Abstract
Parkinson's disease (PD) is a complex multi-factorial neurodegenerative disorder where various altered metabolic pathways contribute to the progression of the disease. Tryptophan (TRP) is a major precursor in kynurenine pathway (KP) and it has been discussed in various in vitro studies that the metabolites quinolinic acid (QUIN) causes neurotoxicity and kynurenic acid (KYNA) acts as neuroprotectant respectively. More studies are also focused on the effects of other KP metabolites and its enzymes as it has an association with ageing and PD pathogenesis. Until now, very few studies have targeted the role of genetic mutations in abnormal KP metabolism in adverse conditions of PD. Therefore, the present review gives an updated research studies on KP in connection with PD. Moreover, the review emphasizes on the urge for the development of biomarkers and also this would be an initiative in generating an alternative therapeutic approach for PD.
Keywords: Ageing, Biomarkers, Kynurenine pathway (KP), Parkinson's disease (PD), Therapeutics
Abbreviations: PD, Parkinson's disease; KP, Kynurenine pathway; QUIN, quinolinic acid; KYNA, kynurenic acid; DA, dopaminergic; α-synuclein, αSyn; AD, Alzheimer's disease; AhR, aryl hydrocarbon receptor; NAD+, nicotinamide adenine dinucleotide; TRP, tryptophan; 3-HK, 3-hydroxykynurenine; KYN, kynurenine; NFK, N′-formylkynurenine; TDO, tryptophan 2,3-dioxygenase; IDO-1, indoleamine-2,3-dioxygenases; FAM, formamidase; KMO, kynurenine −3-monooxygenase; AA, anthranilic acid; KATs, kynurenine aminotransferases; CSF, cerebrospinal fluid; CNS, central nervous system; 3-HAA, 3-hydroxyanthranilic acid; NMDA, N-methyl-d-aspartate; PA, picolinic acid; ATP, adenosine triphosphate; NADPH, nicotinamide adenine dinucleotide phosphate; IFN-γ, interferon-γ; L-DOPA, L-dopamine; MPTP, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; 6-OHDA, 6-hydroxydopamine; SNpc, substantianigra pars compacta; RBCs, red blood cells; LID, L-DOPA-induced dyskinesia; XA, xanthurenic acid; ACMSD, amino-carboxymuconatesemialdehyde decarboxylase; ZNS, zonisamide
Graphical abstract
Highlights
-
•
KP metabolites and enzymes act on NAD+ levels.
-
•
The catabolism of TRP and NAD+ biogenesis are linked through KP.
-
•
KMO and ACMSD genetic alterations involved in PD.
-
•
Genetic link between KP and PD were discussed.
-
•
KP as a therapeutic intervention in PD.
1. Introduction
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease characterized by progressive motor decline due to dopaminergic (DA) neurons deficit [1,2]. It is characterized by tremor, bradykinesia, rigidity and postural instability [3]. Metabolomics studies were performed to identify specific biomarker in PD pathogenesis. Tryptophan (TRP) metabolism along the kynurenine pathway (KP) has been implicated in many diseases, including neurodegenerative and psychiatric disorders [4], inflammation, cancer [5], diabetes and obesity [6]. KP metabolites exert various physiological functions by acting at extra- or intracellular receptors, both in the brain and the periphery. KP metabolites can affect excitatory neurotransmission by modulating activity at glutamate or nicotinic acetylcholine receptors [4] and can affect immune responses by acting at the transcription factor aryl hydrocarbon receptor (AhR) [7]. The end product of the KP is nicotinamide adenine dinucleotide (NAD+), an essential cofactor linked to energy metabolism [8].
Levels of KP metabolites in various tissues regulated by large-neutral amino acid transporters and enzymes [4], and its activities are influenced by exercise [6,9] inflammation [7], and the composition of the gut microbiome [10,11]. KP enzymes have been recognized as promising drug targets to treat neurodegenerative [12] and psychiatric disorders [13], as well as early-stage cancer [5]. Importantly, TRP availability and KP metabolism are altered by inflammation and ageing [[11], [12], [13]], thereby enhancing the risk for age-dependent neurodegenerative disorders, including PD [14,15]. Recently, chronic intestinal inflammation, alterations in the gut microbiome and spreading of α-synuclein (αSyn) aggregates from the gut to the brain via the vagal nerve has been linked to PD pathogenesis [16].
Previous reviews on the possible involvement of the KP in the pathogenesis of PD [17,18] have extensively reviewed relevant experimental and clinical literature until 2015, still, evidence for alterations in KP metabolites and its possible contribution to neuroinflammation and excitotoxicity in PD was predominantly based on experimental studies with a focus on a neurotoxic role for the KP metabolite quinolinic acid (QUIN) and a neuroprotective role for the KP metabolite kynurenic acid (KYNA) within the brain, without taking alterations in KP metabolism in the periphery and the spread of PD pathology from the gut into account. In addition, the referred clinical literature supporting a role for abnormal KP metabolism in the brain (increased KYN/TRP ratio [19] or increased 3-HK/KYNA ratio [20] in cerebrospinal fluid (CSF) or the periphery (increased KYN/TRP ratio in serum [19] was still very limited at that time.
Here we aim to critically review an update on KP in PD research concerning to ageing, inflammation and the microbiota-gut-brain axis with the pathophysiology, genetics and metabolomics of PD. This review is based on recent reviews and original literature on the KP topics and systematic searches in PubMed.
2. TRP degradation via KP in the periphery and the central nervous system (CNS)
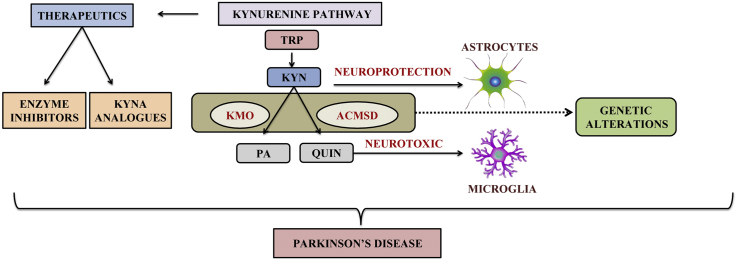
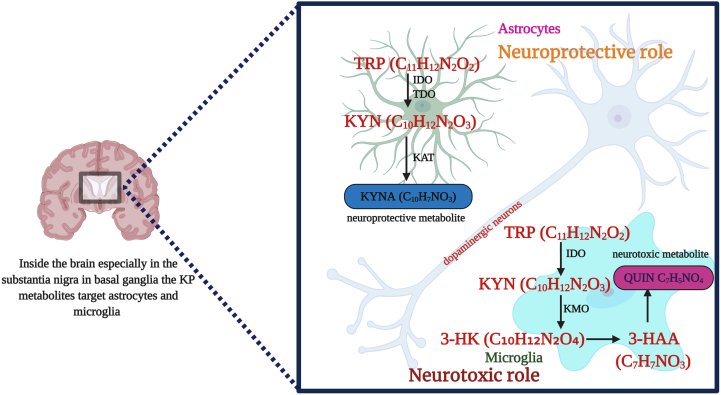
KP is a major degradative pathway that occurs in the liver which synthesizes NAD+ from TRP. TRP is converted to N′-formylkynurenine (NFK) by tryptophan 2,3-dioxygenase (TDO) either in the liver or by indoleamine-2,3-dioxygenases (IDO-1) extra hepatically, which are the major rate-limiting steps, that ends up into several disorders [21]. In this pathway, KYN is synthesized from NFK by the enzyme NFK formamidase (FAM). Further, the catalytic activity results into hydroxylation of KYN to 3-HK by kynurenine -3-monooxygenase (KMO) followed by 3-HK hydrolysis to 3-hydroxyanthranilic acid (3-HAA) by kynureninase, added to 3-HAA, 2-amino-3-carboxymuconoate semialdehyde are also produced in this pathway. Kynureninase can also hydrolyse KYN to anthranilic acid (AA) while kynurenine aminotransferases (I, II, III) (KATs) desalinate KYN to KYNA [22]. Various studies have described the biochemical pathway of TRP metabolism. KP metabolites and its effects in brain have been depicted in Fig. 1.
Fig. 1.
Overview of KP in brain and its effects.
Several KP enzymes have effects in neurotoxicity, neuroprotective or immunomodulatory reactions [14]. In microglia and astrocytes of the brain, most of the KP metabolites synthesize 3-HK [23]. In healthy cells, 3-HK leads to neuronal apoptosis and neurodegeneration by generating free radicals [24]. While in affected cells kynureninase converts 3-HK into QUIN that have the potential role in neurotoxicity and neuronal dysfunction [25]. However, it is seen that KYNA metabolite has the effect of blocking QUIN and other excitotoxins [23,24]. The ratio of KYN metabolites alters glutamatergic signalling and protects against excitotoxicity mediated by N-methyl-d-aspartate (NMDA) receptors. These findings suggest that KP has a major role in physiological conditions.
