Abstract
Objective:
Metabolic syndrome (MetS) includes several cardiovascular (CV) risk factors. This study aimed to assess CV risk of MetS, contribution of its components to the risk, and whether MetS provides additional risk beyond its components.
Methods:
The Prospective Urban Rural Epidemiology (PURE) Turkey cohort included 3933 individuals aged between 35 and 70 years, with a median follow-up of 8.9 years. MetS was diagnosed as the presence of any of the following criteria: high blood pressure, high fasting plasma glucose, abdominal obesity, low HDL-cholesterol, or high triglycerides. The primary outcome was the composite of fatal CV events, non-fatal myocardial infarction, stroke or heart failure, adjusted for age, sex, smoking, family history of CV diseases, and LDL-cholesterol.
Results:
The primary outcome was more common in the MetS group [178 (9.2%) vs. 70 (3.5%); corresponding incidence rate of 11.3 vs. 4.2 per 1000 person-years; log-rank p<0.001]. Each component was significantly associated with the primary outcome; however, when the components were sequentially included in the model, abdominal obesity and high triglycerides did not provide additional risk on top of the other three components. The hazard ratio for MetS for the primary outcome was 2.12 (95% confidence interval 1.59–2.81, p<0.001), and the discriminative ability (c-statistics) of the models with MetS and the components was similar.
Conclusion:
MetS increases the risk of CV events more than two-fold. High blood pressure, high fasting plasma glucose, and low HDL-cholesterol are the top three components of MetS for CV risk. MetS and its components have a similar discriminative ability for CV events.
Keywords: metabolic syndrome, cardiovascular diseases, mortality, cohort study
Introduction
Metabolic syndrome (MetS) is defined as a cluster of several interrelated clinical and laboratory parameters. It is believed that abdominal obesity or insulin resistance is the common denominator in the development of MetS (1). Several societies have defined MetS using different criteria (2-4). Some of them place insulin resistance or abdominal obesity as an essential component, whereas others give equal weight to each component. Moreover, the definitions of abdominal obesity and fasting plasma glucose level are also different. The presence of various definitions causes confusion in terms of assessing the risk attributed to MetS, and also when comparing the findings of different studies. In 2005, a new definition was proposed (5), and a standard definition was endorsed by several societies later on (6). The American Diabetes Association and the European Association for the Study of Diabetes criticized the use of MetS as a unique clinical entity (7). However, considering MetS as a clinical entity may at least provide increased awareness of the importance of a constellation of several key risk factors by health practitioners and patients.
MetS is a common problem in Turkey, but the information is based on studies conducted more than 10 years back (8-10). The Metabolic Syndrome among Turkish Adults (METSAR) study found that the prevalence of MetS defined using the National Cholesterol Education Program Adult Treatment Panel III (ATPIII) criteria was 40% and 20% in women and men, respectively (11). A similar prevalence was observed in a recent meta-analysis of prevalence studies in Turkey (8).
As MetS is composed of several cardiovascular (CV) risk factors, the presence of MetS is expected to increase the risk of CV events. Several studies have assessed the CV risk in MetS and reached inconsistent conclusions (9, 10, 12-16). The Turkish Adult Risk Factor Study showed that the risk of fatal and non-fatal coronary heart disease was increased approximately two-fold in MetS in a cohort with no coronary heart disease at baseline, and the risk was slightly higher in women than in men (10). The INTERHEART study showed that the risk of myocardial infarction is increased in MetS, however, the risk is nearly the same as the risk associated with hypertension and diabetes mellitus alone (12). Post-hoc analysis of the Atherosclerosis Intervention in Metabolic Syndrome with low HDL/High Triglycerides (AIM-HIGH) study suggests that the risk is mainly caused by diabetes but not MetS itself after adjustment for age, sex, and the number of the components (16); however, others report that MetS is an independent risk factor for CV diseases (13, 17, 18). Moreover, the impact of individual components of MetS differs between studies (18-20). The inconsistent results may, in part, be caused by using different definitions of MetS, in different populations, and different approaches to statistical adjustments.
The Prospective Urban Rural Epidemiology (PURE) study is a prospective, multinational, multilevel study assessing the relationship between various clinical and socio-economic factors and non-communicable diseases (21). This study aimed to assess the magnitude of risk of CV events in the PURE Turkey participants with MetS, the contribution of the individual components to the CV risk, and also to evaluate whether the CV risk of MetS is greater than the risk conferred by its components.
