Abstract
Background:
Neuromuscular training (NMT) has been shown to attenuate high-risk biomechanics in uninjured athletes. At the time that athletes return to sport after anterior cruciate ligament (ACL) reconstruction (ACLR), they demonstrate hip biomechanical deficits associated with injury to the reconstructed knee versus the uninjured contralateral knee.
Purpose:
The primary purpose of the study was to examine whether an NMT program can improve single-leg drop (SLD) landing hip biomechanics for athletes after ACLR. Secondarily, we compared the posttraining SLD hip biomechanics of athletes after ACLR with a control group of athletes who also completed the NMT program.
Study Design:
Controlled laboratory study.
Methods:
A total of 18 ACLR and 10 uninjured athletes were recruited and completed a 12-session NMT program. A knee-specific questionnaire and biomechanics of an SLD task was evaluated for each athlete before and after NMT. Paired t tests were used to compare pre- and posttraining International Knee Documentation Committee (IKDC) scores. Repeated-measures analysis of variance (ANOVA) was performed to assess the main effects and interactions of testing session × limb for the ACLR athletes. A 2-way ANOVA was conducted to quantify the interactions and main effects of group × limb.
Results:
There was a significant increase (P = .03) in IKDC scores from pre- to posttraining. For the ACLR athletes, there was a significant session × limb interaction for hip external rotation moment (P = .02) and hip abduction angle (P = .013). Despite increases in hip external rotation moment, no significant changes from pre- to posttraining were observed for the involved limbs. No significant changes were observed for hip abduction angle of the involved limbs between training sessions. Significant main effects of session (P < .05) revealed that athletes landed with greater hip excursion, lower hip flexion moment, and lower ground-reaction force after training. The posttraining comparison between the ACLR and control groups found no significant group × limb interactions for any of the hip kinematic or kinetic variables. A significant main effect of group (P < .05) revealed that the ACLR athletes landed with greater hip flexion angle and hip external rotation moment.
Conclusion:
ACLR athletes demonstrated an improvement in SLD hip biomechanics and neuromuscular control after participating in an NMT program.
Clinical Relevance:
This evidence indicates a potential role for NMT to improve hip biomechanics during an SLD task so as to reduce ACL injury risk.
Keywords: neuromuscular training, hip biomechanics, anterior cruciate ligament
The anterior cruciate ligament (ACL) is one of the most commonly injured structures within the knee. The standard of care within the United States for individuals who desire to return to sport after ACL injury is restoring ligamentous stability through ACL reconstruction (ACLR). However, this treatment paradigm, which includes rigorous postoperative rehabilitation, often results in suboptimal outcomes for athletes with ACL-injured knees. These include a significant number of ACLR athletes not returning to their previous level of activity,2 nearly 1 in 3 athletes sustaining further ACL injuries either to their ACL graft or to the contralateral ACL,1,29 and degenerative changes in the knee as early as 10 to 20 years after injury.5,20,24 Neuromuscular and biomechanical deficits are directly implicated in the etiology of these complications after ACLR.
Biomechanical deficits proximal to the knee joint may expose the knee to vulnerable positions and a greater risk of further knee injury. Several reports11,17,22,29,30,38,39 have described an association between neuromuscular deficits at the trunk and hip and an increased risk of ACL or knee injury. In addition, studies4,13,18,26 that have investigated ACL injury mechanisms through video analysis have found that most ACL tears occur on a single leg with the hip and knee in extension, and there is a lateral displacement of the trunk and a medial collapse of the knee. This combination of landing on a single leg and demonstrating biomechanical deficits proximal to the knee is deleterious and may result in an ACL injury.
The assessment of single-leg drop (SLD) landing mechanics is not included in the current return-to-sport criteria. While commonly used return-to-sport criteria include functional and strength symmetry, recent reports35,37 indicate that these symmetry measures may overestimate knee function and should not be assumed to imply normal landing biomechanics. Therefore, addressing movement-related SLD hip biomechanical deficits may improve knee function and help athletes to safely reintegrate into sports.
