Abstract
Objective:
Acute coronary syndrome (ACS) is a leading cause of death worldwide. There is great interest in defining the risk factors and underlying mechanisms of ACS among young people. The microbiota and its metabolites have recently become a popular research topic, yet there is still no study that investigated microbiota-generated metabolites as a possible risk factor in young patients with ACS. In this study, we aimed to investigate the relationship between microbiota-generated metabolites and ACS in young people.
Methods:
This study included 44 young patients with ACS (<50 years of age), 39 elderly patients with ACS, and 44 patients with normal coronary arteries. Inflammatory parameters and serum trimethylamine N-oxide (TMAO) and choline levels were measured in all patients.
Results:
Young patients with ACS had significantly higher levels of TMAO and choline compared to the control and elderly ACS groups. Also, elderly patients with ACS had a significantly higher level of TMAO than the control group. Linear regression analysis was performed to determine the independent predictors of TMAO. Two regression models were involved. The first model included young ACS and control groups, while the second model included young and elderly ACS groups. In the first model, we found that young ACS (ß=0.399, p=0.004) and smoking ACS (ß=0.211, p=0.046) were significantly associated with TMAO level. In the second model, young ACS was significantly associated with TMAO level (ß=0.230, p=0.035).
Conclusion:
In this study, we have shown that young ACS was significantly associated with increased TMAO level.
Keywords: acute coronary syndrome, young, microbiota, trimethylamine N-oxide
Introduction
Coronary artery disease (CAD) is now the leading cause of death worldwide, and its incidence is increasing (1). The etiology of CAD is multifactorial, including many traditional and nontraditional risk factors (2, 3). Age is one of the most important traditional risk factors for CAD (4). Although the possibility of CAD increases with age, its incidence has recently increased among young people (5). Defining the risk factors and underlying mechanisms of CAD in young people is of great interest. Identifying and modifying risk factors may possibly prevent and/or slow the progression of the disease.
Recently, novel nontraditional risk factors have been reported to explain the pathogenesis of CAD, including procoagulant, homocysteine, and inflammatory markers (2, 3). Microorganisms found on the human body are defined as the “microbiota,” which has also become popular as a possible novel nontraditional risk factor for CAD. These microbiota metabolize overconsumed proteins and metabolites such as cholines and phosphatidylcholines which generate trimethylamine (TMA). TMA is rapidly absorbed and is converted to trimethylamine N-oxide (TMAO) in the liver (6). Increased serum TMAO has been shown to increase the risk of atherosclerosis by increasing proinflammatory cytokines and thrombus formation. Moreover, increased serum TMAO has been found to be associated with adverse cardiac events (6, 7).
To date, no study has investigated microbiota-generated metabolites in young patients with acute coronary syndrome (ACS) as a risk factor for CAD development. We hypothesized that increased microbiota-generated metabolites may be a risk factor for CAD in young patients and therefore investigated the TMAO level in young patients with ACS.
Methods
Study population
This study was designed as a cross-sectional case–control study comprised of 44 young patients with ACS [< 50 years old (8)] and 39 elderly patients with ACS who underwent coronary angiography (cases) and 44 patients who underwent angiography and were found to have normal coronary arteries (controls).
Patients who did not have a final diagnosis of atherosclerotic ACS after coronary angiography (n=6), those with a previous history of CAD, malignancy, heart failure, severe heart valve disease, acute infection, rheumatic or hematological disease, liver disease, renal failure, or intestinal disease, as well as those who recently used an antimicrobial drug, were eliminated from the study. This study was approved by our Local Ethics Committee and was carried out according to the rules of the Declaration of Helsinki. Informed consent was obtained from all patients. ACS was classified as follows: unstable angina pectoris (USAP) if patients have ischemic chest discomfort exceeding 20 min, whose pain was not relieved with nitrates and had emerged within the last 48 h, with normal serum troponin levels, and with or without ST-segment deviation on electrocardiography (ECG); non-ST-elevation myocardial infarction (NSTEMI) if the patient developed a rise in cardiac biomarker levels (troponin I and creatine kinase-MB) during clinical follow-up; STEMI if there was permanent ST and J point elevation in at least 2 contiguous leads [>2 mm (0.2 mV) in men or >1.5 mm (0.15 mV) in women] in leads V2–V3 and/or of >1 mm (0.1 mV) in other contiguous chest or limb leads (9-11).