Methods
The PURE study is an investigator-initiated study led by McMaster University Population Health Research Institute, Hamilton, Canada. It enrolled 202,165 participants in 27 countries. The study design was published elsewhere (21, 22). Briefly, data were collected at multiple levels (individual, household, community, and country levels). The participants were enrolled from countries with four income levels: low, lower-middle, upper-middle, and high income. Turkey was included among the upper-middle income countries. The PURE Turkey study was conducted by the Metabolic Syndrome Society and approved by Marmara University Ethics Committee (approval number: MAR-SBY-2005-0183) and the Republic of Turkey Ministry of Health.
Sampling of participants
For the PURE Turkey cohort, information regarding social and financial data was obtained from the Turkish Statistical Institute, and seven cities (Kocaeli, Aydın, Nevşehir, Antalya, Samsun, Malatya, and Gaziantep) from seven regions were selected using randomization. İstanbul was included as the eighth region.
The objective was to include different geographical areas and income groups, which would represent different lifestyles. For each city, information regarding the income and population of the towns and villages was obtained from local authorities, and a list was created. From this list, a town or village was chosen randomly, and selected households were contacted.
Participants and data collection
In a selected household, participants aged between 35 and 70 years and who were expected to continually reside there for at least the next 4 years were included. Persons with severe mental disorder, severe frailty or immobility, and inadequate communication skills were excluded. Informed consent was obtained from all participants.
Recruitment occurred between 2008 and 2009, and 4056 participants from 2576 households were included in Turkey. The PURE questionnaires were translated into Turkish and used to collect the study data (23). Anthropometric measurements, blood tests, spirometry, and electrocardiography were performed in all participants at baseline and every 3 years. The morbidity and mortality data were obtained yearly by phone calls.
Definitions of variables and end-points
Blood pressure was measured at least twice at baseline at rest in a sitting position, and the second value or mean values of the last two measurements were used for the analyses. Hypertension was defined as blood pressure of ≥140/90 mm Hg or use of antihypertensive drug.
Diabetes mellitus was defined as fasting plasma glucose level of 7.0 mmol/L (126 mg/dL), history of diabetes, or intake of antidiabetic medications. HbA1c level of ≥6.5% was added as a diagnostic criterion for diabetes since 2011.
MetS was defined as the presence of ≥3 of the following criteria (6):
Abdominal obesity: ≥94 cm in men or ≥80 cm in women;
Low HDL-cholesterol: <40 mg/dL (1.0 mmol/L) in men, <50 mg/dL (1.3 mmol/L) in women, or intake of fibrate or nicotinic acid (niacin);
High triglycerides: ≥150 mg/dL (1.7 mmol/L) or intake of fibrate or niacin;
High blood pressure: Systolic blood pressure ≥130 mm Hg and/or diastolic blood pressure ≥85 mm Hg, or intake of antihypertensive medication in a patient with a history of hypertension;
High fasting plasma glucose: ≥100 mg/dL (5.6 mmol/L) or drug treatment for elevated glucose level.
The primary outcome was major CV events, which is a composite of fatal CV events, non-fatal myocardial infarction (MI), stroke, or heart failure. As secondary outcomes, each component of the primary outcome, total mortality, and relative contribution of the MetS components to the CV risk was assessed.
Statistical analyses
Categorical variables were presented as frequency and percentages, and compared using the chi-squared test. Continuous variables were assessed for normal distribution using graphical and analytical methods. Continuous variables with normal distribution were presented as mean±standard deviation and compared using unpaired t test, and those with non-normal distribution were presented as median and interquartile range (IQR) and compared using the Mann–Whitney U test.
Time-to-event data were first assessed using the Kaplan–Meier analysis. Shared frailty Cox regression model, taking the level 2 (community level) as a clustering variable, was used to consider the hierarchical nature of the data. The model was adjusted for age, sex, smoking, family history of CV diseases (coronary artery disease (CAD) or stroke), and low-density lipoprotein (LDL)-cholesterol. To assess a non-linear relationship, restricted cubic splines were applied for age. The models were compared using Akaike information criteria (AIC) and likelihood ratio test. The simple model was presented because the models with restricted cubic spline were not superior to the base model. Interactions of several variables (sex, smoking, and baseline history of CAD or stroke) with MetS were assessed separately.
Proportional hazard assumption was assessed using global test and by plotting Schoenfeld residuals. Log-linearity was assessed by plotting Martingale residuals against each covariate.
Two sensitivity analyses were performed to assess the robustness of the results. These are the analyses conducted after excluding participants with major CV events within 1 year after enrollment and the analysis after multiple imputations for missing values.