Aberrant biomechanics and faulty neuromuscular patterns are modifiable risk factors for ACL injuries. Neuromuscular training (NMT) and injury prevention programs that target the neuromuscular system can effectively correct movement deficits in athletes.12,23 In addition, uninjured athletes participating in preseason NMT programs have dramatically reduced their risk of noncontact ACL injuries and other lower extremity injuries.10,25,31 However, there is limited evidence of NMT programs being implemented in ACLR athletes before returning to sport. The overall aim of this study was to understand what the effect that an NMT program has on SLD hip biomechanics and neuromuscular control in a group of ACLR athletes. Secondarily, we sought to understand how the hip mechanics of ACLR athletes during a single-landing task differed from the mechanics of an uninjured control cohort after they completed the same training program. The primary hypothesis was that ACLR athletes after participating in an NMT would demonstrate greater hip flexion, increased hip excursions, lower hip flexion moments, and greater hip external rotation moment during an SLD. The second hypothesis was that the posttraining hip biomechanics during an SLD task would not differ between athletes after ACLR and a control group of uninjured athletes.
Methods
Athletes, Criteria to Participate in Study, and Self-Reported Questionnaire
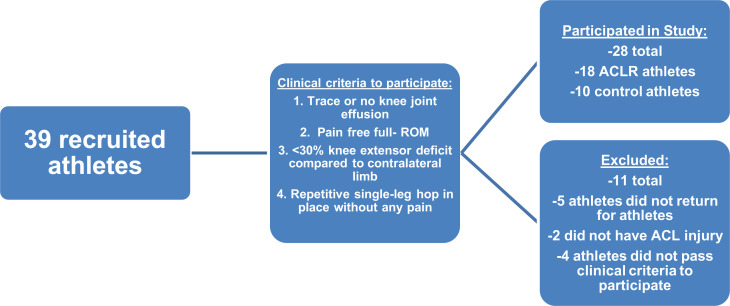
After approval from an institutional review board, we recruited 39 athletes for this study. Of these, 5 athletes did not return for posttraining biomechanics tests and were excluded from the study, 2 athletes were not included because they did not have an ACL injury, and 4 athletes did not pass the clinical examination to participate in the study; these patients were sent back for further rehabilitation and were not again considered for inclusion. Thus, 28 athletes (18 who had undergone ACLR and 10 control athletes) participated in the study. The ACLR athletes (8 male and 10 female—height: 1.7 ± 0.1 m; weight: 72.3 ± 1.53 kg; age: 19.4 ± 7.2 years) were approximately 8 months (7.7 ± 3.7 months) from ACLR, and the control athletes (4 male and 6 female—height: 1.6 ± 0.1 m; weight: 73.1 ± 24.4 kg; age: 16.4 ± 3.6 years) had no history of lower extremity injury. This information is summarized in Figure 1.
Figure 1.
Flowchart of the athletes who participated and were excluded from our study. ACL, anterior cruciate ligament; ACLR, ACL reconstruction; ROM, range of motion.
The ACLR athletes were recruited from the sports medicine clinic of The Ohio State University. A few of them were recommended to participate in the study by their clinical care team, which included a physician and/or physical therapist. The control athletes were recruited from high schools or sports teams that were covered by the department of sports medicine at the university. Written informed consent was received from all athletes over 18 years of age, and written assent and parental permission were received from athletes younger than 18 years. The athletes with ACL-injured knees underwent ACLR with a semitendinosus and gracilis tendon autograft performed by the same group of surgeons using standardized surgical techniques. Postoperative rehabilitation was completed at the same sports medicine clinic where the ACLR occurred.