Coronary angiography
Coronary angiography was performed using the standard Judkins technique via femoral or radial access. Two experienced cardiologists who were blinded to the patient’s data evaluated all digital angiographic images. The presence of 50% stenosis in the left main coronary artery and 70% stenosis in other major coronary arteries was considered as critical stenosis. The number of vessels with critical stenosis is also recorded.
Biochemical analysis and microbiota parameters
Blood samples were taken from all patients on admission. These blood samples were centrifuged for 10 min at 3000 rpm, and the serum was stored at −80°C in aliquots until the day of analysis. The samples were analyzed using standard laboratory methods to determine electrolytes, blood glucose, total cholesterol, low-density lipoprotein, high-density lipoprotein (HDL), and triglyceride levels. Serum TMAO and choline levels were measured using enzyme-linked immunosorbent assays (Shanghai Sunred Biological Technology Co., Ltd., Shanghai, China) according to the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics software (version 22.0; IBM Corp., Armonk, NY, USA). The Kolmogorov–Smirnov test was used to test the normal distribution of the data. Continuous variables were expressed as the mean±standard deviation (SD) and median (first and third quartiles) according to the distribution. The Kruskal–Wallis test was used for three-group comparisons, and the Mann–Whitney U test was used for two-group comparisons. The chi-square test was used to compare categorical variables. The Spearman correlation coefficient was used to determine the correlation of TMAO with other continuous variables. We performed linear regression analysis to determine the significant predictors of TMAO, and a two-tiered approach was used. The first stage consisted of young ACS and young control groups, whereas the second stage consisted of young ACS and elderly ACS groups. A p-value <0.05 was considered statistically significant.
Results
Over-all, 44 young patients with ACS, 39 elderly patients with ACS, and 44 patients with normal coronary arteries were included in this study. The baseline characteristics of the study population are presented in Table 1. There was no significant difference among the groups in terms of baseline characteristics except age (p<0.001).
Table 1.
Comparison of baseline characteristics among groups
| Variables | Control group (n=44) | Young ACS (n=44) | Elderly ACS (n=39) | P | Control vs.young | Control vs.elderly | Young vs.elderly |
|---|---|---|---|---|---|---|---|
| Age, years | 46 (41-55) | 48 (44-50) | 65 (61-70) | <0.001 | 0.827 | <0.001 | <0.001 |
| Male gender (%) | 23 (52) | 30 (68) | 28 (72) | 0.137 | 0.130 | 0.070 | 0.722 |
| BMI (kg/m2) | 26.5±3.2 | 27.6±2.3 | 27.0±2.4 | 0.135 | 0.062 | 0.377 | 0.253 |
| DM (%) | 3 (7) | 4 (9) | 4 (10) | 0.851 | 0.695 | 0.576 | 0.858 |
| HT (%) | 6 (14) | 11 (25) | 5 (13) | 0.880 | 0.179 | 0.388 | 0.37 |
| Smoking (%) | 8 (18) | 6 (14) | 6 (15) | 0.841 | 0.562 | 0.736 | 0.822 |
| SBP (mm Hg) | 126 (120-138) | 120 (110-130) | 124 (120-130) | 0.310 | 0.059 | 0.643 | 0.150 |
| DBP (mm Hg) | 75 (70-83) | 70 (65-80) | 73 (70-80) | 0.305 | 0.239 | 0.743 | 0.153 |
