then, a hole was cut between the two prepared filaments. The catheter was inserted up to the fixing ring and the slipknots were tightened. Finally, the skin was subcutaneously tunneled and the port reservoir of the VAMP was placed at the back of the animal. Postoperatively, all animals were antagonized by subcutaneous injection of a mixture of atipamezole (2.5 mg/kg), flumazenil (0.5 mg/kg) and naloxone (1.2 mg/kg). Throughout the experimental phase, the VAMP was flushed with heparinized solution (25 IU/ml) every other day.
Induction of SAH
The filament perforation model was performed (M.E.M.) as described before by Buhler et al.8,9 Briefly, the animals were anesthetized by subcutaneous injection of a mixture of medetomidine (0.5 mg/kg), midazolam (5 mg/kg) and fentanyl (0.05 mg/kg) (MMF). When sufficient anesthesia depth was achieved, animals were positioned supine on a warming plate at 37°C and fixed with tape. The left neck was depilated, disinfected and a midline incision was made. By separating the underlying subcutaneous tissue in a blunt fashion, the subjacent external, internal and common carotid arteries were exposed. After tracing the external carotid artery (ECA) in direction of the digastric muscle, the ECA was ligated and cut. The pterygopalatine artery (PPA) branching from the internal carotid artery (ICA) was exposed and ligated. Two micro clips were placed at the ICA and common carotid artery (CCA) in order to interrupt blood flow temporarily. An incision was made in the ECA and a blunted 6–0 monofilament thread (Prolene, Ethicon Johnson & Johnson, Belgium) was inserted. After transecting the ECA, the filament was carefully pushed forward into the ICA until the filament tip reached the clipped part of the ICA and was fixed by silk ligatures at the proximal insertion in the ECA stump. Next, the micro vascular clips were removed and the monofilament thread was advanced intracranially within the ICA until a slight resistance was felt. Directly before inducing SAH, 150 µl of pre-warmed contrast agent (Iomeprol; Imeron 400, Bracco Imaging Group, Germany) were administered via the VAMP to optimize visualization of SAH in micro-CT. Then, the filament was pushed another 1–2 mm further to induce vessel perforation. Next, the filament was retracted into the ECA stump, thereby allowing full reperfusion of cerebral vessels via the ICA and inducing SAH. Finally, the filament was withdrawn from the ECA and the ECA was ligated. The wound was closed with a simple interrupted suture (6–0 Vicryl, Ehticon, Norderstedt). Postoperatively, all animals were antagonized by subcutaneous injection of a mixture of atipamezole (2.5 mg/kg), flumazenil (0.5 mg/kg) and naloxone (1.2 mg/kg).
Postoperative monitoring and assessment of neuroscore
Body weight was determined daily as a sensitive indicator for their well-being. Likewise, neurological deficits were assessed on a daily basis using the previously described neuroscore.9 Mice without neurological deficits scored 0 points, while mice with a profound loss of neurological functions could reach up to 33 points. Animals that died immediately after induction of SAH received a score of 34. For post-operative pain management, all animals received carprofen (5 mg/kg) every day subcutaneously and metamizole (200 mg/kg) via drinking water.
Imaging procedures
Intravenous DSA
All animals underwent i.v. DSA directly after catheter implantation (day 0), as well as every other day after SAH.21,22 i.v. DSA was performed using an industrial combined planar X-ray and micro-CT-system (Y.Fox; Yxlon, Garbsen, Germany) equipped with an open multifocus X-ray tube and a 14-bit direct amorphous silicon flat panel detector (Varian PaxScan 2520 D/CL; Varian, Palo Alto, CA, USA). Anaesthetized (MMF) mice were positioned in a mouse cradle23 under the X-ray source. After gain-and-offset calibration, DSA was performed during i.v. infusion of 250 µl of a pre-warmed (37°C) contrast agent bolus (Iomeprol 400; infusion rate 3.4 ml/min) via the VAMP. Using the planar X-ray function, projections were acquired at 30 frames per second (fps) (tube voltage: 80 kV, tube current: 75 µA). The i.v. DSA sequences were recorded in an audio video interleave (AVI) file format.
Micro-CT imaging after induction of SAH
Five to 10 min after induction of SAH, a micro-CT was carried out (V.W.) (day 1) as described before17,23 using the following scan parameters: tube voltage 80 kV (current 75 µA), 360° rotation within 33 s scan time and continuous image acquisition at 30 fps resulting in 990 projections. Raw-data were reconstructed using a filtered back-projection algorithm applying the Shepp–Logan filter and a matrix of 512 × 512 × 512 voxels (Reconstruction Studio Software; TeraRecon). The created DICOM data were assessed using OsiriX imaging software (OsiriX v. 5.0.2, 64-bit; Pixmeo, SARL, Bernex, Switzerland).
SAH-score in mice
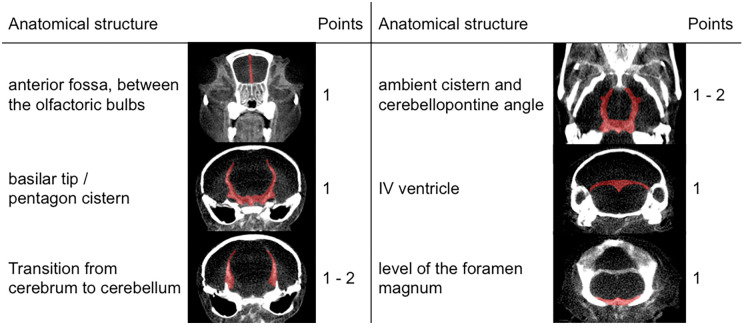
To develop a scoring system for SAH in mice, SAH was analysed on the micro-CT scans using OsiriX imaging software. This SAH-score comprises the presence or absence of subarachnoid blood in defined anatomical structures (Figure 1). Points were scored for blood in (a) the anterior fossa between the olfactoric bulbs (1 point), (b) the basilar tip/pentagon cistern (1 point), (c) the 4th ventricle (1 point), and (d) at the level of the foramen magnum (1 point). One or two points were given, if blood was visible in the ambient cistern/cerebellopontine angle (unilateral = 1 point; bilateral = 2 points) and at the transition from cerebrum to cerebellum adjacent to the lateral skull wall (unilateral = 1 point; bilateral = 2 points). The degree of SAH was finally stratified into four grades: 0 points represented grade 0; 1–3 points, grade 1; 4–6 points, grade 2; and 7–8 points, grade 3, respectively.