All athletes participating in the study underwent a clinical examination by a licensed physical therapist or athletic trainer; the clinical examination included measurements of knee joint health and function. To participate in the study, each participant regardless of the cohort had to demonstrate (1) trace or no knee joint effusion,32 (2) pain-free full range of motion, (3) <30% knee extensor deficit compared with the contralateral limb during isokinetic testing, and (4) repetitive single-leg hop in place without any pain, proper total body control, and without excessive frontal plane collapse of the knee. Athletes who did not meet these criteria were sent back to postoperative rehabilitation to address their impairments. If an athlete demonstrated a lack of body control or excessive frontal plane motion of the knee, the testing clinician had the option of deeming the participant inappropriate for the biomechanics testing based on clinical judgment. Bilateral knee extensor isokinetic testing was completed using a dynamometer (Biodex Medical Systems) at 60 deg/s.
The 2000 International Knee Documentation Committee (IKDC) subjective knee evaluation form was given to all participating ACLR athletes before and after the training program during their visit to the laboratory for their biomechanics evaluation. The form consists of 10 questions that focus on symptoms, sports activities, and function.14 Each athlete with ACLR was asked to complete the questionnaire independently, and the research staff was available to answer questions.
Biomechanics Testing
A pre- and posttraining motion analysis assessment of an SLD task was conducted for all participants. A total of 55 retroreflective markers were placed on the athletes, as described previously,3 and a 12-camera motion capture system (Motion Analysis Corp) collected the motion profiles of the athletes completing the landing task. Marker trajectories were sampled at 240 Hz, and separate ground-reaction force data were collected for each limb at 1200 Hz.
The participating athletes completed 5 SLDs of each lower extremity off of a 30.5-cm plyometric box onto embedded force plates (Bertec Corp). The athletes were allowed at least 2 practice trials to get accustomed to the task. Researchers (C.N., S.W., R.T., A.C.) demonstrated the task, gave verbal commands on how to accomplish the task, and corrected the athletes during the practice trials. The verbal instructions were as follows: (1) the athlete was to stand at the edge of the plyometric box on the leg that they planned to land on, (2) their opposite leg as to be flexed and kept behind them so as not to interfere with the landing, and (3) the athlete was told to slightly hop off and land in front of the box and to stick the landing. These instructions were the same during the pre- and posttest. A trial was deemed successful if the athlete dropped off the box on 1 leg, landed onto the embedded floor plate, and stuck/held the landing for 2 seconds.
Neuromuscular Training
The NMT program included 12 training sessions focused on core stability, posterior chain muscle activation (gluteal and hamstring muscles), and controlled, soft landings. All of the sessions were completed by all study participants. The training program that was implemented in this study to address biomechanical deficits in ACLR athletes was described in detail by Di Stasi et al.8 In short, the program consisted of 7 exercise progressions, with each exercise progression having 4 levels of increasing difficulty. The progression to the next level of exercises was determined based on individual performance rather than training session number. Specifically, the ability of an athlete to progress to the next level of exercise difficulty was determined by the athletic trainer or strength and conditioning coach who administered the training. Because of these performance-based progression criteria, not all athletes had achieved the same performance levels for each progression by their 12th session.
Data Treatment and Statistical Analysis
The IKDC form was scored and totaled using a previously reported method.14 There was 1 missing value from one of the study participants, which was filled out during the participant’s pretraining biomechanics testing session. This was possibly due to an error in filling out the evaluation form, and the answer to the missing question was replaced by the average score of the other ACLR athletes’ answer to the same question. Given that IKDC scores were not normally distributed based on the goodness-of-fit test, a Wilcoxon rank-sum nonparametric test was used to evaluate the significant differences between the pretraining and posttraining IKDC scores.
