Abstract
Objective:
Dysglycemia is prevalent in cystic fibrosis (CF) but screening with annual oral glucose tolerance tests (OGTT) can be burdensome. We investigated alternate glycemic markers—hemoglobin A1c (HbA1c), 1,5-anhydroglucitol (1,5AG), fructosamine (FA), and glycated albumin (GA)—as screening tests for CF-related diabetes (CFRD) and pre-diabetes (CFPD) in youth with CF as defined by the gold-standard OGTT 2-hour glucose (2hG).
Methods:
Youth 10 to 18 years with CF had a 1,5AG, FA, GA, HbA1c, and 2-hour OGTT collected. Correlations between all glycemic markers and 2hG were evaluated. Area under the receiver operative characteristic (ROC-AUC) curves were generated. Optimal cut points for predicting CFPD (2hG ≥ 140 mg/dL) and CFRD (2hG ≥ 200 mg/dL) were determined.
Results:
Fifty-eight youth with CF were included (2hG < 140, n = 16; CFPD, n = 33; CFRD, n = 9; 41% male, mean ± SD age 14.2 ± 3.6 years, BMI z-score 0.0 ± 0.8, % predicted forced expiratory volume in 1 second [FEV1] 89.9 ± 15.1, % predicted forced vital capacity [FVC] 103.2 ± 14.6). ROC-AUC’s for all alternate markers were low for CFPD (0.52–0.67) and CFRD (0.56–0.61). At a cut point of 5.5%, HbA1c had 78% sensitivity (95% CI: 0.45–0.94) and 41% specificity (95% CI: 0.28–0.55) for identifying CFRD, correlating to a ROC-AUC of 0.61 (95% CI: 0.42–0.8).
Conclusions:
All alternate markers tested demonstrate poor diagnostic accuracy for identifying CFRD by 2hG.
Keywords: 1,5-anhydroglucitol; cystic fibrosis-related diabetes; fructosamine; glycated albumin; hemoglobin A1c
1 |. BACKGROUND
Cystic fibrosis-related diabetes (CFRD) is common in the cystic fibrosis (CF) population and is increasing in prevalence as long-term survival rates increase.1 While only 2% of children with CF are estimated to meet criteria for CFRD, this figure increases to 19% in adolescents and 40% to 50% in adults.1 CFRD results from a combination of insulinopenia and to a lesser degree, insulin resistance.2 CFRD is associated with poorer clinical outcomes including diminished lung function, suboptimal nutritional status as demonstrated by poor weight gain, and increased mortality risk.3–6 Early treatment with insulin is associated with improved outcomes; thus, ensuring that CFRD is identified is critical.
The current gold-standard for CFRD screening and diagnosis is the oral glucose tolerance test (OGTT) and guidelines from the Cystic Fibrosis Foundation and the American Diabetes Association recommend annual screening in all patients with CF ≥10 years of age.7 The Cystic Fibrosis Foundation Patient Registry’s Annual Report reveals poor adherence to OGTT screening guidelines, as only 61.7% of individuals aged 10 to 17 years with CF who have not been previously diagnosed with CFRD had documented OGTT screening in 2017.8 This number decreased to only 29.1% in individuals 18 years or older who meet the same criteria.8 Poor adherence to OGTT screening is likely multifactorial and possible contributing factors include the time-consuming nature of the test, the necessary pretest fasting period, the need for serial blood draws, and the lack of familiarity with OGTT’s in the pulmonary clinic setting. For this reason, determining the utility of simpler, non-fasting alternatives for CFRD screening is necessary.
Hemoglobin A1c (HbA1c) has been shown to be insensitive for detecting CFRD, as defined by current diagnostic thresholds, and although lower HbA1c cut points have been proposed to increase sensitivity for detecting CFRD, these cut points are controversial.9,10 Historically, HbA1c has been thought to be an insensitive screening test in CFRD due to increased red blood cell turnover, thus resulting in an underestimation of glycemia.11 However, a recent report by our group demonstrated a HbA1c and average glucose relationship in youth with CF that was, in fact, no different from other diabetes populations.12
Alternate measures of glycemia—fructosamine (FA), glycated albumin (GA), and 1,5-anhydroglucitol (1,5AG)—have been found, in other populations, to be potentially useful screening tests for diabetes.13 A study of FA in a small population of adults with CF found that FA adjusted for total protein exhibited a positive correlation with 2hG on OGTT and a negative correlation with forced expiratory volume in 1 second (FEV1).14 Unlike HbA1c, FA and GA are composed of non-immunoglobulin serum proteins that are not dependent on the half-life of red blood cells.15,16 FA is formed when blood glucose covalently binds to serum proteins to form ketoamines15 while GA is produced through the glycation of serum albumin.16 This process occurs approximately 10-fold faster with albumin than with hemoglobin and may be particularly useful for identifying situations of elevated postprandial hyperglycemia and increased glucose excursions.16 Consequently, both alternative markers are not affected by increased red blood cell turnover and reflect more recent glycemic control over the preceding 14 to 21 days.17 Another biomarker, 1,5AG, a naturally occurring dietary polyol, may also better reflect postprandial hyperglycemia as it competes with glucose for reabsorption in the kidneys.18 Lower 1,5AG levels are seen in the setting of hyperglycemia and glucosuria over the preceding 1 to 2 weeks.18
The aim of this study was to investigate alternative markers of glycemia, specifically HbA1c, FA, GA, and 1,5AG, as screening tests for CFPD and CFRD.