Figure 1.
Our proposed SAH scoring scheme similar to the well-established Fisher's scale in humans. Depending on the distribution of blood throughout the subarachnoid space, each animal is scored between 0 and 8 points. In some regions, bilateral appearance of hemorrhage is scored two points. Regions of interest were dyed red in the exemplary micro-CT scans. According to the sum of points, the SAH-score is stratified into four grades (grade 0 = 0 points, grade 1 = 1–3 points, grade 2 = 4–6 points, grade 3 = 7–8 points). We found the SAH-score to correlate strongly with the neuroscore and reduction of artery diameters.
Image processing of video files and artery diameter measurements
The recorded AVI files were imported using ImageJ (ImageJ 1.47v; Wayne Rasband, National Institutes of Health, USA) and decomposed into single projections. About six to eight single projections of the early arterial phase were then overlaid. The images were flattened and saved as TIF-format. A 27 G (0.4 mm) cannula placed directly under the skull of the mouse was used as a reference diameter.
Artery diameters were measured at the positions specified in Figure 2(a). In order to yield comparable results, each artery was measured at its proximal and distal end, with a distance of approximately 500 µm from the bifurcation. Additionally, a third point was measured in the middle section of all arteries.
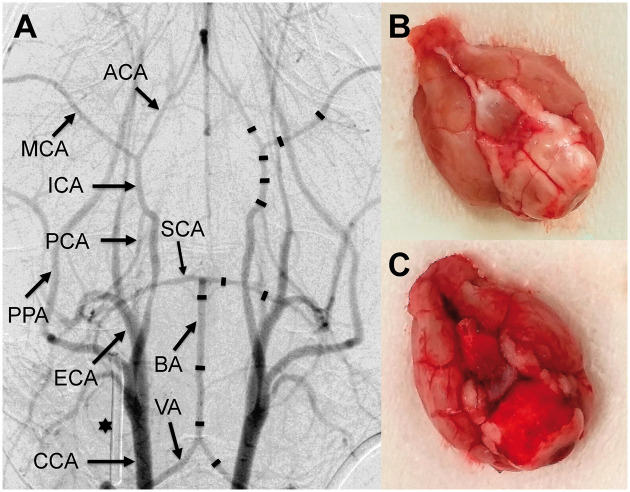
Figure 2.
i.v. DSA of the murine arterial cerebrovascular system (a). On the right side, the measurement points are indicated as black lines. On the left, vessel anatomy is explained: VA (vertebral artery), BA (basilar artery), SCA (superior cerebellar artery), CCA (common carotid artery), ECA (external carotid artery), ICA (internal carotid artery), PCA (posterior cerebral artery), MCA (middle cerebral artery), ACA (anterior cerebral artery), PPA (pterygopalatine artery); 27 G cannula (asterisk) used for calibration. Photograph of an explanted brain of a sham mouse without SAH (b) and a brain of a mouse which died immediately after induction of SAH due to severe SAH (c).
Semiquantitative perfusion determination
The AVI-Files were imported into ImageJ for further analyses. Small regions of interest (ROI) were drawn in both CCA and into the cerebral tissue supplied by the MCA to determine the arterial input function (AIF). Afterwards the images and ROI data were transferred to MATLAB (MATLAB, The MathWorks, Inc., USA) and processed by an in-house developed algorithm. In detail, mean signal intensity (SI)-time curves were extracted from each ROI and transformed into contrast agent concentration (C)-time curves by assuming a linear correlation of absorbance and concentration. The time points of 30% and 70% of the maximum concentration during the rise of the contrast agent concentration were identified and the duration was calculated in both, the tissue ROI and the artery ROI. The duration obtained from the tissue ROI was normalized to the duration obtained from the AIF by division, thus taking into account that different hemodynamic and technical conditions during application of the contrast agent existed.
Histological and immmunhistochemical analyses
Animals were sacrificed not later than 14 days post induction of SAH (for a timeline see Figure 3(e)) by inhalation of CO2 and their brains were extracted; 20 µm coronal cryostat sections at the level of the striatum and the hippocampus were used for hematoxylin and eosin (H&E) staining and immunohistochemical analysis. Immunhistochemistry with antibodies against Iba1, CD68 and MAP2, respectively, was used to detect potential subtle ischemic changes as indicated by activation or transformation of microglia (Iba1 and CD68) or loss of MAP2. Analysis was performed with rabbit polyclonal antibody against Iba1 (1:1000; WAKO Chemicals Europe, Neuss, Germany), rabbit polyclonal antiserum against MAP2 (1:500; Synaptic Systems, Goettingen, Germany) and rat monoclonal antibody against CD68 (1:2000; Abcam, Cambridge, United Kingdom). Brains of the animals that developed pseudoaneurysms were fixed with paraformaldehyd and embedded in paraffin. Serial sections through the pseudoaneurysm were produced and stained with H&E and elastica van gieson. Histological analyses were performed by a neuropathologist (C.S.) who was blinded to the experimental groups.
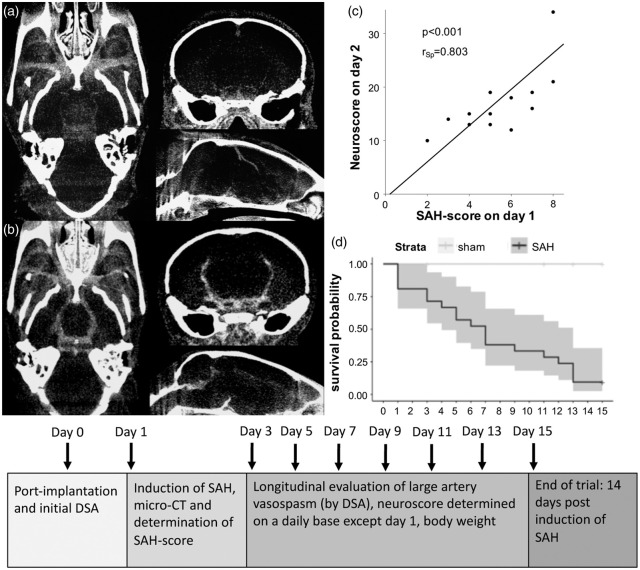
Figure 3.