3. Impact of KP in ageing
Researches have observed that an increased TRP degradation rate was found in ageing people, which revealed that the degradation of TRP accompanies ageing through KP [26,27]. TRP and the levels of its metabolites and enzymes studied in the brain, liver, and kidney of young, middle and old aged female Wistar rats, where it was found that TRP and TDO activity decreased with age in all tissues [17]. In C. elegans model, the TDO activity was reduced that in turn suppressed the toxicity of αSyn and other aggregation-prone proteins which suggested reducing proteotoxicity in ageing and its related disorders [28]. In contradictory, another study showed that the IDO activity was increased in the brain and decreased in liver and kidney with age [17]. Thus, based on the earlier results it is evident that low – grade sustained inflammation and up-regulation of IDO has a role in ageing and its associated diseases [22]. This increased inflammation observed in ageing is acting as a driving force in KP activity and results in over – production of QUIN, which might increase the chances of neurodegenerative diseases [29]. Earlier it was noted that picolini acid (PA) was elevated in human CSF samples [30] and liver [31]. Another prominent study revealed that the levels of KYNA in CSF were significantly increased with progressing age of healthy as well as patients susceptible to neurodegenerative diseases [32]. In a study, enhanced level of KYNA was assessed in the brain of 3 months and 4 months old rats, which correspondingly showed the precursor, KYN with increased levels in cortex and hippocampus regions of these rats whereas KAT increased three-fold in cortex and striatum [33]. In contrast, the level of KYNA was reduced in the CSF of PD and Alzheimer's disease (AD) patients [34].
NAD+ is a major component among the metabolic coenzymes or substrates that found inside every cellular activity and energy production. The importance of the final product NAD+ is essential for the production of adenosine triphosphate (ATP). The major NAD+ biosynthetic pathway includes de novo synthesis pathway, Preiss-Handler pathway and salvage pathway. The de novo is the multi-enzymatic KP eventually producing nicotinic acid mononucleotide from the last enzyme step. Recent study has stated that the role of NAD+ on ageing can prevent from age related diseases [35]. Fang et al. [36] also disclosed the novel role of NAD+ in autophagy and mitophagy. Altered KP activity impacts NAD+ production thereby results in age associated diseases [37]. KP metabolites and enzyme derivatives have an impact on nicotinamide adenine dinucleotide phosphate (NADPH) synthesis and NMDA receptor-mediated transmission that may contribute to the degenerative changes of the aged persons [17]. A study has reported that the decreased TRP metabolism leads to a declined level of nicotinic acid biosynthesis were the NAD coenzymes functions in biogenetic and biosynthetic pathways [38]. In an earlier study, the levels of various KP metabolites such as an increase in KYN level or decrease in TRP concentration and AA concentration there is an evident increase in levels of NAD+ levels in cells [17]. Thus, increased concentrations of KP metabolites and inhibition of KP enzymes can cause KP toxicity and that it is possible to replenish the depleted NAD+ levels with TRP, KYN, nicotinic acid or salvage pathway precursor nicotinamide supplementation [39]. Hence, it is evident from the above studies that, not only KP metabolites but also its enzymes have a pivotal role in the process of ageing. Grant and Kapoor [40] claimed that the activity of IDO with interferon-γ (IFN-γ) could increase the concentration of NAD required for maintaining cellular energy metabolism during inflammation suggesting the use of inhibitors in KP metabolism. Similarly, in macrophages, activation of IFN-γ leads to an increase in the immune-mediated IDO activity, thus promoting an elevation in the production of NAD+ molecules [41]. Conflictingly, another study revealed that the inhibition of IDO and quinolinate phosphoribosyl transferase by 1- methyl-L-tryptophan and phthalic acid gives a dose-dependent decrease in intracellular NAD+ levels and sirtuin deacetylase-1 activity resulting in reduced cell viability [17]. Bellac et al. [42] has studied that the KP inhibitors Ro-61-8048 and o-methoxybenzoylalanine can effectively decrease the NAD+ levels in cells, which gives rise to induced inflammation and increased hippocampal apoptosis; thus it recommends that these inhibitors also act in neuroinflammation and neuroinfectious diseases. Thus, more studies and clinical trials have to be conducted mainly focusing on the development of inhibitors and analogues related to KP metabolites and enzymes.
4. KP and PD
In animal models of PD increased levels of KYNA in brain can protect nigrostriatal dopamine neurons against QUIN-induced excitotoxin damage [43]. Miranda et al. [43] used QUIN infusions in the rat brain that lowered the levels of KYN and KYNA. Low levels of KYNA are also capable of decreasing the limit of excitotoxicity. A decrease in KYNA level in rat brain noticed when administered with L-dopamine (L-DOPA) and D-amphetamine. [44,45]. In PD rats, brain and plasma, were examined for the level of TRP and its metabolites which showed an increased level of TRP, KYN, 3-HK and QUIN [46]. The bilateral injection of KYNA in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) treated PD monkeys and rats lessen the motor symptoms in a dose-dependent manner [47,48]. The level of KYNA and the biosynthetic enzymes KAT-I and KAT-II analyzed in plasma and red blood cells (RBCs) of PD patients. Increase in KYNA along with KAT-II in RBCs of PD patients, may be a sequential pathway against excitatory neurotoxic effects [49]. It has also been shown that reduced KAT-I activity [50] and KYNA activity [51] is responsible for MPTP and 6-hydroxydopamine (6-OHDA) toxicity. Similarly, KAT-I and KAT-II activities were lower in plasma of PD patients with a decrease in KYNA whereas an elevated level of KYNA was associated with an increase in KATs in RBC of PD patients [52]. Besides, KAT-II inhibition increases two to three fold of striatal dopamine level which can be protected by co-administration of KYNA [53]. In 2017, a study in PD patients showed lower TRP concentrations with higher KYN: TRP ratio, KYN, AA and KYNA than in controls [54]. Further studies with reduced levels of KYNA were observed in the cortical regions, caudate, putamen, substantia nigra pars compacta (SNpc), and cerebellum of PD patients [49,55]. The molar ratio of TRP to KYN and KYN to KYNA remain unchanged in PD patients treated with and without L-DOPA. The study also showed a decrease in KYN and KYNA with an increase in 3-HK [56]. A detailed review of the involvement of KP in PD pathogenesis was described by Lim et al. [21] in 2015. Treatment by L-DOPA in PD can alter the levels of kynurenine metabolites and it also affects the glutamatergic transmission that leads to L-DOPA-induced dyskinesia (LID). Havelund et al. [57] conducted a study to look into the possible role of KYN metabolites in LID, where the assessment of KP metabolites in plasma and CSF of PD patients with LID showed a four-fold increase in the 3-HK/KYNA ratio and a decrease in the levels of AA. In neurodegenerative disorders, 3-HAA causes oxidative stress thereby results in the production of reactive oxygen species [58]. In healthy non-smoking subjects, stochastic resonance therapy was found to affect TRP metabolism by reducing the levels of TRP, KYN and KYNA that might induce neuropsychiatric disorders such as AD, PD, depression and schizophrenia [59]. Exposure of BDE-47, a flame retardant in Drosophila PD model, slowed down the formation of KYNA with an increase in 3-HK [60]. Potential antioxidant activity of KYNA and xanthurenic acid (XA) reduces neurodegenerative diseases by maintaining redox homeo dynamics [61].
5. Microbiota-gut-brain association with KP in PD
Historically, neurological diseases have studied within the CNS, but researches proved the association between the gut and the brain. In PD, pathological αSyn accumulation is initiated in the gut and disseminates to the brain through the vagus nerve [62]. The αSyn inclusions called Lewy bodies has been observed in highest frequency in submandibular gland and lower esophagus followed by small intestine, stomach, colon and rectum [63]. The presence of αSyn is normally observed in people with increasing age [64]. Constipation is a non-motor PD symptom that is linked with αSyn accumulation and neurodegeneration with elevated signs of inflammation, oxidative stress and intestinal permeability [65,66]. These pathological changes are found prior to motor symptoms in PD, which confirms that PD pathogenesis is initiated in the gut. Noteworthy, experimental proof for the association between gut microbiome in causing PD is missing.
Studies have reported the intense effect of gut microbiota on KP metabolites. KYN in the gut can cross the blood brain barrier and contribute to the production of KP metabolites in the brain [67]. QUIN and KYNA are the most important KP metabolites in neuro gastroenterology studies were the function is not yet understood, but still, these metabolites act as immunoregulators [68]. The metabolite KYNA concentration in the gastrointestinal tract exerts gastric mucosal defence mechanism and immunoregulatory effects through a G protein-coupled receptor, GPR35 [69]. Moreover, various intestinal bacteria encode KP enzymes that produce KYN and 3-HAA [70], which results in neurotoxic effects during pathological conditions [71]. Many studies have reported the altered plasma levels of KP metabolites due to microbial colonization under pathological conditions [[72], [73], [74], [75]]. Evidence derived from microbiome studies has shown that the gut microbiota is one of the factors in KYN pathway to prevent or alleviate intestinal inflammation. A recent study has suggested that excess level of QUIN due to mutated KP lead to the formation of metabolite assemblies that causes αSyn aggregation, which fallouts with neuronal toxicity and PD [76]. The link between gut microbiome associated with KP in neurodegenerative disorders have been reviewed in previous studies [77,78]. At present, the role of gut linked with brain has attracted prominent attention since the complexity between the gut microbiota and KP would be beneficial in exploring therapeutic strategies in treating neurodegenerative disorders.