| LVEF (%) | 62 (58-65) | 55 (58-60) | 55 (50-57) | 0.058 | 0.056 | 0.080 | 0.062 |
| Type of ACS (%) | 0.556 | ||||||
| USAP | 10 (23) | 12 (31) | |||||
| NSTEMI | - | 11 (25) | 12 (31) | ||||
| STEMI | 23 (52) | 15 (39) | |||||
| Number of vessels with critical stenosis (%) | 0.253 | ||||||
| 1-vessel disease | 18 (41) | 10 (26) | |||||
| 2-vessel disease | 17 (39) | 16 (41) | |||||
| 3-vessel disease | 9 (20) | 13 (33) | |||||
| Final therapy (%) | - | ||||||
| Medical therapy | 0 (0) | 4 (10.3) | 0.029 | ||||
| PCI | 38 (86) | 27 (62.2) | 0.404 | ||||
| CABG | 6 (14) | 8 (20.5) | 0.742 |
ACS - acute coronary syndrome; BMI - body mass index; SBP - systolic blood pressure; DBP - diastolic blood pressure; DM - diabetes mellitus; HT- hypertension; EF- ejection fraction; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI - ST-segment elevation myocardial infarction; USAP - unstable angina pectoris; CAD - coronary artery disease;
PCI - percutaneous coronary intervention; CABG - coronary artery bypass graft
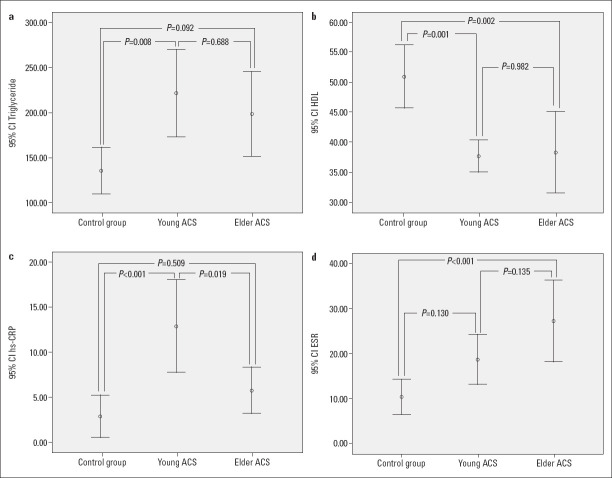
The laboratory parameters of the study groups are listed in Table 2. HDL cholesterol (p=0.001), triglycerides (p=0.003), ESR (p=0.001), and hsCRP (p<0.001) were significantly different among the three groups (Fig. 1).
Table 2.
Comparison of laboratory parameters among groups
| Variables | Control group (n=44) | Young ACS (n=44) | Elderly ACS (n=39) | P | Control vs.young | Control vs.elderly | Young vs.elderly |
|---|---|---|---|---|---|---|---|
| WBC (103/µL) | 10 (9-11) | 11 (10-13) | 10 (8-13) | 0.780 | 0.040 | 0.844 | 0.135 |
| Hemoglobin (g/dL) | 12.5±1.5 | 13.3±1.5 | 13.2±2.1 | 0.129 | 0.152 | 0.275 | 0.442 |
| Platelets (103/µL) | 264 (209-294) | 228 (196-290) | 234 (209-290) | 0.185 | 0.056 | 0.315 | 0.578 |
| Glucose (mg/dL) | 88 (83-95) | 91 (85-96) | 85 (83-93) | 0.132 | 0.162 | 0.598 | 0.062 |
| Urea (mg/dL) | 31.2±10.5 | 29.7±10.0 | 30.5±10.3 | 0.184 | 0.494 | 0.752 | 0.728 |
| Creatinine (mg/dL) | 0.8±0.20 | 0.9±0.19 | 0.9±0.20 | 0.280 | 0.132 | 0.120 | 0.908 |
| Sodium (mEq/L) | 138.6±2.3 | 138.2±4.6 | 139.5±4.1 | 0.274 | 0.738 | 0.078 | 0.288 |
| Potassium (mEq/L) | 4.2±0.4 | 4.2±0.5 | 4.3±0.4 | 0.463 | 0.521 | 0.192 | 0.553 |
| AST (U/L) | 19 (16-25) | 19 (14-29) | 19 (15-25) | 0.658 | 0.235 | 0.603 | 0.210 |
| ALT (U/L) | 16 (13-20 ) | 25 (19-47) | 19 (14-28) | 0.110 | 0.430 | 0.204 | 0.070 |
| Total-C (mg/dL) | 178.5±26.9 | 162.6±39.8 | 168.7±53.2 | 0.184 | 0.030 | 0.285 | 0.554 |
| LDL-C (mg/dL) | 102 (90-122) | 107 (72-115) | 103 (7 -128) | 0.819 | 0.499 | 0.767 | 0.834 |
| HDL-C (mg/dL) | 46 (39-56) | 37 (32-42) | 34 (29-45) | 0.001 | 0.001 | 0.002 | 0.982 |
| TG (mg/dL) | 114 (76-150) | 167 (121-284) | 169 (107-227) | 0.003 | 0.008 | 0.092 | 0.688 |
| hsCRP (mg/dL) | 0.58 (0.20-3.20) | 6.70 (2.70-14.4) | 2.44 (1.60-7.40) | <0.001 | <0.001 | 0.509 | 0.019 |
| ESR (mm/h) | 6 (3-13) | 18 (9-26) | 23 (10-42) | 0.001 | 0.130 | <0.001 | 0.135 |
ACS - acute coronary syndrome; ESR - erythrocyte sedimentation rate; hsCRP - high-sensitivity C-reactive protein; AST -aspartate aminotransferase; ALT - alanine aminotransferase; total-C - total cholesterol; HDL - high-density lipoprotein; LDL - low-density lipoprotein; TG - triglyceride; WBC - white blood cell
Figure 1.