2 |. RESEARCH DESIGN AND METHODS
2.1 |. Study population
Youth aged 10 to 18 years with a known diagnosis of CF were identified from pulmonary and endocrinology clinics and recruited to participate in a larger study on early glucose abnormalities in CF. Those individuals with available OGTT and alternate glycemic marker data who were not on insulin therapy were included in this analysis. Inclusion criteria for a diagnosis of CF included a positive newborn screen, positive sweat chloride test, and/or positive genetic test. Exclusion criteria included a known diagnosis of type 1 or type 2 diabetes mellitus, a history of insulin or other medications affecting glucose metabolism, including steroids, in the preceding 3 months, hospitalizations in the prior 6 weeks, or pregnancy. The study was approved by the Colorado Multiple Institutional Review Board (Aurora, Colorado) and age-appropriate consent and/or assent was obtained.
2.2 |. Study visit
Participants presented to Children’s Hospital Colorado Clinical and Translational Research Center for a single study visit. Height and weight were collected, and body mass index was calculated. A pediatric endocrinologist completed a physical exam including Tanner staging. Participant medical records were reviewed, and the following were recorded: CF genotype and pulmonary function testing from the most recent pulmonary clinic visit.
2.3 |. Laboratory procedures
All participants were asked to fast for a minimum of 8 hours prior to the study visit. Blood was obtained for measurement of HbA1c, 1,5AG, FA, and GA. An OGTT was completed with samples collected for measurement of blood glucose and insulin levels at 0, 60, and 120 minutes after glucola was administered (1.75 g/kg; maximum dose of 75 g). Normoglycemia was defined as a 2hG < 140 mg/dL. CFPD was defined as a 2hG ≥ 140 mg/dL. CFRD was defined as a 2hG ≥ 200 mg/dL.
HbA1c was measured on a DCCT-aligned DCA Vantage Analyzer (Siemens, Deerfield, Illinois) with an inter-day coefficient variation (CV) of 2.8%. Measurements were obtained on a point-of-care device. The normal reference range for HbA1c is <5.7%.19 FA was measured using a colorimetric assay with an inter-assay CV of 3% on the Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation). The normal reference range for FA is 205 to 285 μmol/L.20 Because FA originates from non-enzymatic glycation of proteins, it is both glucose and protein concentration dependent.21 FA was corrected for albumin concentration with the following calculation: albumin-adjusted fructosamine (AAFA) = FA × 4/albumin. GA was measured as a percentage of glycated albumin relative to the total amount of albumin via an enzymatic method adapted to the Roche Analyzer per the Lucica GAL assay (Asahi Kasei Pharma, Tokyo, Japan) with an inter-assay CV of 2.1% and a mean of 22.7%. The normal reference range for GA is 11% to 16%.22 FA and GA were measured in the lab of Dr. Michael Steffes at the University of Minnesota. 1,5AG was measured via a colorimetric assay (GlycoMark, Tomen America, New York) with an inter-assay CV of 4.1% at 4.67 μg/mL. The normal reference range for 1,5AG is 10 to 31 μg/mL.23
Spirometry data were gathered from routine pulmonary clinic visits. Forced expiratory volume in 1 second % predicted (FEV1) and forced vital capacity % predicted (FVC) were collected from the most recent CF clinic visit where the participant was noted to be at baseline health status. Values were calculated as percentages of normal per the Global Lung Initiative (GLI), a standard measure of reporting lung function that is adjusted for age, sex, height, and race/ethnicity.24
2.4 |. Statistical analysis
Mean (±SD) for normally distributed measures and median (IQR) for skewed measures were reported. An overall analysis of variance F test or Cochran-Mantel-Haenszel test was used to assess differences between the three groups of participants. Pearson’s correlation coefficient was used to determine the correlation between estimates of glycemia (HbA1c [%], 1,5-AG [μg/mL], FA [μmol/L], GA [%]) and the 2hG on OGTT. Logistic regression models assessed the ability of the alternate markers to predict 2hG categories (CFPD ≥ 140 mg/dL and CFRD ≥ 200 mg/dL) by comparing area under the receiver operating characteristic curve (ROC-AUC). The cut points for alternate markers that maximized the Youden index for 2hG categories (CFPD and CFRD) were determined. Participants were categorized as either above or below the identified cut points for each alternate glycemic marker. Welch’s t tests compared the lung function between groups to assess for any differences in FEV1 and FVC. Statistical significance level was set at alpha < .05 level. The data analysis was performed using SAS software 9.4 (Cary, North Carolina).