6. Genetic link between KP and PD
Sequencing of genomes associated with KP enzymes encoding regions such as IDO1/2, ACMSD, TDO2, KYNU, 3-HAO, KATII, KMO has been identified in certain neurological diseases [79]. Until now, only two kynurenine enzymes, KMO and amino-carboxymuconate semialdehyde decarboxylase (ACMSD) genes have been found to be with PD (Table 1). KMO mutations have also been studied in bipolar disorder and schizophrenia [15,[80], [81], [82]]. The interesting factor for the significant association between ACMSD in PD is that QUIN with excitotoxic property contributes to the progression and modification in the inflammatory response in PD [83]. The expression of allele-specific differences in ACMSD has to be examined to understand an exact link between ACMSD and PD [83]. From genome-wide association studies, SNPs on ACMSD identified its ability as a risk in PD [[84], [85], [86], [87], [88], [89], [90], [91]]. In a Spanish family, it was found that the family had a history of parkinsonism, epilepsy and myoclonic tremor and also showed mutation in p.Trp26 which is a top codon in ACMSD [92]. In a sporadic PD case, a novel p.Glu298Lys mutation associated with ACMSD enzyme has been examined with no first-degree family history; hence it suggests that one disrupted allele is adequate to cause idiopathic PD [93]. Thus, the above studies will direct for in vivo and in vitro studies to detect the genetic alterations of KP enzymes in PD.
Table 1.
Studies on genetic association of kynurenine pathway in Parkinson's disease.
| Gene | Location | No. of exons | rs number | No. of patients | Mutation | Results | Reference |
|---|---|---|---|---|---|---|---|
| KMO | chr 1 | 17 exons | rs2275163 | 105 cases | No association of SNPs | First investigation in KMO gene and the genetic link in PD is missing | [15] |
| rs1053230 | |||||||
| rs2050518 rs6661244 | |||||||
| ACMSD | chr 2 | 13 exons | rs6430538 | 989 PD cases | NIL | No polymorphism contribute to PD | [84] |
| 599 PD cases | T/C | No significant difference observed between allelic and genotypic frequencies | [85] | ||||
| 6476 PD cases | T/C | GWAS studies identified protein altering variants in 29 PD loci. | [91] | ||||
| Case study | p.Glu298Lys | First study to identify the mutation and this would deregulate kynurenine pathway in some PD cases | [93] | ||||
| 240 | T/C | GWAS studies analyzed for homozygosity and structural genomic variations which resulted in novel characterization in PD | [89] | ||||
| 13,708 cases | T/C | Meta-analysis identified the replication association analysis. | [90] | ||||
| Spanish family | c.77G > A | Study focused on Familial cortical myoclonic tremor and epilepsy along with parkinsonism were the findings implicates KP in neurodegeneration | [92] | ||||
| rs10928513 | 1345 cases | – | The replication study analyzed 11 associated genes were the susceptible loci were well represented | [88] | |||
| rs6723108 | 16,452 PD | From meta-analysis study, ACMSD alone showed genome-wide significance | [86] | ||||
| rs10928513 | 8750 cases | – | No association was observed with ACMSD | [87] |
chr– chromosome; KMO-kynurenine – 3-monooxygenase; SNPs-single nucleotide polymorphisms; ACMSD - aminocarboxymuconate semialdehyde decarboxylase; GWAS-genome wide association studies.
7. KP biomarkers in PD
Several biological fluids are considered as the possible candidate for the detection of biomarkers that include blood, CSF and urine (Table 2) [94]. Burgos et al. [95] found that collection of CSF and serum, along with a complete evaluation of phenotypic conditions from PD patients, concluded that these biofluids reflected the exact cellular changes in diseased neuronal tissue. It was stated that the compounds associated with KP could cross the blood-brain barrier easily and enter into CSF [4]. It is suspected that there is a direct or indirect connection between PD and KP metabolites along with variations in blood. In ageing, up-regulated levels of IDO in the blood can be observed in PD [22]. Moreover, the ratio of KYN/KYNA in αSyn expressing fruit flies and urine of PD patients were found to be increased [96]. In urine, 18 differentially expressed metabolites were detected in PD patients in which the level of KYN seems to be elevated [97]. Another study also showed a higher level of KYN and 3-HK in CSF that may induce oxidative stress in PD patients [98]. In the serum of 18 PD patients and 20 AD patients, the level of KYN: TRP ratio, KYN, AA and KYNA were elevated [54]. Recently, the ratio of QUIN/KYNA and the level of QUIN were found to be higher in the plasma of PD patients [99,100]. Another recent KYN pathway study in PD has found that the level of KYNA was reduced in CSF with an increased level of KYN, KYNA and QUIN in serum [34]. Therefore, biomarker studies are essential to identify the early signs of PD and further profiling are useful to develop pharmacological interventions.
Table 2.
List of biomarker studies in bio-fluidsand its outcome on KP metabolites of Parkinson's disease.
| Biofluid | Metabolites | Model | No. of subjects | Primary outcome | Analytical methods | Pathway | Results | Conclusion | Reference |
|---|---|---|---|---|---|---|---|---|---|
| CSF and serum | Human | 105 patients | Effect of age on KYN metabolites and analysis of the influence of medication use | LC-MS | Tryptophan metabolism | ↓KYNA in CSF | |||
| (n = 33 AD patients | ↑ KYN, KYNA and QUIN in serum | KP has a potential as a marker of disease progression or as a therapeutic target in neurodegeneration | [34] | ||||||
| n = 33 PD patients | |||||||||
| n = 39 control subjects) | |||||||||
| Serum | Knurenines | Human | 18 PD patients | Compare peripheral kynurenines with metabolic syndrome associated kynurenines | HPLC | Tryptophan metabolism | ↓ TRP concentration | ||
| 20 AD patients | ↑KYN: TRP ratio, KYN, AA and KYNA | Association between neurodegenerative disorders and metabolic syndromes | [54] | ||||||
| Plasma | 184 metabolites | Human | 82 PD patients | Quantified the plasma levels of metabolites | LC-TOFMS | Pathomechanistic pathways | ↓KYNA/KYN ratio | ||
| ↑ QUIN/KYNA ratio | Plasma biomarkers are available for predicting PD | [99] | |||||||
| Human | Catabolism of tryptophan and tyrosine in relation to the isoprenoid pathway in some neurological and psychiatric disorder | HPLC | ↑ QUIN | Hypercatabolism of tryptophan could be a consequence of the modifying effect of hypothalamic digoxin on amino acid transport. | [100] | ||||
| Urine | urinary metabolites | Human | |||||||
| Drosophila melanogaster | 106 PD | Identified the correlation between clinical phenotype and urinary metabolite profile | GC–MS | ||||||
| LC-MS | Tryptophan metabolism | ↑α-synuclein and modified tryptophan metabolism. | Urinary metabolites were assessed in PD | ||||||
| Urinary metabolomics profiling would benefit in relating metabolic pathway deviations and metabolites signatures to characterize PD | [96] | ||||||||
| Characterize the urinary metabolites in PD patients | – | ↑KYN | Alterations in metabolic profiling is associated with PD | [97] | |||||
| CSF | Human | 92 PD patients | Examine the function of KYN pathway and its interaction between oxidative stress and neuroinflammation | HPLC | |||||
| Human | 20 PD patients | ELISA | KYN pathway | ↑KYN and 3-HK | Dysfunctional KYN pathway may induce oxidative stress and neuroinflammation | [98] |
PD-Parkinson's disease; CSF-cerebrospinal fluid; KYNA - Kynurenic acid; KYN – kynreunine; QUIN – quinolinic acid; AA –Anthranilic acid; GC–MS-Gas chromatography mass spectrometry; LC-MS- Liquid chromatography–mass spectrometry; LC-TOFMS - liquid chromatography time-of-flight mass spectrometry; HPLC – High performance liquid chromatography; ELISA – Enzyme Linked Immunosorbent Assay.
8. Targeting KP and its associated enzymes in PD
Modification of endogenous concentrations of KYN, QUIN and KYNA has evolved as a possible therapeutic approach in animal models of PD. The KP pathway can be targeted in two ways, namely: 1) enzyme inhibitors and 2) KYNA analogues [101].
9. Enzyme inhibitors for KP
Enzyme inhibitors facilitate neuroprotective effects and inhibit QUIN induced neurotoxicity and other neurotoxic substances. Nicotinylalanine, an inhibitor of KMO and kynureninase, prevent the accumulation of QUIN and 3-HK in models of neurological disorders [102,103] which in turn increases the concentration of KYNA [101]. When administered along with L-kynurenine and probenecid, which is an inhibitor of organic acid transport, it further increases KYNA and prevents seizures along with excitotoxic damage. Earlier it was also found that meta-nitro benzoyl alanine inhibits kynurenine-3-hydroxylase whereas ortho-methoxy benzoyl alanine inhibits kynureninase [104]. Zonisamide (ZNS) is an antiepileptic drug administered in cultured astrocytes showed an increased level of KYNA, KYN, XA, cinnabarinic acid but not QUIN. This shows the effectiveness of ZNS as an additional treatment for PD during L-DOPA therapy [105].