To assess the CV risk of each component and their additional effect, each component was modeled separately with an adjustment for the same variables used in the main model. Subsequently, their chi-squared values were ordered, and variables with the highest to lowest chi-squared value were sequentially added to the model one at a time. The significance of the inclusion of additional components was assessed with the likelihood ratio test at each step, comparing the likelihood value of a model with the previous one.
In order to assess whether a model including MetS is better than the model that includes the components, we first obtained an adjusted model for MetS, then we replaced MetS with its components. The two models were compared using AIC (with smaller values indicating a better model), and c-statistics (value closer to 1.0 is better).
Analyses were performed using Stata v.15 (StataCorp, TX, USA), and p<0.05 was considered significant.
Results
Analyses in this study were made on 3933 (97%) of 4056 participants with complete data.
Approximately half of the population had MetS (1944, 49.4%). Patients with MetS, compared to those without MetS, were older (mean age, 52.3±8.9 vs. 47.9±8.8 years, p<0.001) and were more often to be women (63.6% vs. 58.7%; p=0.002). The two most common components of MetS were abdominal obesity (93.6%) and low HDL-cholesterol (84.4%). As expected, many CV risk factors were more common in participants with MetS (Table 1). However, the frequency of smoking was lower in patients with MetS (40.9% vs. 47.2%; p<0.001).
Table 1.
Baseline characteristics
| Variables | Overall n=3933 | MetS (+) n=1944 (49.4%) | MetS (–) n=1989 (50.6%) | P-value |
|---|---|---|---|---|
| Age, years | 50.1±9.2 | 52.3±8.9 | 47.9±8.8 | <0.001 |
| Female, n (%) | 2404 (61.1) | 1236 (63.6) | 1168 (58.7) | 0.002 |
| Individual components of MetS, n (%) | ||||
| High fasting plasma glucose | 811 (20.6) | 722 (37.1) | 89 (4.47) | <0.001 |
| Abdominal obesity | 2834 (72.1) | 1820 (93.6) | 1014 (50.1) | <0.001 |
| High blood pressure | 2078 (52.8) | 1488 (76.5) | 590 (29.7) | <0.001 |
| Low HDL-cholesterol | 2285 (58.1) | 1641 (84.4) | 644 (32.4) | <0.001 |
| High triglycerides | 1671 (42.5) | 1407 (72.4) | 264 (13.3) | <0.001 |
| Systolic BP, mm Hg | 129.3±22.1 | 136.9±22.0 | 121.8 (19.5) | <0.001 |
| Diastolic BP, mm Hg | 80.2±11.9 | 83.9±11.8 | 76.6±10.7 | <0.001 |
| BMI, kg/m2 | 29.8 (26.6-33.8) | 32.0 (29.0-35.7) | 27.7 (24.6-31.2) | <0.001 |
| Hypertension, n (%) | 1560 (39.7) | 1150 (59.2) | 410 (20.6) | <0.001 |
| Diabetes mellitus, n (%) | 546 (13.9) | 492 (25.3) | 54 (2.7) | <0.001 |
| History of CAD or ischemic stroke, n (%) | 288 (7.3) | 209 (10.8) | 79 (4.0) | <0.001 |
| Tobacco use (Current/former vs. never) | 1734 (44.1) | 795 (40.9) | 939 (47.2) | <0.001 |
| Glucose (mmol/L), median (IQR)* | 4.82 (4.44-5.28) | 5.05 (4.60-5.89) | 4.66 (4.38-4.94) | <0.001 |
| LDL-cholesterol, mmol/L** | 3.33±0.93 | 3.42±0.95 | 3.24±0.90 | <0.001 |
| HDL-cholesterol, mmol/L** | 1.17±0.36 | 1.03±0.27 | 1.31 (0.38) | <0.001 |
| Triglycerides, mmol/L, median (IQR)*** | 1.56 (1.25-2.04) | 1.93 (1.58-2.39) | 1.31 (1.12-1.56) | <0.001 |
| Location: urban vs. rural, n (%) | 2566 (65.2) | 1255 (64.6) | 1311 (65.9) | 0.372 |
To convert to mg/dL multiply by
18,
38.67,
88.57.
BMI - body mass index; BP - blood pressure; CAD - coronary artery disease, CV - cardiovascular; IQR - interquartile range
Abdominal obesity was the most common component in both women and men and was present in 97.1% and 87.6% of women and men with MetS, respectively (Table 2). Low HDL-cholesterol was the second most common component of MetS in women (87.6%). In men, low HDL-cholesterol or high triglycerides was the second most common component, and each was present in approximately 80% of participants with MetS. By contrast, the least common component was high fasting plasma glucose in women and men (38.2% and 35.3%, respectively).
Table 2.