3 |. RESULTS
Fifty-eight youth with CF were included in this analysis. Demographic characteristics are presented in Table 1. Participants had a mean age of 14.2 ± 3.6 years and a mean BMI = z-score − 0.02 ± 0.82. Forty-one percent were male, 91% were white, 7% were Hispanic, and 2% were black. Sixteen participants had normal glucose tolerance (28%), 33 had CFPD (57%), and 9 had CFRD (15%). Table 1 presents mean values for each alternate marker when classified by 2hG (normoglycemia [NG] vs CFPD vs CFRD). HbA1c, FA, and GA correlated with 2hG (0.38, 0.27, 0.28; p < .05); however, only HbA1c was significantly different among each of the three 2hG groups.
TABLE 1.
Demographics and baseline mean values for alternate markers categorized by 2-hour glucose (2hG)
| Mean for all (N = 58) | NG: 2hG < 140 mg/dL (N = 16) | CFPD: 2hG ≥ 140 mg/dL (N = 33) | CFRD: 2hG ≥ 200 mg/dL (N = 9) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 14.2 ± 3.6 | 13.9 ± 3.8 | 14.3 ± 3.7 | 13.9 ± 3.2 | .91 |
| Male, N (%) | 24 (41.4) | 6 (37.5) | 16 (48.5) | 2 (22.2) | .34 |
| Race, N (%) | .47 | ||||
| White | 53 (91.4) | 14 (87.5) | 30 (90.9) | 9 (100.0) | |
| Hispanic | 4 (6.9) | 1 (6.2) | 3 (9.1) | 0 (0.0) | |
| Black | 1 (1.7) | 1 (6.2) | 0 (0.0) | 0 (0.0) | |
| BMI z-score | −0.02 ± 0.82 | −0.20 ± 0.77 | 0.15 ± 0.84 | −0.29 ± 0.82 | .22 |
| FEV1 (%) | 89.89 ± 15.14 | 93.39 ± 11.03 | 88.42 ± 16.71 | 89.01 ± 16.29 | .60 |
| FVC (%) | 103.19 ± 14.60 | 104.50 ± 10.66 | 101.52 ± 16.39 | 107.00 ± 14.14 | .56 |
| Pancreatic sufficiency status, n (% sufficient) | 4 (6.9) | 3 (18.8) | 1 (3.0) | 0 (0.0) | .09 |
| G-tube feedings present, n (% yes) | 8 (13.8) | 2 (12.5) | 4 (12.1) | 2 (22.2) | .73 |
| Genotype class, n (%) | .02 | ||||
| Class I-III | 44 (77.2) | 8 (53.3) | 28 (84.8) | 8 (88.9) | |
| Class IV-V | 6 (10.5) | 5 (33.3) | 1 (3.0) | 0 (0.0) | |
| OGTT glucose (mg/dL) | |||||
| 0 min | 95.81 ± 9.95 | 86.56 ± 6.61 | 98.39 ± 7.35 | 102.78 ± 12.43 | <.01 |
| 60 min | 197.28 ± 59.75 | 142.31 ± 30.67 | 206.64 ± 46.09 | 260.67 ± 65.19 | <.01 |
| 120 min | 141.71 ± 44.62 | 107.25 ± 17.15 | 137.61 ± 32.52 | 218.00 ± 20.32 | <.01 |
| OGTT insulin (mclU/ml) | |||||
| 0 min | 4.52 ± 3.03 | 3.50 ± 1.63 | 4.73 ± 3.39 | 5.56 ± 3.32 | .23 |
| 60 min | 45.35 ± 36.15 | 32.89 ± 30.94 | 51.19 ± 40.57 | 38.86 ± 17.45 | .38 |
| 120 min | 46.02 ± 35.96 | 24.11 ± 15.51 | 48.67 ± 37.45 | 64.00 ± 39.02 | .07 |
| Hemoglobin A1c (%) | 5.5 ± 0.3 (min-max 4.6–6.5) | 5.34 ± 0.26 | 5.62 ± 0.29 | 5.64 ± 0.27 | <.01 |
| 1,5-Anhydroglucitol (mcg/ml) | 19.14 ± 6.63 (min-max 4.9–39.8) | 19.24 ± 7.53 | 18.64 ± 5.91 | 20.78 ± 7.97 | .70 |
| Fructosamine (μmol/L) | 233.1 ± 18.79 (min-max 182–267) | 228 ± 21.06 | 234 ± 18.76 | 238.89 ± 13.49 | .35 |
| Glycated albumin (%) | 12.29 ± 1.04 (min-max 10–15) | 12.19 ± 0.75 | 12.24 ± 1.12 | 12.67 ± 1.22 | .51 |
| Total albumin-adjusted fructosamine | 55.49 ± 4.68 (min-max 45.5–67.37) | 55.03 ± 3.74 | 55.36 ± 5.07 | 56.08 ± 5.35 | .87 |
Note: Pulmonary function data were available for 49 of the 58 total participants. Data are presented as mean ± SD or number, N (%).