Customized software was used to reduce and analyze the kinematic and kinetic data. Data collection was stopped after the athlete had completed a successful landing task and held that position for at least 2 seconds. Marker position gaps that were within the 24 consecutive frames during the SLD task were filled using a cubic spline function in Cortex—a motion capture software program (Cortex Version 4.1; Motion Analysis Corp). After all the markers were properly labeled and the gaps were filled, the marker position data and the ground-reaction force data were low-pass filtered using a bidirectional Butterworth filter at 12 and 50 Hz, respectively. Next, the marker position data were exported to calculate the kinematic and kinetic variables. These calculations were processed using custom codes in Visual 3D (C-motion Inc) and Matlab (Mathworks Inc). Hip kinematic variables were calculated using Cardan-Euler sequence for local coordinate systems (X-Y-Z). Hip kinetic variables were calculated using inverse dynamics. Hip joint excursions were calculated as the difference between peak and initial contact (IC). IC was defined as when the vertical component of the ground-reaction force exceeded 10 N. All data were time normalized to 100% of stance, which was from IC to final stabilization.
Hip kinematic and kinetic variables at IC, hip excursion, and peak vertical ground-reaction force during the SLD task are reported. The focus of the analysis was on hip biomechanical variables at IC because ACL injuries typically occur during the first 20-50 ms.18 Herein, all reported biomechanical variables are at IC, unless otherwise suggested, and all reported moments are external moments. Since IC is based on vertical ground-reaction force exceeding 10 N, we report peak vertical ground-reaction force.
First, to understand the effect of the NMT in ACLR athletes on hip kinematics and kinetics, a repeated-measures analysis of variance (ANOVA) was performed to assess the interactions and main effects of session (pre- and posttraining) and limb (involved and uninvolved). Second, to compare the posttraining hip kinematics and kinetics between the ACLR and control cohorts, a 2-way ANOVA was conducted to assess the interactions and main effects of group (ACLR and control) and limb (involved/dominant and uninvolved/nondominant). The dominant and nondominant limbs of the control group were matched with the involved and uninvolved limbs of the ACLR group, respectively. If significant interactions were found, post hoc paired and independent t tests were used to test for significant differences. The alpha level was set to .05 a priori to determine significant results.
Results
Study Participants and Self-Reported Function
There was a significant increase (P = .03) in IKDC scores from pretraining (84.93 ± 12.4) to posttraining (91.1 ± 6.3) for the ACLR athletes.
Effect of NMT on Single-Leg Landing Hip Biomechanics in ACLR Athletes
Table 1 summarizes the pre- and posttraining SLD hip kinematics and kinetics for the ACLR group. There were no significant session × limb interactions for hip flexion angle or hip excursion. However, a significant main effect of session (P = .01) was identified for hip excursion. The ACLR athletes went through greater hip excursion during the SLD task after participating in the training program. In addition, a significant main effect of limb (P < .01) was found for hip flexion angle. The involved limbs landed with greater hip flexion angle than the uninvolved limbs. There was no significant session × limb interaction for hip flexion moment. However, a significant main effect of session (P < .01) and limb (P = .03) was observed for hip flexion moment. The ACLR athletes landed with less hip flexion moment after training, and the involved limbs demonstrated less hip flexion moment than the uninvolved limbs.
Table 1.
Pre- and Posttraining Hip Kinematic and Kinetic Variables of ACLR Athletesa
| ACLR group | |||||
|---|---|---|---|---|---|
| Variable | Pretraining: Involved | Pretraining: Uninvolved | Posttraining: Involved | Posttraining: Uninvolved | Interactions and Main Effects: P |
| Hip flexion angle, deg | 26.2 ± 5.4 | 21.6 ± 6.7 | 26.6 ± 6.3 | 23.0 ± 5.8 | Limb: P = .0002 |
| Hip excursion, deg | 26.2 ± 9.7 | 25.3 ± 6.9 | 31.1 ± 9.3 | 30.7 ± 9.4 | Session: P = .01 |
| Hip flexion moment, N·m/kg | 0.54 ± 0.33 | 0.61 ± 0.39 | 0.17 ± 0.18 | 0.22 ± 0.15 | Session: P < .01 Limb: P = .03 |
| Hip abduction angle, deg | 10.4 ± 3.9 | 12.7 ± 3.5 | 11.6 ± 4.9 | 9.6 ± 2.8 | Session × Limb: P = .013 |
| Hip abduction moment, N·m/kg | 0.016 ± 0.16 | 0.09 ± 0.14 | 0.009 ± 0.09 | 0.04 ± 0.08 | Limb: P = .029 |
| Hip rotation moment, N·m/kg | 0.005 ± 0.04 | 0.055 ± 0.06 | 0.037 ± 0.05 | 0.023 ± 0.047 | Session × Limb: P = .015 |
| Peak vertical ground-reaction force, N/kg | 37.2 ± 8.2 | 37.0 ± 8.1 | 33.0 ± 5.3 | 32.6 ± 4.6 | Session: P = .003 |
aACLR, anterior cruciate ligament reconstruction.