Distribution of the individual components of MetS
| Overall (n=1944) | Women (n=1236, 63.6%) | Men (n=708, 36.4%) | P-value | |
|---|---|---|---|---|
| Abdominal obesity | 1820 (93.6) | 1200 (97.1) | 620 (87.6) | <0.001 |
| Low HDL-cholesterol level | 1641 (84.4) | 1083 (87.6) | 558 (78.8) | <0.001 |
| High blood pressure | 1488 (76.5) | 960 (77.7) | 528 (74.6) | 0.121 |
| High triglycerides | 1407 (72.4) | 846 (68.5) | 561 (79.2) | <0.001 |
| High fasting plasma glucose level | 722 (37.1) | 472 (38.2) | 250 (35.3) | 0.206 |
Follow-up and CV events
The median (IQR) follow-up time was 8.83 years (8.68–8.99 years) and 8.88 years (8.70–9.01 years) in patients with and without MetS, respectively. The primary outcome was observed in 178 (9.2%) and 70 (3.5%) patients with and without MetS, respectively (log-rank p<0.001, Fig. 1). The corresponding incidence rate was 11.3/1000 person-years vs. 4.2/1000 person-years, respectively. Also, the risk of primary outcome was significantly increased with the cumulative number of the MetS components (p for trend <0.001; Fig. 2).
Figure 1.
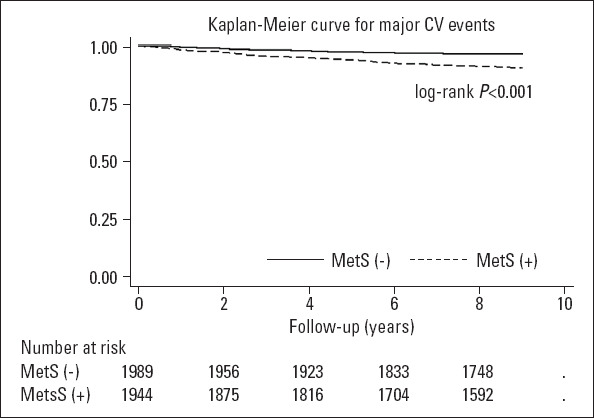
Kaplan–Meier plot for major cardiovascular events
Figure 2.
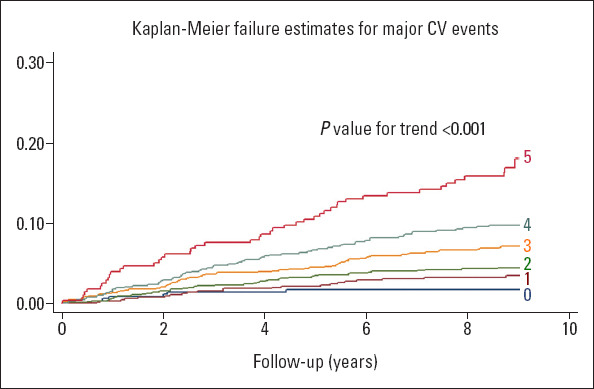
Effect of the cumulative number of the component of metabolic syndrome on major cardiovascular events
CV risk of MetS components
Each MetS component increased the risk of major CV events after adjustment for age, sex, smoking, family history of CV diseases, and LDL-cholesterol (Table 3).
Table 3.
Risk of each component of MetS for major cardiovascular events
| HR (95% CI)* | P-value | Chi-square | AIC | c-statistics | |
|---|---|---|---|---|---|
| High blood pressure | 2.10 (1.52-2.91) | <0.001 | 185.67 | 3870.3 | 0.756 |
| High fasting plasma glucose | 1.82 (1.40-2.37) | <0.001 | 193.55 | 3873.8 | 0.754 |
| Low HDL-cholesterol | 1.72 (1.30-2.25) | <0.001 | 184.55 | 3876.9 | 0.752 |
| High triglycerides | 1.56 (1.21-2.02) | 0.001 | 179.55 | 3880.9 | 0.748 |
| Abdominal obesity | 1.56 (1.13-2.15) | 0.007 | 176.98 | 3884.9 | 0.750 |
*Each component was separately modeled and adjusted for age, sex, smoking, family history of CV diseases, and LDL-cholesterol.