Micro-CT of a sham operated mouse (a) without SAH (SAH grade 0) but contrast enhanced vessels, and of a mouse with pronounced SAH (b) after filament perforation (SAH grade 3). Highest neuroscore (c) within the first two days after SAH correlated strongly with the SAH score (r = 0.803, p < 0.001). SAH score was also negatively associated with survival probability in the following days (p = 0.0032). Altogether, 22% (n = 5) of mice subjected to SAH died immediately after induction of SAH and loss rate during the first three days after SAH was 30% (n = 7). Only two animals reached up to day 15, whereas all sham-operated mice survived during the observation period (d). To better understand the experimental setup, a timeline gives an overview (e).
Statistical analyses
Only nonparametric statistical methods were used because of the small number of animals per group (nsham = 7 vs. nSAH = 23). It was not possible to properly plan and statistically power the study as no data exist regarding longitudinal imaging of cerebral artery diameter changes in mice. Analyses were descriptive and performed (M.E.M.) within the R statistical programming environment (v.3.5.2, R Core Team 2018, Vienna Austria; RStudio IDE v.1.1.463). The difference of artery diameter (measured in percentage points) compared to the initial baseline i.v. DSA was chosen as the primary outcome parameter. Secondary outcomes were neuro-, SAH-score and weight. Additionally, semiquantitative perfusion parameters derived from DSA were also investigated. To properly model the longitudinal effect of SAH and time on cerebral vasospasm, we applied nonparametric one-factorial longitudinal data analysis design (F1-LD-F1) using the nparLD package.24 Here, the respective outcomes (including all measured artery calibres, weight, neuroscore and perfusion) between the groups of SAH vs. sham mice were compared during repeated measurements for days 0–15. F1-LD-F1 models were fitted for the time range of maximal difference (depending on the respective outcome on days 3–5) between SAH and sham groups. P-values <0.05 were considered significant. In case of multiple testing, the alpha-level was adjusted using the Holm–Bonferroni correction.
Results
SAH-score, neuroscore and survival
The SAH-score of sham (Figure 3(a)) and of SAH-animals (Figure 3(b)) was assessed using micro-CT. All animals in the sham group scored 0 points (SAH grade 0, n = 6), whereas mice in the SAH group had a significant higher median score of 6 points (LQ-UQ: 4–7, n = 21; W = 9, pWMW<0.001), which resulted in the following distribution of SAH-grades: grade 0: n = 3 (14.28%), grade 1: n = 2 (9.52%), grade 2: n = 8 (38.1%) and grade 3: n = 8 (38.1%). Notably, all mice (n = 4) with a grade 3 scoring and a maximum of 8 points died within first 15 min after induction of SAH.
SAH-score (overall median = 5, LQ-UQ = 0–7, range = 0–8, n = 27) and neuroscore one day after induction of SAH (median = 8, LQ-UQ = 0–12, range = 0-34, n = 27) showed a highly significant positive association (rsp = 0.803, p < 0.001; Figure 3(c)). In addition, SAH-score and survival showed a negative significant association (rsp = −0.71, p < 0.001).
Neuroscore was highest within the first three days after SAH ranging between 8 and 21 points and declined during the course of the study. All sham-operated mice survived, while 91% (n = 21) of mice subjected to SAH died during the two-week observation period and only 9% (n = 2) survived (median survival time t = 7 days, 95% CI: 4–13 days, nevents/deaths = 19, compared to the sham group (pLogRank = 0.0016, n = 27), as shown in the Kaplan–Meier plot (Figure 3(d)). The mortality rate was 22% shortly after induction of SAH and about 30% of the animals died within the first three days. In three animals, we were not able to determine the SAH-score. In one of these animals, the VAMP was occluded at this particular time. In the remaining two animals, both with suspected filament perforation but without any contrast agent enhancement in micro-CT despite VAMP patency, however, vasospasm and neurological deficits were comparable to all other animals with confirmed SAH, and one of these two animals developed a dissecting pseudoaneurysm of the ICA (Figure 4(a)).
Figure 4.
Intravenous DSA of three different mice (a, b, c) which developed pseudoaneurysm after vessel perforation (black arrows). Pseudoaneurysms arose from the proximal intracranial ICA (n = 2; a, b) and the proximal ACA (n = 1; c). Both ICA aneurysms showed a faster growth rate than of the ACA over several days with sudden death of both animals probably due to aneurysm rupture.