Abbreviations: BMI, body mass index; CFPD, cystic fibrosis-related pre-diabetes; CFRD, cystic fibrosis-related diabetes; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NG, normoglycemia.
ROC curves were generated to determine optimal cut points that maximize sensitivity and specificity for detecting a 2hG ≥ 200 mg/dL and ≥140 mg/dL (Figure 1A,B). ROC-AUCs for all alternate markers were low for both CFPD (AUC’s 0.52–0.67) and CFRD (AUC’s 0.56–0.61). HbA1c had the highest ROC-AUC of 0.61 (95% CI: 0.42–0.8) for identifying CFRD and 0.67 (95% CI: 0.53–0.8) for CFPD. Optimal cut points for identifying CFRD by 2hG for each alternate marker are as follows: HbA1c = 5.5%, FA = 225 μmol/L, AAFA = 59 μmol/g, GA = 14%, and 1,5AG = 20.4 mcg/mL (Table 2).
FIGURE 1.
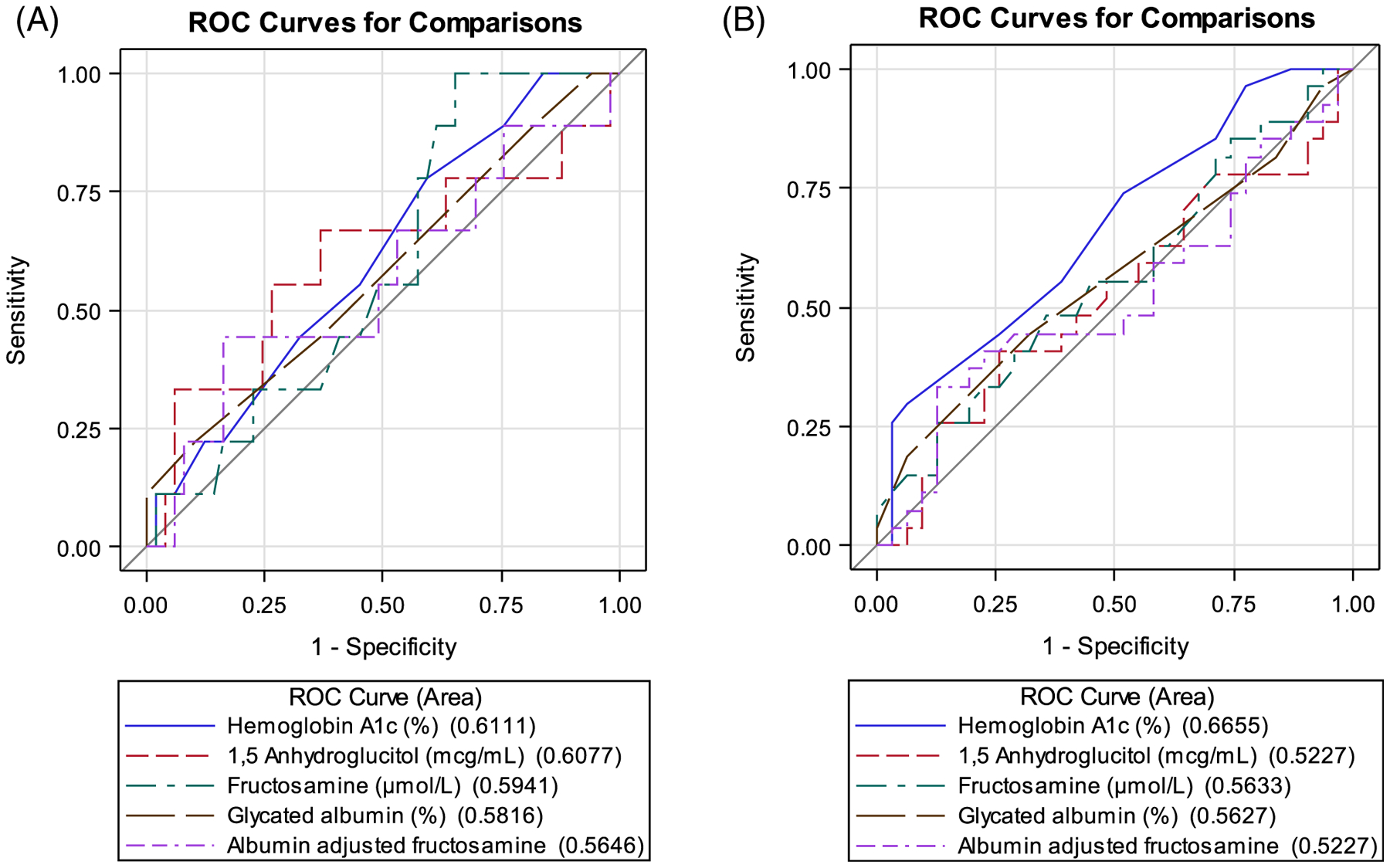
(A) Oral glucose tolerance test 2-hour glucose ≥200 mg/dL. Area under the receiver operating characteristic (ROC-AUC) curve measurements for alternative glycemic markers at predicting cystic fibrosis-related diabetes. ROC-AUC values (95% CI): hemoglobin A1c 0.61 (0.42, 0.80), 1,5-anhydroglucitol 0.61 (0.37, 0.85), fructosamine 0.59 (0.42, 0.77), glycated albumin 0.58 (0.37, 0.79), and albumin-adjusted fructosamine 0.56 (0.33, 0.80). All associated p-values were non-significant. (B) Oral glucose tolerance test 2-hour glucose ≥140 mg/dL. Area under the receiver operating characteristic (ROC-AUC) curve measurements for alterative glycemic markers at predicting cystic fibrosis-related pre-diabetes. ROC-AUC values (95% CI): hemoglobin A1c 0.67 (0.53, 0.8), 1,5-anhydroglucitol 0.52 (0.37, 0.68), fructosamine 0.56 (0.41, 0.71), glycated albumin 0.56 (0.42, 0.71), and albumin-adjusted fructosamine 0.52 (0.37, 0.69). All associated p-values were non-significant
TABLE 2.