10. KYNA analogues
The effort of developing antagonists that bind at glutamate sites have provided great therapeutic strategies in PD. Neuroprotection by KP can be achieved through the activity of KYNA with analogues such as glutamate mimics since a raise in glutamate receptor activation leads to stroke or neurodegeneration [106]. Interestingly, KYNA analogues such as chloro-KYNA can easily cross the blood-brain barrier when compared to KYNA. The effectiveness of these analogues is increased further by substituting acetic acid or amido- and thio- compounds. Moreover, NMDA receptors antagonists are processed from synthetic KYNA derivatives for anticonvulsive effects and neuroprotection [101]. These derivatives crossed the blood-brain barrier [106] and showed protection against cerebral ischemia in vitro [107]. Furthermore, the prodrug form of 4-chloro-KYN entered the brain through the blood-brain barrier and inhibited QUIN neurotoxicity in rat hippocampus, and striatum [[108], [109], [110]] and intraperitoneal administration of pro-drugs delivered potential effects in epilepsy and neurodegenerative disorders [111]. Hence, the use of enzyme inhibitors and KYNA analogues reduce the excitatory amino acid receptors activation, thereby ensuring increased production of KYNA in CNS, which might stop or alter the progress of neurodegenerative diseases.
11. KP therapeutic interventions in PD
The most commonly used drug for PD is L-DOPA and DA agonists which are only capable of relieving the symptoms and does not assist in slowing down the neurodegenerative process. The prolonged usage of this therapeutics leads to serious side effects such as LID and motor fluctuations [112] hence, it is necessary to develop medications that reduce these side effects. Thus, as a better alternative, targeting KP metabolites as a therapeutic approach would reduce the side effects of long term L-DOPA usage (Table 3).
Table 3.
Therapeutic approaches of kynurenine pathway in in vitro and in vivo of Parkinson's disease.
| Therapy | Aim of the study | Model | Dosage | Effects | Results | Ref |
|---|---|---|---|---|---|---|
| Ro- 61-8048 | Prolong administration of KMO inhibitor reduces LID | Four MPTP treated monkeys received L-dopa (100 mg) and benserazide (25 mg) for a month to develop LID | 50 mg/kg daily before treating with L-Dopa/benserazide for a month | ↑serum kynurenine | [119] | |
| ↑KYNA reverted after last treatment | LID was reduced whereas no effects no L-Dopa | |||||
| First experiment | ||||||
| Assess the NMDA receptor in L-DOPA + MPTP treated monkeys | Two experiments were conducted in MPTP treated monkeys | 1)MPTP treated group (0.5 mg in saline) | [120] | |||
| 2) L-Dopa/benserazide treated (100/25 mg) | Glutamate receptors analyzed by autoradiography using [3H]CGP-39653 (NR1/NR2A antagonist) and [3H]Ro25–6981 (NR1/NR2B antagonist). | Striatal [3H]CGP-39653 was unaltered↓ MPTP lesion [3H]Ro25–6981 binding seen in L-Dopa alone treated MPTP monkeys ↓antidyskinetic drugs treated monkeys | ||||
| 3) L-Dopa + CI1041 (10 mg/kg) | ||||||
| 4) L-Dopa + cabergoline (0.015 to .035 mg/kg) | ||||||
| Second experiment | ||||||
| 1) MPTP treated | ||||||
| 2) L-Dopa/benserazide (100/25 mg) | ||||||
| 3) Ro- 61-8048 (50 mg/kg) + L-Dopa | ||||||
| To examine the biochemical and behavioral effects of systemic Ro 61–8048 administration in MPTP parkinsonian monkeys | Four to six female monkeys | MPTP was administered about 40 ml by oral gavage Prolopa (a mixture of 100 mg L-Dopa and 25 mg benserazide)administered orally | ↑ KYNA concentration in serum and CSF when administered Ro 61–8048 alone or with L-Dopa | Ro 61–8048 + L-Dopa administration reduced dyskinesias | [121] | |
| Elevation of KYNA levels through inhibition of kynurenine 3-hydroxylase constitutes a novel approach for managing LID in PD | ||||||
| KYNA pre-treatment | induced neuronal cell death cause increase mitochondrial dysfunction and caspase9/3 activity | SH-SY5Y and SK-N-SH human neuroblastoma cells | Cell lines treated with KYNA 2 h before MPP+ treatment | ↑Bax expression and Δψm ↑ cytochrome c release and caspase9/3 activity | KYNA plays a protective role by declining Bax expression and maintaining mitochondrial dysfunction | [50] |
| L-Kyn + probenecid | Treatment with L-KYN and probenecid evaluated behavioral, morphological and neurochemical alterations in 6-OHDA rats | Rodents | probenecid (50 mg/kg, i.p.) + L-KYN (75 mg/kg, i.p.) and injected with 6-OHDA (20 μg/2 μl) | ↑rotation behavior, ↑striatal reactive gliosis, ↑neurodegeneration, ↓ dopamine levels | Therapy modulated glutamatergic and cholinergic activities by increasing endogenous KYNA levels | [51] |
| Nicotinylalanine+ L-kynurenine and probenecid | Protection of dopaminergic neurons against QUIN toxicity through endogenous KYNA | Rats | nicotinylalanine (5.6 nmoles), kynurenine (450 mg/kg i.p.) and probenecid (200 mg/kg i.p.) induced | ↑ KYNA levels with ↓ QUIN levels in substantia nigra and whole brain | The ↑ KYNA levels prevent the loss of dopaminergic neurons from QUIN infusion | [42] |
| Synthetic kynurenines | Effects of four synthetic kynurenines on MPTP treated PD model | Mice | Seven doses of MPTP (7 × 15 mg/kg) injected: four doses of 15 mg/kg MPTP injected (sc) on the first day and three additional doses after 24 h. Later, kynurenine (20 mg/kg)injected | ↓iNOS/i-mtNOS activity, complex I activity | synthetic kynurenines hasneuroprotective properties against PD | [114] |
PD – Parkinson's disease; MPTP - 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; L-Dopa – Levodopa; LID – L-Dopa induced dyskinesia; KMO – kynurenine -3-monooxygenase; KYNA – kynurenic acid; NMDA - N-methyl-d-aspartate; [3H] – tritium radioactive; NR1,NR2A,NR2B – subunits of NMDA receptors; MPP+ − 1-methyl-4-phenylpyridinium; Δψm– mitochondrial membrane potential; L-Kyn – L-Kynurenine; 6-OHDA - 6-hydroxydopamine; QUIN –quinolinic acid; iNOS – inducible nitric oxide synthase; i-mtNOS – mitochondrial iNOS; AMPA- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.
KP metabolites are associated with neurotransmission of DA, glutamatergic and cholinergic regulation, thus targeting KP might relieve motor and non-motor symptoms in PD [113] It has been observed that pre-treatment of KYNA in a human DA neuroblastoma cell line reduced MPP+ induced neuronal cell death [50] and in combination with NMDA or QUIN in SNpc, it conserved striatal tyrosine hydroxylase activity [42]. Additionally, KYN along with probenecid revealed protective effects [51], and when the nicotinylalanine supplement was also added, it presented elevated KYNA concentration, reduced effects of QUIN excitotoxicity and beneficial effects against NMDA in SNpc [42]. Additionally, synthetic kynurenines unveiled positive effects in MPTP model [114] and 4-Cl-hydroxyanthranilate, a metabolized form of 4-chloro-KYN showed neuroprotective action in rat hippocampus and striatum [109,115]. Currently, FK506 is used as an immunosuppressant in PD and inhibits MPP+ and 3-nitropropionic acid-induced KYNA synthesis improving the formation of KYNA in the cortex [116]. Similarly, another inhibitor UPF648 is responsible for the enhanced synthesis of KYNA in excitotoxically lesioned neuro-depleted striata [117]. Further, the inhibitor Ro-61-8048 increased the levels of L-KYN and KYNA and decreased LIDs without affecting the anti-parkinsonian effect of L-DOPA [[118], [119], [120], [121]]. Inhibition of TDO and KMO in fruit fly models of AD, PD and Huntington's disease reversed disease phenotype, emphasizing the therapeutic promise in neurodegenerative disorders [12].
Various researches in KP has led to the speculation that metabolites in the downstream pathway lead to the development of disorders like PD, hence these metabolites serve as a potential therapeutic target. Besides, the major component of KP is TRP, targeting TRP enzyme can reduce the toxicity in neurons and provide neuroprotection in PD. The possibility of targeting KP to reduce QUIN and further increase the level of KYNA in the brain offers a new approach to decrease excitotoxicity and enhance neuroprotection.
12. Future directions
The catabolism of the essential amino acid TRP and biogenesis of NAD+ are linked through KP. KP was originally implicated in psychiatric disorders, cancers, and neuroinflammatory disorders, but in recent years KP has garnered much attention due to its promising therapeutic aspects in neurodegenerative diseases. NAD+ levels decreases with age but during neuroinflammatory and neuroinfectious conditions, NAD+ concentration has shown to increase. In these conditions, the use of NAD+ inhibitors would control the level, which led to the hope of achieving promising outcomes in PD. KP and its intermediates are believed to play a pivotal role in the synthesis of NAD+ and cellular bioenergetics in PD prognosis. On the other hand, it is essential to validate the importance of the metabolites KYNA and QUIN in the regulation of KP. Inhibitors and analogues to these metabolites could be the key to slow down the advancement of PD. Depending on their influence in the pathway, these metabolites could also serve as prognostic markers which will reveal the impending development of PD. The possible interventions by these metabolites in PD, their inhibitors and the impact on NAD+ have not been widely studied. At the clinical level, the field of stem cell research in KP associated diseases are already actively emerging. With the help of stem cell research and animal models, it is possible to understand the influence of the metabolites and their inhibitors in the pathway. To overcome the crucial hindrances, the impact of stem cell therapy might help in understanding the function of KP metabolites for future therapeutic interventions.