Longitudinal monitoring of artery diameter by intravenous DSA
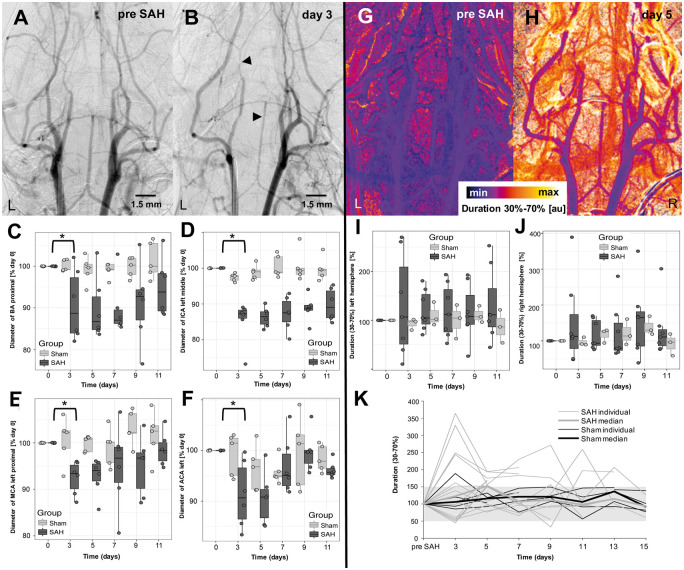
Overall, n = 22 (16 SAH, 6 sham) mice were available for longitudinal follow-up studies for a median of 11 days (LQ-UQ = 7–13, range = 1–15). A reduction of artery diameter was detected in all animals with filament perforation (n = 16), including the aforementioned three animals without proven SAH in micro-CT. Non-parametric modeling showed highly significant main effects of SAH on artery diameter reduction for all measured arteries. This effect was significant regardless of the effects of time and the interaction between SAH and time on reduction of artery diameter (for details see Table 1), meaning that the main effect of SAH could be interpreted also independently from time during the modeled period. Reduction of artery diameter affected all artery sections on both sides of the circle of Willis with a trend towards the left side (i.e. side ipsilateral to the perforation). Maximum artery diameter reduction occurred three to five days after induction of SAH, with a decrease of mean arterial vessel diameter of (i) 12.09% on day 3 and 13.17% on day 5 in the proximal BA, (ii) 14.52% on day 3 and 17.04% on day 5 in the left middle ICA, (iii) 9.69% on day 3 and 9.62% on day 5 in the left proximal MCA and (iv) 9.22% on day 3 and 12.18% on day 5 in the left ACA (Figure 5 and Table 1). Most severe diameter constriction occurred at the (i) left middle ICA (Δmax = 30.5%, p < 0.001), (ii) the left ACA (Δmax = 21.2%, p = 0.014), (iii) the left proximal MCA (Δmax = 28.16%, p < 0.001) and (iv) the proximal BA (Δmax = 23.49%, p < 0.001). Reduction of artery diameter normalized between days 7 and 15 and showed an intra-individual progress (Figure 5(c) to (f)).
Table 1.
Summary table of statistics of one-factorial longitudinal data analyses (F1-LD-F1) on reduction of artery diameters and on secondary outcomes between day 0 and day 5.
| Primary and secondary | Group (SAH vs. SHAM) |
Time (days) |
Interaction (group vs. time) |
p* (Bonf.) | |||
|---|---|---|---|---|---|---|---|
| Outcome | Stat. | p-value | Stat. | p-value | Stat. | p-value | |
| ACA_L | 6.04 | 0.014 | 8.78 | 1.91e-4 | 2.94 | 0.0547 | 0.00263 |
| ICA_L_M | 35.85 | 2.13e-9 | 69.30 | 2.44e-3 | 3.85 | 1.46e-6 | 0.00263 |
| ICA_L_D | 9.77 | 1.77e-3 | 28.38 | 8.47e-12 | 6.62 | 2.13e-3 | 0.00263 |
| MCA_L_P | 22.75 | 1.85e-6 | 4.16 | 0.0262 | 5.92 | 6.61e-3 | 0.00263 |
| BA_P | 19.94 | 8.00e-6 | 6.42 | 4.73e-3 | 3.85 | 0.0341 | 0.00263 |
| SAH-score | 71.26 | 3.12e-17 | 71.26 | 3.12e-17 | 71.26 | 3.12e-17 | 0.00263 |
| Neuroscore | 141.44 | 1.29e-32 | 95.61 | 4.55e-30 | 95.61 | 4.55e-30 | 0.00263 |
| Weight | 1.63 | 0.201 | 17.51 | 1.77e-6 | 15.07 | 1.01e-5 | 0.00263 |
p*: p-star level of significance according to Bonferroni correction by repeated testing (16 reduction of artery diameter + 3 s. outcome), p*=0.05/19=0.00263.
ACA_L: anterior cerebral artery left; ICA_L_M/D: internal cerebral artery left middle/distal; MCA_L_P: middle cerebral artery left proximal; BA_P: basilar artery proximal; Stat.: test statistics of F1-LD-F1 using robust nonparametric ANOVA-type tests; e-6: 10 to the power of the respective negative number.
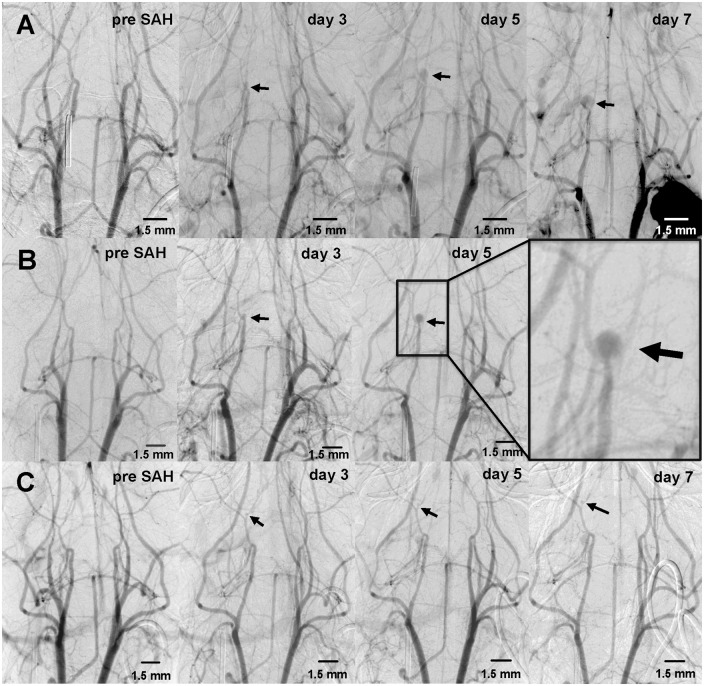
Figure 5.
DSA was performed prior (a) the induction of SAH (day 0) and afterwards every other day beginning two days after induction of SAH. In all mice, large artery vasospasm peaked on days 3 to 5 in the proximal BA and left ICA (b, black arrowheads). Vasospasm normalized individually after approximately two weeks (c–f). Changes in artery diameter were also reflected in the semiquantitative perfusion evaluation. Individual pixel evaluation of the 30%–70%-duration before (g) and after induction of SAH (h) showed an increase from baseline to day 3. Furthermore, perfusion values showed bimodal distributions with large variance including outliers (i–j). Largest perfusion deficits occurred around days 3–5 and days 9–11. Individual perfusion curves showed that only some of the SAH animals developed relevant perfusion delays, whereas the sham animals all ranged around the initial values (k).