Comparisons of the optimal cutoffs, sensitivities, and specificities of five alternative markers for predicting cystic fibrosis-related diabetes as defined by the gold-standard oral glucose tolerance test 2-hour glucose ≥200 mg/dL
| Measure | Optimal cutoff | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|
| Hemoglobin A1c (%) | 5.5 | 0.78 (0.45, 0.94) | 0.41 (0.28, 0.55) |
| 1,5-Anhydroglucitol (mcg/mL) | 20.4 | 0.67 (0.35, 0.88) | 0.63 (0.49, 0.75) |
| Fructosamine (μmol/L) | 225 | 1.0 (0.7, 1.0) | 0.35 (0.23, 0.49) |
| Glycated albumin (%) | 14 | 0.22 (0.06, 0.55) | 0.9 (0.78, 0.96) |
| Albumin-adjusted fructosamine | 59 | 0.44 (0.19, 0.73) | 0.84 (0.71, 0.91) |
FEV1 and FVC measurements for individuals who fell above and below the identified cut points for each alternate glycemic marker were compared. Individuals who fell above the HbA1c cut point of 5.5% demonstrated a significant difference in FVC when compared to individuals who fell below the cut point (105% vs. 96%, p = .02). FEV1 was higher in those with HbA1c levels below this cut point vs. those with HbA1c levels above this point; however, the difference was not statistically significant (96% vs. 88%, p = .06). Pulmonary function data reflected a relatively healthy cohort of youth with CF with an average FEV1 = 89.9 ± 15.1% and a FVC = 103.2 ± 14.6%.
4 |. DISCUSSION
This is the first study to examine four simple glycemic estimates as potential screening tools for CFPD and CFRD. In contrast to studies in non-CF populations, these tests demonstrated a poor ability to identify CFPD and CFRD as defined by the gold-standard 2hG on OGTT. HbA1c had the highest sensitivity and specificity for detecting CFPD and CFRD, but at a lower cut point than previously reported and with a modest ROC-AUC of 0.61 and 0.67, respectively. Therefore, in this population of youth with CF and high rates of early dysglycemia, none of these simpler glycemic estimates appear suitable for replacing OGTT for diabetes screening.