For peak vertical ground-reaction force, there was no significant session × limb interaction, but there was a significant main effect of session (P = .003). After completing the training, the ACLR athletes landed with lower peak vertical ground-reaction force.
A significant session × limb interaction (P = .013) was observed for hip abduction angle. The post hoc analysis revealed a significant reduction (P < .001) of hip abduction angle for the uninvolved limbs from pre- to posttraining. There was no significant change (P = .25) of the involved limb hip abduction angle despite an increase from pre- to posttraining. There was no significant interaction of session × limb for hip abduction moment, but a main effect of limb (P = .015) was observed. The involved limbs demonstrated lower hip abduction moment than the uninvolved limbs.
Furthermore, there was a significant session × limb interaction (P = .015) for hip external rotation moment. After the training, the uninvolved limbs demonstrated significantly lower (P < .01) hip external rotation moment than the involved limbs. The involved limbs did not demonstrate a significant change (P = .06) from pre- to posttraining despite the involved limbs landing with more hip external rotation moment.
Posttraining Comparison of Hip Biomechanics Between the ACLR and Control Groups
There were no significant group × limb interactions for any of the kinematic or kinetic variables.
A significant main effect of group was observed for hip flexion angle (P < .001) and hip external rotation moment (P = .021). The ACLR athletes posttraining landed with greater hip flexion angle and hip external rotation moment compared with the control group of athletes. Otherwise, there were no other significant main effects of group or limb found for any of the other variables.
See Table 2 for a summary of the posttraining hip kinematic and kinetics for the athletes with ACLR and the control athletes.
Table 2.
Posttraining Hip Kinematic and Kinetic Variables of the ACLR and Control Groupa
| ACLR Group Posttraining | Control Group Posttraining | Interactions and Main Effects: P | |||
|---|---|---|---|---|---|
| Variable | Involved | Uninvolved | Dominant | Nondominant | |
| Hip flexion angle at initial contact, deg | 26.5 ± 6.3 | 23.0 ± 5.8 | 17.9 ± 5.2 | 18.1 ± 7.5 | Group: P < .001 |
| Hip excursion, deg | 31.1 ± 9.3 | 30.7 ± 9.4 | 25.9 ± 8.8 | 26.3 ± 7.4 | |
| Hip abduction angle at initial contact, deg | 11.6 ± 4.9 | 9.6 ± 2.8 | 11.2 ± 6.2 | 8.85 ± 5.5 | |
| Hip flexion moment at initial contact, N·m/kg | 0.39 ± 0.2 | 0.44 ± 0.2 | 0.43 ± 0.2 | 0.44 ± 0.1 | |
| Hip abduction moment at initial contact, N·m/kg | 0.32 ± 0.15 | 0.31 ± 0.13 | 0.32 ± 0.12 | 0.33 ± 0.12 | |
| Hip external rotation moment at initial contact, N·m/kg | 0.046 ± 0.07 | 0.035 ± 0.05 | 0.0001 ± 0.06 | 0.0027 ± 0.04 | Group: P = .021 |
| Peak vertical ground-reaction force, N/kg | 33.1 ± 5.4 | 32.6 ± 4.64 | 33.8 ± 5.6 | 35.5 ± 3.1 | |
aACLR, anterior cruciate ligament reconstruction.