Comparing the sham and SAH group on a day-by-day basis showed that mean arterial diameters of the proximal BA, the left ICA, MCA, and ACA were significantly (all p < 0.05) smaller on days 3 and 5 in the SAH group. Furthermore, the left MCA of SAH animals showed significantly smaller values also on days 9 and 11 (both p < 0.05) in comparison to the MCA of sham mice. Arterial diameter reduction of the left ICA (p = 0.057, rPe = 0.519) and proximal BA (p = 0.077, rPe = 0.47) determined a weak positive correlation with survival time. As for the secondary outcomes, neuro- and SAH-scores were significantly higher in SAH mice than in sham animals (Table 1), and they were significantly influenced by- and interacted with time (pNeuroscore<0.001; pTime = pInteraction<0.001) and (pSAHscore = pTime = pInteraction<0.001), respectively, but no significant correlation between SAH-score and arterial diameter was detected.
Semiquantitative perfusion analyses
Seven SAH and three sham mice survived at least 11 days and were eligible for robust non-parametric longitudinal analyses. For these animals, perfusion values for all consecutive time points were generated from DSA images as described in the methods section. Perfusion values derived from the timepoints of 30% and 70% of the maximum contrast agent concentration (duration values) showed bimodal distributions with some variance including outliers, especially for the SAH group (Figure 5(g) to (j)). Largest perfusion deficits (i.e. delays in transit time) were observed around days 3–5 and days 9–11, which was more pronounced for the left hemisphere (i.e. ipsilateral to the site of vessel perforation) than for the contralateral (right) side. Up to day 5, there was a significant time-effect with delayed ipsilateral cerebral perfusion times in mice of the SAH group compared to sham animals (pTime = 0.0088), while the group effect (pGroup = 0.67) and interaction (pInteraction = 0.89) were not relevant. This time-effect, however, diminished during longitudinal follow-up until day 11 (pTime = 0.75). Interestingly, the contralateral hemisphere showed no relevant difference between sham and SAH group (pGroup = 0.95, pTime = 0.30, pInteraction = 0.42) within any time range up to 11 days, which corresponds with the angiographically observed differences in artery diameter. Furthermore, a statistically significant negative correlation between perfusion values on day 5 and survival time (p = 0.04, rPe = −0.54), but no relevant correlation between perfusion and neuroscore as well as perfusion and SAH severity was observed. However, arterial diameter reduction of the left MCA showed a significant negative correlation with perfusion values on day 3 (rPe = −0.72, p = 0.005). Examining individual perfusion curves, only some of the SAH animals developed relevant perfusion delays, whereas the sham animals all ranged around the initial values (Figure 5(k)).
Pseudoaneurysms
Filament perforation-associated pseudoaneurysms arising from the proximal intracranial ICA were detected in two, and from the proximal ACA in one animal (Figure 4). The latter was visible 2 days after filament-perforation (Figure 4(c)). It showed a slow growth rate (9% from days 3–5, 14% from days 5–7 and 1% from days 7–9). This animal had to be taken from trial on day 9 due to a combination of weight loss and an increased neuroscore. The two ICA pseudoaneurysms demonstrated considerably higher growth rates of 49% and 95% between day 3 and day 5 (Figure 4(a) and (b)), and of 43% between days 5 and 7 (Figure 4(a)). Both mice died over night and showed excessive intracranial blood clots at autopsy.
Histology and immunhistochemistry
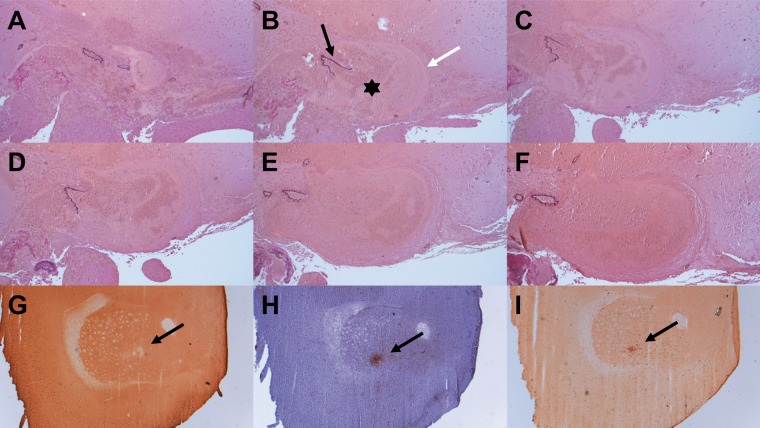
For histological analyses, 26 out of 30 mice were available (nsham = 6; nSAH = 20). ICA-pseudoaneurysms observed in i.v. DSA were clearly detectable in histological slices (Figure 6(a) to (f)). One animal presented a small area of basal necrotic brain parenchyma, which we believed to be due to filament perforation. Histology and immunohistochemistry revealed small infarctions of the striatum in n = 2 mice (Figure 6(g) to (i)), both with SAH-grade 2 and a high neuroscore (median = 12.5/9.8) over the complete test duration, compared to other mice. Two animals from the SAH group presented large hemispheric infarcts, both taken out of trial ahead of time (one on day 2, the other on day 3), due to a profound loss of neurological functions (neuroscore of 19 on day 2, 16 on day 3, respectively) and weight loss. In one case, the infarction might be caused by a long operation time while trying to induce SAH, whereby the filament remains too long into the vessel. In four animals, which died immediately after induction of SAH and reached the maximum SAH-score in CT, a considerable amount of blood was detected on the basal brain surface in histological analyses. Likewise, two of these animals showed severe intraventricular hemorrhage (including the lateral 3rd, and 4th ventricle), which was also visible in micro-CT. Furthermore, one mouse with a pseudoaneursym presented intraventricular hemorrhage in CT and histology.
Figure 6.