Comparison of the triglyceride (a), HDL-cholesterol (b), hsCRP (c), and ESR (d) values among the groups
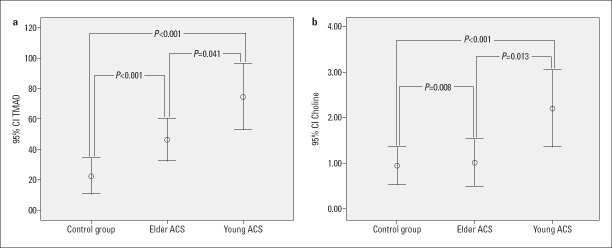
The microbiota parameters of the study groups are shown in Table 3. Serum TMAO and choline levels were significantly different among the three groups. To determine the clinical importance of these parameters in young patients with ACS, a comparison of the three study groups was made. It was found that young patients with ACS had significantly higher levels of TMAO and choline compared to both the control and elderly ACS groups and elderly patients with ACS had significantly higher levels of TMAO than the control group (Fig. 2).
Table 3.
Comparison of microbiota parameters among groups
| Variables | Control group (n=44) | Young ACS (n=44) | Elderly ACS (n=39) | P | Control vs.young | Control vs.elderly | Young vs.elderly |
|---|---|---|---|---|---|---|---|
| Choline (ng/mL) | 0.45 (0.43-0.61) | 0.62 (0.52-4.10) | 0.54 (0.51-0.59) | 0.001 | <0.001 | 0.008 | 0.013 |
| TMAO (µg/mL) | 10 (8.60-13.31) | 57 (13-123) | 21 (10-95) | 0.001 | <0.001 | <0.001 | 0.041 |
TMAO - trimethylamine N-oxide; ACS - acute coronary syndrome
Figure 2.
Comparison of the TMAO (a) and choline levels (b) among the groups
The Spearman correlation coefficient was used to determine the correlation between microbiota parameters and continuous variables. When all participants were included in the analysis (control group and ACS patients), TMAO was positively correlated with hsCRP (r=0.311, p<0.001), ESR (r=0.324, p=0.006), and BMI (r=0.200, p=0.024), while choline was positively correlated with hsCRP (r=0.222, p=0.012) and BMI (r=0.235, p=0.008). When only ACS patients were included in the analysis (young and elderly ACS patients), choline was negatively correlated with age (r=–0.243, p=0.027).
Linear regression analysis was performed to determine the statistically significant prognostic factors (significant predictors) of TMAO. Two regression models were involved. The first model included young ACS and control groups, while the second model included young ACS and elderly ACS groups. In the first model, we found that young ACS (ß=0.399, p=0.004) and smoking ACS (ß=0.211, p=0.046) were significantly associated with TMAO level. In the second model, young ACS was significantly associated with TMAO level (ß=0.230, p=0.035) (Table 4).
Table 4.
