Abstract
Objective:
To study the prevalence of cardiovascular (CV) risk factors (RFs) in the rural population of the Chui region of Kyrgyzstan (Central Asia).
Methods:
The sample was representative of the population in terms of age and sex and included at least 10% of the population aged 18-65 y. Of the 1,672 people included in the cohort, 1.330 people responded to the invitation (79.5% of the total sample population). All study participants were interviewed using standardized questionnaires and examined by a cardiologist. Blood pressure (BP), weight, height, waist circumference (WC), fasting serum glucose, and fasting lipid level were measured.
Results:
The prevalence of major CV RFs in the examined sample was as follows: arterial hypertension 34.1%, obesity 25.7%, and abdominal obesity 52.3%; the factors were significantly more prevalent in women (68.2%) and increased with age. The frequency of lipid metabolism disorders was 88.4% in the examined subjects, and an increased level of low-density cholesterol (70.5%) was common. Hypodynamia was detected in 15.6% of the subjects, diabetes mellitus in 3.76%, and a family history of cardiovascular disease was present in 34.8% of the examined subjects. The frequency of smoking was 24.6% and was significantly higher in men (46.9%).
Conclusion:
Abdominal obesity, followed by hypercholesterolemia and arterial hypertension were the most common RFs among the rural population of the Chui region of Kyrgyzstan. Smoking was the most common RF among men. The prevalence of traditional CV RFs, except smoking, increased with age.
Keywords: epidemiology, cardiovascular risk factors, arterial hypertension, hyperlipidemia, obesity, diabetes mellitus, smoking
Introduction
Cardiovascular diseases (CVD) are the leading cause of mortality in the world. According to the World Health Organization (WHO), 17.5 million people die of CVD annually, accounting for about 30% of all deaths (1, 2). In Kyrgyzstan, CVD ranks first in the structure of total mortality, accounting for more than half (50.1%) of all deaths each year (3). Furthermore, since 1991, there has been a progressive increase in the CVD mortality. In particular, in 1991, CVDs accounted for 261.9 deaths per 100.000 population, and in 2011, this number was 326.3 cases per 100,000 people; it then increased by 24.6%, representing the most significant increase in CVD deaths among working-age population (40–65 y old) (3).
Cardiovascular (CV) mortality is directly related to the prevalence of risk factors (RFs); the main RFs are arterial hypertension (AH), dyslipidemia, obesity, smoking, and diabetes mellitus (DM) (4–8). In Kyrgyzstan, studies on the prevalence of CV RF were mainly conducted during the Soviet Union era. However, since then, the socio-economic way of life has changed significantly. Therefore, there are no updated statistics on the CV RFs prevalence in Kyrgyzstan.
It is well known that the lifestyles of people living in rural and urban areas are different, and such differences can affect the prevalence of CV RFs. In Kyrgyzstan, after the collapse of the Soviet Union, a health care reform took place that changed the system of health services. This reform led to the abolishment of many positions, resulting in the downsizing of several primary medical services and reduction in the number of practicing cardiologists. Thus, there is a lack of preventive measures for control of CV RFs in rural areas, resulting in increased prevalence of CVs, and the diagnosis of CVs is usually established as per the patients’ referral.
Thus, this study aimed to investigate the prevalence of the leading CV RFs among the working-age population living in the rural areas of the Chui region of Kyrgyzstan (Central Asia).
Methods
To study the prevalence of CV RF, a cross-sectional epidemiological study was conducted on the working-age rural population of the Chui region of Kyrgyzstan.
Enrollment of subjects was performed jointly with the National Research Center for Preventive Medicine of the Ministry of Health, Moscow, Russia. Two settlements in the Chuy region of Kyrgyzstan were randomly selected (Kant and Orlovka with population 16.785 of age 18–65 years) to participate in the study. Further, the electoral data of these settlements was requested from the National Statistical Committee of Kyrgyzstan. The population data were then divided as per age subgroups and sex, and the samples were selected with computer randomization.
The study population comprised 1672 subjects and was representative of the population in terms of age and sex and included at least 10% of the population aged 18–65 y. Further, the selected individuals were invited to undergo a medical examination at a local medical center. Of the 1672 study subjects, 1330 (79.5%) responded to the invitation. All participants provided written consent for study participation, and the study was approved by the Ethics Committee of the National Center of Cardiology and Internal Medicine (NCCIM)–Protocol N4 of 13.03.2012.
The study protocol included the following:
Filling in a standardized questionnaire, consisting of nine sections of information that included passport information, past medical history, family medical history, presence of CVD and RF, information on nutrition, physical activity, and disability (see Appendix). Moreover, objective data on height, weight, waist circumference (WC), blood pressure (BP), and heart rate were collected.
The following biochemical parameters were evaluated: glucose and lipid spectrum levels [total cholesterol (TC), triglycerides (TG), high (HDL-C), and low-density lipoprotein cholesterol (LDL-C)]. Blood sampling was performed once from the cubital vein after a 12-h fasting period from the following after the survey. After the blood specimen was collected, serum was separated, frozen, and stored in a container with liquid nitrogen. The sample was then transported to the biochemistry laboratory of the National Collaborating Centre for Infectious Diseases (NCCID). Biochemical blood parameters were evaluated using a photometric method using the auto analyzer Respons 920. The concentration of LDL-cholesterol was calculated by Friedewald’s formula: LDL-C=TC-HDL-cholesterol-TG/2.2.