AIC - Akaike information criteria (lower value is better); HR - hazard ratio. c-statistics measures the discriminative ability of the model, and values closer to 1.0 are better
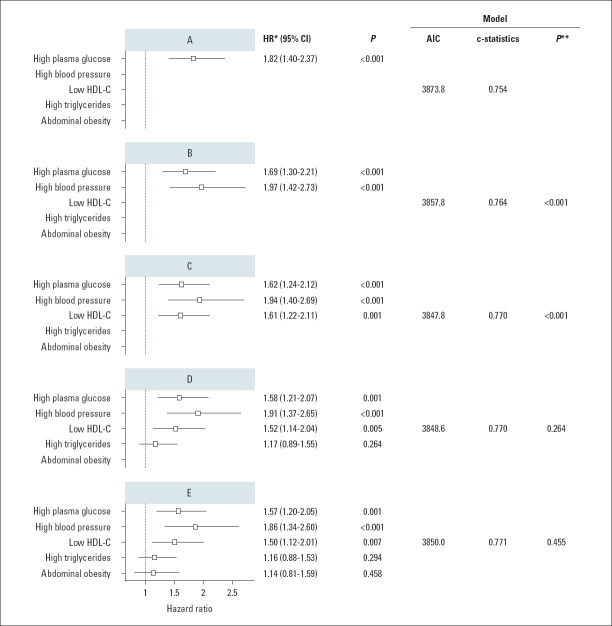
When each component was added to the model in a sequence based on their chi-squared values, the inclusion of fasting plasma glucose, high blood pressure, and low HDL-cholesterol created a better model in terms of discriminative ability; however, the inclusion of high triglycerides and abdominal obesity on these three components did not increase the CV risk further, suggesting that these two components did not provide a significant contribution to the risk when they are added on top of the three components (Fig. 3).
Figure 3.
Comparison of the models that sequentially includes MetS components based on their chi-squared values.
*: Each model was adjusted for age, sex, smoking, family history of CV diseases, and LDL-cholesterol. **: P-value for model comparison using likelihood ratio test (in comparison with the model applied in the previous step). AIC - Akaike information criteria (lower value is better); BP - blood pressure; HDL-C - high-density lipoprotein cholesterol; HR - hazard ratio
CV risk of MetS
The unadjusted hazard ratio (HR) for MetS in predicting the primary endpoint was 2.59 [95% confidence interval (CI), 1.98–3.40, p<0.001]. The adjusted risk of major CV events was 2.1 times higher compared with those without MetS (HR, 2.12; 95% CI, 1.59–2.81, p<0.001; Model 1 in Table 4). When the two models, one including MetS and the other including its components, were compared, the first model was not better compared with the second model, and the difference between the c-statistics was trivial (Model 2 in Table 4).
Table 4.
Risk of major CV events for MetS (Model 1) and for the combination of its components (Model 2)
| HR (95% CI)* | P-value | AIC** | c-statistics | |
|---|---|---|---|---|
| Model 1: Risk of MetS for major CV events | ||||
| MetS | 2.12 (1.59-2.81) | <0.001 | 3863.6 | 0.761 |
| Model 2: Risk of the components of MetS modeled together | ||||
| High fasting plasma glucose | 1.57 (1.20-2.05) | 0.001 | 3850.0 | 0.771 |
| High BP | 1.86 (1.34-2.60) | <0.001 | ||
| Low HDL-cholesterol | 1.50 (1.12-2.01) | 0.007 | ||
| High triglycerides | 1.16 (0.88-1.53) | 0.294 | ||
| Abdominal obesity | 1.14 (0.81-1.59) | 0.458 |
Adjusted for age, sex, smoking, family history of CV diseases, and LDL-cholesterol.
AIC - Akaike information criteria (lower value denotes better model); BP - blood pressure.
c-statistics measures the discriminative ability of the model, and values closer to 1.0 are better
Several interactions were assessed separately. The increased risk of major CV events due to MetS was similar between men and women and between smoker and non-smoker (p for interaction=0.686 and 0.157, respectively).
The risk of MetS for severe CV events in patients with a history of CAD or stroke was 1.74 times higher compared to those without that history (HR 1.74; 95% CI, 0.71–4.28; p for interaction=0.226), but was not statistically significant.
Two sensitivity analyses were performed to assess the robustness of the results. First, an analysis was conducted after excluding participants with major CV events within 1 year after enrollment. Second, analysis after multiple imputation for missing values was applied. These analyses provided similar results; therefore, the results were not presented.
Secondary outcomes
Along with total mortality, each element of the primary outcome was assessed separately as secondary endpoints (Table 5). Because of the low number of events, only age- and sex-adjusted survival analyses were applied. The HR for total mortality was 1.28 but was not statistically significant. By contrast, the risks of CV mortality, MI, stroke, and heart failure were significantly higher in patients with MetS (Table 5).
Table 5.