13. Conclusion
The principle mechanism behind the pathological conditions of PD is yet to be fully understood. It is evident that KP metabolites can be used as novel prognostic markers and modulators for targeting therapy in PD. In order to provide better clinical intervention and treatment at the onset of PD, it is imperative to find accurate biomarkers for early diagnosis, including prodromal diagnosis and preclinical diagnosis. At the same time, KP biomarkers can also be utilized to monitor the progress of the disease. Accurate and early diagnoses are significant for successful treatment in PD patients, and sensitive and selective biochemical biomarkers will play an essential role in the detection of prodromal PD. Hence, we would propose that targeting KP and its associated enzymes using inhibitors or novel compounds would be an effective therapeutic means by which other agents can interact and possibly emerge as a promising treatment in PD.
Funding
This work was supported by ICMR IF (grant no. INDO/FRC/4 52 N-47 1201 8–19-lHD; dated: 09.10.2018) and ICMR SRF (grant no. 45/2/2018-Hum/BMS; dated: 31.05.2018) to complete the review work.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Acknowledgement
The authors thank Indian Council of Medical Research (ICMR) for awarding International Fellowship (IF) and Senior Research Fellowship (SRF) to complete the review article successfully. Also, the authors thank Bharathiar University, Coimbatore, India for the support and providing the necessary infrastructural facilities to carry out the review work.
Fig. 1 description: Conversion of tryptophan (TRP) to kynurenine (KYN) requires rate limiting enzymes. Peripheral KP influences in brain thereby the kynurenine metabolites in the blood affects KP metabolism in brain since it crosses the blood-brain barrier. In the brain, KYN is converted to kynurenic acid (KYNA) by the enzyme kynurenine aminotransferase (KAT) in astrocytes which exhibits neuroprotective role. In microglia, KYN is converted to 3-hydroxykynurenine (3−HK) by kynurenine monooxygenase (KMO) which is further metabolized non-enzymatically to quinolinic acid (QUIN) which unveils neurotoxic effects.
Contributor Information
Dhivya Venkatesan, Email: dhivi893@gmail.com.
Balachandar Vellingiri, Email: geneticbala@buc.edu.in.
References
- 1.Kaavya J., Mahalaxmi I., Dhivya V., Venkatesh B., Arul N., Devi S.M., Cho S.G., Balachandar V. Unraveling correlative roles of dopamine transporter (DAT) and parkin in Parkinson’s disease (PD)-a road to discovery? Brain Res. Bull. 2020;157:169–179. doi: 10.1016/j.brainresbull.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Mohana D.S., Mahalaxmi I., Aswathy N.P., Dhivya V., Balachandar V. Does retina play a role in Parkinson’s disease? Acta Neurol. Belg. 2020;120:257–265. doi: 10.1007/s13760-020-01274-w. [DOI] [PubMed] [Google Scholar]
- 3.Dhivya V., Ramkumar S., Illakiyapavai D., Sangeetha M., Ganesan S., Devi S.M., Sasikala K., Balachandar V. Screening of genetic mutations in early onset parkinsonism patients: a family based study in Tamil Nadu population. Int. J. Hum. Genet. 2016;16:158–165. doi: 10.1080/09723757.2016.11886293. [DOI] [Google Scholar]
- 4.Schwarcz R., Bruno J.P., Muchowski P.J., Wu H.Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platten M., Nollen E.A., Röhrig U.F., Fallarino F., Opitz C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019;18:379–401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 6.Cervenka I., Agudelo L.Z., Ruas J.L. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794. doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 7.Munn D.H., Mellor A.L. Indoleamine 2, 3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Opitz C.A., Heiland I. Dynamics of NAD-metabolism: everything but constant. Biochem. Soc. Trans. 2015;43:1127–1132. doi: 10.1042/BST20150133. [DOI] [PubMed] [Google Scholar]
- 9.Agudelo L.Z., Ferreira D.M., Cervenka I., Bryzgalova G., Dadvar S., Jannig P.R., Pettersson-Klein A.T., Lakshmikanth T., Sustarsic E.G., Porsmyr-Palmertz M., Correia J.C. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metab. 2018;27:378–392. doi: 10.1016/j.cmet.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L.S., Davies S.S. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome. Med. 2016;8:46. doi: 10.1186/s13073-016-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacol. 2017;112:399–412. doi: 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Breda C., Sathyasaikumar K.V., Idrissi S.S., Notarangelo F.M., Estranero J.G., Moore G.G., Green E.W., Kyriacou C.P., Schwarcz R., Giorgini F. Tryptophan-2, 3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc. Natl. Acad. Sci. U. S. A. 2016;113:5435–5440. doi: 10.1073/pnas.1604453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erhardt S., Schwieler L., Imbeault S., Engberg G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacol. 2017;112:297–306. doi: 10.1016/j.neuropharm.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Lim C.K., Fernández-Gomez F.J., Braidy N., Estrada C., Costa C., Costa S., Bessede A., Fernandez-Villalba E., Zinger A., Herrero M.T., Guillemin G.J. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog. Neurobiol. 2017;155:76–95. doi: 10.1016/j.pneurobio.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Török N., Török R., Szolnoki Z., Somogyvári F., Klivényi P., Vécsei L. The genetic link between Parkinson’s disease and the kynurenine pathway is still missing. Parkinsons Dis. 2015 doi: 10.1155/2015/474135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houser M.C., Tansey M.G. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 2017;3:1–9. doi: 10.1038/s41531-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braidy N., Guillemin G.J., Mansour H., Chan-Ling T., Grant R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011;278:4425–4434. doi: 10.1111/j.1742-4658.2011.08366.x. [DOI] [PubMed] [Google Scholar]
- 18.Braidy N., Berg J., Clement J., Khorshidi F., Poljak A., Jayasena T., Grant R., Sachdev P. Role of nicotinamide adenine dinucleotide and related precursors as therapeutic targets for age-related degenerative diseases: rationale, biochemistry, pharmacokinetics, and outcomes. Antioxid. Redox Signal. 2019;30:251–294. doi: 10.1089/ars.2017.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widner B., Leblhuber F., Fuchs D. Increased neopterin production and tryptophan degradation in advanced Parkinson's disease. J. Neural Transm. 2002;109:181–189. doi: 10.1007/s007020200014. [DOI] [PubMed] [Google Scholar]
- 20.LeWitt P.A., Li J., Lu M., Beach T.G., Adler C.H., Guo L., Arizona Parkinson's Disease Consortium 3-hydroxykynurenine and other Parkinson's disease biomarkers discovered by metabolomic analysis. Mov. Disord. 2013;28:1653–1660. doi: 10.1002/mds.25555. [DOI] [PubMed] [Google Scholar]
- 21.Lim C.K., Fernández-Gomez F.J., Braidy N., Estrada C., Costa C., Costa S., Bessede A., Fernandez-Villalba E., Zinger A., Herrero M.T., Guillemin G.J. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog. Neurobiol. 2017;155:76–95. doi: 10.1016/j.pneurobio.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Sas K., Szabó E., Vécsei L. Mitochondria, oxidative stress and the kynurenine system, with a focus on ageing and neuroprotection. Mol. 2018;23:191. doi: 10.3390/molecules23010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillemin G.J., Kerr S.J., Smythe G.A., Smith D.G., Kapoor V., Armati P.J., Croitoru J., Brew B.J. Kynurenine pathway metabolism in human astrocytes;a paradox for neuronal protection. J. Neurochem. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 24.Chiarugi A., Meli E., Moroni F. Similarities and differences in the neuronal death processes activated by 3OH-kynurenine and quinolinic acid. J. Neurochem. 2001;77:1310–1318. doi: 10.1046/j.1471-4159.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- 25.Guidetti P., Schwarcz R. 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur. J. Neurosci. 1999;11:3857–3863. doi: 10.1046/j.1460-9568.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 26.Frick B., Schroecksnadel K., Neurauter G., Leblhuber F., Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin. Biochem. 2004;37:684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Pertovaara M., Raitala A., Lehtimäki T., Karhunen P.J., Oja S.S., Jylhä M., Hervonen A., Hurme M. Indoleamine 2, 3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech. Ageing Dev. 2006;127:497–499. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Van Der Goot A.T., Zhu W., Vázquez-Manrique R.P., Seinstra R.I., Dettmer K., Michels H., Farina F., Krijnen J., Melki R., Buijsman R.C., Silva M.R. Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation. PNAS. 2012;109:14912–14917. doi: 10.1073/pnas.1203083109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Cruz V.P., Carrillo-Mora P., Santamaría A. Quinolinic acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. IJTR. 2012;5:S8158. doi: 10.4137/IJTR.S8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coggan S.E., Smythe G.A., Bilgin A., Grant R.S. Age and circadian influences on picolinic acid concentrations in human cerebrospinal fluid. J. Neurochem. 2009;108:1220–1225. doi: 10.1111/j.1471-4159.2009.05868.x. [DOI] [PubMed] [Google Scholar]