Identification of RF:
AH was defined as an increase in systolic BP (SBP) ≥140 mm Hg and/or diastolic BP (DBP) ≥90 mm Hg or ongoing antihypertensive therapy. If a subject was detected with elevated BP for the first time, it was measured again after 2–3 d of the first measurement. The grading of AH was performed according to the European Society of Cardiology guidelines (9).
Lipid levels were considered abnormal if TC was ≥5.2 mmol/L, TG was ≥1.8 mmol/L; LDL-C ≥2.6 mmol/L, and/or HDL-C was <1.29 mmol/L in women and <1.03 mmol/L in men (10).
The presence of obesity was defined as per a body mass index (BMI) ≥30 kg/m2, calculated as weight (kg)/height (m)2. WC was measured at the midpoint of the distance between the costal arch and the iliac crest. Abdominal obesity (AO) was defined as WC ≥94 cm in men and ≥80 cm in women (11).
Those who reported sitting at work for ≥5 h in the absence of active leisure (walking or exercise 30–40 min/d for ≥5 times a week) were considered to have a low physical activity level (12).
Those who smoked at least 1 cigarette every day or had a history of smoking were categorized as smokers. Moreover, the presence of passive smoking was evaluated (12).
Diabetes was diagnosed as per the generally accepted criteria (13). Subjects with elevated glucose levels were subsequently invited to the NCCID for further examination to clarify the diagnosis.
Statistical analysis
Statistical analyses were performed using the STATISTICA 6.0 software. The Kolmogorov–Smirnov test was used for checking the normality of continuous variables. Normal variables were compared using student’s t-test; data are presented as mean±standard deviation values. Non-normal continuous variables were compared using the Mann–Whitney U test, and the data are presented as median (25%–75%) values. The relationships between the qualitative variables were assessed using the two-sided test for the difference between two proportions. The significance of CV RFs as per age and sex was determined using multiple logistic regression analysis. For all statistical analyses, p<0.05 was considered to indicate statistical significance.
Results
The age and sex distribution of the studied population is presented in Table 1. The socio-demographic characteristics of the study population were as follows; 68.3% were married, 16.1% were not married, 7.6% were divorced, and 8% were widowed. Further, 37.1% had higher education, while the remaining 62.9% had either secondary or primary education. At the time of the study, 780 people (58.6%) were employed, 148 (11.1%) had never worked, 246 (18.5%) were temporarily unemployed, 134 were retired (10.1%), and 22 (1.7%) were receiving disability benefits. The prevalence rates of the main CV RFs in the study population are presented in Table 2.
Table 1.
Age and sex distribution of the study population
| Age (years) | Total (n=1330) | Men (n=567) | Women (n=763) |
|---|---|---|---|
| Before 30 | 277 (20.8%) | 148 (26.1%) | 129 (16.9%) |
| 30-39 | 279 (21.0%) | 139 (24.5%) | 140 (18.4%) |
| 40-49 | 328 (24.7%) | 112 (19.8%) | 216 (28.3%) |
| 50-59 | 285 (21.4%) | 100 (17.6%) | 185 (24.2%) |
| Over 60 | 161 (12.1%) | 68 (12.0%) | 93 (12.2%) |
Table 2.
Prevalence of cardiovascular risk factors among the population of Chiu valley in Kyrgyzstan
| Risk factor | All (n=1330) | Men (n=567) | Women (n=763) | P** |
|---|---|---|---|---|
| Arterial hypertension; n (%) | 453 (34.1%) | 208 (36.7%) | 233 (30.5%) | 0.021 |
| Age standardized | 39.7% | 28.3% | ||
| SBP/DBP; mm Hg | 126±22/79±12 | 126±19/80±11 | 126±24/79±12 | 0.998/0.123 |
| Obesity; n (%) | 342 (25.7%) | 89 (15.7%) | 253 (33.2%) | <0.001 |
| Age standardized | 17.2% | 31.4% | ||
| Abdominal obesity; n (%) | 696 (52.3%) | 176 (31.0%) | 520 (68.2%) | <0.001 |
| Age standardized | 34.1% | 65.9% | ||
| Hypodinamia; n (%) | 208 (15.6%) | 82 (14.5%) | 126 (16.5%) | 0.320 |
| Age standardized | 14.8% | 16.7% | ||
| Dyslipidemia | ||||
| High TC; n (%) | 550 (41.4%) | 206 (36.3%) | 344 (45.1%) | 0.001 |
| Age standardized | 38.6% | 43.5% | ||
| TC; mmol/L* | 4.96 (4.2-5.79) | 4.9 (4.08-5.75) | 5.0 (4.3-5.84) | 0.006 |
| High TG; n (%) | 349 (26.2%) | 197 (34.7%) | 152 (19.9%) | <0.001 |
| Age standardized | 35.8% | 19.4% | ||
| TG; mmol/L* | 1.1 (0.82-1.74) | 1.33 (0.9-1.94) | 1.1 (0.76-1.55) | <0.001 |
| High LDL-C; n (%) | 937 (70.5%) | 370 (65.3%) | 567 (74.3%) | <0.001 |
| Age standardized | 66.9% | 73.6% | ||
| LDL-C; mmol/L* | 3.11 (2.47-3.8) | 2.93 (2.35-3.72) | 3,22 (2.57-3.9) | <0.001 |
| Low HDL-C; n (%) | 759 (57.1%) | 321 (56.6%) | 438 (57.4%) | 0.772 |
| Age standardized | 56.2% | 57.2% | ||
| HDL-C; mmol/L* | 1.1 (0.9-1.3) | 1.0 (0.9-1.2) | 1.2 (1.0-1.4) | <0.001 |
| Diabetes mellitus; n (%) | 50 (3.76%) | 21 (3.70%) | 29 (3.80%) | 0.923 |
| Age standardized | 3.6% | 3.6% | ||
| Smoking; n (%) | 327 (24.6%) | 266 (46.9%) | 61 (8.0%) | <0.001 |
| Age standardized | 47% | 8.3% | ||
| Cigarettes per day | 12.7±8.7 | 13.5±8.8 | 7.5±5.5 | <0.001 |
| Passive smoking; n (%) | 814 (61.2%) | 437 (77%) | 434 (56.9%) | <0.001 |
| Age standardized | 77.3% | 60.1% |
Data presented as Median (25%–75%);
P–between men and women groups
SBP - systolic blood pressure; DBP - diastolic blood pressure; TC - total cholesterol; TG - triglycerides; LDL-C - low-density lipoprotein cholesterol; HDL-C - high-density lipoprotein cholesterol; CAD - coronary artery disease
Arterial hypertension
AH was diagnosed in 453 participants (34.1%) (Table 2), with 20.8% having grade-I AH, 7.8% having grade-II AH, and 5.5% having grade-III AH. The prevalence of AH was associated with older age, and in those aged ≥60 y, it reached 75.2% (Fig. 1). In our study, the AH prevalence was slightly higher among women than among men (36.7% vs. 30.5%, p=0.021). However, no significant sex-based differences were identified in the prevalence of AH or BP in the different age groups.