Multivariate linear regression analyses showing significant predictor of the TMAO
| Model 1 (young ACS and control group) | Unstandardized coefficients | Standardized coefficients | |||
|---|---|---|---|---|---|
| B | SE | β | t | P | |
| Age | 0.641 | 0.877 | 0.080 | 0.732 | 0.467 |
| Gender | 19.616 | 14.392 | 0.153 | 1.363 | 0.177 |
| BMI | 4.688 | 2.427 | 0.212 | 1.931 | 0.057 |
| HT | 7.526 | 17.229 | 0.047 | 0.437 | 0.664 |
| DM | 14.469 | 24.692 | 0.062 | 0.586 | 0.560 |
| Smoking | 36.184 | 17.811 | 0.211 | 2.032 | 0.046 |
| SBP | 0.462 | 0.518 | 0.093 | 0.891 | 0.376 |
| Creatinine | 43.358 | 36.584 | 0.139 | 1.185 | 0.240 |
| Total-C | 0.148 | 0.220 | 0.082 | 0.674 | 0.502 |
| LDL | 0.188 | 0.242 | 0.094 | 0.776 | 0.440 |
| HDL | 0.036 | 0.470 | 0.009 | .077 | 0.939 |
| Triglyceride | 0.013 | 0.055 | 0.028 | 0.242 | 0.809 |
| hsCRP | 0.260 | 0.550 | 0.054 | 0.473 | 0.637 |
| Young ACS | 50.046 | 16.748 | 0.399 | 2.988 | 0.004 |
| Model 2 (young and elderly ACS) | |||||
| Age | 2.309 | 1.870 | 0.387 | 1.235 | 0.221 |
| Gender | 4.627 | 15.389 | 0.035 | 0.301 | 0.765 |
| BMI | 0.664 | 3.329 | 0.026 | 0.200 | 0.842 |
| HT | 3.896 | 19.549 | 0.024 | 0.199 | 0.843 |
| DM | 10.991 | 22.386 | 0.053 | 0.491 | 0.625 |
| Smoking | 1.832 | 21.310 | 0.011 | 0.086 | 0.932 |
| SBP | 0.476 | 0.530 | 0.100 | 0.899 | 0.371 |
| Creatinine | 13.381 | 36.058 | 0.041 | 0.371 | 0.712 |
| Total-C | 0.362 | 0.210 | 0.272 | 1.727 | 0.088 |
| LDL | 0.383 | 0.221 | 0.274 | 1.736 | 0.087 |
| Triglyceride | 0.033 | 0.046 | 0.083 | 0.718 | 0.475 |
| hsCRP | 0.180 | 0.546 | 0.074 | 0.346 | 0.536 |
| Young ACS | 28.275 | 13.139 | 0.230 | 2.152 | 0.035 |
B - unstandardized regression coefficient; SE - standard error; β- standardized β coefficient; BMI - body mass index; DM - diabetes mellitus; HT - hypertension; hsCRP - high-sensitivity C-reactive protein; total-C - total cholesterol; SBP - systolic blood pressure; TMAO - trimethylamine N-oxide; LDL - low-density lipoprotein; HDL - high-density lipoprotein;
ACS - acute coronary syndrome
Discussion
In this present study, we found that young patients with ACS had significantly higher levels of TMAO and choline compared to the control and elderly ACS groups. To our knowledge, this is the first study demonstrating an independent relationship between TMAO and young ACS.
Atherosclerosis is the main cause of CAD, and many traditional and nontraditional risk factors play a role in its etiology (12). Recent studies have demonstrated that other factors such as the immune system, inflammatory process, and vascular changes may play important roles in the pathogenesis of CAD (13, 14). The microbiota is also a novel nontraditional risk factor (6). Several studies have shown the relation of the microbiota and its imbalance to CAD as well as its major risk factors. Microbiota as a potential diagnostic marker for CAD was also reported (15, 16).
There are many beneficial bacteria on various parts of the body, including the skin, mouth, and intestines. The bacteria in these regions are identified as microbiota and consist of various microorganisms in healthy individuals. The microbiota begins to develop immediately after birth and varies according to nutrition, genetics, age, and geographical region. These microorganisms play an important role in the physiological, metabolic, and immune processes of our bodies. Therefore, the microbiota has been defined as a new metabolic organ (17, 18).