- 31.Moroni F. Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur. J. Pharmacol. 1999;375:87–100. doi: 10.1016/S0014-2999(99)00196-X. [DOI] [PubMed] [Google Scholar]
- 32.Kepplinger B., Baran H., Kainz A., Ferraz-Leite H., Newcombe J., Kalina P. Age-related increase of kynurenic acid in human cerebrospinal fluid–IgG and β2-microglobulin changes. Neurosignals. 2005;14:126–135. doi: 10.1159/000086295. [DOI] [PubMed] [Google Scholar]
- 33.Gramsbergen J.B., Schmidt W., Turski W.A., Schwarcz R. Age-related changes in kynurenic acid production in rat brain. Brain Res. 1992;588:1–5. doi: 10.1016/0006-8993(92)91337-E. [DOI] [PubMed] [Google Scholar]
- 34.Sorgdrager F.J., Vermeiren Y., Van Faassen M., Van Der Ley C., Nollen E.A., Kema I.P., De Deyn P.P. Age-and disease-specific changes of the kynurenine pathway in Parkinson’s and Alzheimer’s disease. J. Neurochem. 2019;151:656–668. doi: 10.1111/jnc.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang E.F., Lautrup S., Hou Y., Demarest T.G., Croteau D.L., Mattson M.P., Bohr V.A. NAD+ in aging: molecular mechanisms and translational implications. Trends Mol. Med. 2017;23:899–916. doi: 10.1016/j.molmed.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang E.F., Scheibye-Knudsen M., Brace L.E., Kassahun H., SenGupta T., Nilsen H., Mitchell J.R., Croteau D.L., Bohr V.A. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD+/SIRT1 reduction. Cell J. 2014;157:882–896. doi: 10.1016/j.cell.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro-Portuguez R., Sutphin G.L. Kynurenine pathway, NAD+ synthesis, and mitochondrial function: targeting tryptophan metabolism to promote longevity and healthspan. Exp. Gerontol. 2020;132 doi: 10.1016/j.exger.2020.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bender D.A., McCreanor G.M. The preferred route of kynurenine metabolism in the rat. BBA–Gen Sub. 1982;717:56–60. doi: 10.1016/0304-4165(82)90379-8. [DOI] [PubMed] [Google Scholar]
- 39.Grant R., Nguyen S., Guillemin G. Kynurenine pathway metabolism is involved in the maintenance of the intracellular NAD+ concentration in human primary astrocytes. IJTR. 2010;3:S4779. doi: 10.4137/ijtr.s4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant R., Kapoor V. Inhibition of indoleamine 2, 3-dioxygenase activity in IFN-γ stimulated astroglioma cells decreases intracellular NAD levels. Biochem. Pharmacol. 2003;66:1033–1036. doi: 10.1016/S0006-2952(03)00464-7. [DOI] [PubMed] [Google Scholar]
- 41.Grant R.S. Indoleamine 2, 3-Dioxygenase activity increases NAD+ production in IFN-γ–stimulated human primary mononuclear cells. IJTR. 2018;11 doi: 10.1177/1178646917751636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellac C.L., Coimbra R.S., Christen S., Leib S.L. Inhibition of the kynurenine-NAD+ pathway leads to energy failure and exacerbates apoptosis in pneumococcal meningitis. J. Neuropathol. Exp. Neurol. 2010;69:1096–1104. doi: 10.1097/NEN.0b013e3181f7e7e9. [DOI] [PubMed] [Google Scholar]
- 43.Miranda A.F., Boegman R.J., Beninger R.J., Jhamandas K. Protection against quinolinic acid-mediated excitotoxicity in nigrostriatal dopaminergic neurons by endogenous kynurenic acid. Neuroscience. 1997;78:967–975. doi: 10.1016/S0306-4522(96)00655-0. [DOI] [PubMed] [Google Scholar]
- 44.Zinger A., Barcia C., Herrero M.T., Guillemin G.J. The involvement of neuroinflammation and kynurenine pathway in Parkinson's disease. Parkinsons Dis. 2011 doi: 10.4061/2011/716859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu H.Q., Rassoulpour A., Schwarcz A. Effect of systemic L-DOPA administration on extracellular kynurenate levels in the rat striatum. J. Neural Transm. 2002;109:239–249. doi: 10.1007/s007020200020. [DOI] [PubMed] [Google Scholar]
- 46.Rassoulpour A., Wu H.Q., Poeggeler B., Schwarcz R. Systemic d-amphetamine administration causes a reduction of kynurenic acid levels in rat brain. Brain Res. 1998;802:111–118. doi: 10.1016/S0006-8993(98)00577-0. [DOI] [PubMed] [Google Scholar]
- 47.Kubesova A., Tejkalova H., Syslova K., Kacer P., Vondrousova J., Tyls F., Fujakova M., Palenicek T., Horacek J. Biochemical, histopathological and morphological profiling of a rat model of early immune stimulation: relation to psychopathology. PLoS One. 2015;10 doi: 10.1371/journal.pone.0115439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham W.C., Robertson R.G., Sambrook M.A., Crossman A.R. Injection of excitatory amino acid antagonists into the medial pallidal segment of a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) treated primate reverses motor symptoms of parkinsonism. Life Sci. 1990;47:PL91–7. doi: 10.1016/0024-3205(90)90376-3. [DOI] [PubMed] [Google Scholar]
- 49.Brotchie J.M., Mitchell I.J., Sambrook M.A., Crossman A.R. Alleviation of parkinsonism by antagonism of excitatory amino acid transmission in the medial segment of the globus pallidus in rat and primate. J. Mov. Disord. 1991;6:133–138. doi: 10.1002/mds.870060208. [DOI] [PubMed] [Google Scholar]
- 50.Knyihár-Csillik E., Chadaide Z., Mihály A., Krisztin-Péva B., Fenyő R., Vécsei L. Effect of 6-hydroxydopamine treatment on kynurenine aminotransferase-I (KAT-I) immunoreactivity of neurons and glial cells in the rat substantia nigra. Acta Neuropathol. 2006;112:127–137. doi: 10.1007/s00401-006-0086-4. [DOI] [PubMed] [Google Scholar]
- 51.Lee K.S., Lee H.J., Noh Y.H., Lee J.Y., Cho S.H., Yoon O.J., Lee W.B., Kim K.Y., Chung Y.H., Kim S.S. Kynurenic acid attenuates MPP+-induced dopaminergic neuronal cell death via a Bax-mediated mitochondrial pathway. Eur. J. Cell Biol. 2008;87:389–397. doi: 10.1016/j.ejcb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Silva-Adaya D., Pérez-De La Cruz V., Villeda-Hernández J., Carrillo-Mora P., González-Herrera I.G., García E., Colín-Barenque L., Pedraza-Chaverrí J., Santamaría A. Protective effect of L-kynurenine and probenecid on 6-hydroxydopamine-induced striatal toxicity in rats: implications of modulating kynurenate as a protective strategy. Neurotoxicol. Teratol. 2011;33:303–312. doi: 10.1016/j.ntt.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Hartai Z., Klivenyi P., Janaky T., Penke B., Dux L., Vecsei L. Kynurenine metabolism in plasma and in red blood cells in Parkinson’s disease. J. Neurol. Sci. 2005;239:31–35. doi: 10.1016/j.jns.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Wu H.Q., Rassoulpour A., Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J. Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 55.Oxenkrug G., van der Hart M., Roeser J., Summergrad P. Peripheral tryptophan-kynurenine metabolism associated with metabolic syndrome is different in Parkinson’s and Alzheimer’s diseases, Endocrinol. Diabetes Metab. J. 2017;1 http://researchopenworld.com/wp-content/uploads/2017/11/EDMJ-2017 [PMC free article] [PubMed] [Google Scholar]
- 56.Beal M.F., Matson W.R., Storey E., Milbury P., Ryan E.A., Ogawa T., Bird E.D. Kynurenic acid concentrations are reduced in Huntington’s disease cerebral cortex. J. Neurol. Sci. 1992;108:80–87. doi: 10.1016/0022-510X(92)90191-M. [DOI] [PubMed] [Google Scholar]
- 57.Ogawa T., Matson W.R., Beal M.F., Myers R.H., Bird E.D., Milbury P., Saso S. Kynurenine pathway abnormalities in Parkinson's disease. J. Neurol. 1992;42:1702. doi: 10.1212/WNL.42.9.1702. [DOI] [PubMed] [Google Scholar]
- 58.Havelund J.F., Andersen A.D., Binzer M., Blaabjerg M., Heegaard N.H., Stenager E., Faergeman N.J., Gramsbergen J.B. Changes in kynurenine pathway metabolism in Parkinson patients with L-DOPA-induced dyskinesia. J. Neurochem. 2017;142:756–766. doi: 10.1111/jnc.14104. [DOI] [PubMed] [Google Scholar]