Secondary outcomes adjusted for age and sex
| MetS (+) | MetS (–) | HR (95% CI) | P-value | |
|---|---|---|---|---|
| Total mortality, n (%) | 89 (4.6) | 56 (2.8) | 1.28 (0.91-1.80) | 0.150 |
| CV mortality, n (%) | 36 (1.85) | 16 (0.8) | 1.89 (1.04-3.43) | 0.037 |
| Non-CV mortality | 53 (2.73) | 40 (2.01) | 1.05 (0.69-1.59) | 0.829 |
| MI, n (%) | 94 (4.8) | 42 (2.1) | 2.03 (1.40-2.93) | <0.001 |
| Stroke, n (%) | 56 (2.9) | 15 (0.8) | 2.83 (1.59-5.06) | <0.001 |
| Heart failure, n (%) | 37 (1.9) | 11 (0.6) | 2.61 (1.31-5.18) | 0.006 |
Discussion
This cohort of the PURE study showed that approximately half of the population aged 35–70 years in Turkey have MetS, and it is more common in women than in men. The adjusted risk of major CV events is two-fold higher in participants with MetS compared with those without MetS. High plasma glucose, high blood pressure, and low HDL-cholesterol were the most important risk predictors among the components, and inclusion of abdominal obesity and high triglycerides on top of these three components did not increase the risk further. Moreover, no difference was found between the discriminative ability of MetS and its components in predicting CV outcomes.
Because of the global epidemic of obesity, the prevalence of MetS is expected to be high. The METSAR study was conducted approximately 15 years ago to assess the prevalence of MetS in a sample representative of Turkey’s population (11). In that study, the overall prevalence of MetS was 33.9%. The prevalence is higher (49.4%) in our study; however, there some differences exist between the studies. First, the METSAR included participants aged ≥20 years, but PURE included individuals aged 35–70 years. Second, the METSAR study used the cut-off value of 110 mg/dL for fasting plasma glucose level and 102 cm for men and 88 cm for women for waist circumference, but the cut-off values in this study were 100 mg/dL, and 94 and 80 cm, respectively. Despite these differences, the results of the two studies show that MetS is an important public health problem in Turkey. MetS is more common in women, which is probably due to the higher prevalence of abdominal obesity in women (24). It may be expected that the prevalence of MetS is different in rural and urban areas due to differences in lifestyle. However, both METSAR and this PURE cohort showed that the prevalence of MetS is similar in rural and urban areas. This may be because the lifestyle in rural areas already resembles that in urban areas in Turkey.
The risk of major CV events increases as the number of components are increased. This is an expected finding and gives a stimulus for clinicians and patients in terms of awareness of the problem and may motivate them in changing their lifestyle and decision of treatment threshold.
The contribution of each component to the CV risk was assessed. In separate models, each MetS component was found a significant predictor of CV events. However, when each component was sequentially added to the model based on their chi-squared values, the inclusion of high fasting plasma glucose, high blood pressure, and low HDL-cholesterol were significant, but the inclusion of high triglycerides and abdominal obesity on top of these three components did not provide a significant additional risk. These findings suggest that each component is a predictor of CV risk; however, a relative contribution to the risk may change depending on what components are present in a given patient. The Third National Health and Nutrition Examination Survey (NHANES-III) showed that high blood pressure and low HDL-cholesterol are important predictors of risk, and diabetes mellitus, but not high fasting plasma glucose, was another significant predictor (19). Few studies also suggest that diabetes mellitus or high fasting plasma glucose is the main factor predicting CV events in patients with MetS (20, 25). Our findings imply that providing equal weight to each component to define MetS is not appropriate, and different combinations of components may lead to different risk profiles. Nevertheless, the risk of abdominal obesity should not be undervalued based on these results because it is significantly associated with other risk factors (26). Also, the present study showed that abdominal obesity is significantly associated with major CV events, but its additional effect becomes negligible on top of the three components in the context of MetS.
The secondary outcomes of this study were to assess each component of primary outcome and total mortality separately. Age- and sex-adjusted risk of MetS for total mortality and non-CV mortality were not significant. However, MetS was a significant predictor for CV mortality, MI, stroke, and heart failure. As the number of events is low, we adjusted only for age and sex, therefore, the results should be assessed cautiously and as hypothesis-generating findings.
MetS increased the risk of major CV events two-folds. Although MetS is a valuable entity to define the cluster of closely related cardiometabolic risk factors, our results suggest that the CV risk of MetS is not higher than the risk associated with its components.