The identification of new microbiota-generated metabolites and pathways associated with cardiovascular risk in humans is the most interesting evidence for the role of the intestinal microbiota in cardiovascular disease. Choline is an essential nutrient for the human body, but people cannot synthesize enough choline for their metabolic needs. Therefore, a sufficient amount of choline should be taken through diet (19, 20). However, overconsumption of choline may lead to the production of metabolites that are harmful to the human body. The most harmful dead-end metabolite of the intestinal microbiota is TMAO, which is derived from choline. In addition to choline, choline-containing compounds such as red meat, eggs, and dairy products, as well as lecithin and carnitine, are potential precursors of TMAO. Fish and other seafood also contain an important amount of free TMAO. However, the association between increased TMAO and atherosclerosis seems like a paradox because seafoods are generally accepted as cardioprotective foods. The main reason for this paradox is that the role of free TMAO in seafood has commonly been overlooked. Free TMAO in seafood is directly absorbed from the gastrointestinal tract, and its level in plasma increases rapidly, whereas TMAO derived from choline and other precursors is produced in the liver by the oxidation of TMA by the microbiota, and its levels in plasma increase more slowly. Therefore, whether TMAO is taken up directly or as TMA after bacterial reduction is important (21). Studies have demonstrated that TMAO derived from hepatic oxidation by the microbiota is associated with the risk of atherosclerosis (22). Landfald et al. (21) related this paradox to a red herring.
Previous studies proposed that increased serum TMAO was associated with an increased risk of atherosclerosis and adverse cardiac events (7). The main possible underlying mechanism linking increased TMAO with atherosclerosis risk has been explained as follows. First, TMAO increases pro-inflammatory cytokines but decreases anti-inflammatory cytokines. Second, it promotes lipid accumulation and foam cell formation. Third, it increases platelet activation and aggregation. Fourth, it causes endothelial cell dysfunction and vascular inflammation via oxidative stress (7, 23). Therefore, an increased TMAO level is associated with pro-atherogenic and prothrombotic events that result in ACS. Unlike in these studies, a recent study testing the association between TMAO and coronary artery calcium (CAC) and carotid intima–media thickness (cIMT) found that TMAO was not associated with measures of atherosclerosis (CAC incidence, CAC progression, or cIMT) (24). The main reason for this unexpected result may be that the subjects in this study were relatively young and healthy.
In our study, young patients with ACS had significantly higher levels of TMAO and choline compared to the control and elderly ACS groups. This impressive finding may be attributed to a higher ratio of unhealthy and fast-food habits among young patients with ACS since unhealthy diets among young people have become more popular worldwide has been recognized (25). Indeed, the fact that we found a negative correlation between age and choline level supports our hypothesis that unhealthy eating habits are more common in young ACS patients. Moreover, young ACS was significantly associated with increased TMAO level. Therefore, it may be concluded that microbiota-generated metabolites might be causally related with ACS in younger patients, but further studies with longitudinal follow-up protocols are needed to see whether such a causal relationship exists. Although previous studies showed that microbiota-generated metabolites were associated with the development of many cardiovascular diseases (6, 26), to our knowledge this is the first study to investigate the role of the intestinal microbiota in young patients with ACS, showing the important role the intestinal microbiota may play in the pathogenesis of young ACS. We believe that future studies that focus on manipulating the microbiota to lessen cardiovascular events may lead to a breakthrough in this area.
Evidence from animal studies supports the concept that the microbiota can mechanistically impact host lipid levels. The possible mechanisms have been explained as follows. First, bile salts in the gut are converted into secondary bile acids by the microbiota. Bile acids entering the blood can activate hepatic or systemic lipid metabolism through certain receptors. The fermentation of nondigestible carbohydrates could be another potential mechanism through which the microbiota could affect lipid metabolism. Short-chain fatty acids, mainly n-butyrate, acetate, and propionate, are produced during the fermentation of these food components and provide rich sources of energy for the host and influence lipid metabolism. Third, TMAOs can directly affect lipid levels by reversing cholesterol transport, cholesterol and sterol metabolism, and/or the degradation of bile acids (27).
CAD is a chronic inflammatory disease. Studies have demonstrated that hsCRP is a good inflammatory biomarker for CV risk classification. This effect of CRP has been reported to be independent from traditional risk factors (28). In this study, we evaluated hsCRP and found significantly higher levels in patients with ACS compared to the control group. In addition, we observed that hsCRP was positively correlated with TMAO. These findings suggest that both TMAO and hsCRP have major clinical importance in patients with young ACS.
