Abstract
Objective
Adipose tissue inflammation and fibrosis appear to contribute to insulin resistance in obesity. Vitamin D receptor (Vdr) genes are expressed by adipocytes, macrophages, and fibroblasts, all of which could potentially play a role in adipose tissue inflammation and fibrosis. As vitamin D has been shown to have direct anti-inflammatory effects on adipocytes, we determined whether specific vitamin D receptor-mediated effects on adipocytes could impact adipose tissue inflammation and fibrosis and ultimately insulin resistance.
Methods
We examined the effects of repleting vitamin D in 25(OH)D-deficient, insulin resistant, overweight-to-obese human subjects (n = 19). A comprehensive assessment of whole-body insulin action was undertaken with stepped euglycemic (∼90 mg/dL) hyperinsulinemic clamp studies both before and after the administration of vitamin D or placebo. Adipose tissue fibrosis and inflammation were quantified by real-time rt-PCR and immunofluorescence in subcutaneous abdominal adipose tissue.
To determine whether vitamin D's effects are mediated through adipocytes, we conducted hyperinsulinemic clamp studies (4 mU/kg/min) and adipose tissue analysis using an adipocyte-specific vitamin D receptor knockout (VDR-KO) mouse model (adiponectin-Cre + VDR+/fl) following high-fat diet feeding for 12 weeks.
Results
25(OH)D repletion was associated with reductions in adipose tissue expression of pro-inflammatory and pro-fibrotic genes, decreased collagen immunofluorescence, and improved hepatic insulin sensitivity in humans. Worsening trends after six months on placebo suggest progressive metabolic effects of 25(OH)D deficiency. Ad-VDR-KO mice mirrored the vitamin D-deficient humans, displaying increased adipose tissue fibrosis and inflammation and hepatic insulin resistance.
Conclusions
These complementary human and rodent studies support a beneficial role of vitamin D repletion for improving hepatic insulin resistance and reducing adipose tissue inflammation and fibrosis in targeted individuals, likely via direct effects on adipocytes. These studies have far-reaching implications for understanding the role of adipocytes in mediating adipose tissue inflammation and fibrosis and ultimately impacting insulin sensitivity.
Keywords: Vitamin D, Insulin sensitivity, Inflammation, Fibrosis, Adipocyte, Macrophage
Highlights
-
•
Vitamin D repletion improved hepatic insulin sensitivity in obese insulin-resistant and vitamin D deficient human.
-
•
Correcting vitamin D deficiency concomitantly reduced adipose tissue expression of pro-inflammatory and pro-fibrotic genes.
-
•
Worsening trends in these metabolic parameters were observed following 6 months of uncorrected vitamin D deficiency.
-
•
Adipocyte-specific depletion of VDR in mice induced adipose tissue inflammation and fibrosis and hepatic insulin resistance.
1. Introduction
Adipose tissue regulates numerous physiological processes, and its expansion in obese humans is associated with disrupted metabolic homeostasis, insulin resistance, and type 2 diabetes mellitus (T2D) [1]. Adipose tissue expansion in obesity is accompanied by adipose tissue dysfunction characterized by adipocyte hypertrophy and hyperplasia, increased inflammation, impaired extracellular matrix remodeling, and upregulation of pro-fibrotic signaling pathways mediated by fibroblasts together with altered secretion of adipokines [[2], [3], [4], [5], [6]]. Obesity is also characterized by increased macrophage infiltration into fat tissue, which is another important source of adipose-derived pro-inflammatory factors [[7], [8], [9], [10]]. An inter-relationship among adipose tissue inflammation, adipose fibrosis, and insulin resistance has been established in rodents [11] and may also be relevant in humans [9,[12], [13], [14], [15]].
Low 25-hydroxyvitamin D [25(OH)D] levels are associated with glucose intolerance, insulin resistance, metabolic syndrome, and increased risk of T2D development [[16], [17], [18]], although the causal effects of vitamin D deficiency on these conditions have yet to be clearly established. The prevalence of vitamin D deficiency has increased globally, with an estimated 1 billion people worldwide qualifying as vitamin D deficient (defined as a 25(OH)D level of less than 20 ng/ml or 50 nmol/L [19,20]) or insufficient (defined as a 25(OH)D level of 21–29 ng/ml or 52–72 nmol/L). Of note, a recent large clinical trial demonstrated that vitamin D repletion reduced the risk of developing T2D by 62% in individuals with initial vitamin D levels <12 ng/ml [21]. Adipose tissue is a reservoir for vitamin D in humans, and adipose 25(OH)D content appears to correlate to 25(OH)D plasma levels [[22], [23], [24]]. Vitamin D receptor (Vdr) genes are expressed by adipocytes, macrophages, and fibroblasts [[25], [26], [27], [28], [29], [30], [31]], all of which could potentially play a role in adipose tissue inflammation and fibrosis. Vitamin D has inhibitory effects on adipose tissue inflammation and on leukocyte infiltration both in vivo and in vitro, in part by upregulating the expression of anti-microbial peptides such as LL-37 [32]. 1,25(OH)2D inhibits the nuclear factor kappa light-chain-enhancer of activated B cells (NF-κB) signaling pathway, which in turn leads to decreased release of interleukin-8 (IL-8), monocyte chemoattractant protein-1 (MCP-1), and IL-6 by human preadipocytes and MCP-1 by human adipocytes [33,34]. Recent studies have shown that vitamin D reduces hepatic and renal fibrosis by suppressing TGF-β-SMAD signal transduction [3,31,[35], [36], [37]]. As vitamin D has been shown to have direct anti-inflammatory effects on adipocytes [28,38], we hypothesized that vitamin D could mediate beneficial effects on adipose tissue inflammation and fibrosis by working through adipocyte vitamin D receptors. Therefore, studying an adipocyte-specific Vdr knockout model, we determined whether specific vitamin D receptor-mediated effects on adipocytes could impact adipose tissue inflammation and fibrosis and ultimately insulin resistance.
The objectives of the present study were to evaluate the effects of two levels of vitamin D repletion (to 30 ng/ml and greater than 50 ng/ml, respectively) on insulin sensitivity and adipose tissue biology in overweight or obese insulin-resistant humans with vitamin D deficiency and conduct parallel rodent studies establishing the impact of adipocyte vitamin D signaling on systemic glucose metabolism. Distinct from prior research into vitamin D repletion in humans, the current studies employed a placebo-controlled dose- and duration-dependent study design at different vitamin D repletion levels and state-of-the art stepped hyperinsulinemic clamps to separately quantify hepatic vs peripheral insulin sensitivity.
2. Materials and methods
2.1. Experimental design: human studies
This randomized, double-blind, placebo-controlled study examining the dose-dependent effects of vitamin D repletion on insulin sensitivity and adipose tissue biology in individuals at risk of T2D was conducted at the Clinical Research Center of the Albert Einstein College of Medicine (Einstein) in the Bronx, New York. The study was approved by Einstein's Institutional Review Board, and all of the participants provided written informed consent. The inclusion criteria included 19 (N = 19) overweight or obese (BMI range 25–39 kg/m2) participants with insulin resistance by homeostasis model assessment-estimated insulin resistance (HOMA-IR ≥ 3.0) and 25-hydroxyvitamin D levels less than 20 ng/ml who were randomly assigned to receive vitamin D3 or a placebo matched in taste and appearance once a week for up to 6–8 months. The exclusion criteria included a history of diabetes, major psychiatric disorders on medication, HIV/AIDS, cancer, sarcoidosis, alcohol or substance abuse, Cushing's syndrome, primary hyperparathyroidism, nephrolithiasis, pregnancy or breast-feeding, regular visits to a tanning salon, hypercalcemia or hypocalcemia, uncontrolled hypertension, any chronic illness requiring medication other than arthritis, hypertension and hyperlipidemia, and a positive urine toxicology screen at any of the visits.
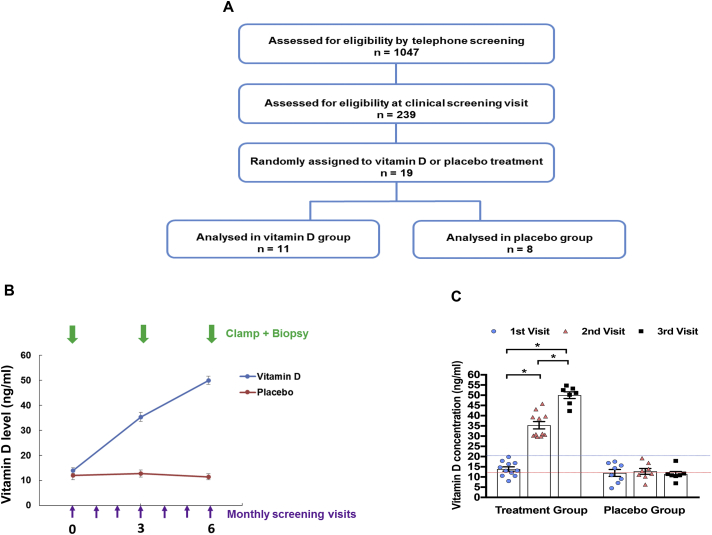
The participants were recruited through print advertisements beginning in March 2009. Potential participants underwent screening first by telephone and then via an in-person clinical visit. The study flow diagram is shown in Figure 1A, which indicates the number of subjects who needed to be screened to identify the study population based on BMI, HOMA-IR, and 25(OH)D criteria. A detailed medical history and physical examination, routine clinical biochemistry, and electrocardiogram were performed at the clinical visit. The participants were screened for vitamin D deficiency (25(OH)D < 20 ng/ml). Serum 25(OH)D levels were measured using a Diasorin chemiluminescent assay (Diasorin, Stillwater, MN, USA). Measuring 25(OH)D levels is more clinically applicable as it is stable with a longer half-life (15–50 days) than 1,25(OH)D with a half-life of 15 h [39,40]. The subjects' baseline characteristics are included in Supplementary Table 1.
Figure 1.
Identification and treatment of humans with vitamin D deficiency and insulin resistance. (A) Recruitment process. (B) Schematic Vitamin D repletion protocol for both vitamin D and placebo groups. (C) Vitamin D concentrations at the three clamp study visits in both groups.
The enrolled participants were instructed to maintain their body weight and usual diet and exercise lifestyle for the study duration. The participants were assigned an intervention (either vitamin D or placebo) that was logged and dispensed by the hospital pharmacy in a manner that kept the research team and participants blinded. The randomization sequence used in this study was created following a biostatistics consultation and was kept in a secure, password-protected file that was only accessed by the study coordinator and research pharmacist. The subjects in the treatment arm were given weekly doses of vitamin D3 (Ddrops Company, Woodbridge, ON, Canada) to reach a target level of 30 ng/ml (second visit) during the first three months of treatment and thereafter continued on weekly vitamin D3 doses until a level of at least 50 ng/ml (third visit) was achieved. The vitamin D3 dose was calculated based on the formula “change in serum 25(OH)D levels = 44.5 x vitamin D dose (in μg/kg/day)” as reported by Barger-Lux et al. [41]. The last study visit and subject follow-up was concluded by September 2015. Four patients in the vitamin D group and one patient in the placebo group were not analyzed during the third clamp due to loss of intravenous access during the study, inability to achieve target vitamin D levels, weight loss, and unsuccessful attempts to reach the patient.