Histological analysis confirmed pseudoaneurysm observed in DSA (a–f, elastica van gieson, 100× magnification): a round structure, filled with an appreciable amount of erythrocytes (b – asterisk), surrounded by a fibrinogen coat (b – white arrow) and in some sections in contact with a sustentative vessel (b – black arrow). Initially, the nearby vessel is closed (a), afterwards the ruptured vessel section is visible (b–e) and at the last slice, the vessel is intact again (f). Additionally, histology showed smaller infarctions of the striatum after staining with MAP2 (g; black arrow, 25× magnification). Microglia and macrophages were found in the CD68-staining (i; black arrow, 25× magnification) and microglia in Iba1-staining (h; black arrow, 25× magnification).
Discussion
In this study, we provide for the first time a score to quantify the degree of SAH in a mouse filament perforation model, as well as a detailed longitudinal characterization of SAH-induced artery diameter changes in living mice using micro-CT and DSA. We observed an association between SAH severity in mice with survival and with reduction of artery diameter and validated it against well-established functional outcome tests, such as the neuroscore. Furthermore, we used robust non-parametric statistical frameworks to properly quantify the effect of SAH and time on diameter reductions and on perfusion curves. Additionally, this study is the first to visualize growing pseudoaneurysms at the site of filament perforation in mice. Thus, this work provides valuable insights into the longitudinal characteristics of SAH-induced artery diameter changes in living mice and emphasizes the key differences between human and murine pathophysiology, which is of high relevance for subsequent animal studies particularly for research on new therapeutic strategies.
Cerebral vasospasm, among others, has been recognized as a key complication of SAH.10 Despite intensive research, sufficient therapy for delayed cerebral vasospasm after SAH is not available in all cases. Therefore, ongoing research aims at gaining further insights into the pathomechanisms of SAH-associated vasospasm and to evaluate novel drugs for its effective treatment. Although we tend to interpret the reduction in artery diameter observed in our study as large artery vasospasm (LAV), we cannot definitely proof this as no dynamic measurements during changes in blood pressure or neuronal activation measurements were performed. Nevertheless, longitudinal in vivo quantification of cerebral vasospasm in mice is of high relevance for subsequent animal studies including the investigation of new therapeutic strategies. Former studies mostly used either male or both sexes to study SAH-related effects including cerebral vasospasm. In the underlying study, however, only female mice were used in order to reduce social stress while keeping the animals in groups during the observation period. We are aware that in rats gender influences regarding SAH have been reported. More exactly, SAH has been reported to be associated with greater bleeds and early brain injury in male animals.25 Friedrich et al. also observed an increased 24 h mortality in male rats. However, we did not find any corresponding literature describing similar gender differences for mice.
Until now, only a few studies performed DSA in mice in vivo16,26 and none of these studies used DSA to assess SAH-associated vasospasm. Previous studies described delayed cerebral vasospasm ex vivo performing histological analyses on fixed tissue27,28 or by using casting techniques.15 Besides examination of histological slices from fixed tissue, in some studies animals were sacrificed by perfusion with ink or opacifying compounds at pre-defined endpoints at, e.g. 12, 24 or 48 h after induction of SAH and the whole brains were used for evaluation of artery diameters. These methods, however, may have the disadvantage that vasospasm in some vascular segments may be missed, because artery diameter may only be evaluated at certain predefined anatomical positions. Additionally, these techniques are highly dependent on the person executing them. Friedrich et al.7 accomplished intravital visualization of microvasospasm by using in vivo fluorescence microscopy in a cranial window model. Whereas this method is restricted to the investigation of superficial arteries, our method (with the currently available flat panel detector technology) is restricted to the visualization of the larger arteries of the skull base. In their study, Friedrich et al. analysed microvasospasm 3 h, 6 h and 72 h after induction of SAH and graded vasospasm according to the percentage of vessel constriction. They observed a distinct reduction of vasospasm from 3 h to 6 h and finally to 72 h after induction of SAH. The degree of vasospasm observed by Friedrich et al. after 72 h is roughly comparable to our results on day 3 (see also supplemental material Figure 7).
Whereas in several studies detailed histological analyses of the brain were performed to investigate the effect of SAH, we did not initially plan to perform histological analyses in the underlying study. Detection of the aneurysms in mice, however, motivated us to perform some histological analyses, mainly to verify the pseudoaneurysms. A detailed comparison of DSA at different time points with histology would have led to a significant increase of the limited number of mice used in this study.
We considered it to be of interest to validate the successful induction of SAH. For this purpose, we used the built-in computed tomographic function of the industrial X-ray inspection system used for DSA. Unfortunately, in our pilot experiments, SAH was barely visible due to the well-known insufficient signal-to-noise ratio (SNR) of (older) flat-panel detectors. We therefore decided to apply contrast agent just before induction of SAH, which enabled us to clearly identify SAH in CT and to establish the described grading system (i.e. the SAH-score). SAH-score was not only significantly associated with the neurological outcome, but also an independent positive predictor for worse overall survival – similar to the Fisher scale30 used to classify severity of SAH in humans. The combination of the SAH-score and established neurological scoring systems allowed statistical stratification of SAH severity in our study, hereby allowing better comparison of new treatment approaches that require reproducible and comparable SAH severity.
A limitation of our study is that i.v. DSA was only applied as a 2D-imaging method (i.e. dorso-ventral beam path) and thus vasospasm could be visualized only in a single plane. It would have been possible to add another i.v. DSA run in a second projection (e.g. latero-lateral beam path), but this would have required another bolus of contrast agent within a short time resulting in a volume overload and cardiac stress for the animals. Notably i.v. DSA images have limited resolution (13 µm pixel size), which allows only sharp delineation of larger, proximal arteries. Imaging of smaller, more peripherally located arteries, however, would have been desirable. X-ray devices comprising the latest detector technology might offer higher resolutions and optimized SNR, hereby leading to an increased image quality. Motion artifacts during imaging also need to be controlled, as already minor movements of the mouse (e. g. deep breathing) may cause significant artifacts at high spatial resolutions. Therefore, every mouse was positioned in a mouse cradle and additionally fixed with ear- and a tooth bar in order to minimize breathing artifacts.23
The decision to assess cerebral perfusion in mice in 2D i.v.-DSA-datasets was made at a relatively late time point in this study, as the initial goal was to measure LAV. Due to the retrospective nature of the perfusion analyses, data in some cases were insufficient as contrast agent boli were too long (which resulted in saturation effects) or measurements were too short to analyse the area under the curve. Although perfusion measurements using 2D-datasets might be less sensitive compared to using 3D-datasets (like e.g. in clinical CT perfusion in patients), we managed to find some relevant correlations. We however have to confess that the 2D-perfusion analyses as performed in our study were quite difficult to evaluate (in contrast to evaluation of vessel diameter). Prospective measurements with shorter boli and longer onset- and offset-curves would be helpful in this context.