Our understanding of the meaning of HbA1c, and what it reflects in CF, has evolved over time. Traditionally, HbA1c has been shunned as a screening test for CFRD due to concerns for inaccuracies in the CF population speculated to be secondary to shortened red blood cell lifespan, chronic iron deficiency anemia, and/or alterations in hemoglobin glycation rates.11,25 A recent publication by our group, however, found that the relationship between HbA1c and mean plasma glucose in a cohort of youth with CFRD was similar to that reported in landmark studies in individuals with type 1 and type 2 diabetes.12 A previously published small study involving 20 adults with CF came to similar conclusions.26 Reports on the utility of HbA1c as a screening test for CFRD, defined by the OGTT, have been conflicting. Burgess et al found that screening for CFRD using a HbA1c cut point of 5.8% had a 93.8% sensitivity and reduced the need for an OGTT by 50%; however, it is notable that their study methods differed from ours as the mean age of their participants was older at 31.7 ± 10.4 years and HbA1c methodology was different.9 In contrast, Boudreau et al found that the same HbA1c cut point of 5.8% only had a 68.2% sensitivity for identifying CFRD and decreasing the HbA1c cut point to 5.5% was noted to increase the sensitivity to 95%.10 Our study demonstrated a similar optimal HbA1c cut point of 5.5%; however, it was associated with a lower sensitivity of 80% and a poor specificity. One explanation for these discrepancies may be the different analytical performances of the assays used.27,28
Additionally, for each alternate glycemic marker we completed a dichotomous assessment of pulmonary function of individuals who fell above the cut point maximizing sensitivity and specificity for identifying either CFRD or CFPD vs. those who fell below the cut point. Notably, the only cut point that identified individuals with better vs. worse pulmonary function was the HbA1c cut point of 5.5% for identifying CFRD. However, there was no difference in pulmonary function between glycemic groups categorized by the gold-standard 2hG on OGTT. Therefore, the significance of the HbA1c and its relationship to pulmonary function requires further study. Of note, other studies have shown that earlier time points on the OGTT (i.e., 30 minutes and 1 hour) may more accurately identify early clinical changes in pulmonary function than the 2hG.29,30
Fructosamine is a ketoamine formed secondary to non-enzymatic protein glycation and it has traditionally been used in situations where HbA1c is unreliable.18 Studies have shown that FA correlates with total protein and albumin concentrations; consequently, correction for total protein or albumin improves both diagnostic performance and correlation with HbA1c in identifying diabetes.14,31 A small study recently examined the utility of FA in CFRD and found that FA appeared to be a reliable tool for CFRD screening and correlated inversely with FEV1 in individuals with CFRD.14 The findings in our population, however, differ and this may be secondary to differences in demographics as our population was younger and included a higher proportion of individuals with CFPD and CFRD. Our population was also healthier overall with less variability in pulmonary function.
Like FA, GA is produced through glycation of albumin and reflects mean plasma glucose over the preceding 2 to 3 weeks.16,17 This study is the first to evaluate the use of GA in the CF population. Studies in non-CF populations have suggested utility of GA as a screening test,32 but no studies have previously assessed this marker in the CF population. GA has been described to more accurately identify situations of increased BG variability and postprandial hyperglycemia,16 and both glucose patterns are seen in early CFRD. However, GA was not identified as a useful tool for CFPD or CFRD screening in our cohort.
Last, 1,5AG, a naturally occurring dietary polyol that competes with glucose for reabsorption in the kidneys, reflects BG control over the preceding few days to 2 weeks.18 In a study of glycemic excursions in individuals with type 1 and type 2 diabetes, Dungan et al found that 1,5AG better identified postprandial glucose excursions on CGM than either HbA1c or FA.33 A study in Japanese adults found that 1,5AG maximized sensitivity and specificity in predicting type 2 diabetes as defined by OGTT when compared to both HbA1c and FA.34 Only one prior study has assessed 1,5AG as a screening test for CFRD, but this study was limited to 10 adults with CF and found no correlation with 2hG or HbA1c.35 Similarly, we did not demonstrate clinical utility for 1,5AG in diagnosing CFRD.
While we specifically evaluated the utility of 1,5AG, FA, GA, and HbA1c as screening markers for detecting dysglycemia in the CF population, further study is required to determine whether these tools may have value for monitoring glycemia over time in individuals with CFRD. Additionally, it is important to note that all four alternative markers estimate average glycemia over various time periods. In early CFRD, where postprandial glycemic excursions are the most common abnormality, simple estimates of average glycemia may not reveal abnormalities that OGTT or other screening tools such as continuous glucose monitoring can detect. Continued examination of these markers and their relationship to prospective clinical outcomes is needed.