2.1.1. Euglycemic stepped hyperinsulinemic pancreatic clamp studies
Euglycemic stepped hyperinsulinemic clamp studies were conducted in the vitamin D-deficient participants at baseline [25(OH)D level < 20 ng/ml] and after repletion to two different 25(OH)D levels [level I ≃ 30 ng/ml (second visit), and level II ≃ 50 ng/ml (third visit)] (Figure 1B,C). Euglycemic stepped hyperinsulinemic clamp studies were also performed in the placebo group at baseline and two different time points based on the expected time necessary for vitamin D repletion, and monthly screening visits were conducted to check their vitamin D levels. On the day of the study, the participants presented to the Clinical Research Center (CRC) at 8:00 a.m. after an overnight fast. Two intravenous cannulas were established, one for infusions and another for blood sampling in a dorsal vein of the opposite arm. A primed continuous infusion of [6,6-2H2] glucose (bolus 200 mg/m2 for 3 min) was started at t = 0 min, followed by continuous infusion of 2 mg/min/m2 for the entire study. From 0 to 120 min, basal insulin infusion rates were maintained at 15 mU/m2/min. From 120 to 240 min, insulin infusion rates were increased to 30 mU/m2/min to mimic physiologic hyperinsulinemia (the clamp's “low-insulin” step), and insulin infusion rates were then further increased to 80 mU/m2/min for 240–360 min (the clamp's “high-insulin” step) as previously described [9,42]. Fixed levels of glucoregulatory hormones were maintained throughout the 360 min clamp studies using somatostatin infusion (250 μg/h) and replacement of glucagon (0.6 ng/kg/min) and growth hormone (3 ng/kg/min). Euglycemia (∼90 mg/dl) was maintained by a variable infusion of 20% dextrose (the specific activity of infused dextrose was maintained equivalent to the plasma glucose-specific activity by adding D-[6,6-2H2] glucose to the infusate), and plasma glucose levels were measured every 5 min for the study duration. Blood samples were obtained at hourly intervals to determine the plasma levels of insulin, C-peptide, glucagon, cortisol, lactate, free fatty acids (FFA), and glycerol. Samples for D-[6,6-2H2] glucose determinations were obtained every 15 min during the steady-state periods (t = 180–240 and 300–360 min). At the end of the study, all of the infusions except dextrose were stopped at t = 360 min. To avoid hypoglycemia, dextrose infusion was continued for ∼30 min after the completion of the study. The participants were given a standard meal and had their plasma glucose concentrations checked for an additional ∼1 h before being discharged home.
2.1.2. Analytical procedures
2.1.2.1. Plasma hormones and substrates
Plasma glucose levels were measured using a Beckman glucose analyzer (glucose oxidase method; Fullerton, CA, USA). Plasma insulin, C-peptide, glucagon, and cortisol concentrations were measured by radioimmunoassay at the Diabetes Research Center Hormone Assay Core as previously reported [42]. Plasma lactate, free fatty acids, and glycerol were measured using spectrophotometric techniques.
2.1.2.2. Glucose turnover
6,6-2H2 glucose concentrations were measured by gas chromatography mass spectrometry (GCMS) as previously described [43]. Rates of glucose appearance (Ra) and glucose disappearance (Rd, or glucose uptake) were calculated using Steele's steady–state equation [44]. Rates of endogenous glucose production (EGP) were determined by subtracting the rates of glucose infusion from the tracer-determined Ra. Data for glucose turnover, plasma hormones, and substrate concentrations represent the mean values during the final 60 min of the clamp's low-insulin phase (t = 180–240 min) and the final 60 min of the clamp's high-insulin phase (t = 300–360 min).
2.1.2.3. Adipose tissue studies
At t = 60 min of the clamp studies, adipose tissue biopsies were obtained from the periumbilical region. A small 0.25-cm periumbilical cutaneous incision was made under local anesthesia (1% lidocaine), and 1–2 g of adipose tissue was obtained by aspiration [45]. Biopsy specimens (∼300 mg) were homogenized immediately in TRIzol reagent (Invitrogen Technologies) on ice at the bedside to inhibit any RNAase activity and subsequently stored at −80 °C for future real-time RT-PCR analysis.
2.1.2.4. Adipose tissue separation
Adipose tissue samples from all the participants were immediately washed at least three times with saline. The samples were then digested with collagenase type 1 (0.05 g per 30 mL of Hanks Balanced Salt solution with 4% BSA; Worthington Biochemical) for 30 min at 37 °C with intermittent shaking, followed by extensive washing with Dulbecco's phosphate-buffered solution depleted of magnesium and calcium (Mediatech). The adipocytes were separated from the stromal-vascular fraction (SVF) by centrifugation at 3,000 rpm for 10 min. Macrophages were isolated from the SVF using CD14+ antibody-coated magnetic Dynabeads (Dynal Biotech) following the manufacturer's protocol. The adipose tissue samples and macrophages were washed with PBS, stored in TRIzol, and analyzed by real-time RT-PCR.
2.1.2.5. Quantitative real-time RT-PCR
Total RNA was extracted from the adipose tissue samples and macrophages following the protocol of a Qiagen RNeasy Mini kit (Cat# 74104). A SuperScript III First-Strand Synthesis System (Life Technologies, Cat# 18080051) was used to synthesize first-strand cDNA, the real-time RT-PCR template. As previously described [15], to verify that the relative expression value of the housekeeping gene provided an accurate reflection of cDNA loading, we first correlated the relative expression values of three housekeeping genes [glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ribosomal proteins 19 (RPL19) and 18S], B2M, and beta-actin with each other and the geometric mean of the three values. Pearson's correlation coefficients were consistently ≥0.92, indicating that the expression of the five genes provided a reasonably accurate reflection of cDNA loading. GAPDH was chosen as the housekeeping gene to be included in every RT-PCR reaction because it was consistently expressed among the various cell types and study conditions [15]. Gene expression of inflammatory factors (TNF = tumor necrosis factor alpha, IL-6 = interleukin-6, iNOS = inducible nitric, and PAI-1 = plasminogen activator inhibitor-1), pro-fibrotic factors (TGFB1 = transforming growth factor beta 1, HIF1A = hypoxia-inducible factor-1 alpha, COL1,5,6 = collagen I, V, and VI, respectively, MMP7 = matrix metalloproteinase-7, and TSP1 = thrombospondin 1), and VDR target genes in the adipose tissue samples (DUSP10 = dual-specific phosphatase 10, TRAK1 = trafficking protein, kinesin binding 1, NRIP1 = nuclear receptor interacting protein 1, and THBD = thrombomodulin) were determined by quantitative real-time RT-PCR using the specific protocol for LightCycler (Roche Diagnostics, Indianapolis, IN, USA) as previously described [42]. Primers used for the VDR target genes [46] and pro-fibrotic genes were as follows: (DUSP10: forward GAGGCTTTTGAGTTCATTGAG, reverse ATCCGAGTGTGCTTCATC; TRAK1: forward AGATCACACACCTGCTATCGC, reverse GTATTGGGCATGGTTTTGTTCC; NRIP1: forward TGGAATGCAGTCATCCATGT, reverse CTGGTTCAGGACCTGTTGGT; THBD: forward GACCTTCCTCAATGCCAGTC, reverse GCCGTCGCCGTTCAGTAG; TSP-1: forward TCAGGACCCATCTATGATAAAACCTA, reverse TCAGGTCAGAGAAGAAGAACACATTTC; MMP-7: forward AGCCAAACTCAAGGAGATGC, reverse ACTCCACATCTGGGCTTCTG; collagen 6a: forward GACCTCGGACCTGTTGGGT, reverse TACCCCATCTCCCCCTTCAC; collagen 5a: forward GATTGAGCAGATGAAACGG, reverse CCTTGGTTAGGATCGACCC; collagen 1a: forward TACAGCGTCACTGTCGATG, reverse TCAATCACTGTCTTGCCCCAG; HIF1A: forward TGCATCTCCATCTCCTACCC, reverse CGTTAGGGCTTCTTGGATA; and TGF-b1: forward AACCGGCCTTTCCTGCTTCTCA, reverse CGCCCGGGTTATGCTGGTTGTA). Reaction conditions were as follows: 40 cycles of denaturation at 95 °C for 2 s, annealing at 60 °C for 5 s, and elongation at 74 °C for 12 s and 82 °C for 3 s. Results are expressed as the relative change by determining the ratio of the genes of interest a given individual 2 and 5 h after IL infusion vs SAL infusion corrected for the relative expression of GAPDH in the same pair of samples. To calculate the mRNA copy number, we generated standard curves for GAPDH and the genes of interest using plasmid standards with known concentrations. Melting curves were used to determine the specificity of the gene products, which were subsequently confirmed by running the PCR products on agarose gels. All the reactions were performed at least three times [15].
2.1.2.6. Endotrophin immunofluorescence staining
Adipose tissue was prepared for immunofluorescence staining in the following manner. Formalin-fixed, paraffin-embedded adipose tissue samples were cut into 5 μm sections, deparaffinized in xylene, and rehydrated through a series of decreasing concentrations of ethanol. After regular antigen retrieval, the sections were incubated with rabbit polyclonal anti-endotrophin antibody (kindly provided by Professor Philipp Scherer, UT Southwestern Medical Center) [47] diluted at 1:500 (v/v) for 1 h at room temperature. After incubation with primary antibodies, the sections were repeatedly rinsed with PBS and incubated for 1 h at room temperature with Alexa Fluor 568 donkey anti-rabbit IgG (Molecular Probes, Cat# A10042, dilution 1:250 (v/v)) as the secondary antibody. All of the antibodies were diluted in Dako Ready-to-use Antibody Diluent (Cat# S080981-2). Incubation steps were carried out with Dako Auto Stainer Plus. Negative control slides were run in parallel with positive slides by applying rabbit IgG isotype control antibody (Cat# 02-6120) diluted at the same protein concentration as the antibody. The sections were sealed with ProLong Gold Antifade Mountant (Cat# 36935) with 4' 6-diamidino-2-phenylindole (DAPI).
The slides were digitally scanned on a 3DHISTECH Panoramic 250 Whole Slide Scanner (3DHISTECH, Budapest, Hungary). They were taken with a multi-color LED source Lumencor Spectra with a 20X/0.8 air objective. A low-noise high-speed 5.5 megapixel scientific CMOS camera (PCO, edge 4.2 m, PCO Inc.) was used for image acquisition. The exposure time of each channel was selected to avoid saturation and maintained constant for all of the slides. The acquisition software Panoramic Scanner automatically stitched the individual field of view of the tissues, creating one image. The HistoQuant module from QuantCenter software (3DHISTECH, Budapest, Hungary) was used for the analysis. The criteria for segmentation of positive signals were extensively tested and selected based on user inspection. After the criteria were established, the analysis parameters were maintained constant for all of the slides.
2.1.2.7. Collagen VI immunofluorescence staining
Adipose tissue was prepared for immunofluorescence staining in the following manner. Formalin-fixed, paraffin-embedded adipose tissue samples were cut into 5 μm sections, deparaffinized in xylene, and rehydrated through a series of decreasing concentrations of ethanol. The sections were pretreated with Dako Cytomation Target Retrieval Solution pH 9.0 buffer (Cat# S236884-2). The sections were incubated with rabbit polyclonal anti-collagen VI antibody (Abcam, Cat# 6588, dilution 1:250 (v/v)) for 1 h at room temperature. After incubation with primary antibodies, the sections were repeatedly rinsed with PBS and incubated for 1 h at room temperature with Alexa Fluor 568 donkey anti-rabbit IgG (Molecular Probes, Cat# A10042, dilution 1:250 (v/v)) as the secondary antibody. All the antibodies were diluted in Dako Ready-to-use Antibody Diluent (Cat# S080981-2). The incubation steps were carried out with Dako Auto Stainer Plus. Negative control slides were run in parallel with positive slides by applying rabbit IgG isotype control antibody (Cat# 02-6120) diluted at the same protein concentration as the antibody. The sections were sealed with ProLong Gold Antifade Mountant (Cat# 36935) with 4' 6-diamidino-2-phenylindole (DAPI). Subsequent scanning, imaging, and analysis of the slides were performed as previously described.