Figure 1.
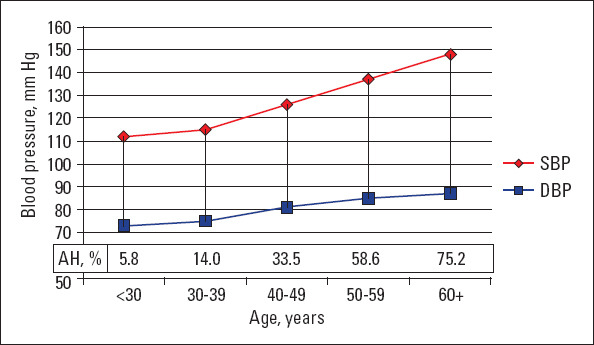
Blood pressure level and prevalence of arterial hypertension in different age groups
SBP - systolic blood pressure; DBP - diastolic blood pressure; AH - arterial hypertension
The mean BP in the studied population was 126±22/79±12 SBP (mean±SD)/DBP (mean±SD) mm Hg, with no significant differences between men and women (126±20/80±11 mm Hg and 126±24/79±13 mm Hg, respectively). Both, SBP and DBP increased progressively with age (Fig. 1).
Results of multiple logistic regressions for AH are reported in Table 3. Participants with AH were more likely to have obesity, especially AO. Association with other CV RFs after adjustment for age and sex was not significant.
Table 3.
Logistic regression modeling the factors, associated with arterial hypertension in rural Kyrgyz population, adjusting for age and sex
| OR (95% CI) | P | |
|---|---|---|
| Demographic factors | ||
| Each 10 years of age | 2.38 (2.09– 2.71) | <0.001 |
| Sex (male) | 1.14 (0.82–1.16) | 0.472 |
| Risk factors | ||
| Obesity | 1.57 (1.21–2.19) | 0.009 |
| Abdominal obesity | 1.79 (1.26–2.53) | 0.001 |
| Lipid abnormalities | ||
| High cholesterol level | 1.2 (0.87–1.66) | 0.264 |
| High LDL-C level | 1.15 (0.78–1.69) | 0.481 |
| Low HDL-C level | 0.9 (0.68–1.19) | 0.462 |
| High TG | 1.17 (0.83–1.65) | 0.363 |
| DM | 0.61 (0.31–1.22) | 0.167 |
| Current smoking | 1.2 (0.84–1.71) | 0.334 |
TC - total cholesterol; TG - triglycerides; LDL-C - low-density lipoprotein cholesterol; HDL-C - high-density lipoprotein cholesterol; DM – diabetes mellitus
Obesity and low physical activity
Obesity (BMI ≥30 kg/m2) was present in 342 (25.7%) patients. The frequency of obesity in women was twice of that in men. It is noteworthy that the higher degrees of obesity (BMI >35 kg/m2) were identified primarily in women. The AO prevalence was even higher, reaching 52.3%, especially in women (Table 2, Fig. 2). The frequency of obesity as per BMI and AO progressively increased with age. This pattern was traced in both men and women.
Figure 2.
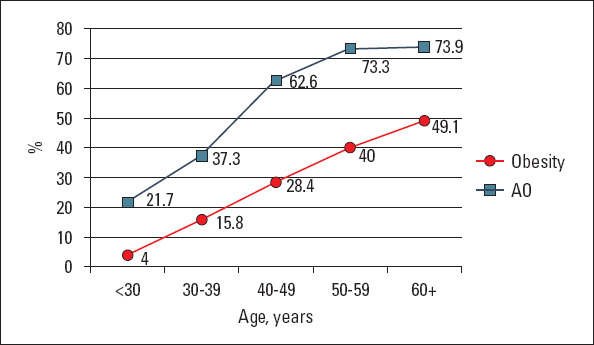
Prevalence of obesity and abdominal obesity in different age groups
AO - abdominal obesity
Low physical activity was diagnosed in 208 respondents (15.6%) and had a similar frequency in men and women (14.5% and 16.5% respectively, p-ns) (Table 2). The prevalence ranged from 14%–16% in all age groups without significant sex-based differences. The exception was the age group <30 y, where men had the minimum value (10.8%) and women had maximum value (21.7%; p=0.021).