- 59.Guillemin G.J., Williams K.R., Smith D.G., Smythe G.A., Croitoru-Lamoury J., Brew B.J. Developments in Tryptophan and Serotonin Metabolism. Spinger; 2003. Quinolinic acid in the pathogenesis of Alzheimer’s disease; pp. 167–176. [DOI] [PubMed] [Google Scholar]
- 60.Kepplinger B., Baran H., Sedlnitzky-Semler B., Badawi N.R., Erhart H. Stochastic resonance activity influences serum tryptophan metabolism in healthy human subjects. IJTR. 2011;4:IJTR–S7986. doi: 10.4137/IJTR.S7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji F., Wei J., Luan H., Li M., Cai Z. Study of metabolic disorders associated with BDE-47 exposure in Drosophila model by MS-based metabolomics. Ecotoxicol. Environ. Saf. 2019;184 doi: 10.1016/j.ecoenv.2019.109606. [DOI] [PubMed] [Google Scholar]
- 62.Kubicova L., Hadacek F., Bachmann G., Weckwerth W., Chobot V. Coordination complex formation and redox properties of kynurenic and xanthurenic acid can affect brain tissue homeodynamics. Antioxidants. 2019;8:476. doi: 10.3390/antiox8100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Del Tredici K., Braak H. A not entirely benign procedure: progression of Parkinson’s disease. Acta Neuropathol. 2008;115:379–384. doi: 10.1007/s00401-008-0355-5. [DOI] [PubMed] [Google Scholar]
- 64.Mulak A., Bonaz B. Brain-gut-microbiota axis in Parkinson's disease. World J. Gastroenterol. 2015;21:10609. doi: 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Böttner M., Zorenkov D., Hellwig I., Barrenschee M., Harde J., Fricke T., Deuschl G., Egberts J.H., Becker T., Fritscher-Ravens A., Arlt A. Expression pattern and localization of alpha-synuclein in the human enteric nervous system. Neurobiol. Dis. 2012;48:474–480. doi: 10.1016/j.nbd.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 66.Forsyth C.B., Shannon K.M., Kordower J.H., Voigt R.M., Shaikh M., Jaglin J.A., Estes J.D., Dodiya H.B., Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devos D., Lebouvier T., Lardeux B., Biraud M., Rouaud T., Pouclet H., Coron E., Varannes S.B. des, Naveilhan P., Nguyen J.M., Neunlist M. Colonic inflammation in Parkinson's disease. Neurobiol. Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 68.Westfall S., Lomis N., Kahouli I., Dia S.Y., Singh S.P., Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell. Mol. Life Sci. 2017;74:3769–3787. doi: 10.1007/s00018-017-2550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dehhaghi M., Kazemi Shariat Panahi H., Guillemin G.J. Microorganisms, tryptophan metabolism, and kynurenine pathway: a complex interconnected loop influencing human health status. IJTR. 2019;12 doi: 10.1177/1178646919852996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao J., Xu K., Liu H., Liu G., Bai M., Peng C., Li T., Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect. Mi. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vujkovic-Cvijin I., Dunham R.M., Iwai S., Maher M.C., Albright R.G., Broadhurst M.J., Hernandez R.D., Lederman M.M., Huang Y., Somsouk M., Deeks S.G. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 2013;5:193ra91. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Farrell K., Harkin A. Stress-related regulation of the kynurenine pathway: relevance to neuropsychiatric and degenerative disorders. Neuropharmacol. 2017;112:307–323. doi: 10.1016/j.neuropharm.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Shoaie S., Ghaffari P., Kovatcheva-Datchary P., Mardinoglu A., Sen P., Pujos-Guillot E., De Wouters T., Juste C., Rizkalla S., Chilloux J., Hoyles L. Quantifying diet-induced metabolic changes of the human gut microbiome. Cell Metab. 2015;22:320–331. doi: 10.1016/j.cmet.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 74.El Aidy S., Dinan T.G., Cryan J.F. Immune modulation of the brain-gut-microbe axis. Front. Microbiol. 2014;5:146. doi: 10.3389/fmicb.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clarke S.F., Murphy E.F., O’Sullivan O., Ross R.P., O’Toole P.W., Shanahan F., Cotter P.D. Targeting the microbiota to address diet-induced obesity: a time dependent challenge. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tavassoly O., Sade D., Bera S., Shaham-Niv S., Vocadlo D.J., Gazit E. Quinolinic acid amyloid-like fibrillar assemblies seed α-synuclein aggregation. J. Mol. Biol. 2018;430:3847–3862. doi: 10.1016/j.jmb.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Gheorghe C.E., Martin J.A., Manriquez F.V., Dinan T.G., Cryan J.F., Clarke G. Focus on the essentials: tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharmacol. 2019;48:137–145. doi: 10.1016/j.coph.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 79.Anderson G., Seo M., Berk M., Carvalho A.F., Maes M. Gut permeability and microbiota in Parkinson’s disease: role of depression, tryptophan catabolites, oxidative and nitrosative stress and melatonergic pathways. Curr. Pharm. Des. 2016;22:6142–6151. doi: 10.2174/1381612822666160906161513. https://doi [DOI] [PubMed] [Google Scholar]
- 80.Boros F.A., Bohár Z., Vécsei L. Genetic alterations affecting the genes encoding the enzymes of the kynurenine pathway and their association with human diseases. Mutat. Res. Rev. Mutat. 2018;776:32–45. doi: 10.1016/j.mrrev.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Ekelund J., Hovatta I., Parker A., Paunio T., Varilo T., Martin R., Suhonen J., Ellonen P., Chan G., Sinsheimer J.S., Sobel E. Chromosome 1 loci in finnish schizophrenia families. Hum. Mol. Genet. 2001;10:1611–1617. doi: 10.1093/hmg/10.15.1611. [DOI] [PubMed] [Google Scholar]
- 82.Blackwood D.H., Fordyce A., Walker M.T., Clair D.S., Porteous D.J., Muir W.J. Schizophrenia and affective disorders—cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am. J. Hum. Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gurling H.M., Kalsi G., Brynjolfson J., Sigmundsson T., Sherrington R., Mankoo B.S., Read T., Murphy P., Blaveri E., McQuillin A., Petursson H. Genome-wide genetic linkage analysis confirms the presence of susceptibility loci for schizophrenia, on chromosomes 1q32. 2, 5q33. 2, and 8p21–22 and provides support for linkage to schizophrenia, on chromosomes 11q23. 3–24 and 20q12. 1–11.23. Am. J. Hum. Genet. 2001;68:661–673. doi: 10.1086/318788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thirtamara-Rajamani K., Li P., Escobar Galvis M.L., Labrie V., Brundin P., Brundin L. Is the enzyme ACMSD a novel therapeutic target in Parkinson’s disease? Parkinson’s Dis. 2017;7:577–587. doi: 10.3233/JPD-171240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L., Li N.N., Lu Z.J., Li J.Y., Peng J.X., Duan L.R., Peng R. Association of three candidate genetic variants in ACMSD/TMEM163, GPNMB and BCKDK/STX1B with sporadic Parkinson’s disease in Han Chinese. Neurosci. Lett. 2019;703:45–48. doi: 10.1016/j.neulet.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 86.Chang K.H., Chen C.M., Chen Y.C., Fung H.C., Wu Y.R. Polymorphisms of ACMSD-TMEM163, MCCC1, and BCKDK-STX1B are not associated with Parkinson’s disease in Taiwan. Parkinson’s Dis. 2019 doi: 10.1155/2019/3489638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lill C.M., Roehr J.T., McQueen M.B., Kavvoura F.K., Bagade S., Schjeide B.M., Schjeide L.M., Meissner E., Zauft U., Allen N.C., Liu T. Comprehensive research synopsis and systematic meta-analyses in Parkinson’s disease genetics: the PDGene database. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharma M., Ioannidis J.P., Aasly J.O., Annesi G., Brice A., Van Broeckhoven C., Bertram L., Bozi M., Crosiers D., Clarke C., Facheris M. Large-scale replication and heterogeneity in Parkinson disease genetic loci. Neurology. 2012;79:659–667. doi: 10.1212/WNL.0b013e318264e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pihlstrøm L., Axelsson G., Bjørnarå K.A., Dizdar N., Fardell C., Forsgren L., Holmberg B., Larsen J.P., Linder J., Nissbrandt H., Tysnes O.B. Supportive evidence for 11 loci from genome-wide association studies in Parkinson's disease. Neurobiol. Aging. 2013;34:1708–e7. doi: 10.1016/j.neurobiolaging.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 90.Bandrés-Ciga S., Price T.R., Barrero F.J., Escamilla-Sevilla F., Pelegrina J., Arepalli S., Hernández D., Gutiérrez B., Cervilla J., Rivera M., Rivera A. Genome-wide assessment of Parkinson’s disease in a southern Spanish population. Neurobiol. Aging. 2016;45:213–e3. doi: 10.1016/j.neurobiolaging.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nalls M.A., Pankratz N., Lill C.M., Do C.B., Hernandez D.G., Saad M., DeStefano A.L., Kara E., Bras J., Sharma M., Schulte C. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat. Genet. 2014;46:989. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang D., Nalls M.A., Hallgrímsdóttir I.B., Hunkapiller J., van der Brug M., Cai F., Kerchner G.A., Ayalon G., Bingol B., Sheng M., Hinds D. A meta-analysis of genome-wide association studies identifies 17 new Parkinson's disease risk loci. Nat. Genet. 2017;49:1511. doi: 10.1038/ng.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martí-Massó J.F., Bergareche A., Makarov V., Ruiz-Martinez J., Gorostidi A., De Munain A.L., Poza J.J., Striano P., Buxbaum J.D., Paisán-Ruiz C. The ACMSD gene, involved in tryptophan metabolism, is mutated in a family with cortical myoclonus, epilepsy, and parkinsonism. J. Mol. Med. 2013;91:1399–1406. doi: 10.1007/s00109-013-1075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vilas D., Fernandez-Santiago R., Sanchez E., Azcona L.J., Santos-Montes M., Casquero P., Argandona L., Tolosa E., Paisan-Ruiz C. A novel p. Glu298Lys mutation in the ACMSD gene in sporadic Parkinson’s disease. J. Parkinsons Dis. 2017;7:459–463. doi: 10.3233/JPD-171146. [DOI] [PubMed] [Google Scholar]