2.1.2.8. Hydroxyproline assay
A hydroxyproline kit (Catalog #K226-100, BioVision, Milpitas, CA, USA) was used to measure the hydroxyproline levels in the human adipose tissue following the protocol provided by BioVision. The plates were read using an Enspire Multilabel Reader from the Einstein Biomarker Analytic Research Core Facility. Then 100 μl of distilled H2O was added for every 10 mg of tissue, which was homogenized with an ultrasonic probe homogenizer. Then 100 μl of 10 N concentrated NaOH was added to 100 μl of the sample, which was heated at 120 °C for 1 h. Following alkaline hydrolysis, the vials were placed on ice to cool briefly, then 100 μl of 10 N concentrated HCl was added to neutralize the residual NaOH. The vials were vortexed and centrifuged at 10,000×g for 5 min to pellet any insoluble debris following hydrolysis. The samples were placed on a heating plate at 65 °C for evaporation, then chloramine-T concentrate was added to the wells and they were incubated at room temperature for 20 min. Then 50 μl of developer solution was added to each well and incubated at 37 °C for 5 min, and 50 μl of DMAB concentrate solution was added to each well and incubated at 65 °C for 45 min. The absorbance of all the samples was measured at 560 nm (OD 560) in endpoint mode. The plates were read using the Enspire Multilabel Reader from the Einstein Biomarker Analytic Research Core Facility.
2.2. Experimental design: adipose VDRKO mice
Adipocyte-specific VDR knockout (Ad-VDRKO) mice were generated by breeding mice with the VDR gene flanked by loxP sites (gift of David Gardner, UCSF) with mice expressing the Cre recombinase under the control of the adiponectin promoter [48]. The resulting Adipoq-Cre:Vdrfl/fl mice were intercrossed to generate mice with loxP-flanked VDR alleles with and without Cre recombinase, and the latter served as littermate controls. The mice were maintained on a C57BL/6 background and housed in a pathogen-free facility with a 12 h light–dark cycle and free access to water and irradiated rodent chow.
Seven wild-type (WT) and six adipose-specific vitamin D receptor (VDR) knockout (KO) mice were fed a high-fat diet (HFD) (58 kcal% fat w/sucrose, D12331; Research Diets, New Brunswick, NJ, USA) for 12 weeks from 6 weeks of age. The mice were housed in a temperature- and humidity-controlled environment (light cycle 6:00 a.m. to 6:00 p.m.). All of the procedures were approved by the Beth Israel Deaconess Medical Center and Vanderbilt University Institutional Animal Care and Use Committee. Body composition was determined using ECHO-MRI.
2.2.1. Hyperinsulinemic-euglycemic clamps (mice)
One week before performing the hyperinsulinemic-euglycemic clamps, carotid artery and jugular vein catheters were surgically placed for sampling and infusions. The mice were fasted for 5 h and then clamped as previously described [49]. The clamp was initiated at t = 0 min with a continuous insulin infusion (4 mU/kg/min) for 2 h. Primed continuous infusions of [3-3H]glucose (1.5 μ Ci bolus, 50 n Ci/min infusion) were administered to assess glucose uptake and production. [3-3H]glucose (0.06 μ Ci/μL) was added to the glucose infusate to clamp both arterial glucose and glucose-specific activity. At 120 min, a 5 μ Ci bolus of 14C-2-deoxyglucose was administered to assess the tissue-specific glucose metabolic rate (Rg). Arterial glucose levels were monitored every 10 min to provide feedback for adjusting the glucose infusion rate (GIR) (50% dextrose + [3-3H]glucose). Erythrocytes were replaced to prevent a decline in hematocrits with repeated blood sampling. [3-3H]glucose kinetics were determined at −10 min and from 80 to 120 min. Blood was collected at 2, 5, 15, 25, and 35 min after injection to measure the disappearance of [14C]2-DG from the plasma. At t = 155 min, the mice were anesthetized with pentobarbital and the tissues were freeze-clamped for subsequent analyses. Whole-body glucose appearance (Ra) and endogenous glucose production (EGP), a measure of hepatic glucose production, were calculated as described using non-steady-state equations [49]. Radioactivity of [3-3H]glucose, [14C]2DG, and [14C]2DG-6-phosphate were determined by liquid scintillation counting. EGP was determined by subtracting the GIR from the total Ra. The glucose metabolic index (Rg) was calculated as previously described [50]. Plasma insulin was determined by ELISA. Plasma non-esterified fatty acids (FFAs) and tissue triglycerides were measured spectrophotometrically.
2.2.2. Hydroxyproline assay in rodents
Tissue hydroxyproline content was measured by assessing 4-hydroxyproline levels using a Hydroxyproline Calorimetric Assay kit from BioVision (Cat# K555-100, BioVision). One hundred milligrams of epididymal WATs were homogenized in distilled water and then mixed with 12 N of HCl at 120 °C for 3 h. After centrifugation at 10,000×g for 3 min, the supernatants were dried, incubated with chloramine-T at room temperature for 5 min, and then incubated with p-dimethylaminobenzaldehyde (DMAB) for 90 min at 60 °C. The absorbance was measured at 560 nm using a microplate reader (SpectraMax i3, Molecular Devices) as suggested by the manufacturer. Hydroxyproline content was normalized to the total protein measured by a BCA Assay (Cat# 23227, Pierce BCA Protein Assay kit, Thermo Fisher Scientific).
2.3. Statistical analysis
Several glucose metabolism parameters were measured in each subject, with the primary endpoint being the percent change in endogenous glucose production (EGP) during the final hour of the clamp's “low-insulin” (30 mU/m2/min) step as a measure of the insulin-sensitizing effects of vitamin D. Peripheral glucose uptake (Rd) was examined as a secondary endpoint. The results are expressed as mean ± standard error of the mean. The data were tested for normal distribution using the Shapiro–Wilk test. Differences between the study groups (vitamin D and placebo groups) were assessed using a two-sample t-test. Paired Student's t-tests were used to compare the baseline and endpoint measures within the groups. For the mouse clamps, Student's t-tests or two-way ANOVA followed by Tukey's post hoc tests were conducted. All the human and rodent data are presented as mean ± standard error of the mean. P < 0.05 was considered significant for all of the analyses. Statistical analysis of the data over time was performed using the Statistical Package for the Social Sciences 11.5 software (SPSS Inc., Chicago, IL, USA).
2.4. Sample size
Our main endpoint measurement was the percent change in endogenous glucose production (EGP) during the final hour of the clamp's low-insulin phase. The null hypothesis was that the percent change in EGP would be the same in both groups (vitamin D and placebo). The EGP rates were 1.64 ± 0.6 and 1.03 ± 0.5 mg/kg/min at baseline and following vitamin D repletion, respectively, in our preliminary studies. The sample size calculation was based on a 2-sided, paired t-test assuming a mean difference in EGP of 0.61, a standard deviation of 0.5, and no change with placebo. This resulted in a sample size of 8 per group to reach a power of 80%.
3. Results
3.1. Identification and treatment of humans with vitamin D deficiency and insulin resistance
Following a recruitment process, 19 obese non-diabetic, insulin-resistant (HOMA-IR > 3) subjects with 25(OH)D levels < 20 ng/ml were enrolled in the study (Figure 1A). Of these, 11 subjects were randomized to the treatment group (vitamin D) and eight received placebo in a double-blinded study design (Figure 1B). The baseline subject characteristics, including age, weight, height, body mass index (BMI), fasting plasma glucose and insulin levels, HOMA-IR, and 25(OH)D levels were similar in the two groups at the first (baseline) visit (Supplementary Table 1). In the group that received vitamin D treatment, the average weekly vitamin D dose was 38,107 ± 3,185 IU. Vitamin D repletion significantly increased serum 25(OH)D levels, successfully attaining target levels within ∼3 months for the second visit and ∼6 months for the third visit (Figure 1C). All the participants in both study groups were initially vitamin D deficient. Serum 25(OH)D levels remained consistently reduced over the same six-month period in the placebo group (Figure 1C).
3.2. Vitamin D improves hepatic insulin sensitivity in vitamin D-deficient insulin-resistant humans
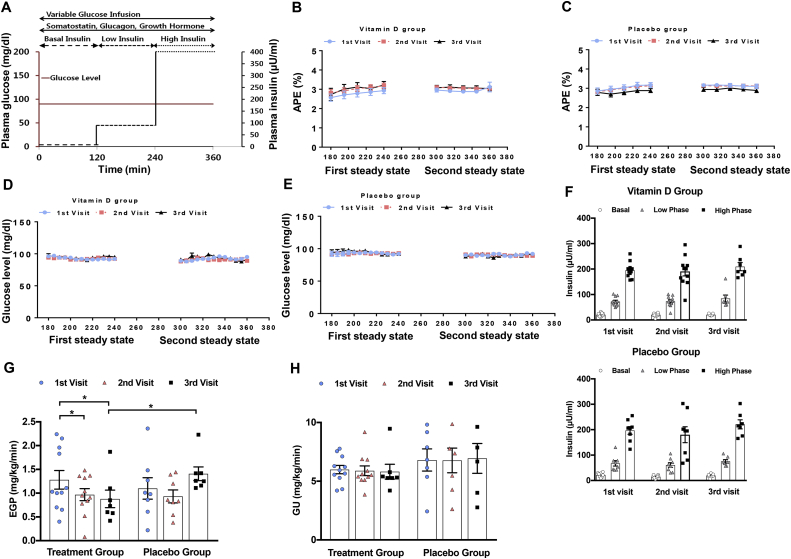
To determine whether vitamin D repletion would improve insulin action in insulin-resistant individuals, a comprehensive study of whole-body insulin action was undertaken with stepped euglycemic (∼90 mg/dL) hyperinsulinemic clamp studies (Figure 2A) both before and after the administration of vitamin D or placebo (Figure 1B). Specifically, a low-insulin phase (∼60 uU/ml) was used to optimally study hepatic insulin sensitivity, while peripheral glucose uptake was maximized during a high-insulin phase (∼400 uU/ml). Steady-state dynamics were attained during these two phases in all of the studies as demonstrated by comparable atom percent excess (APE) during these phases of the vitamin D (Figure 2B) and placebo studies (Figure 2C) and by comparable glucose levels during these two phases for both the vitamin D (Figure 2D) and placebo studies (Figure 2E). There was a trend toward a small increase in GIR in the treatment group, consistent with the decrease in EGP (Supplementary Fig. 1A). Given the high variability, this did not achieve significance. There were no changes in the placebo group (Supplementary Fig. 1B and C).
Figure 2.