The results of the logistic regression showed that obese participants were more likely to have AH, raised TG level, and low HDL-C level. AO was also associated with raised TC and LDL-C levels (Table 4).
Table 4.
Logistic regression, modeling the factors, associated with obesity and abdominal obesity in rural Kyrgyz population, adjusting for age and sex
| Obesity OR (95% CI) | P | Abdominal obesity OR (95% CI) | P | |
|---|---|---|---|---|
| Demographic factors | ||||
| Each 10 years of age | 1.61 (1.41–1.83) | <0.0001 | 1.61 (1.42–1.81) | <0.001 |
| Sex (male) | 0.38 (0.27–0.53) | <0.0001 | 0.19 (0.14–0.25) | <0.001 |
| Risk factors | ||||
| Arterial hypertension | 1.92 (1.41–2.61) | <0.0001 | 2.11 (1.54–2.90) | <0.001 |
| Lipid abnormalities | ||||
| High cholesterol level | 1.29 (0.93–1.79) | 0.13 | 1.60 (1.17–2.18) | 0.003 |
| High LDL-C level | 1.28 (0.85–1.91) | 0.23 | 1.43 (1.02–1.99) | 0.036 |
| Low HDL-C level | 1.57 (1.81–2.10) | 0.002 | 1.41 (1.07–1.85) | 0.014 |
| High TG | 1.68 (1.20–2.36) | 0.003 | 1.78 (1.26–2.53) | 0.001 |
| DM | 0.75 (0.39–1.43) | 0.38 | 0.84 (0.38–1.84) | 0.662 |
| Current smoking | 0.85 (0.58–1.24) | 0.40 | 0.75 (0.55–1.04) | 0.810 |
TC - total cholesterol; TG - triglycerides; LDL-C - low-density lipoprotein cholesterol; HDL-C - high-density lipoprotein cholesterol; DM – diabetes mellitus
Dyslipidemia
Lipid metabolism disorders were common. The overall prevalence of dyslipidemia with at least one disorder in the lipid spectrum was recorded in 88.4% subjects without significant sex-based differences (86.8 in men and 89.6% in women). Increased LDL-C level was the most common form of dyslipidemia (70.5%), followed by lower HDL-C level (57.1%), increased TC level (41.4%), and hypertriglyceridemia (26.2%) (Table 2, Fig. 3). Overall, mixed disorders of lipid metabolism were found to be the most common.
Figure 3.
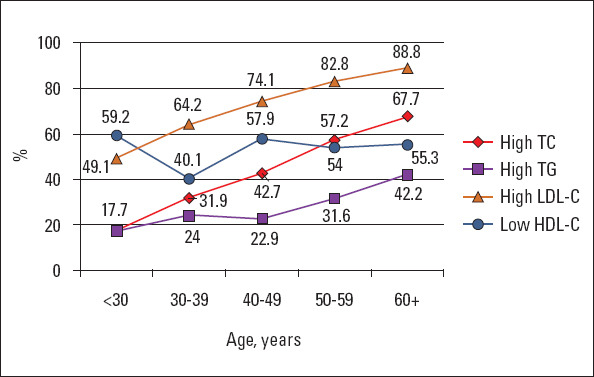
Prevalence of lipid disorders in different age groups
TC - total cholesterol; TG - triglycerides; LDL-C - low-density lipoprotein cholesterol; HDL-C - high-density lipoprotein cholesterol
When assessing the age dynamics of lipid parameters, there was a clear association of the prevalence of lipid disorders with older age, regardless of sex. Thereby, at the age of 30 y, the frequency of hypercholesterolemia was 17.7%, sharply increasing in the fourth decade of life to 42.7%. Consequently, the rate of increase in the prevalence of hypercholesterolemia slowed down, and in people aged >60 y, it reached 67.7%. A similar trend was observed in subjects with high LDL-C level and hypertriglyceridemia. In our study, there was no clear association between reduced HDL-C and age. A decrease in HDL-C was observed with almost equal frequency in all age groups, with the exception of the subgroup of those aged 30–39 y, where the frequency of this indicator decreased to 40.1% [mainly in the men subgroup: 21.6% in men vs. 58.6% in women (p<0.001)] (Fig. 3).
In spite of the high frequency of lipid metabolism disorders, the severity of hypercholesterolemia in the studied population was moderate; the TC level was 4.96 (4.2–5.79) mmol/L, LDL-C was 3.11 (2.47–3.81) mmol/L, TG was 1.11 (0.82–1.74) mmol/L, and HDL-C was 1.1 (0.9–1.3) mmol/L. Increased TC level >6.2 mmol/L was detected in 16% of the patients.
When studying sex-based differences in the frequency of various lipid metabolism disorders, we found that women had significantly higher prevalence of hypercholesterolemia with higher TC and LDL-C levels. Moreover, the frequency of hypertriglyceridemia was higher in men. In terms of the prevalence of reduced HDL-C, there were no significant sex-based differences, with the exception of the subgroup aged 30–39 y.
Participants with a TC level >5.2 mmol/L had a strong association with AO and were less likely to be smokers at the time of the study. They had a mild association with AH. Only AO was associated with elevated LDL-C after adjustment for age and gender (Table 5).