Another problem that we encountered was the occlusion of the vascular access port, which in some cases happened despite frequent flushing with heparinized saline solution. Several studies discussed different ways on how to resolve this issue.21 Repetitive tail vein injections, however, cannot be considered an appropriate alternative here as (i) they may cause chronic tail injury and necrosis and (ii) the bolus of contrast agent is insufficient for cerebral i.v. DSA (although it has been found to be sufficient for cerebral CTA in mice17). Alternatively, magnetic resonance angiography (MRA) has been used before to image murine cerebral arteries at 9.4 T. However, even using a high resolution gradient system combined with a cryo-coil to optimize image resolution and quality, did not yield sufficient image quality for grading of cerebral vasospasm in mice.16
In the present study, SAH was induced by filament perforation. Although methods like single or double injection of blood into the cisterna magna10 or the prechiasmatic cistern11 have been discussed to provide better control over the amount of SAH and to yield stronger vasospasm,31 perforation of an intracranial vessel using a filament has been thought to come closest to acute cerebral aneurysm rupture. Therefore, the filament perforation model is most commonly used to induce SAH in mice14 and studies using this method demonstrated neurological dysfunctions,32 delayed cerebral vasospasm,13,18 brain edema formation and a clinically relevant mortality of about 30% in the first three days,32 which is comparable to our results. Similarly, Scholler et al.29 reported mortality rates of 60% for wild-type and 82% for B1R−/− mice seven days after filament perforation. In our study, mice reached a mortality rate of 91% over the entire observation period of two weeks after induction of SAH. Only two animals (9%) subjected to SAH survived until day 15, which was our defined end of trial. All other animals had to be sacrificed if the neuroscore was too high or a relevant weight loss was observed (which is in accordance with our local animal protection laws). Mortality rate after three days reached 30%, and was 61% after seven days in our study, which thus is comparable with the other studies.
Other methods lack vessel rupture and/or endothelial damage, which are also important factors for the pathology of SAH.5 Additionally, for the first time, pseudoaneurysms of increasing size were documented in three mice, which most probably would not have been possible by most other methods. Animals with fast growing ICA-pseudoaneurysms died abruptly on days 6 and 8 most likely because of spontaneous aneurysm rupture. This assumption is supported by the fact, that in one of the animals a large amount of blood was found within the ventricular system at histological analyses, but not in CT directly after induction of SAH.
Combination of the SAH-score and established neurological scoring systems allows statistical stratification of SAH severity and can support correct the comparison of new treatment approaches that require reproducible and comparable SAH severity.
The present study thus demonstrates that longitudinal imaging and quantification of LAV in animals as small as mice are feasible, hereby establishing a novel protocol to monitor treatment effects in a frequently used animal model. We expanded the use of X-ray-based imaging methods and established a micro-CT-based grading system to analyse the severity of SAH in mice, which we found to be significantly associated with neuroscore, the extent of cerebral vasospasm and an independent predictor of overall survival. In addition, this study is the first to visualize growing pseudoaneurysms in mice at the site of filament perforation. The described methods hold promise to improve further longitudinal investigations on SAH-associated complications and LAV in living mice.
Supplemental Material
Supplemental material, JCB887052 Supplemetal Material for Longitudinal imaging and evaluation of SAH-associated cerebral large artery vasospasm in mice using micro-CT and angiography by Vanessa Weyer, Máté E Maros, Andrea Kronfeld, Stefanie Kirschner, Christoph Groden, Clemens Sommer, Yasemin Tanyildizi, Martin Kramer and Marc A Brockmann in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The acquisition of the micro-CT (Yxlon Y.Fox) was funded by the Federal Ministry of Education and Research and the Land Baden-Württemberg (HBFG Grant#125-648).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
VW and MEM carried out the experiments, while VW generated data and MEM additionally performed the statistical analyses. VW, MEM and MB wrote the manuscript. AK evaluated semiquantitative perfusion and CS performed histological analyses. SK, CG and YT advised the study. MK supervised the experimental aspects of the work. MB designed the experiments and supervised the study. All authors critically reviewed the manuscript and approved the final version.
ORCID iD
Máté E Maros https://orcid.org/0000-0002-1589-8699
Supplemental material
Supplemental material for this article is available online.