Clamp studies in humans. (A) Stepped hyperinsulinemic euglycemic clamp protocol. (B) Average atom percent excess (APE) during two steady states for vitamin D group and (C) placebo group per each visit. (D) Average glucose level during two steady states for vitamin D group and (E) placebo group per each visit. (F) Insulin levels during each clamp per each visit for vitamin D and placebo group. (G) Endogenous glucose production (EGP) and (H) glucose uptake (GU) during the three clamp studies. Data are represented as means ± SEM. ∗p < 0.05; ∗ = significantly different.
Comparable plasma levels of insulin (Figure 2F), C-peptide, glucagon, cortisol, lactate, free fatty acids, and glycerol were attained at the three phases of the euglycemic stepped hyperinsulinemic clamp studies: basal, during the “low-insulin” phase (120–240 min), and during the “high-insulin” phase (240–360 min) (Supplementary Table 2A and B).
Repletion of vitamin D levels to the therapeutic range resulted in a significant improvement in hepatic insulin sensitivity. Endogenous glucose production (EGP) during the clamp studies' low-insulin phase decreased at both the second and third visits compared to the first visit baseline, with no significant difference in EGP between the second and third visits (Figure 2G). Intriguingly, in the placebo group, there were upward trends in EGP from the baseline (first visit) to the third visits (Figure 2G), such that EGP was 37% higher following 6 months of placebo administration than with optimal vitamin D replacement (p = 0.04). However, neither level of vitamin D repletion affected peripheral glucose uptake (GU) (Figure 2H). Taken together, these findings may suggest that vitamin D deficiency over a longer period of time could be associated with worsening hepatic insulin resistance, and that this hepatic insulin resistance can be improved by vitamin D repletion.
3.3. The expression of pro-inflammatory genes (whole fat and adipose tissue macrophages) is reduced after vitamin D repletion
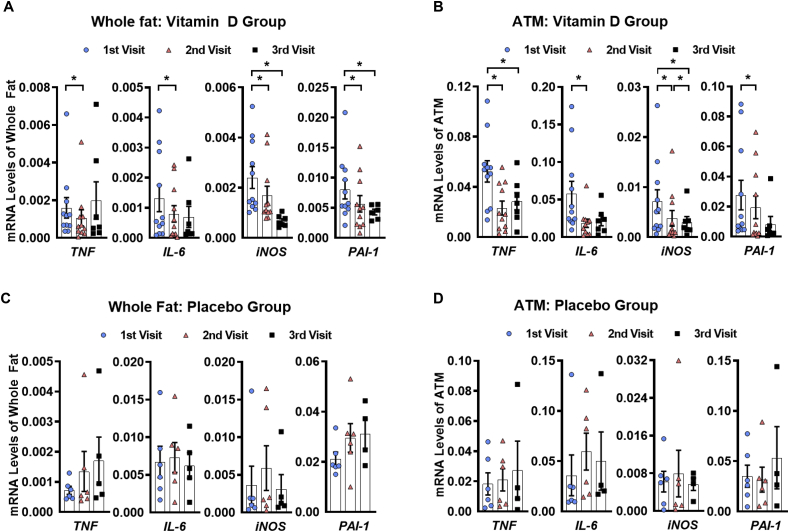
We next investigated the effects of vitamin D administration on adipose tissue inflammation. The expression of the pro-inflammatory genes TNF, IL-6, iNOS, and PAI-1 was significantly reduced in the periumbilical subcutaneous adipose tissue following vitamin D repletion into the normal range (Figure 3A). Interestingly, the expression of the same pro-inflammatory factors in the adipose tissue macrophages also decreased to a similar degree (Figure 3B). Only a few pro-inflammatory gene markers in either whole fat or adipose tissue macrophages were significantly reduced when the vitamin D levels increased from the second to third visits (Figure 3A,B). In the placebo group, no significant differences were observed from the first (baseline) visit, although there was a trend toward the elevated expression of some pro-inflammatory genes at the end of the 6-month study period (Figure 3C,D). These results suggest that vitamin D administration has significant modulatory effects on the gene expression of inflammatory proteins within adipose tissue.
Figure 3.
Adipose tissue gene expression of pro-inflammatory markers in humans. (A) Fold decreases in gene expression of various pro-inflammatory markers in whole fat and (B) adipose tissue macrophages for vitamin D group. (C) Fold decreases in gene expression of various pro-inflammatory markers in whole fat and (D) adipose tissue macrophages for placebo group. Data are represented as means ± SEM. ∗p < 0.05; ∗ = significantly different. TNF = tumor necrosis factor alpha, IL-6 = interleukin-6, iNOS = inducible nitric, PAI-1 = plasminogen activator inhibitor-1.
3.4. Vitamin D repletion reduces the expression of pro-fibrotic genes in adipose tissue
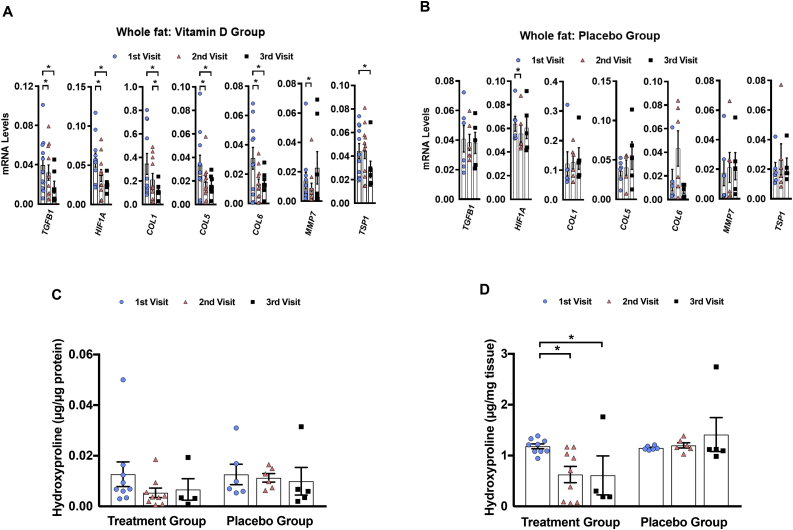
Given the metabolic importance of adipose fibrosis, the gene expression of various pro-fibrotic factors was examined to determine the effects of vitamin D on adipose tissue fibrosis. After vitamin D repletion to ∼30 ng/ml, the expression of the pro-fibrotic genes TGFB1, HIF1A, COL1,5,6 (collagen I, V, and VI), and MMP7 decreased in the whole fat (Figure 4A). Vitamin D repletion to ∼50 ng/ml was associated with reduced gene expression of only some of these pro-fibrotic factors (Figure 4A). In the placebo group, no significant differences were observed except for HIF1A from the first to second visits, although there was a trend toward worsening adipose tissue fibrosis at the end of the 6-month study period (Figure 4B). These results suggest that vitamin D repletion has modulatory effects on the expression of pro-fibrotic genes in adipose tissue.
Figure 4.
Adipose tissue gene expression of pro-fibrotic markers in humans. (A) Fold decreases in gene expression of various pro-fibrotic markers in whole fat samples from vitamin D group and (B) placebo group. (C) Adipose tissue hydroxyproline concentration in µg per µg of protein and in (D) µg per mg of tissue. Data are represented as means ± SEM. ∗p < 0.05; ∗ = significantly different. TGFB1 = transforming growth factor beta 1, HIF1A = hypoxia-inducible factor-1 alpha, COL1,5,6 = Collagen I, V, VI respectively, MMP7 = matrix metalloproteinase-7, TSP1 = thrombospondin1.
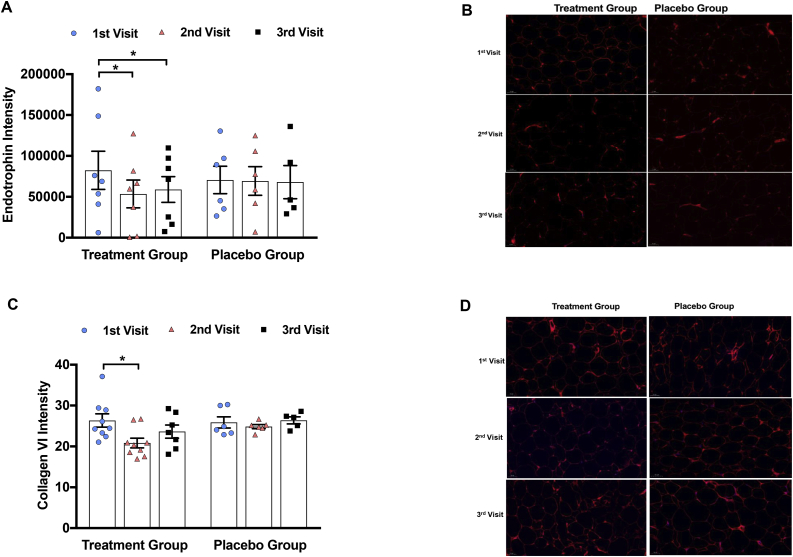
As a quantitative indicator of the overall collagen tissue content, we assayed the hydroxyproline content of whole adipose tissue and found that it significantly decreased after vitamin D repletion [51] (Figure 4C,D). To further determine the effect of vitamin D repletion on adipose tissue fibrosis, we assessed immunofluorescence staining for endotrophin and collagen VI. Endotrophin is a cleavage product of collagen VI in adipose tissue that is involved in fibrosis and inflammation; human studies revealed a strong upregulation of endotrophin in the adipose tissue of diabetic human patients [[52], [53], [54]]. Endotrophin intensity was found to be significantly decreased by approximately 50% in the vitamin D group at the second visit with no further decrease at the third visit, whereas in the placebo group, there was no significant difference between the three visits (Figure 5A,B). In the vitamin D group, there was also a significant reduction in collagen VI immunofluorescence by 19% from the first (baseline) visit to the second visit, with no further reduction from the second to third visits (Figure 5C,D), consistent with the change in gene expression. In the placebo group, collagen VI immunofluorescence did not significantly change throughout the study period, although there was a trend toward worsening of adipose tissue fibrosis at the third visit compared to the baseline when the subjects remained vitamin D deficient for 6 months (Figure 5C,D).
Figure 5.
Adipose tissue immunofluorescence staining in humans. (A) Endotrophin immunofluorescence relative to baseline at each clamp visit in both vitamin D treatment group and placebo group. (B) Endotrophin immunofluorescence: representative images at each clamp visit in both vitamin D treatment group and placebo group. (C) Collagen VI immunofluorescence relative to baseline at each clamp visit in both vitamin D treatment group and placebo group. (D) Collagen VI immunofluorescence in vitamin D and placebo group. Data are represented as means ± SEM. ∗p < 0.05; ∗ = significantly different from the first visit (1st visit).
3.5. Expression profiling of primary vitamin D receptor target genes
We examined the expression of four primary VDR target genes in the adipose tissue samples: nuclear receptor interacting protein 1 (NRIP1), thrombomodulin (THBD), dual-specific phosphatase 10 (DUSP10), and trafficking protein kinesin binding 1 (TRAK1) as representative examples. NRIP1, THBD, and DUSP10 gene expression significantly increased from the first to second visits (p = 0.003, p = 0.03, and p = 0.04, respectively) (Supplementary Fig. 2). There was also a trend toward the increased expression of TRAK1 from the first to the second visits (p = 0.06) (Supplementary Fig. 2). These findings are consistent with the results of a study in which vitamin D was repleted over a similar time course, indicating that these genes can serve as biomarkers for vitamin D3 response in adipose tissue of human subjects [46].