Table 5.
Logistic regression modeling the factors, associated with raised level of TC and LDL-C in rural Kyrgyz population, adjusting for age and sex
| Raised level of TC OR (95% CI) | P | Raised level of LDL-C OR (95% CI) | P | |
|---|---|---|---|---|
| Demographic factors | ||||
| Each 10 years of age | 1.51 (1.35–1.68) | <0.0001 | 1.48 (1.30–1.67) | <0.001 |
| Sex (male) | 1.16 (0.87–1.56) | 0.31 | 1.0 (0.73–1.36) | 0.990 |
| Risk factors | ||||
| Arterial hypertension | 1.31 (0.99–1.73) | 0.06 | 1.23 (0.87–1.74) | 0.241 |
| Obesity | 1.12 (0.82–1.52) | 0.48 | 1.15 (0.76–1.73) | 0.514 |
| Abdominal obesity | 1.86 (1.38–2.50) | <0.0001 | 1.68 (1.21–2.36) | 0.002 |
| DM | 0.96 (0.52–1.79) | 0.91 | 0.86 (0.37–2.03) | 0.742 |
| Current smoking | 0.71 (0.52–0.96) | 0.028 | 0.83 (0.61–1.14) | 0.252 |
TC - total cholesterol; LDL-C - low-density lipoprotein cholesterol; DM – diabetes mellitus
Hyperglycemia
Carbohydrate metabolism disorders in the studied population were less common than dyslipidemias. All patients with increased blood glucose levels during the study were subsequently were invited to NCCIM to confirm the DM diagnosis. DM was observed in 50 (3.76%) of the examined subjects, without significant sex-based differences (3.70% in men vs. 3.80% in women). The prevalence of DM increased with age. In particular, it was 1.39% in men aged <40 y, 2.83% in those aged 40–59 y, and 16.7% in those aged >60 y. In women, there was a similar dynamic in the prevalence of DM; those aged <40 y showed a prevalence of 0.37%, those aged 40–59 y had a prevalence of 4.99%, and those aged >60 y had a prevalence of 8.6%. The glucose fasting level (5.5–6.9 mmol/L) was detected in 4.58% men and 2.49% women (p-ns).
Participants with DM were more likely to have hypertriglyceridemia [OR - 2.88 (1.52–5.46)]. An association with other CV RFs after adjustment for age and sex was not significant (Table 6).
Table 6.
Logistic regression modeling the factors, associated with diabetes in rural Kyrgyz population, adjusting for age and sex
| OR (95% CI) | P | |
|---|---|---|
| Demographic factors | ||
| Each 10 years of age | 1.70 (1.25–2.31) | 0.001 |
| Sex (male) | 0.94 (0.45–2.0) | 0.870 |
| Risk factors | ||
| Arterial hypertension | 1.60 (0.80–3.21) | 0.181 |
| Obesity | 1.37 (0.67–2.80) | 0.393 |
| Abdominal obesity | 1.96 (0.51–2.78) | 0.683 |
| Lipid abnormalities | ||
| High TC level | 0.78 (0.39–1.58) | 0.492 |
| High LDL-C level | 1.23 (0.49–3.07) | 0.661 |
| Low HDL-C level | 0.97 (0.53–1.79) | 0.930 |
| High TG | 2.88 (1.52–5.46) | 0.001 |
| Current smoking | 1.46 (0.68–3.14) | 0.331 |
TC - total cholesterol; TG - triglycerides; LDL-C - low-density lipoprotein cholesterol; HDL-C - high-density lipoprotein cholesterol
Smoking
Overall, the prevalence of smoking was 24.6% (327 respondents), and 68 people (5.1%) reportedly smoked previously. The prevalence of this RF was significantly higher in men than in women (p<0.001). On an average, the number of cigarettes smoked per day was 12.7±8.7 cigarettes/d (13.5±8.8 cigarettes/d in men and 7.5±5.5 cigarettes/d in women, p<0.001) (Fig. 4).
Figure 4.
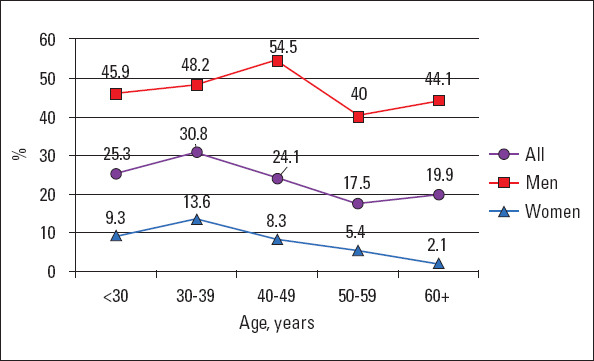
Prevalence of smoking in different age groups
The average age of smoking initiation in the group was 18.8±5.2 y; men started smoking earlier than women (17.8±4.4 y in men vs. 22.6±6.8 y in women, p<0.001). The highest incidence of smoking was observed in those aged 30–39 y (30.8% of the respondents). Consequently, the frequency of smoking was progressively decreasing, reaching 17.5% in those aged 50–59 y (p<0.001). This trend was observed both in men and women. However, it is noteworthy that, if the peak of smoking in women was in the fourth decade of life (13.6%), then in men, it was at the age of 40–49 y (54.5%). It is interesting that the smoking prevalence was higher (≥40%) even in older men (50–59 y old: 40% and ≥60 y: 44.1%).