In this study, we demonstrated a possible association between microbiota and young ACS. However, it should be taken into consideration that the microbiota varies considerably among individuals. Better understanding of the effect of the microbiota on CAD may provide novel approaches for the prevention of CAD and become a novel potential therapeutic target. Future prospective studies focusing on the microbiota of individuals and even manipulating the microbiota may reduce cardiovascular diseases.
Study limitations
Our study had some limitations, the most significant limitation being its small sample size and single-center nature. Second, we evaluated only baseline TMAO levels and did not perform serial TMAO measurements. Third, we did not investigate the eating habits of the study population. It could be interesting to demonstrate an association between eating habits and TMAO levels. Fourth, although we included a control group in this study, the age of the control group represented only young ACS subjects, not elderly ACS subjects. Adding an elderly control group could be useful. In this study, the control group consisted of young people (age and gender matched). However, the fact that a young control group is used against young ACS may affect the results negatively. Because it cannot be guaranteed that people in the control group will not have a myocardial infarction in the future, therefore, control group consisting of people who have completed the age limit (above 50 years of age our study) could be more reliable (29). However, we think that performing a two-step regression analysis may partially solve this problem. Finally, we included patients with USAP, NSTEMI, and STEMI in this study. It could be more beneficial to include only patients with acute myocardial infarction to provide a more homogeneous population. Additional prospective studies with larger numbers of participants are required to better explain the relationship between TMAO and young ACS.
Conclusion
The microbiota and its metabolites have recently become a popular research topic. TMAO is the most harmful dead-end metabolite of the microbiota. In this study, we found that young ACS was significantly associated with increased TMAO level.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – M.B.T., Z.T., F.G., İ.K.; Design – M.B.T., Z.T., A.G.; Supervision – M.B.T., F.B., M.E.E., İ.K.; Fundings – M.B.T., Z.T., F.G., F.B., M.E.E., İ.K., A.G., G.G.T.; Materials – M.B.T., F.G., G.G.T.; Data collection and/or processing – M.B.T., Z.T., G.G.T.; Analysis and/or interpretation – M.B.T., Z.T., F.G., F.B., M.E.E., G.G.T.; Literature search – Z.T., F.G., F.B., İ.K.; Writing – M.B.T., Z.T., M.E.E., İ.K.; Critical review – Z.T., F.G., F.B., İ.K., A.G.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Mack M, Gopal A. Epidemiology, Traditional and Novel Risk Factors in Coronary Artery Disease. Heart Fail Clin. 2016;12:1–10. doi: 10.1016/j.hfc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Kim HL, Kim MA, Oh S, Kim M, Yoon HJ, Park SM, et al. Sex Differences in Traditional and Nontraditional Risk Factors for Obstructive Coronary Artery Disease in Stable Symptomatic Patients. J Womens Health (Larchmt) 2019;28:212–9. doi: 10.1089/jwh.2017.6834. [DOI] [PubMed] [Google Scholar]
- 4.Hajar R. Risk Factors for Coronary Artery Disease:Historical Perspectives. Heart Views. 2017;18:109–14. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_106_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, et al. Twenty Year Trends and Sex Differences in Young Adults Hospitalized With Acute Myocardial Infarction. Circulation. 2019;139:1047–56. doi: 10.1161/CIRCULATIONAHA.118.037137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin M, Qian Z, Yin J, Xu W, Zhou X. The role of intestinal microbiota in cardiovascular disease. J Cell Mol Med. 2019;23:2343–50. doi: 10.1111/jcmm.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haghikia A, Li XS, Liman TG, Bledau N, Schmidt D, Zimmermann F, et al. Gut Microbiota-Dependent Trimethylamine N-Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arterioscler Thromb Vasc Biol. 2018;38:2225–35. doi: 10.1161/ATVBAHA.118.311023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lautamäki A, Airaksinen KE, Kiviniemi T, Vinco G, Ribichini F, Gunn J, et al. Prognosis and disease progression in patients under 50 years old undergoing PCI: the CRAGS (Coronary aRtery diseAse in younG adultS) study. Atherosclerosis. 2014;235:483–7. doi: 10.1016/j.atherosclerosis.2014.05.953. [DOI] [PubMed] [Google Scholar]