5 |. STRENGTHS AND LIMITATIONS
Our study presents novel data evaluating the use of four alternative glycemic markers not previously studied concurrently in the CF population. These markers, however, demonstrated poor utility in identifying CFRD and CFPD, as defined by the 2-hour OGTT glucose, in our group of CF youth. Studies including larger sample sizes and a wider age range of individuals with CF are needed. However, it is important to remember that the OGTT criteria for diagnosing CFRD were adapted from cut points established to detect microvascular disease in individuals at risk for type 2 diabetes36,37 and are not CF-population specific. Additional studies to identify the optimal OGTT cut points for predicting CF-relevant clinical outcomes are needed.
ACKNOWLEDGEMENTS
The authors thank the patients and their families that were involved in this study and the CHC Cystic Fibrosis Center and the CCTSI personnel. This research was supported by NIH grants DK094712-04, UL1 TR 000154 (CCTSI), UL1 TR 001082 (REDCap), and T32DK063687 and Cystic Fibrosis Foundation Therapeutics grants CHAN16A0 and CHAN16GE0. The National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health supported the research provided in this publication. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Materials for the glycated albumin assay were provided by Asahi Kasei Pharma; however, industry contributors played no role in the design, data collection, or reporting of this study.
Funding information
Cystic Fibrosis Foundation Therapeutics, Grant/Award Numbers: CHAN16A0, CHAN16GE0; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Numbers: DK094712-04, T32DK063687, UL1 TR 000154, UL1 TR 001082
Abbreviations:
- 1,5AG
1,5-anhydroglucitol
- 2hG
2-hour glucose
- AAFA
albumin-adjusted fructosamine
- BMI
body mass index
- CF
cystic fibrosis
- CFPD
cystic fibrosis related prediabetes
- CFRD
cystic fibrosis related diabetes
- FA
fructosamine
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- GA
glycated albumin
- HbA1c
hemoglobin A1c
- OGTT
oral glucose tolerance test
- ROC-AUC
area under the receiver operating characteristic curve
Footnotes
CONFLICT OF INTEREST
No potential conflicts of interest relevant to this article were reported from any of the authors.
REFERENCES
- 1.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32(9):1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battezzati A, Mari A, Zazzeron L, et al. Identification of insulin secretory defects and insulin resistance during oral glucose tolerance test in a cohort of cystic fibrosis patients. Eur J Endocrinol. 2011;165(1): 69–76. [DOI] [PubMed] [Google Scholar]
- 3.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162(3 Pt 1): 891–895. [DOI] [PubMed] [Google Scholar]
- 4.Bismuth E, Laborde K, Taupin P, et al. Glucose tolerance and insulin secretion, morbidity, and death in patients with cystic fibrosis. J Pediatr. 2008;152(4):540–545. 545 e1. [DOI] [PubMed] [Google Scholar]
- 5.Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care. 2005;28(9):2141–2144. [DOI] [PubMed] [Google Scholar]
- 6.Chamnan P, Shine BSF, Haworth CS, Bilton D, Adler AI. Diabetes as a determinant of mortality in cystic fibrosis. Diabetes Care. 2010;33(2): 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran A, Brunzell C, Cohen RC, et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2017 Annual Report; 2017.
- 9.Burgess JC, Bridges N, Banya W, et al. HbA1c as a screening tool for cystic fibrosis related diabetes. J Cyst Fibros. 2016;15(2):251–257. [DOI] [PubMed] [Google Scholar]
- 10.Boudreau V, Coriati A, Desjardins K, Rabasa-Lhoret R. Glycated hemoglobin cannot yet be proposed as a screening tool for cystic fibrosis related diabetes. J Cyst Fibros. 2016;15(2):258–260. [DOI] [PubMed] [Google Scholar]
- 11.Wagener JS, GC MN, Taussig LM, et al. Ferrokinetic and hematologic studies in cystic fibrosis patients. Am J Pediatr Hematol Oncol. 1983;5 (2):153–160. [PubMed] [Google Scholar]
- 12.Chan CL, Hope E, Thurston J, et al. Hemoglobin A1c accurately predicts continuous glucose monitoring-derived average glucose in youth and young adults with cystic fibrosis. Diabetes Care. 2018;41(7): 1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan CL, Pyle L, Kelsey M, Newnes L, Zeitler PS, Nadeau KJ. Screening for type 2 diabetes and prediabetes in obese youth: evaluating alternate markers of glycemia - 1,5-anhydroglucitol, fructosamine, and glycated albumin. Pediatr Diabetes. 2016;17(3):206–211. [DOI] [PubMed] [Google Scholar]
- 14.Lam GY, Doll-Shankaruk M, Dayton J, et al. The use of fructosamine in cystic fibrosis-related diabetes (CFRD) screening. J Cyst Fibros. 2018;17(1):121–124. [DOI] [PubMed] [Google Scholar]
- 15.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33(12):2153–2163. [PubMed] [Google Scholar]
- 16.Rondeau P, Bourdon E. The glycation of albumin: structural and functional impacts. Biochimie. 2011;93(4):645–658. [DOI] [PubMed] [Google Scholar]
- 17.Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol. 2008;2 (6):1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JE. Alternative biomarkers for assessing glycemic control in diabetes: fructosamine, glycated albumin, and 1,5-anhydroglucitol. Ann Pediatr Endocrinol Metab. 2015;20(2):74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zercher A, Schulman L, Boone J Quantitative measurement of hemoglobin A1c on the DCA vantage point-of-care analyzer as a diagnostic test for diabetes: an internal validation study; 2014. https://static.healthcare.siemens.com/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/groups/public/@global/@lab/@poc/documents/download/mday/nzg2/~edisp/130890-gc1_dca_hba1c_precision_study_white_paper_to_support_ous_dx_claim_final_web-01360756.pdf. Accessed July 15, 2019.