A high prevalence of passive smoking was noted in our study, which was 3 times more frequent than active tobacco use. In particular, passive smoking was recorded in 61.2% of the respondents. Moreover, men were mostly exposed to tobacco smoke at work, while women were exposed to it at home.
Participants who smoked at the time of the study were less likely to have a raised TC level. An association with other CV RFs after adjustment for age and sex showed no significant results (Table 7).
Table 7.
Logistic regression modeling the factors, associated with current smoking in rural Kyrgyz population, adjusting for age and sex
| OR (95% CI) | P | |
|---|---|---|
| Demographic factors | ||
| Each 10 years of age | 0.96 (0.84–1.09) | 0.521 |
| Sex (male) | 10.03 (7.35–13.68) | <0.001 |
| Risk factors | ||
| Arterial hypertension | 1.14 (0.81–1.62) | 0.452 |
| Obesity | 0.92 (0.61–1.40) | 0.701 |
| Abdominal obesity | 1.76 (0.53–1.09) | 0.132 |
| Lipid abnormalities | ||
| High TC level | 0.68 (0.49–0.97) | 0.033 |
| High LDL-C level | 0.97 (0.69–1.37) | 0.883 |
| Low HDL-C level | 0.91 (0.68–1.21) | 0.510 |
| High TG | 1.04 (0.74–1.48) | 0.815 |
| DM | 0.72 (0.34–1.48) | 0.372 |
TC - total cholesterol; TG - triglycerides; LDL-C - low-density lipoprotein cholesterol; HDL-C - high-density lipoprotein cholesterol; DM – diabetes mellitus
Discussion
In our study, the prevalence of AH was noted in 34.1% of the population, mainly owing to the considerable prevalence of grade-I AH (20.8%), without any significant sex-based differences. Compared to earlier epidemiological studies performed in Kyrgyzstan, our work has demonstrated a substantial increase in the AH prevalence. Initial epidemiological studies have shown a prevalence of AH at 15.2% among the inhabitants of the lowlands and 3.44% among the mountain-dwelling subjects (14). Thereafter, at the end of the last century, the frequency of AH among the urban population was 25%–28.1% (15, 16). At the beginning of the present century, there was a significant increase in the prevalence of AH that reached 41% in urban areas and 38.5% in rural regions. Those differences may be related to the changes in the public health system in Kyrgyzstan after the collapse of the Soviet Union.
The AH prevalence in other populations varies significantly. The proportion of the world’s population with high BP, or uncontrolled hypertension, fell modestly between 1980 and 2008. However, because of population growth and aging, the number of people with hypertension rose from 600 million in 1980 to nearly 1 billion in 2008. The prevalence of hypertension was highest in the African Region, where it was 46% for the entire population (both sexes combined). Across countries belonging to different income groups, the prevalence of hypertension was consistently high, with low-income countries (around 40% for both sexes) showing a higher rate than high-income countries (35% for both sexes) (17). The studies conducted by Wolf–Maier showed that the mean BP in subjects living in Europe was 136/83 (SBP/DBP) mm Hg in Canada 122/77 (SBP/DBP) mm Hg in the USA (18). The lowest level of BP was recorded in the USA and the highest in Germany. The NHANES research program showed that 29% of the adult USA population had AH (19), the presence of which was associated with the intake of contraceptives, older age, and obesity. According to Fields et al. (20), every third resident of the USA has AH. Data on the prevalence of CV RFs in the neighboring countries are limited or unavailable for public access, especially for post-Soviet countries. Based on the available data, the prevalence of AH in Kazakhstan was 23.2% in 2013 (21), 33.8% in the Russian Federation (22), and 26% in Turkey (23). In fact, the problem of AH is relevant globally, and the recent increase in the AH prevalence may be partly attributable to the changes in the lifestyle as well as an increase in the frequency of other metabolic risk factors (RFs), such as insulin resistance, obesity, and DM.
Our study showed a prevalence of obesity at 25.7%; however, the prevalence of AO was even higher at 52.3%. Moreover, the prevalence of obesity increased with age, and obesity was more common among women. Compared to recent studies conducted in Kyrgyzstan, there has been an increase in the prevalence of obesity by 30%–40%. In particular, in the earlier studies, the prevalence of obesity was 16.3%–17.3%, and in 2007, it was 19.6% (10.8% in men and 24.8% in women) among the residents in rural areas (14, 15). This fact relates to an increase in the calorie intake and a simultaneous decrease in physical activity in the population (24). The same trend has been observed in other communities. According to the data of the European Society of Cardiology, 50% of the adult population of the European region is overweight (BMI ≥25 kg/m2), and 1/3rd of the adult population has obesity (BMI ≥30 kg/m2) (17, 25). In the Russian Federation, the age-standardized prevalence of obesity in 2012–2013 was 29.7% (26.6% among men vs. 30.8% among women) (22). The prevalence was 21% and 25% in Kazakhstan and 23% and 36% in Turkey for men and women, respectively (23).
In our study, when studying the frequency of physical inactivity, we found that its prevalence across all age groups ranged from 14%–16% without significant sex-based differences. At the same time, it is noteworthy that the lack of physical activity in this study was evaluated using questionnaires; thereby, the assessment involved subjectivity. Moreover, the questionnaires could not clarify the severity of hypodynamia.