References
- 1.Azurmendi L, Degos V, Tiberti N, et al. Neopterin plasma concentrations in patients with aneurysmal subarachnoid hemorrhage: correlation with infection and long-term outcome. J Neurosurg 2016; 124: 1287–1299. [DOI] [PubMed] [Google Scholar]
- 2.Etminan N, Macdonald RL.Management of aneurysmal subarachnoid hemorrhage. Handb Clin Neurol 2017; 140: 195–228. [DOI] [PubMed] [Google Scholar]
- 3.Guvenc Tuna B, Lachkar N, de Vos J, et al. Cerebral artery remodeling in rodent models of subarachnoid hemorrhage. J Vasc Res 2015; 52: 103–115. [DOI] [PubMed] [Google Scholar]
- 4.Muroi C, Seule M, Mishima K, et al. Novel treatments for vasospasm after subarachnoid hemorrhage. Curr Opin Crit Care 2012; 18: 119–126. [DOI] [PubMed] [Google Scholar]
- 5.Vergouwen MD, Ilodigwe D, Macdonald RL.Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke 2011; 42: 924–929. [DOI] [PubMed] [Google Scholar]
- 6.Chaichana KL, Pradilla G, Huang J, et al. Role of inflammation (leukocyte-endothelial cell interactions) in vasospasm after subarachnoid hemorrhage. World Neurosurg 2010; 73: 22–41. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich B, Muller F, Feiler S, et al. Experimental subarachnoid hemorrhage causes early and long-lasting microarterial constriction and microthrombosis: an in-vivo microscopy study. J Cereb Blood Flow Metab 2012; 32: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhler D, Schuller K, Plesnila N.Protocol for the induction of subarachnoid hemorrhage in mice by perforation of the Circle of Willis with an endovascular filament. Transl Stroke Res 2014; 5: 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feiler S, Friedrich B, Scholler K, et al. Standardized induction of subarachnoid hemorrhage in mice by intracranial pressure monitoring. J Neurosci Methods 2010; 190: 164–170. [DOI] [PubMed] [Google Scholar]
- 10.Lin CL, Calisaneller T, Ukita N, et al. A murine model of subarachnoid hemorrhage-induced cerebral vasospasm. J Neurosci Methods 2003; 123: 89–97. [DOI] [PubMed] [Google Scholar]
- 11.Sabri M, Jeon H, Ai J, et al. Anterior circulation mouse model of subarachnoid hemorrhage. Brain Res 2009; 1295: 179–185. [DOI] [PubMed] [Google Scholar]
- 12.Altay T, Smithason S, Volokh N, et al. A novel method for subarachnoid hemorrhage to induce vasospasm in mice. J Neurosci Methods 2009; 183: 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamii H, Kato I, Kinouchi H, et al. Amelioration of vasospasm after subarachnoid hemorrhage in transgenic mice overexpressing CuZn-superoxide dismutase. Stroke 1999; 30: 867–871; discussion 872. [DOI] [PubMed] [Google Scholar]
- 14.Muroi C, Fujioka M, Marbacher S, et al. Mouse model of subarachnoid hemorrhage: technical note on the filament perforation model. Acta Neurochir Suppl 2015; 120: 315–320. [DOI] [PubMed] [Google Scholar]
- 15.Neulen A, Pantel T, Kosterhon M, et al. A segmentation-based volumetric approach to localize and quantify cerebral vasospasm based on tomographic imaging data. PLoS One 2017; 12: e0172010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueiredo G, Brockmann C, Boll H, et al. Comparison of digital subtraction angiography, micro-computed tomography angiography and magnetic resonance angiography in the assessment of the cerebrovascular system in live mice. Clin Neuroradiol 2012; 22: 21–28. [DOI] [PubMed] [Google Scholar]
- 17.Schambach SJ, Bag S, Steil V, et al. Ultrafast high-resolution in vivo volume-CTA of mice cerebral vessels. Stroke 2009; 40: 1444–1450. [DOI] [PubMed] [Google Scholar]
- 18.Parra A, McGirt MJ, Sheng H, et al. Mouse model of subarachnoid hemorrhage associated cerebral vasospasm: methodological analysis. Neurol Res 2002; 24: 510–516. [DOI] [PubMed] [Google Scholar]
- 19.Ghanavati S, Lerch JP, Sled JG.Automatic anatomical labeling of the complete cerebral vasculature in mouse models. Neuroimage 2014; 95: 117–128. [DOI] [PubMed] [Google Scholar]
- 20.Xie B, Miao P, Sun Y, et al. Micro-computed tomography for hemorrhage disruption of mouse brain vasculature. Transl Stroke Res 2012; 3: 174–179. [DOI] [PubMed] [Google Scholar]
- 21.Fiebig T, Figueiredo G, Boll H, et al. A low cost metal-free vascular access mini-port for artifact free imaging and repeated injections in mice. PLoS One 2013; 8: e65939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueiredo G, Fiebig T, Kirschner S, et al. Minimally invasive monitoring of chronic central venous catheter patency in mice using digital subtraction angiography (DSA). PLoS One 2015; 10: e0130661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schambach SJ, Bag S, Groden C, et al. Vascular imaging in small rodents using micro-CT. Methods 2010; 50: 26–35. [DOI] [PubMed] [Google Scholar]
- 24.Forster A, Bohme J, Maros ME, et al. Longitudinal MRI findings in patients with newly diagnosed glioblastoma after intraoperative radiotherapy. J Neuroradiol. Epub ahead of print 16 January 2019. DOI: 10.1016/j.neurad.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich V, Bederson JB, Sehba FA.Gender influences the initial impact of subarachnoid hemorrhage: an experimental investigation. PLoS One 2013; 8: e80101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidoguchi K, Tamaki M, Mizobe T, et al. In vivo X-ray angiography in the mouse brain using synchrotron radiation. Stroke 2006; 37: 1856–1861. [DOI] [PubMed] [Google Scholar]
- 27.Sehba FA, Mostafa G, Friedrich V, Jr., et al. Acute microvascular platelet aggregation after subarachnoid hemorrhage. J Neurosurg 2005; 102: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 28.Muroi C, Fujioka M, Okuchi K, et al. Filament perforation model for mouse subarachnoid hemorrhage: surgical-technical considerations. Br J Neurosurg 2014; 28: 722–732. [DOI] [PubMed] [Google Scholar]
- 29.Scholler K, Feiler S, Anetsberger S, et al. Contribution of bradykinin receptors to the development of secondary brain damage after experimental subarachnoid hemorrhage. Neurosurgery 2011; 68: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 30.Fisher CM, Kistler JP, Davis JM.Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980; 6: 1–9. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Zhu Y, Zhang Y, et al. Ultrasound guided double injection of blood into cisterna magna: a rabbit model for treatment of cerebral vasospasm. Biomed Eng Online 2016; 15: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J, Wang H, Sheng H, et al. A novel apoE-derived therapeutic reduces vasospasm and improves outcome in a murine model of subarachnoid hemorrhage. Neurocrit Care 2006; 4: 25–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JCB887052 Supplemetal Material for Longitudinal imaging and evaluation of SAH-associated cerebral large artery vasospasm in mice using micro-CT and angiography by Vanessa Weyer, Máté E Maros, Andrea Kronfeld, Stefanie Kirschner, Christoph Groden, Clemens Sommer, Yasemin Tanyildizi, Martin Kramer and Marc A Brockmann in Journal of Cerebral Blood Flow & Metabolism