3.6. Generation and characterization of an adipocyte-specific vitamin D receptor-deficient mouse model (Ad-VDRKO mice)
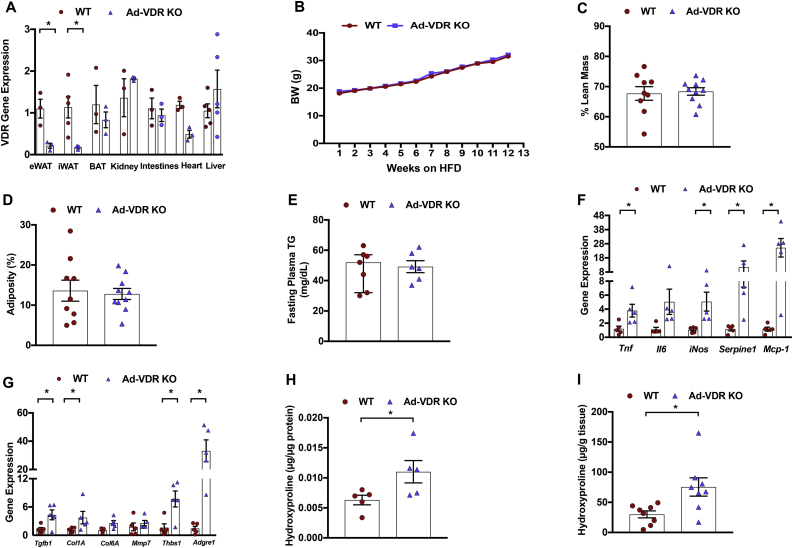
Since adipocytes express the vitamin D receptor (Vdr) and may modulate adipose tissue inflammation and fibrosis [55,56], we hypothesized that the observed actions of vitamin D were mediated through the VDR in adipocytes. Therefore, we next examined the impact of adipose-specific vitamin D receptor knockout on hepatic insulin resistance and adipose tissue inflammation and fibrosis. Confirming the efficiency of adipocyte-specific VDR deletion, Vdr mRNA levels decreased by approximately 81% in the epididymal white adipose tissue (eWAT) and 84% in the inguinal white adipose tissue (iWAT) of the Ad-VDRKO compared with WT control mice (Figure 6A) and trended lower in the brown adipose tissue (p = 0.067). In contrast, Vdr expression was not reduced in the kidney, intestine, heart, or liver (Figure 6A). Following 12 weeks of high-fat diet (HFD) feeding, there were no differences in body weight, percentage lean mass, or adiposity between the wild-type (WT) and adipose-specific vitamin D receptor (VDR) knockout (KO) mice (Figure 6B,C, and D). The WT and Ad-VDRKO mice also did not differ with respect to fasting plasma triglycerides (Figure 6E).
Figure 6.
Adipose tissue gene expression of pro-inflammatory and pro-fibrotic markers in adipose-specific vitamin D receptor knockout mice. Seven wild type (WT) and six adipocyte-specific vitamin D receptor knockout (Ad-VDR KO) mice were fed a high-fat diet (HFD) for twelve weeks from 3 weeks of age. (A) VDR gene expression was significantly decreased in white adipose tissue (eWAT) and inguinal white adipose tissue (iWAT) of Ad-VDR KO compared to WT mice but VDR expression was not reduced in brown adipose tissue (BAT), kidney, intestines, heart or liver. (B) Body weight (BW) of WT and Ad-VDR KO mice fed with HFD for 12 weeks. (C) After 12 weeks on the HFD, percentage lean mass, and (D) adiposity did not differ between WT and Ad-VDR KO mice. (E) WT and Ad-VDR KO mice were not different with respect to basal fasting plasma triglycerides. (F) Adipose tissue expression of pro-inflammatory and (G) pro-fibrotic genes in WT and Ad-VDR KO mice. (H) Adipose tissue hydroxyproline concentration in µg per µg of protein tissue and in (I) µg per gram of tissue. Tnf = tumor necrosis factor alpha, Il6 = interleukin-6, iNos = inducible nitric oxide synthase, Serpine1 (PAI-1) = plasminogen activator inhibitor-1, Mcp-1 = monocyte chemoattractant protein-1. Tgfb1 = transforming growth factor beta 1, Col1A = Collagen I, Col6A = Collagen VI,Mmp7 = matrix metalloproteinase-7, Thbs1 (Tsp1) = thrombospondin1, Adgre1 = F4/80. Data are represented as means ± SEM. ∗p < 0.05.
3.7. Ad-VDRKO mice on a high-fat diet develop adipose tissue inflammation and fibrosis
We next determined whether and how Ad-VDRKO would impact adipose tissue inflammation and fibrosis. As expected, the expression of several pro-inflammatory genes, including Tnf, iNOS, Serpine1 (PAI-1), Mcp-1 and Adgre1 (F4/80) was significantly higher in adipose tissue of Ad-VDRKO mice compared to WT mice, with a trend toward higher expression of Il6 in the former (Figure 6F). Additionally, the expression of pro-fibrotic genes TGFB1, Col6A (collagen VI), and THBS1 (TSP1) in fat was significantly higher in the adipose tissue of the Ad-VDRKO mice compared to the WT mice, with an upward trend in collagen I in the former (Figure 6G). These data suggest that modulating the VDR within the adipocytes may regulate adipose tissue inflammation and fibrosis. As observed in the human studies, adipose tissue hydroxyproline content increased in the Ad-VDRKO mice compared to the WT mice [57] (Figure 6H,I).
3.8. Ad-VDRKO mice on a high-fat diet exhibit increased hepatic insulin resistance
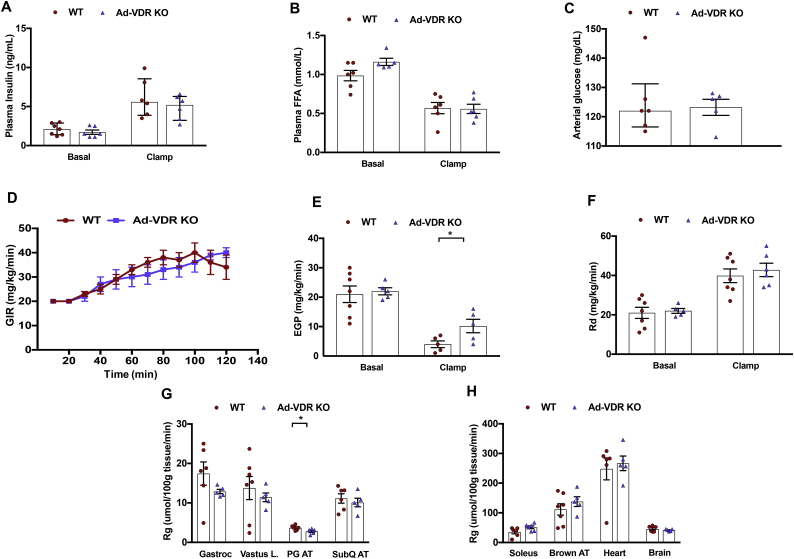
The impact of adipocyte VDR ablation on systemic glucose metabolism was then examined. To delineate the contributions of individual tissues to insulin action in this model, hyperinsulinemic-euglycemic clamps were performed on WT and Ad-VDRKO mice. Euglycemia was maintained (∼120 mg/dl) throughout the clamp studies, and the insulin concentrations increased to comparable levels in both groups. The WT and Ad-VDRKO mice did not differ with respect to fasting plasma insulin, FFA concentrations, and arterial glucose levels (Figure 7A,B, and C). Additionally, circulating FFAs were suppressed in all of the groups, suggesting equivalent insulin-induced suppression of lipolysis.
Figure 7.
Clamp studies in mice. At 12 weeks of age wild type (WT) and adipocyte-specific vitamin D receptor knockout (Ad-VDR KO) mice underwent hyperinsulinemic-euglycemic clamps. Mice were fasted 5 h before the onset of the hyperinsulinemic-euglycemic clamps. WT and Ad-VDR KO mice were not different with respect to (A) basal insulin, and (B) basal plasma free fatty acids (FFA). (A) Insulin concentrations was raised to comparable levels in both WT and Ad-VDR KO mice during the clamps. (B) Circulating FFA were suppressed in all groups during the clamps. (C) Baseline arterial glucose levels. (D) The glucose infusion rates (GIR) in the venous catheter needed to maintain euglycemia in WT and Ad-VDR KO mice. (E) Endogenous glucose production (EGP), a marker of hepatic glucose production, in WT and Ad-VDR KO mice, determined by administration of [6-6 H] glucose during the steady-state period of the insulin clamp. (F) Whole-body disappearance (Rd) were determined during the steady-state period of the insulin clamp. (G, H) Tissue-specific 2-DG uptake (Rg) after the insulin clamp in the gastrocnemius (Gastroc), vastus lateralis (Vastus L.), paragonadal adipose tissue (PG AT), subcutaneous adipose tissue (SubQ AT), soleus, brown adipose tissue (Brown AT), heart tissue and brain in WT and Ad-VDR KO mice. Data are represented as means ± SEM. ∗p < 0.05.
The glucose infusion rate (GIR) necessary to maintain euglycemia trended lower in the Ad-VDRKO mice for most of the time points during the clamp (Figure 7D), suggestive of insulin resistance. Endogenous glucose production (EGP) and rates of glucose disappearance (Rd) did not differ between the WT and Ad-VDRKO mice under basal conditions (Figure 7E,F). EGP was markedly higher in the Ad-VDRKO mice compared to the WT mice during the insulin clamp (Figure 7E). This indicates diminished suppression of EGP by insulin in the Ad-VDRKO mice, that is, increased hepatic insulin resistance. However, there was no difference in Rd between the WT and Ad-VDRKO mice under clamped conditions (Figure 7F).
In addition to hepatic insulin resistance, there were trends toward reduced skeletal muscle and adipose tissue glucose uptake (decreased Rg) in the Ad-VDRKO mice, with small but significant reductions in perigonadal adipose glucose uptake (Figure 7G). In contrast, tissue-specific Rg was no different between the Ad-VDRKO and WT mice in gastrocnemius, vastus lateralis, subcutaneous adipose tissue, soleus, brown adipose tissue, heart tissue, and brain, suggesting that adipocyte VDR is not a critical determinant of insulin action in these tissues (Figure 7G,H). Taken together, these results indicate that vitamin D exerts effects on hepatic insulin sensitivity through its effects on adipose tissue.
4. Discussion
Using a parallel design in rodents and humans, these studies are the first to comprehensively delineate the effects of vitamin D on systemic insulin sensitivity and adipose tissue inflammation and fibrosis. This prospective, randomized, double-blinded placebo-controlled study showed significant improvement in hepatic insulin sensitivity following vitamin D repletion in vitamin D-deficient, insulin-resistant humans in concert with reduced adipose tissue expression of pro-inflammatory and pro-fibrotic genes as well as direct adipose tissue markers of fibrosis. Further elevations of serum vitamin D levels above the normal target range were not associated with additional improvements. Of note, there was a worsening trend in hepatic insulin sensitivity and adipose tissue fibrosis and inflammation in the placebo group over the course of 6 months, suggesting that vitamin D deficiency over a long period of time could be associated with worsening hepatic insulin resistance. Importantly, adipocyte-specific VDRKO mice on a high-fat diet phenocopy the vitamin D deficient humans, exhibiting increased adipose tissue fibrosis, inflammation, and hepatic insulin resistance, suggesting that vitamin D modulates insulin sensitivity via specific effects on adipocytes.