Strengths and limitations
This study has several limitations: 1) The study included only the Turkish cohort of the PURE study; hence, the results may not be generalized to other populations. 2) A low number of events precluded to do several additional analysis and adjustments, and may have reduced the power of the study. We tried to perform a propensity score analysis to avoid potential problems caused by the low number of events; however, the common support region was too shallow, which severely reduced the matched sample size, and propensity scores were aggregated at two extreme probability values, which may lead to highly biased estimation. Therefore, we preferred to continue with the current analysis. Despite these limitations, our study has some strengths. The study sample is representative of the Turkish population, and we have very low missing values, and approximately 9 years of follow-up. Several sensitivity analyses also provided similar results to the main analysis. We think these factors increase the reliability of our findings.
Conclusion
MetS is a major public health problem in Turkey, affecting approximately half of the population aged 35–70 years, and the presence of MetS in this population doubles the risk of CV events. Each component of MetS increases the CV event risk. However, high blood pressure, high fasting plasma glucose, and low HDL-cholesterol are the main predictors of CV risk associated with MetS. The CV risk of MetS reflects the risk of its components.
Acknowledgment:
This study was mainly supported by Metabolic Syndrome Society in Turkey. The Population Health Research Institute of McMaster University provided financial and scientific support during the study. We also thank Astra Zeneca and Sanofi companies for their financial support given at the beginning of the study. SY, SR, and AO were involved in the design and conducting of the PURE international study. In the present study, all the authors were involved in the concept, design, critical review, and preparation of the manuscript. Statistical analyses were performed by MK, and reviewed by SY, AO, and SG.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – A.O., M.K., S.G., Y.A., K.K., A.T., B.Ç.T., M.V.K., S.R., S.Y.; Design – A.O., M.K., S.G., Y.A., K.K., A.T., B.Ç.T., M.V.K., S.R., S.Y.; Supervision – A.O., M.K., S.G., Y.A., K.K., A.T., B.Ç.T., M.V.K., S.R., S.Y.; Fundings – Metabolic Syndrome Society (Turkey), The Population Health Research Institute of McMaster University (Canada); Materials – A.O., M.K., S.G., Y.A., K.K., A.T., B.Ç.T., M.V.K., S.R., S.Y.; Data collection and/or processing – A.O., M.K., S.G., Y.A., K.K., A.T., B.Ç.T., M.V.K., S.R., S.Y.; Analysis and/or interpretation – A.O., M.K., S.G., Y.A., K.K., A.T., B.Ç.T., M.V.K., S.R., S.Y.; Literature search – A.O., M.K., S.G., Y.A., K.K., A.T., B.Ç.T., M.V.K., S.R., S.Y.; Writing – A.O., M.K., S.G., Y.A., K.K., A.T., B.Ç.T., M.V.K., S.R., S.Y.; Critical review – A.O., M.K., S.G., Y.A., K.K., A.T., B.Ç.T., M.V.K., S.R., S.Y.
References
- 1.Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006;47:1093–100. doi: 10.1016/j.jacc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1:diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–52. [PubMed] [Google Scholar]
- 4.Expert Panel on Detection E Treatment of High Blood Cholesterol in A Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. International Diabetes Federation Task Force on Epidemiology and Prevention;Hational Heart, Lung, and Blood Institute;American Heart Association;World Heart Federation;International Atherosclerosis Society;International Association for the Study of Obesity. Harmonizing the metabolic syndrome:a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention;National Heart, Lung, and Blood Institute;American Heart Association;World Heart Federation;International Atherosclerosis Society;and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 7.Kahn R, Buse J, Ferrannini E, Stern M. American Diabetes Association; European Association for the Study of Diabetes. The metabolic syndrome:time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 8.Abacı A, Kılıçkap M, Göksülük H, Karaaslan D, Barçın C, Kayıkçıoğlu M, et al. Data on prevalence of metabolic syndrome in Turkey:Systematic review, meta-analysis and meta-regression of epidemiological studies on cardiovascular risk factors. Turk Kardiyol Dern Ars. 2018;46:591–601. doi: 10.5543/tkda.2018.00878. [DOI] [PubMed] [Google Scholar]
- 9.Onat A, Ceyhan K, Basar O, Erer B, Toprak S, Sansoy V. Metabolic syndrome:major impact on coronary risk in a population with low cholesterol levels--a prospective and cross-sectional evaluation. Atherosclerosis. 2002;165:285–92. doi: 10.1016/s0021-9150(02)00236-8. [DOI] [PubMed] [Google Scholar]