Lipid metabolism disorders are one of the most important RFs associated with CVD development. In our study, the frequency of hypercholesterolemia was 36.2% in men and 45.1% in women. However, disorders of at least 1 of the 4 lipid parameters were observed in 88.4% of the subjects, and most respondents had combined dyslipidemias. As per the results of studies conducted in our country during the Soviet era, the prevalence of hypercholesterolemia was 17.0%–19.6% and was lower among highlanders (14, 15, 26). According to the STEPS study conducted by the WHO, the frequency of hypercholesterolemia was 23.3%, where the mean cholesterol level was 4.4 (95% CI 4.3–4.7) mmol/L and the TC level was >6.2 mmol/L in 4.7% of the examined cohort (27); this was significantly lower than the analogous indicators identified in our study. In the Russian Federation, as per the epidemiologic study, ECVD-RF prevalence of raised TC level was 57.6% (22). The frequency of significant hyperlipidemia with a TC level >6.2 in Kazakhstan in 2014 was 10% and 13% and that in Turkey was 8% and 10% in men and women, respectively (23).
Numerous studies have been conducted to study the prevalence of lipid metabolism disorders in different regions of the world. In particular, according to the National Institutes of Health (USA), the prevalence of hypercholesterolemia among Americans is 25% (19). The results from the NHANES study showed that the mean level of TC in adults aged >20 y was 201 mg/dL in men and 203 mg/dL in women (28). However, the introduction of CVD preventive programs and the active identification of high-risk individuals together with the prescription of lipid-lowering drugs in Europe and the USA have lowered the prevalence of hyperlipidemia in the population (10).
A confirmed diagnosis of DM was established in 3.76% of the study population, without significant sex-based differences. According to the official statistical data, the prevalence of DM in Kyrgyzstan is unreliable because it reflects the data as per referral of patients to the doctor. However, it is well known that DM can be asymptomatic for a long time and is often diagnosed in the later, more advanced stages. In the STEPS study, DM was found in 8.8% of the surveyed population, and another 4.5% of the respondents had carbohydrate metabolism disorders (27). According to WHO data, there has been a steady worldwide increase in the number of patients with DM, and >70% of these patients live in low and middle-income countries. This problem is particularly evident in the South Asian region where the number of DM patients has been rising rapidly each year (29). In Europe, the highest prevalence of DM was recorded in Germany (10.2%), Bulgaria (10%), and Spain (9.9%), while the lowest incidence was in Iceland (2.0%), Ireland (3.4%), and the Netherlands (3.7%). The prevalence of DM increases with age in both sexes. Thus, 10% of subjects <60 y old, 10%–20% of those aged 60–69 y old, and 15%–20% of those aged >70 y previously had DM; newly diagnosed DM was found in a similar number of subjects (29). Among Americans >20 y old, DM was found in 9.6%, and among those aged >60 y, it was present in 21%. The prevalence is higher in men than in women (11% vs. 9%) (30). In neighboring countries, the prevalence of diabetes in 2014 was 5% in the Russian Federation and Kazakhstan, while that in Turkey was almost 3 times higher at 14.8% (22, 23).
In our study, we found a high frequency of smoking among the rural population. In the first epidemiological studies in our country, smoking was prevalent among highlanders (32.7%) and among the urban population (48%) (14). In the 80s, the prevalence of smoking among men reached 58.5% and decreased with age (26). In the early 2000s, the prevalence of smoking among the rural population of Kyrgyzstan was 50.1% in men and 0.5% in women (24). In our study, the frequency of smoking among men was comparable to that reported previously (46.9%). Moreover, the prevalence of smoking among women was significantly higher at 8.0% (24, 26).
Smoking has now become a pervasive epidemic (31). The prevalence of daily tobacco smoking varied widely among different regions. The highest overall prevalence for smoking is estimated at nearly 29% in the European region, and the lowest was in the African Region (8%). The highest prevalence of smoking among men was in the Western Pacific Region (46%) and among women in the European region (20%). In all regions, men smoke more than women, with the exception of Sweden (18% in women vs. 15% in men). Smoking is more common among men in central and eastern Europe and among women in northern and western Europe (17, 31).
Study limitations
One study limitation was that the determination of laboratory values (lipid panel, glucose) was only performed once for all subjects. Therefore, in the subsequent analysis of lipid and glycemic disorders, the individual risk was not studied. Our sample had both, low-risk and high-risk individuals. In this regard, the level of LDL-cholesterol <2.6 mmol/L (<100 mg/dL) was accepted as the normal level. Repeated determination of the lipid spectrum in individuals with impaired lipid metabolism was not performed.
Moreover, the 2-h oral glucose tolerance test was not performed. Therefore, subjects who were diagnosed with DM with fasting glucose ranging from 5.5–6.9 mmol/L were classified into the group without DM that may distort the prevalence of glycemic disturbances in the study population.
Finally, based on our questionnaires, we were unable to quantify the lack of physical activity as well as first-hand smoking and second-hand smoking.
Conclusion
AO (especially among women), hypercholesterolemia, and AH were the most common RFs among the rural population of the Chui region of Kyrgyzstan. In the male subgroup, the most frequent risk factor was smoking. The prevalence of traditional CV RFs, except smoking, increased with age.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – A.G.P., A.V.K., E.M.M.; Design – E.M.M.; Supervision – I.S.S., A.S.D., E.M.M.; Fundings – This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors; Materials – A.G.P., A.N.K., A.T.A.; Data collection and/or processing – A.G.P., A.N.K., A.T.A.; Analysis and/or interpretation – A.G.P., O.S.L., A.E.M., I.S.S.; Literature search – A.G.P., O.S.L.; Writing – A.G.P., O.S.L., A.E.M.; Critical review – A.V.K., A.S.D., E.M.M.