- 95.Chahine L.M., Stern M.B., Chen-Plotkin A. Blood-based biomarkers for Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20:S99–103. doi: 10.1016/S1353-8020(13)70025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burgos K., Malenica I., Metpally R., Courtright A., Rakela B., Beach T., Shill H., Adler C., Sabbagh M., Villa S., Tembe W. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer's and Parkinson's diseases correlate with disease status and features of pathology. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luan H., Liu L.F., Meng N., Tang Z., Chua K.K., Chen L.L., Song J.X., Mok V.C., Xie L.X., Li M., Cai Z. LC–MS-based urinary metabolite signatures in idiopathic Parkinson’s disease. J. Proteome Res. 2015;14:467–478. doi: 10.1021/pr500807t. [DOI] [PubMed] [Google Scholar]
- 98.Luan H., Liu L.F., Tang Z., Zhang M., Chua K.K., Song J.X., Mok V.C., Li M., Cai Z. Comprehensive urinary metabolomic profiling and identification of potential noninvasive marker for idiopathic Parkinson’s diseas. Sci. Rep. 2015;5:13888. doi: 10.1038/srep13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iwaoka K., Otsuka C., Maeda T., Yamahara K., Kato K., Takahashi K., Takahashi K., Terayama Y. Impaired metabolism of kynurenine and its metabolites in CSF of parkinson’s disease. Neurosci. Lett. 2020;714 doi: 10.1016/j.neulet.2019.134576. [DOI] [PubMed] [Google Scholar]
- 100.K.H. Chang, M.L. Cheng, H.Y. Tang, C.Y. Huang, Y.R. Wu, C.M. Chen, Alternations of metabolic profile and kynurenine metabolism in the plasma of Parkinson's disease, Mol. Neurobiol. 55 (2018) 6319–2638. doi: 10.1007/s12035-017-0845-3. [DOI] [PubMed]
- 101.Ravikumar A., Deepadevi K.V., Arun P., Manojkumar V., Kurup P.A. Tryptophan and tyrosine catabolic pattern in neuropsychiatric disorders. Neurol. India. 2000;48:231–238. [PubMed] [Google Scholar]
- 102.Nemeth H., Toldi J., Vécsei L., 70 Transm.Suppl. Kynurenines, Parkinson’s disease and other neurodegenerative disorders: preclinical and clinical studies. J. Neurol. 2006:285–304. doi: 10.1007/978-3-211-45295-0_45. [DOI] [PubMed] [Google Scholar]
- 103.Stachowski E.K., Schwarcz R. Regulation of quinolinic acid neosynthesis in mouse, rat and human brain by iron and iron chelators in vitro. J. Neural Transm. 2012;119:123–131. doi: 10.1007/s00702-011-0694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malherbe P., Köhler C., Da Prada M.O., Lang G., Kiefer V., Schwarcz R., Lahm H.W., Cesura A.M. Molecular cloning and functional expression of human 3-hydroxyanthranilic-acid dioxygenase. J. Biol. Chem. 1994;269:13792–13797. [PubMed] [Google Scholar]
- 105.Natalini B., Mattoli L., Pellicciari R., Carpenedo R., Chiarugi A., Moroni F. Synthesis and activity of enantiopure (S)(m-nitrobenzoyl) alanine, potent kynurenine-3-hydroxylase inhibitor. Bioorg. Med. Chem. Lett. 1995;5:1451–1454. doi: 10.1016/0960-894X(95)00255-R. [DOI] [PubMed] [Google Scholar]
- 106.Fukuyama K., Tanahashi S., Hoshikawa M., Shinagawa R., Okada M. Zonisamide regulates basal ganglia transmission via astroglial kynurenine pathway. Neuropharmacol. 2014;76:137–145. doi: 10.1016/j.neuropharm.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 107.Stone T.W., Forrest C.M., Darlington L.G. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J. 2012;279:1386–1397. doi: 10.1111/j.1742-4658.2012.08487.x. [DOI] [PubMed] [Google Scholar]
- 108.Jackson P.F., Davenport T.W., Garcia L., McKinney J.A., Melville M.G., Harris G.G., Chapdelaine M.J., Damewood J.R., Pullan L.M., Goldstein J.M. Synthesis and biological activity of a series of 4-aryl substituted benz [b] azepines: antagonists at the strychnine-insensitive glycine site. Bioorg. Med. Chem. Lett. 1995;5:3097–3100. doi: 10.1016/0960-894X(95)00544-0. [DOI] [Google Scholar]
- 109.Wu H.Q., Salituro F.G., Schwarcz R. Enzyme-catalyzed production of the neuroprotective NMDA receptor antagonist 7-chlorokynurenic acid in the rat brain in vivo. Eur. J. Pharmacol. 1997;319:13–20. doi: 10.1016/S0014-2999(96)00829-1. [DOI] [PubMed] [Google Scholar]
- 110.Wu H.Q., Lee S.C., Schwarcz R. Systemic administration of 4-chlorokynurenine prevents quinolinate neurotoxicity in the rat hippocampus. Eur. J. Pharmacol. 2000;390:267–274. doi: 10.1016/S0014-2999(00)00024-8. [DOI] [PubMed] [Google Scholar]
- 111.Lee S.C., Schwarcz R. Excitotoxic injury stimulates pro-drug-induced 7-chlorokynurenate formation in the rat striatum in vivo. Neurosci. Lett. 2001;304:185–188. doi: 10.1016/S0304-3940(01)01791-8. [DOI] [PubMed] [Google Scholar]
- 112.Battaglia G., La Russa M., Bruno V., Arenare L., Ippolito R., Copani A., Bonina F., Nicoletti F. Systemically administered D-glucose conjugates of 7-chlorokynurenic acid are centrally available and exert anticonvulsant activity in rodents. Brain Res. 2000;860:149–156. doi: 10.1016/S0006-8993(00)01962-4. [DOI] [PubMed] [Google Scholar]
- 113.Widnell K. Pathophysiology of motor fluctuations in Parkinson’s disease. J Mov. Disord. 2005;20:S17–S22. doi: 10.1002/mds.20459. [DOI] [PubMed] [Google Scholar]
- 114.Zádori D., Klivényi P., Toldi J., Fülöp F., Vécsei L. Kynurenines in Parkinson’s disease: therapeutic perspectives. J. Neural Transm. 2012;119:275–283. doi: 10.1007/s00702-011-0697-3. [DOI] [PubMed] [Google Scholar]
- 115.Acuña-Castroviejo D., Tapias V., López L.C., Doerrier C., Camacho E., Carrión M.D., Mora F., Espinosa A., Escames G. Protective effects of synthetic kynurenines on 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced parkinsonism in mice. Brain Res. Bull. 2011;85:133–140. doi: 10.1016/j.brainresbull.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 116.Guidetti P., Wu H.Q., Schwarcz R. In situ produced 7-chlorokynurenate provides protection against quinolinate-and malonate-induced neurotoxicity in the rat striatum. Exp. Neurol. 2000;163:123–130. doi: 10.1006/exnr.1999.7284. [DOI] [PubMed] [Google Scholar]
- 117.Sas K., Robotka H., Toldi J., Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 2007;257:221–239. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 118.Perez-De La Cruz V., Santamaria A. Integrative hypothesis for Huntington’s disease: a brief review of experimental evidence. Physiol. Res. 2007;56:513–526. doi: 10.33549/physiolres.931049. [DOI] [PubMed] [Google Scholar]
- 119.Grégoire L., Rassoulpour A., Guidetti P., Samadi P., Bédard P.J., Izzo E., Schwarcz R., Di Paolo T. Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behav. Brain Res. 2008;186:161–167. doi: 10.1016/j.bbr.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 120.Ouattara B., Belkhir S., Morissette M., Dridi M., Samadi P., Grégoire L., Meltzer L.T., Di Paolo T. Implication of NMDA receptors in the antidyskinetic activity of cabergoline, CI-1041, and Ro 61-8048 in MPTP monkeys with levodopa-induced dyskinesias. J. Mol. Neurosci. 2009;38:128–142. doi: 10.1007/s12031-008-9137-8. [DOI] [PubMed] [Google Scholar]
- 121.Samadi P., Grégoire L., Rassoulpour A., Guidetti P., Izzo E., Schwarcz R., Bédard P.J. Effect of kynurenine 3-hydroxylase inhibition on the dyskinetic and antiparkinsonian responses to levodopa in parkinsonian monkeys. J. Mov. Disord. 2005;20:792–802. doi: 10.1002/mds.20596. [DOI] [PubMed] [Google Scholar]