The VDR is expressed in several insulin-responsive metabolic tissues, including the liver, skeletal muscle, and adipose tissue. Adipose tissue-derived inflammatory factors, which increase in proportion to the degree of adiposity, are believed to contribute to insulin resistance [6]. Adipocytes have been shown to initiate and/or sustain a pro-inflammatory response within adipose tissue [15,56]. This is consistent with the demonstrated regulatory role of the adipocytes, potentially through peroxisome proliferator-activated receptor-γ (PPAR-γ) to modulate adipose tissue inflammation and systemic insulin sensitivity [55]. It was recently determined that certain adipocyte progenitors modulate obesity-induced white adipose tissue fibrogenesis and are therefore associated with loss of metabolic fitness [58].
As inflammation impairs insulin sensitivity, it was likely that reduced adipose tissue inflammation and fibrosis observed in our studies contributed to improving hepatic insulin sensitivity. Consistent with the current research, there is a substantial body of rodent studies linking adipose tissue inflammation with insulin resistance, particularly of the liver [59]. Activation of hepatic inflammatory pathways involving IKK-β and NF-κB by adipocyte-derived cytokines has been shown to cause hepatic insulin resistance, suggesting a connection between adipose (particularly visceral) inflammation and hepatic insulin resistance [31,60].
Despite the improvements in adipose tissue inflammation and fibrosis in the current studies, peripheral insulin sensitivity measured by GU did not change. Of note, increasing adiposity does not appear to activate inflammatory cascades in skeletal muscle as it does in adipose tissue and the liver [59]. This may suggest why hepatic, but not peripheral, insulin sensitivity was associated with adipose tissue inflammation and fibrosis in both the human and rodent studies. Vitamin D deficiency is associated with increased circulating matrix metalloproteinase (MMP-2 and MMP-9) and C-reactive protein (CRP), which were shown in a few studies to be reduced by vitamin D supplementation [[61], [62], [63]]. Additionally, the active form of vitamin D [1,25(OH)2D] may restrain macrophage cytokine production by suppressing the NF-кB inflammatory pathway [64]. Vitamin D is a fat-soluble vitamin that is stored in adipose tissue and therefore could exert important paracrine effects within this tissue [65,66]. Thus, these studies in adipocyte-specific VDRKO mice point to important effects of vitamin D on adipose tissue, with systemic metabolic sequelae.
Along with our observation of significant reductions in adipose tissue inflammation, normalizing vitamin D levels led to a significant reduction in adipose tissue fibrotic gene expression and collagen (by immunofluorescence), while the placebo group trended toward a worsening of these parameters over six months of continued vitamin D deficiency. Complementary findings of increased adipose tissue fibrosis markers were observed in the Ad-VDRKO mice. Pathological expansion of adipose tissue in obesity results in inflammation, fibrosis, and insulin resistance [67]. To the best of our knowledge, this is the first study establishing a connection between vitamin D deficiency and adipose tissue fibrosis and among the first to make a connection between adipose tissue fibrosis and insulin resistance in humans [[12], [13], [14],68]. There is substantial evidence in rodents that adipose tissue fibrosis is linked to metabolic dysfunction, further supported by the current findings in Ad-VDRKO mice [13,14,69,70]. PPARγ agonists and adiponectin both reduce adipose tissue fibrosis, inflammation, and insulin resistance [4]. With the activation of vitamin D receptors and inhibition of the TGF-β/SMAD signaling pathway [3,[35], [36], [37]], it is likely that vitamin D repletion can prevent the development of adipose tissue fibrosis and offer additional metabolic benefits.
Discrepancies in the results of previous human studies investigating the relationship between vitamin D and insulin resistance highlight the need for carefully designed research with measurement of serum 25(OH)D levels before and after adequate dosing. This is particularly noteworthy given a recent clinical trial in which administering vitamin D to 2,423 pre-diabetic participants, the vast majority of whom were vitamin D replete, did not impact progression to T2D [21]. Of note, in those subjects with initial vitamin D levels <12 ng/ml, vitamin D repletion reduced the hazard ratio of developing T2D to 0.38 (CI: 0.18 to 0.80) [21]. The lack of benefit in the Pittas study from increasing mean vitamin D levels from 27.7 to 54.3 ng/ml is consistent with the lack of further benefit of raising vitamin D levels to ∼50 ng/ml in the current study. Furthermore, there is a clear need for more sensitive metabolic measures [[71], [72], [73]] as used in the current research. Some studies have shown no effect of vitamin D supplementation on insulin sensitivity [[74], [75], [76], [77]]. This includes a meta-analysis of prospective randomized clinical trials [77] employing HOMA-IR, which is recognized to have limitations as a longitudinal measure of insulin sensitivity [78]. Conversely, several studies showed positive associations between serum vitamin D levels and peripheral insulin sensitivity in both pre-diabetic and T2D subjects [79,80]. Four out of six cross-sectional studies using euglycemic stepped hyperinsulinemic clamps, considered the gold standard for measuring insulin sensitivity, showed positive associations between vitamin D levels and insulin sensitivity in non-diabetic subjects [[81], [82], [83], [84], [85], [86]]. Prospective studies also revealed beneficial effects of vitamin D supplementation on insulin sensitivity [87,88], including a meta-analysis of prospective cohort studies [17,89,90]. Intriguingly, prospective studies comparing euglycemic-hyperinsulinemic clamps at baseline and after 12 and 24 weeks of vitamin D supplementation noted improved insulin sensitivity in obese insulin-resistant subjects [91,92] but not in T2D subjects [74], suggesting that the established metabolic defects of T2D are not easily reversed by vitamin D repletion.
Further elevations in serum vitamin D levels above the normal range were not associated with additional improvements in inflammatory and fibrotic markers nor with further improvement in EGP or increases in VDR-related gene expression. Our findings are consistent with those of Pittas et al. in which increasing vitamin D levels from 27.7 to 54.3 ng/ml failed to impact the progression to type 2 diabetes [21]. This suggests that increasing vitamin D levels above normal/replete levels is not associated with further metabolic benefits. Additionally, in our study, not all of the subjects included in the first visit completed the third visit; hence, separate analyses were conducted to compare the first vs second and first vs third visits. The reduced sample number at the third visit might explain instances in which there was significant improvement at the second but not third visit.
Our results may appear to be at odds with our prior work demonstrating that the VDR promotes insulin resistance in cultured adipocytes [93]. In those studies, the VDR was shown to be induced by both dexamethasone and TNF-α, and knockdown of the VDR protected against the development of insulin resistance induced by those agents. Conversely, the overexpression of the VDR in adipocytes caused reduced insulin-stimulated glucose uptake, yet the addition of vitamin D did not affect insulin action [93]. Although the reasons for the discrepancy between those in vitro studies and the in vivo work presented herein are not entirely clear, we speculate that the effects of vitamin D/VDR on fibrosis and inflammation, not modeled in the culture system, would predominate over any direct effects of the VDR on adipocyte insulin action. An additional potential limitation is that our study lacked sufficient power to detect potential sex- or ethnicity-specific 25(OH)D thresholds or dose–response relationships. It is unclear how long the corrective effects of vitamin D would last post-treatment. Finally, these results cannot be extrapolated to patients with diabetes, because such subjects were excluded.
5. Conclusions
In summary, increasing vitamin D concentrations in the normal range improved hepatic insulin resistance and reduced adipose expression of pro-inflammatory and pro-fibrotic genes in concert with diminished tissue fibrosis markers. These findings were accentuated by the worsening trends in insulin resistance and adipose tissue fibrosis observed following 6 months of uncorrected vitamin D deficiency. Further elevations in vitamin D concentrations had no additional impact on metabolism or adipose tissue characteristics. Importantly, parallel rodent studies demonstrated that adipocyte-specific depletion of the vitamin D receptor induced adipose tissue inflammation and fibrosis as well as worsened hepatic insulin resistance, suggesting that the latter could be attributed to the effects of vitamin D on adipose tissue. Thus, correcting vitamin D deficiency in targeted individuals may have favorable metabolic effects and could potentially offer substantial public health benefits given the magnitude of the global obesity epidemic. These studies have far-reaching implications for understanding the role of adipocytes in mediating adipose tissue inflammation and fibrosis, ultimately impacting insulin sensitivity.
Data access and responsibility
The principal investigator, Dr. Meredith Hawkins, had full access to all of the data in this study and takes responsibility for the data integrity and the data analysis accuracy.
Author contributions
E.R., P.K., and M.K. conceived the study, designed and conducted the experiments, analyzed the results, and drafted the manuscript. E.L-Y., S.K. A.G., and K.Z. conducted the experiments, analyzed the results, and drafted the manuscript. J.Y.Y., M.C., S.J., and S.K. analyzed the results and drafted the manuscript. S.B. and P.G. conducted the experiments. All of the authors edited and approved the final manuscript.
Funding
This study was supported by grants from the American Diabetes Association (1-12-CT-41 to P.K. and M.H.), the National Institutes of Health (DK069861, DK079974 and DK48321, to M.H.), and by the Einstein-Montefiore NIH CTSA Grant UL1TR001073 from the National Center for Research Resources (NCRR). E.D.R. was supported by NIH R01 DK 085171, DK 102173, and DK 102170. So.K. was supported by NIH R01 DK 116008. E.L-Y. was supported by the American Diabetes Association (1-18-PMF-024) and the National Center for Advancing Translational Science Einstein-Montefiore Clinical and Translational Science Awards (UL1 TR002556).
Acknowledgments
We acknowledge the assistance of Robin Sgueglia, Roger Maginley, Pooja Ragavan, Vera DesMarais, Snema Damal Villivallam and Hillary Guzik and thank the Ddrops Company for supplying vitamin D and placebo for the studies. The imaging was conducted at the Analytical Imaging Facility, which is funded by NCI Cancer Grant P30CA013330. The mouse phenotyping studies were conducted at Beth Israel Deaconess Medical Center except for the clamp studies, which were performed at the Vanderbilt University Medical Center Mouse Metabolic Phenotyping Center (DK059637). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views or policies of the FDA, NCRR, or NIH.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.101095.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Figure 1. (A) Endogenous glucose production (EGP) and (B and C) glucose infusion rates (GIR). Data are represented as means ± SEM. Supplementary Figure 2. Expression profiling of the primary VDR target genes. qPCR was performed to determine the relative changes in the genes' mRNA expression (A) Dual-specific phosphatase 10 (DUSP10), (B) trafficking protein, kinesin binding 1 (TRAK1), (C) nuclear receptor interacting protein 1 (NRIP1), and (D) thrombomodulin (THBD) normalized by the reference genes GAPDH and RPLP0. The average of the reference genes RPLP0 and GAPDH was set at 1. Data are represented as means ± SEM. ∗P < 0.05; ∗ = significantly different from the first visit. #P = 0.06. Supplementary Table 1. Baseline subject characteristics. Supplementary Table 2. Hormone levels during the clamp studies. Table 2a. Hormone levels during the clamp studies (vitamin D group). Table 2b. Hormone levels during the clamp studies (placebo group).