References
- 1.Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJ, et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1483–92. doi: 10.1161/CIRCULATIONAHA.113.004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazzini A, Lazzini S. Cardiovascular disease:an economical perspective. Curr Pharm Des. 2009;15:1142–56. doi: 10.2174/138161209787846883. [DOI] [PubMed] [Google Scholar]
- 3.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 5.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–8. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 7.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102:1082–5. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 8.Preis SR, Pencina MJ, Hwang SJ, D'Agostino RB, Sr, Savage PJ, Levy D, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation. 2009;120:212–20. doi: 10.1161/CIRCULATIONAHA.108.846519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 10.European Association for Cardiovascular Prevention &Rehabilitation. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 11.Alberti MM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. International Diabetes Federation Task Force on Epidemiology and Prevention;Hational Heart, Lung, and Blood Institute;American Heart Association;World Heart Federation;International Atherosclerosis Society;International Association for the Study of Obesity. Harmonizing the metabolic syndrome:a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention;National Heart, Lung, and Blood Institute;American Heart Association;World Heart Federation;International Atherosclerosis Society;and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 12.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice:The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention &Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–81. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Authors/Task Force Members. Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, et al. ESC Committee for Practice Guidelines (CPG). ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD:the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–87. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 14.Mirrakhimov MM. Several aspects of clinical cardiology in Kirghizia. Kardiologiia. 1972;12:17–22. [PubMed] [Google Scholar]
- 15.Meĭmanaliev TS, Shleĭfer EA, Madaminov IaK, Aĭtbaev KA, Ismailova ChS, Eroshenko VSh, et al. Nutrition and the incidence of ischemic heart disease and risk factor for its occurrence among men 20-59 years of age depending on their ethnic group affiliation. Vopr Pitan. 1989;4:28–32. [PubMed] [Google Scholar]
- 16.Mirrakhimov MM, Rafibekova ZhS, Dzhumagulova AS, Meimanaliev TS, Murataliev TM, Shatemirova KK. Prevalence and clinical peculiarities of essential hypertension in a population living at high altitude. Cor Vasa. 1985;27:23–8. [PubMed] [Google Scholar]
- 17.World Health Organization. Global status report on non-communicable diseases 2010. Available online: URL;http://apps.who.int/iris/bitstream/10665/44579/1/97892406⇅8_eng.pdf .
- 18.Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–9. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2009 update:a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 20.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States, 1999–2000:a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 21.Aringazina A, Kuandikov T, Arkhipov V. Burden of the Cardiovascular Diseases in Central Asia. Cent Asian J Glob Health. 2018;7:321. doi: 10.5195/cajgh.2018.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muromtseva GA, Kontsevaya AV, Konstantinov VV, Artamonova GV, Gatagonova TM, Duplyakov DV, et al. The prevalence of non-infectious diseases risk factors in Russian population in, 2012-2013 years. The results of ECVD-RF. Cardiovascular Therapy and Prevention. 2014;13:4–11. [Google Scholar]
- 23.Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, et al. ESC Scientific Document Group, uropean Society of Cardiology:Cardiovascular Disease Statistics 2017. Eur Heart J. 2018;39:508–79. doi: 10.1093/eurheartj/ehx628. [DOI] [PubMed] [Google Scholar]
- 24.Romanova TA, Nyshanova ST, Polupanov AG. Prevalence of arterial hypertension and other cardiovascular risk factors in the rural population of Kyrgyzstan. Prevention of diseases and health promotion. 2007;3:14–7. eLIBRARY ID:13333840. [Google Scholar]
- 25.De Bacquer D, De Backer G Cokkinos K, Keil U, Montaye M, Ostör E, et al. Overweight and obesity in patients with coronary heart disease: are we meeting the challenge? Eur Heart J. 2004;25:121–8. doi: 10.1016/j.ehj.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Meimanaliev TS, Shleifer EA, Aitbaev KA, Aitmurzaeva GT, Gil'fanova VSh, Podgurskaya LP, et al. Prevalence of ischaemic heart disease risk factors among the male population in Frunze aged 40-59 years and results of a five-year prevention programme. Cor Vasa. 1991;33:451–7. [PubMed] [Google Scholar]
- 27.A TA, Makhmutkhodzhaev SA, Kydyralieva RB, Altymysheva AT, Dzhakipova RS, Zhorupbekova KS, et al. Prevalence of Risk Factors of Non-Communicable Disease in Kyrgyzstan: Assessment using WHO STEPS Approach. Kardiologiia. 2016;56:86–90. doi: 10.18565/cardio.2016.11.86-90. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) Disparities in screening for and awareness of high blood cholesterol:United States, 1999–2002. MMWR Morb Mortal Wkly Rep. 2005;54:117–9. [PubMed] [Google Scholar]
- 29.World Health Organization. Global Report on WHO of Diabetes. World Health Organization. 2016. Available online: URL;https://apps.who.int/
- 30.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes mellitus: the Framingham Offspring Study. Diabetes. 2000;49:2201–7. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 31.Scholte op Reimer W, de Swart E, De Bacquer D, Pyörälä K, Keil U, Heidrich J, et al. Smoking behaviour in European patients with established coronary heart disease. Eur Heart J. 2006;27:35–41. doi: 10.1093/eurheartj/ehi497. [DOI] [PubMed] [Google Scholar]