- 20.Selvin E, Warren B, He X, Sacks DB, Saenger AK. Establishment of community-based reference intervals for Fructosamine, glycated albumin, and 1,5-Anhydroglucitol. Clin Chem. 2018;64(5):843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunika K, Itakura M, Yamashita K. Correction of fructosamine value for serum albumin and globulin concentrations. Diabetes Res Clin Pract. 1991;13(1–2):37–44. [DOI] [PubMed] [Google Scholar]
- 22.Corporation AKP. What’s glycated albumin (GA)?. https://www.asahikasei.co.jp/shindan/en/ga-I/intro02.html. Accessed July 10, 2019
- 23.GlycoMark. For the quantitative measurement of 1,5-anhydroglucitol (1,5-AG) in serum or plasma. https://glycomark.com/wp-content/uploads/2018/01/GlycoMark-insert-Rev-G-2017.pdf. Accessed July 10, 2019.
- 24.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ater JL, Herbst JJ, Landaw SA, O’Brien RT. Relative anemia and iron deficiency in cystic fibrosis. Pediatrics. 1983;71(5):810–814. [PubMed] [Google Scholar]
- 26.Brennan AL, Gyi KM, Wood DM, Hodson ME, Geddes DM, Baker EH. Relationship between glycosylated haemoglobin and mean plasma glucose concentration in cystic fibrosis. J Cyst Fibros. 2006;5(1): 27–31. [DOI] [PubMed] [Google Scholar]
- 27.Lam GY, Sissons S, Smith MP, Brown NE, Leung WM, Estey MP. How reliable is your HbA1c test? Revisiting the use of HbA1c in cystic fibrosis-related diabetes (CFRD) screening. J Cyst Fibros. 2019;18(2):e14–e15. [DOI] [PubMed] [Google Scholar]
- 28.Chan CL, McFann K, Newnes L, Nadeau KJ, Zeitler PS, Kelsey M. Hemoglobin A1c assay variations and implications for diabetes screening in obese youth. Pediatr Diabetes. 2014;15(8):557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hameed S, Morton JR, Jaffe A, et al. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care. 2010; 33(2):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brodsky J, Dougherty S, Makani R, Rubenstein RC, Kelly A. Elevation of 1-hour plasma glucose during oral glucose tolerance testing is associated with worse pulmonary function in cystic fibrosis. Diabetes Care. 2011;34(2):292–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Segade S, Rodriguez J, Camina F. Corrected Fructosamine improves both correlation with HbA1C and diagnostic performance. Clin Biochem. 2017;50(3):110–115. [DOI] [PubMed] [Google Scholar]
- 32.Furusyo N, Koga T, Ai M, et al. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa population study (KOPS). Diabetologia. 2011;54(12):3028–3036. [DOI] [PubMed] [Google Scholar]
- 33.Dungan KM, Buse JB, Largay J, et al. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29(6):1214–1219. [DOI] [PubMed] [Google Scholar]
- 34.Yamanouchi T, Akanuma Y, Toyota T, et al. Comparison of 1,5-anhydroglucitol, HbA1c, and fructosamine for detection of diabetes mellitus. Diabetes. 1991;40(1):52–57. [DOI] [PubMed] [Google Scholar]
- 35.Kinnaird KE, Sauerwein TJ. Lack of correlation between 1,5-anhydroglucitol assay and oral glucose tolerance test in patients with cystic fibrosis. Endocr Pract. 2010;16(2):167–170. [DOI] [PubMed] [Google Scholar]
- 36.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–1197. [DOI] [PubMed] [Google Scholar]
- 37.International Expert, C. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]