References
- 1.Kusminski C.M., Bickel P.E., Scherer P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nature Reviews Drug Discovery. 2016;15(9):639–660. doi: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 2.Ding N., Yu R.T., Subramaniam N., Sherman M.H., Wilson C., Rao R. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153(3):601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito I., Waku T., Aoki M., Abe R., Nagai Y., Watanabe T. A nonclassical vitamin D receptor pathway suppresses renal fibrosis. Journal of Clinical Investigation. 2013;123(11):4579–4594. doi: 10.1172/JCI67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buechler C., Krautbauer S., Eisinger K. Adipose tissue fibrosis. World Journal of Diabetes. 2015;6(4):548–553. doi: 10.4239/wjd.v6.i4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crewe C., An Y.A., Scherer P.E. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. Journal of Clinical Investigation. 2017;127(1):74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonakdaran S., Varasteh A.R. Correlation between serum 25 hydroxy vitamin D3 and laboratory risk markers of cardiovascular diseases in type 2 diabetic patients. Saudi Medical Journal. 2009;30(4):509–514. [PubMed] [Google Scholar]
- 8.Desai H.R., Sivasubramaniyam T., Revelo X.S., Schroer S.A., Luk C.T., Rikkala P.R. Macrophage JAK2 deficiency protects against high-fat diet-induced inflammation. Scientific Reports. 2017;7(1):7653. doi: 10.1038/s41598-017-07923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koppaka S., Kehlenbrink S., Carey M., Li W., Sanchez E., Lee D.E. Reduced adipose tissue macrophage content is associated with improved insulin sensitivity in thiazolidinedione-treated diabetic humans. Diabetes. 2013;62(6):1843–1854. doi: 10.2337/db12-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makkonen J., Westerbacka J., Kolak M., Sutinen J., Corner A., Hamsten A. Increased expression of the macrophage markers and of 11beta-HSD-1 in subcutaneous adipose tissue, but not in cultured monocyte-derived macrophages, is associated with liver fat in human obesity. International Journal of Obesity (Lond) 2007;31(10):1617–1625. doi: 10.1038/sj.ijo.0803635. [DOI] [PubMed] [Google Scholar]
- 11.Halberg N., Khan T., Trujillo M.E., Wernstedt-Asterholm I., Attie A.D., Sherwani S. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Molecular and Cellular Biology. 2009;29(16):4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer M., Yao-Borengasser A., Unal R., Rasouli N., Gurley C.M., Zhu B. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. American Journal of Physiology. Endocrinology and Metabolism. 2010;299(6):E1016–E1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer M., Unal R., Zhu B., Rasouli N., McGehee R.E., Jr., Peterson C.A. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. Journal of Clinical Endocrinology & Metabolism. 2011;96(12):E1990–E1998. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawler H.M., Underkofler C.M., Kern P.A., Erickson C., Bredbeck B., Rasouli N. Adipose tissue hypoxia, inflammation, and fibrosis in obese insulin-sensitive and obese insulin-resistant subjects. Journal of Clinical Endocrinology & Metabolism. 2016;101(4):1422–1428. doi: 10.1210/jc.2015-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishore P., Li W., Tonelli J., Lee D.E., Koppaka S., Zhang K. Adipocyte-derived factors potentiate nutrient-induced production of plasminogen activator inhibitor-1 by macrophages. Science Translational Medicine. 2010;2(20) doi: 10.1126/scitranslmed.3000292. [DOI] [PubMed] [Google Scholar]
- 16.Pittas A.G., Dawson-Hughes B., Li T., Van Dam R.M., Willett W.C., Manson J.E. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29(3):650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 17.Song Y., Wang L., Pittas A.G., Del Gobbo L.C., Zhang C., Manson J.E. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36(5):1422–1428. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scragg R., Sowers M., Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the third national health and nutrition examination survey. Diabetes Care. 2004;27(12):2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine . Committee to review dietary reference intakes for vitamin, D. And calcium, the national academies collection: Reports funded by national Institutes of health. In: Ross A.C., editor. Dietary reference intakes for calcium and vitamin D. National Academies Press (US) National Academy of Sciences; Washington (DC): 2011. [Google Scholar]
- 20.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. Journal of Clinical Endocrinology & Metabolism. 2012;97(4):1153–1158. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 21.Pittas A.G., Dawson-Hughes B., Sheehan P., Ware J.H., Knowler W.C., Aroda V.R. Vitamin D supplementation and prevention of type 2 diabetes. New England Journal of Medicine. 2019;381(6):520–530. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccolo B.D., Dolnikowski G., Seyoum E., Thomas A.P., Gertz E.R., Souza E.C. Association between subcutaneous white adipose tissue and serum 25-hydroxyvitamin D in overweight and obese adults. Nutrients. 2013;5(9):3352–3366. doi: 10.3390/nu5093352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blum M., Dolnikowski G., Seyoum E., Harris S.S., Booth S.L., Peterson J. Vitamin D(3) in fat tissue. Endocrine. 2008;33(1):90–94. doi: 10.1007/s12020-008-9051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcotorchino J., Tourniaire F., Landrier J.F. Vitamin D, adipose tissue, and obesity. Hormone Molecular Biology and Clinical Investigation. 2013;15(3):123–128. doi: 10.1515/hmbci-2013-0027. [DOI] [PubMed] [Google Scholar]
- 25.Dretakis O.E., Tsatsanis C., Fyrgadis A., Drakopoulos C.G., Steriopoulos K., Margioris A.N. Correlation between serum 25-hydroxyvitamin D levels and quadriceps muscle strength in elderly cretans. Journal of International Medical Research. 2010;38(5):1824–1834. doi: 10.1177/147323001003800530. [DOI] [PubMed] [Google Scholar]
- 26.Baz-Hecht M., Goldfine A.B. The impact of vitamin D deficiency on diabetes and cardiovascular risk. Current Opinion in Endocrinology Diabetes and Obesity. 2010;17(2):113–119. doi: 10.1097/MED.0b013e3283372859. [DOI] [PubMed] [Google Scholar]
- 27.Yiu Y.F., Chan Y.H., Yiu K.H., Siu C.W., Li S.W., Wong L.Y. Vitamin D deficiency is associated with depletion of circulating endothelial progenitor cells and endothelial dysfunction in patients with type 2 diabetes. Journal of Clinical Endocrinology & Metabolism. 2011;96(5):E830–E835. doi: 10.1210/jc.2010-2212. [DOI] [PubMed] [Google Scholar]
- 28.Ding C., Gao D., Wilding J., Trayhurn P., Bing C. Vitamin D signalling in adipose tissue. British Journal of Nutrition. 2012;108(11):1915–1923. doi: 10.1017/S0007114512003285. [DOI] [PubMed] [Google Scholar]
- 29.Oh J., Riek A.E., Darwech I., Funai K., Shao J., Chin K. Deletion of macrophage Vitamin D receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell Reports. 2015;10(11):1872–1886. doi: 10.1016/j.celrep.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song L., Papaioannou G., Zhao H., Luderer H.F., Miller C., Dall'Osso C. The vitamin D receptor regulates tissue resident macrophage response to injury. Endocrinology. 2016;157(10):4066–4075. doi: 10.1210/en.2016-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong B., Zhou Y., Wang W., Scott J., Kim K.H., Sun Z. Vitamin D receptor activation in liver macrophages ameliorates hepatic inflammation, steatosis, and insulin resistance in mice. Hepatology. 2019;71(5):1559–1574. doi: 10.1002/hep.30937. [DOI] [PubMed] [Google Scholar]
- 32.Zasloff M. Fighting infections with vitamin D. Nature Medicine. 2006;12(4):388–390. doi: 10.1038/nm0406-388. [DOI] [PubMed] [Google Scholar]
- 33.Mutt S.J., Karhu T., Lehtonen S., Lehenkari P., Carlberg C., Saarnio J. Inhibition of cytokine secretion from adipocytes by 1,25-dihydroxyvitamin D(3) via the NF-kappaB pathway. The FASEB Journal. 2012;26(11):4400–4407. doi: 10.1096/fj.12-210880. [DOI] [PubMed] [Google Scholar]
- 34.Gao D., Trayhurn P., Bing C. 1,25-Dihydroxyvitamin D3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. International Journal of Obesity (Lond) 2013;37(3):357–365. doi: 10.1038/ijo.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song B.J., Rockey D.C. Status of research on vitamin D supplementation in treating or preventing liver fibrosis. Liver International. 2013;33(5):653–655. doi: 10.1111/liv.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palumbo-Zerr K., Zerr P., Distler A., Fliehr J., Mancuso R., Huang J. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nature Medicine. 2015;21(2):150–158. doi: 10.1038/nm.3777. [DOI] [PubMed] [Google Scholar]
- 37.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinology and Metabolism Clinics of North America. 2010;39(2):365–379. doi: 10.1016/j.ecl.2010.02.010. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szymczak-Pajor I., Sliwinska A. Analysis of association between vitamin D deficiency and insulin resistance. Nutrients. 2019;11(4) doi: 10.3390/nu11040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones G. Pharmacokinetics of vitamin D toxicity. American Journal of Clinical Nutrition. 2008;88(2):582s–586s. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 40.Holick M.F. Vitamin D status: measurement, interpretation, and clinical application. Annals of Epidemiology. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barger-Lux M.J., Heaney R.P., Dowell S., Chen T.C., Holick M.F. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporosis International. 1998;8(3):222–230. doi: 10.1007/s001980050058. [DOI] [PubMed] [Google Scholar]
- 42.Tonelli J., Li W., Kishore P., Pajvani U.B., Kwon E., Weaver C. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53(6):1621–1629. doi: 10.2337/diabetes.53.6.1621. [DOI] [PubMed] [Google Scholar]
- 43.Venkatesan P., Tiwari A., Dasgupta R., Carey M., Kehlenbrink S., Wickramanayake A. Surrogate measures of insulin sensitivity when compared to euglycemic hyperinsulinemic clamp studies in Asian Indian men without diabetes. Journal of Diabetic Complications. 2016;30(2):287–291. doi: 10.1016/j.jdiacomp.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 44.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 45.Mauriege P., Imbeault P., Langin D., Lacaille M., Almeras N., Tremblay A. Regional and gender variations in adipose tissue lipolysis in response to weight loss. The Journal of Lipid Research. 1999;40(9):1559–1571. [PubMed] [Google Scholar]
- 46.Ryynänen J., Neme A., Tuomainen T.P., Virtanen J.K., Voutilainen S., Nurmi T. Changes in vitamin D target gene expression in adipose tissue monitor the vitamin D response of human individuals. Molecular Nutrition & Food Research. 2014;58(10):2036–2045. doi: 10.1002/mnfr.201400291. [DOI] [PubMed] [Google Scholar]