- 9.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 10.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. Linee guida ESC 2015 per il trattamento delle sindromi coronariche acute nei pazienti senza sopraslivellamento persistente del tratto ST alla presentazione. Task Force per il Trattamento delle Sindromi Coronariche Acute nei Pazienti senza Sopraslivellamento Persistente del Tratto ST alla Presentazione della SocietàEuropea di Cardiologia (ESC) [2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC)] G Ital Cardiol (Rome) 2016;17:831–72. doi: 10.1714/2464.25804. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 12.European Association for Cardiovascular Prevention &Rehabilitation. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 13.Fioranelli M, Bottaccioli AG, Bottaccioli F, Bianchi M, Rovesti M, Roccia MG. Stress and Inflammation in Coronary Artery Disease: A Review Psychoneuroendocrineimmunology-Based. Front Immunol. 2018;9:2031. doi: 10.3389/fimmu.2018.02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorat MV, Tabrizi R, Kolahdooz F, Akbari M, Salami M, Heydari ST, et al. The effects of coenzyme Q10 supplementation on biomarkers of inflammation and oxidative stress in among coronary artery disease: a systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology. 2019;27:233–48. doi: 10.1007/s10787-019-00572-x. [DOI] [PubMed] [Google Scholar]
- 15.Emoto T, Yamashita T, Kobayashi T, Sasaki N, Hirota Y, Hayashi T, et al. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism:gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels. 2017;32:39–46. doi: 10.1007/s00380-016-0841-y. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita T, Emoto T, Sasaki N, Hirata KI. Gut Microbiota and Coronary Artery Disease. Int Heart J. 2016;57:663–71. doi: 10.1536/ihj.16-414. [DOI] [PubMed] [Google Scholar]
- 17.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bull MJ, Plummer NT. Part 1: The Human Gut Microbiome in Health and Disease. Integr Med (Encinitas) 2014;13:17–22. [PMC free article] [PubMed] [Google Scholar]
- 19.Altuntaş Y, Batman A. Microbiota and metabolic syndrome. Turk Kardiyol Dern Ars. 2017;45:286–96. doi: 10.5543/tkda.2016.72461. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landfald B, Valeur J, Berstad A, Raa J. Microbial trimethylamine-N-oxide as a disease marker:something fishy? Microb Ecol Health Dis. 2017;28:1327309. doi: 10.1080/16512235.2017.1327309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, et al. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition:A randomized controlled trial. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201600324. [DOI] [PubMed] [Google Scholar]
- 23.Roncal C, Martínez-Aguilar E, Orbe J, Ravassa S, Fernandez-Montero A, Saenz-Pipaon G, et al. Trimethylamine-N-Oxide (TMAO) Predicts Cardiovascular Mortality in Peripheral Artery Disease. Sci Rep. 2019;9:15580. doi: 10.1038/s41598-019-52082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer KA, Benton TZ, Bennett BJ, Jacobs DR, Jr, Lloyd-Jones DM, Gross MD, et al. Microbiota-Dependent Metabolite Trimethylamine N-Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA) J Am Heart Assoc. 2016;5:e003970. doi: 10.1161/JAHA.116.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sogari G, Velez-Argumedo C, Gómez MI, Mora C. College Students and Eating Habits:A Study Using An Ecological Model for Healthy Behavior. Nutrients. 2018;10:1823. doi: 10.3390/nu10121823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Li X, Yang F, Zhao R, Pan X, Liang J, et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease:Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front Pharmacol. 2019;10:1360. doi: 10.3389/fphar.2019.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghazalpour A, Cespedes I, Bennett BJ, Allayee H. Expanding role of gut microbiota in lipid metabolism. Curr Opin Lipidol. 2016;27:141–7. doi: 10.1097/MOL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 29.Kayıkçıoğlu M. How to design studies on premature myocardial infarction? Turk Kardiyol Dern Ars. 2017;45:495–7. doi: 10.5543/tkda.2017.24668. [DOI] [PubMed] [Google Scholar]