- 10.Onat A, Hergenc G, Can G. Prospective validation in identical Turkish cohort of two metabolic syndrome definitions for predicting cardiometabolic risk and selection of most appropriate definition. Anatol J Cardiol. 2007;7:29–34. [PubMed] [Google Scholar]
- 11.Kozan O, Oguz A, Abaci A, Erol C, Ongen Z, Temizhan A, et al. Prevalence of the metabolic syndrome among Turkish adults. Eur J Clin Nutr. 2007;61:548–53. doi: 10.1038/sj.ejcn.1602554. [DOI] [PubMed] [Google Scholar]
- 12.Mente A, Yusuf S, Islam S, McQueen MJ, Tanomsup S, Onen CL, et al. Metabolic syndrome and risk of acute myocardial infarction a case-control study of 26,903 subjects from 52 countries. J Am Coll Cardiol. 2010;55:2390–8. doi: 10.1016/j.jacc.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 13.Simons LA, Simons J, Friedlander Y, McCallum J. Is prediction of cardiovascular disease and all-cause mortality genuinely driven by the metabolic syndrome, and independently from its component variables? The Dubbo study. Heart Lung Circ. 2011;20:214–9. doi: 10.1016/j.hlc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Mayer O Jr, Bruthans J, Seidlerova J, Karnosova P, Vanek J, Hronova M, et al. Prospective study of metabolic syndrome as a mortality marker in chronic coronary heart disease patients. Eur J Intern Med. 2018;47:55–61. doi: 10.1016/j.ejim.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Church TS, Thompson AM, Katzmarzyk PT, Sui X, Johannsen N, Earnest CP, et al. Metabolic syndrome and diabetes, alone and in combination, as predictors of cardiovascular disease mortality among men. Diabetes Care. 2009;32:1289–94. doi: 10.2337/dc08-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyubarova R, Robinson JG, Miller M, Simmons DL, Xu P, Abramson BL, et al. Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH) Investigators. Metabolic syndrome cluster does not provide incremental prognostic information in patients with stable cardiovascular disease: A post hoc analysis of the AIM-HIGH trial. J Clin Lipidol. 2017;11:1201–11. doi: 10.1016/j.jacl.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–9. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 18.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP San Antonio Heart Study. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110:1251–7. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 19.Alexander CM, Landsman PB, Teutsch SM, Haffner SM Third National Health and Nutrition Examination Survey (NHANES III); National Cholesterol Education Program (NCEP) NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–4. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 20.Younis A, Goldkorn R, Goldenberg I, Geva D, Tzur B, Mazu A, et al. Impaired Fasting Glucose Is the Major Determinant of the 20-Year Mortality Risk Associated With Metabolic Syndrome in Nondiabetic Patients With Stable Coronary Artery Disease. J Am Heart Assoc. 2017;6:e006609. doi: 10.1161/JAHA.117.006609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S PURE Investigators-Writing Group. The Prospective Urban Rural Epidemiology (PURE) study:examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am Heart J. 2009;158:1–7. doi: 10.1016/j.ahj.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Oğuz A, Telci Çaklılı Ö, Tümerdem Çalık B PURE Investigators. The Prospective Urban Rural Epidemiology (PURE) study: PURE Turkey. Turk Kardiyol Dern Ars. 2018;46:613–23. doi: 10.5543/tkda.2018.32967. [DOI] [PubMed] [Google Scholar]
- 23.Gunes FE, Imeryuz N, Akalin A, Bekiroglu N, Alphan E, Oguz A, et al. Development and validation of a semi-quantitative food frequency questionnaire to assess dietary intake in Turkish adults. J Pak Med Assoc. 2015;65:756–63. [PubMed] [Google Scholar]
- 24.Ural D, Kılıçkap M, Göksülük H, Karaaslan D, Kayıkçıoğlu M, Özer N, et al. Data on prevalence of obesity and waist circumference in Turkey: Systematic review, meta-analysis and meta regression of epidemiological studies on cardiovascular risk factors. Turk Kardiyol Dern Ars. 2018;46:577–90. doi: 10.5543/tkda.2018.62200. [DOI] [PubMed] [Google Scholar]
- 25.Udell JA, Steg PG, Scirica BM, Eagle KA, Ohman EM, Goto S, et al. Reduction of Atherothrombosis for Continued Health (REACH) Registry Investigators. Metabolic syndrome, diabetes mellitus, or both and cardiovascular risk in outpatients with or at risk for atherothrombosis. Eur J Prev Cardiol. 2014;21:1531–40. doi: 10.1177/2047487313500541. [DOI] [PubMed] [Google Scholar]
- 26.Oğuz A, Temizhan A, Abaci A, Kozan O, Erol C, Ongen Z, et al. Obesity and abdominal obesity;an alarming challenge for cardio-metabolic risk in Turkish adults. Anatol J Cardiol. 2008;8:401–6. [PubMed] [Google Scholar]