- 47.Park J., Morley T.S., Scherer P.E. Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Molecular Medicine. 2013;5(6):935–948. doi: 10.1002/emmm.201202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eguchi J., Wang X., Yu S., Kershaw E.E., Chiu P.C., Dushay J. Transcriptional control of adipose lipid handling by IRF4. Cell Metabolism. 2011;13(3):249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lantier L., Williams A.S., Williams I.M., Yang K.K., Bracy D.P., Goelzer M. SIRT3 is crucial for maintaining skeletal muscle insulin action and protects against severe insulin resistance in high-fat-fed mice. Diabetes. 2015;64(9):3081–3092. doi: 10.2337/db14-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraegen E.W., James D.E., Jenkins A.B., Chisholm D.J. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. American Journal of Physiology. 1985;248(3 Pt 1):E353–E362. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- 51.Khan T., Muise E.S., Iyengar P., Wang Z.V., Chandalia M., Abate N. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Molecular and Cellular Biology. 2009;29(6):1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Y., Gu X., Zhang N., Kolonin M.G., An Z., Sun K. Divergent functions of endotrophin on different cell populations in adipose tissue. American Journal of Physiology. Endocrinology and Metabolism. 2016;311(6):E952–E963. doi: 10.1152/ajpendo.00314.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun K., Park J., Gupta O.T., Holland W.L., Auerbach P., Zhang N. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nature Communications. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karsdal M.A., Henriksen K., Genovese F., Leeming D.J., Nielsen M.J., Riis B.J. Serum endotrophin identifies optimal responders to PPARgamma agonists in type 2 diabetes. Diabetologia. 2017;60(1):50–59. doi: 10.1007/s00125-016-4094-1. [DOI] [PubMed] [Google Scholar]
- 55.Li P., Fan W., Xu J., Lu M., Yamamoto H., Auwerx J. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell. 2011;147(4):815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer-Posovszky P., Wang Q.A., Asterholm I.W., Rutkowski J.M., Scherer P.E. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology. 2011;152(8):3074–3081. doi: 10.1210/en.2011-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo T., Nocon A., Fry J., Sherban A., Rui X., Jiang B. AMPK activation by metformin suppresses abnormal extracellular matrix remodeling in adipose tissue and ameliorates insulin resistance in obesity. Diabetes. 2016;65(8):2295–2310. doi: 10.2337/db15-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marcelin G., Ferreira A., Liu Y., Atlan M., Aron-Wisnewsky J., Pelloux V. A PDGFRalpha-mediated switch toward CD9(high) adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metabolism. 2017;25(3):673–685. doi: 10.1016/j.cmet.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 59.Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. Journal of Clinical Investigation. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai D., Yuan M., Frantz D.F., Melendez P.A., Hansen L., Lee J. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nature Medicine. 2005;11(2):183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shab-Bidar S., Neyestani T.R., Djazayery A., Eshraghian M.R., Houshiarrad A., Kalayi A. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metabolism Research Review. 2012;28(5):424–430. doi: 10.1002/dmrr.2290. [DOI] [PubMed] [Google Scholar]
- 62.Tabesh M., Azadbakht L., Faghihimani E., Tabesh M., Esmaillzadeh A. Calcium-vitamin D cosupplementation influences circulating inflammatory biomarkers and adipocytokines in vitamin D-insufficient diabetics: a randomized controlled clinical trial. Journal of Clinical Endocrinology & Metabolism. 2014;99(12):E2485–E2493. doi: 10.1210/jc.2014-1977. [DOI] [PubMed] [Google Scholar]
- 63.Sharifi N., Amani R., Hajiani E., Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47(1):70–80. doi: 10.1007/s12020-014-0336-5. [DOI] [PubMed] [Google Scholar]
- 64.Wang Q., He Y., Shen Y., Zhang Q., Chen D., Zuo C. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. Journal of Biological Chemistry. 2014;289(17):11681–11694. doi: 10.1074/jbc.M113.517581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veldhoen M., Ferreira C. Influence of nutrient-derived metabolites on lymphocyte immunity. Nature Medicine. 2015;21(7):709–718. doi: 10.1038/nm.3894. [DOI] [PubMed] [Google Scholar]
- 66.Pandolfi F., Franza L., Mandolini C., Conti P. Immune modulation by vitamin D: special emphasis on its role in prevention and treatment of cancer. Clinical Therapeutics. 2017;39(5):884–893. doi: 10.1016/j.clinthera.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Sun K., Kusminski C.M., Scherer P.E. Adipose tissue remodeling and obesity. Journal of Clinical Investigation. 2011;121(6):2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caer C., Rouault C., Le Roy T., Poitou C., Aron-Wisnewsky J., Torcivia A. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Scientific Reports. 2017;7(1):3000. doi: 10.1038/s41598-017-02660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. International Journal of Obesity (Lond) 2009;33(1):54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiological Reviews. 2013;93(1):1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 71.Seida J.C., Mitri J., Colmers I.N., Majumdar S.R., Davidson M.B., Edwards A.L. Clinical review: effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. Journal of Clinical Endocrinology & Metabolism. 2014;99(10):3551–3560. doi: 10.1210/jc.2014-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pilz S., Verheyen N., Grubler M.R., Tomaschitz A., Marz W. Vitamin D and cardiovascular disease prevention. Nature Reviews Cardiology. 2016;13(7):404–417. doi: 10.1038/nrcardio.2016.73. [DOI] [PubMed] [Google Scholar]
- 73.Angellotti E., Pittas A.G. The role of vitamin D in the prevention of type 2 diabetes. To D or not to D? Endocrinology. 2017;158(7):2013–2021. doi: 10.1210/en.2017-00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kampmann U., Mosekilde L., Juhl C., Moller N., Christensen B., Rejnmark L. Effects of 12 weeks high dose vitamin D3 treatment on insulin sensitivity, beta cell function, and metabolic markers in patients with type 2 diabetes and vitamin D insufficiency - a double-blind, randomized, placebo-controlled trial. Metabolism. 2014;63(9):1115–1124. doi: 10.1016/j.metabol.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 75.Davidson M.B., Duran P., Lee M.L., Friedman T.C. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care. 2013;36(2):260–266. doi: 10.2337/dc12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barengolts E., Manickam B., Eisenberg Y., Akbar A., Kukreja S., Ciubotaru I. Effect of high-dose vitamin D repletion on glycemic control in african-american males with prediabetes and hypovitaminosis D. Endocrine Practice. 2015;21(6):604–612. doi: 10.4158/EP14548.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poolsup N., Suksomboon N., Plordplong N. Effect of vitamin D supplementation on insulin resistance and glycaemic control in prediabetes: a systematic review and meta-analysis. Diabetic Medicine. 2016;33(3):290–299. doi: 10.1111/dme.12893. [DOI] [PubMed] [Google Scholar]
- 78.Xiang A.H., Watanabe R.M., Buchanan T.A. HOMA and Matsuda indices of insulin sensitivity: poor correlation with minimal model-based estimates of insulin sensitivity in longitudinal settings. Diabetologia. 2014;57(2):334–338. doi: 10.1007/s00125-013-3121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kayaniyil S., Vieth R., Retnakaran R., Knight J.A., Qi Y., Gerstein H.C. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33(6):1379–1381. doi: 10.2337/dc09-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Al-Daghri N.M., Al-Attas O.S., Alokail M.S., Alkharfy K.M., Al-Othman A., Draz H.M. Hypovitaminosis D associations with adverse metabolic parameters are accentuated in patients with Type 2 diabetes mellitus: a body mass index-independent role of adiponectin? Journal of Endocrinological Investigation. 2013;36(1):1–6. doi: 10.3275/8183. [DOI] [PubMed] [Google Scholar]
- 81.Morisset A.S., Tardio V., Weisnagel J., Lemieux S., Bergeron J., Gagnon C. Associations between serum 25-hydroxyvitamin D, insulin sensitivity, insulin secretion, and beta-cell function according to glucose tolerance status. Metabolic Syndrome and Related Disorders. 2015;13(5):208–213. doi: 10.1089/met.2014.0159. [DOI] [PubMed] [Google Scholar]
- 82.Muscogiuri G., Policola C., Prioletta A., Sorice G., Mezza T., Lassandro A. Low levels of 25(OH)D and insulin-resistance: 2 unrelated features or a cause-effect in PCOS? Clinical Nutrition. 2012;31(4):476–480. doi: 10.1016/j.clnu.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 83.Muscogiuri G., Sorice G.P., Prioletta A., Policola C., Della Casa S., Pontecorvi A. 25-Hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity (Silver Spring) 2010;18(10):1906–1910. doi: 10.1038/oby.2010.11. [DOI] [PubMed] [Google Scholar]
- 84.Joham A.E., Teede H.J., Cassar S., Stepto N.K., Strauss B.J., Harrison C.L. Vitamin D in polycystic ovary syndrome: relationship to obesity and insulin resistance. Molecular Nutrition & Food Research. 2016;60(1):110–118. doi: 10.1002/mnfr.201500259. [DOI] [PubMed] [Google Scholar]
- 85.Wallace I.R., McKinley M.C., McEvoy C.T., Hamill L.L., Ennis C.N., McGinty A. Serum 25-hydroxyvitamin D and insulin resistance in people at high risk of cardiovascular disease: a euglycaemic hyperinsulinaemic clamp study. Clin Endocrinology (Oxf) 2016;85(3):386–392. doi: 10.1111/cen.13100. [DOI] [PubMed] [Google Scholar]
- 86.Pramono A., Jocken J.W.E., Essers Y.P.G., Goossens G.H., Blaak E.E. Vitamin D and tissue-specific insulin sensitivity in humans with overweight/obesity. Journal of Clinical Endocrinology & Metabolism. 2019;104(1):49–56. doi: 10.1210/jc.2018-00995. [DOI] [PubMed] [Google Scholar]
- 87.von Hurst P.R., Stonehouse W., Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. British Journal of Nutrition. 2010;103(4):549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 88.Pittas A.G., Harris S.S., Stark P.C., Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30(4):980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 89.Afzal S., Bojesen S.E., Nordestgaard B.G. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clinical Chemistry. 2013;59(2):381–391. doi: 10.1373/clinchem.2012.193003. [DOI] [PubMed] [Google Scholar]
- 90.Asbaghi O., Khosroshahi M.Z., Kashkooli S., Abbasnezhad A. Effect of CalciumVitamin D CoSupplementation on insulin, insulin sensitivity, and glycemia: a systematic review and meta-analysis of randomized clinical trials. Hormone and Metabolic Research. 2019;51(5):288–295. doi: 10.1055/a-0887-0205. [DOI] [PubMed] [Google Scholar]
- 91.Cefalo C.M.A., Conte C., Sorice G.P., Moffa S., Sun V.A., Cinti F. Effect of vitamin D supplementation on obesity-induced insulin resistance: a double-blind, randomized, placebo-controlled trial. Obesity (Silver Spring) 2018;26(4):651–657. doi: 10.1002/oby.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lemieux P., Weisnagel J.S., Caron A.Z., Julien A.S., Morisset A.S., Carreau A.M. Effects of 6-month vitamin D supplementation on insulin sensitivity and secretion: a randomized, placebo-controlled trial. European Journal of Endocrinology. 2019;181(3):287–299. doi: 10.1530/EJE-19-0156. [DOI] [PubMed] [Google Scholar]
- 93.Kang S., Tsai L.T., Zhou Y., Evertts A., Xu S., Griffin M.J. Identification of nuclear hormone receptor pathways causing insulin resistance by transcriptional and epigenomic analysis. Nature Cell Biology. 2015;17(1):44–56. doi: 10.1038/ncb3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. (A) Endogenous glucose production (EGP) and (B and C) glucose infusion rates (GIR). Data are represented as means ± SEM. Supplementary Figure 2. Expression profiling of the primary VDR target genes. qPCR was performed to determine the relative changes in the genes' mRNA expression (A) Dual-specific phosphatase 10 (DUSP10), (B) trafficking protein, kinesin binding 1 (TRAK1), (C) nuclear receptor interacting protein 1 (NRIP1), and (D) thrombomodulin (THBD) normalized by the reference genes GAPDH and RPLP0. The average of the reference genes RPLP0 and GAPDH was set at 1. Data are represented as means ± SEM. ∗P < 0.05; ∗ = significantly different from the first visit. #P = 0.06. Supplementary Table 1. Baseline subject characteristics. Supplementary Table 2. Hormone levels during the clamp studies. Table 2a. Hormone levels during the clamp studies (vitamin D group). Table 2b. Hormone levels during the clamp studies (placebo group).