Discussion
In this study, we found that ACLR athletes who had participated in a 12-session NMT program demonstrated significant improvements in SLD hip biomechanics compared with pretraining values, and posttraining SLD hip biomechanics were almost similar to those of a control group of athletes with no history of lower extremity injury.
The training program improved hip biomechanical deficits related to increased risk of ACL injury. Paterno et al29 found that a deficit in hip rotation moment during a bilateral jump-landing task independently predicted a second ACL injury—either a reinjury of the graft or a new injury to the intact, contralateral ACL—with excellent sensitivity and specificity in ACLR athletes. Furthermore, a 2018 systematic review and meta-analysis15 on lower extremity biomechanics during SLD after ACLR found that athletes demonstrate less flexion of the lower limbs, which results in stiff landing patterns during a unilateral landing task. Our study demonstrated that these biomechanical risk factors may be modified. While an increase in hip external rotation moment was observed from pre- to posttraining for the involved limbs of the cohort of ACLR athletes, this change did not reach statistical significance.
In addition, the ACLR athletes in our study demonstrated greater hip excursion and lower peak vertical ground-reaction force after participating in the training program, which possibly indicates that these athletes were avoiding these stiff landing patterns. These changes in hip biomechanics may protect the knee as these athletes transition back to sport. Deficits proximal to the knee have a significant effect on the biomechanical and neuromuscular control of the knee, including the forces absorbed by the joint.30 Focusing on addressing hip deficits before returning to sport may prevent further knee injuries in these athletes.
There has been a paucity of studies investigating the role of NMT or injury prevention programs in ACLR athletes. A majority of the focus of this work has been on preventing primary ACL injury in uninjured athletes. A 2018 meta-analysis34 of ACL injury reduction training programs found that these programs reduce the risk of ACL injuries by half in all athletes and reduce noncontact ACL injuries in female athletes by two-thirds. These training programs mitigate the risk of primary ACL injury by attenuating deleterious kinematic and kinetic movement patterns.12,21 The evidence from the current study indicates that an NMT program can alleviate biomechanical and neuromuscular risk factors in ACLR athletes and establish normative SLD hip biomechanics when compared with a group of healthy athletes. Implementing these training programs is imperative because ACLR athletes who return to sport are at a 30- to 40-times greater risk for an ACL injury than their uninjured counterparts.36 The evidence in the literature19,27,28,33 indicates that it is no longer acceptable to assume that ACLR athletes who complete postoperative rehabilitation and pass return-to-sport testing are at an equivalent or lower risk for a subsequent ACL injury than before the injury. Our current study, in combination with the unacceptably high risk of second ACL injuries, suggests that injury prevention research should shift its focus on these injured athletes who are returning to sport.
The high risk of second ACL injury has been associated with the pervasive biomechanical deficits reported in these athletes. Movement deficits and asymmetries persist for nearly 5 years after ACLR for higher demand activities such as unilateral or bilateral jump-landing tasks.6,7 Additionally, recent reviews9,16 on gait mechanics have found that athletes demonstrate altered ambulatory mechanics for at least 5 years or longer after ACLR. While no studies to date have investigated the effects of a training program on SLD biomechanics in a cohort of injured athletes, a recent systematic review and meta-analysis investigated the effect of injury prevention programs on the biomechanics of landing tasks in uninjured athletes.21 This review found that athletes after participating in a training program demonstrated greater hip flexion during these dynamic tasks. Despite the data in the current study not observing a significant change in hip flexion angle from pre- to posttraining, the athletes did demonstrate more hip joint excursion after training during the unilateral task. A training program that addresses modifiable risk factors such as biomechanical and neuromuscular deficits may potentially be a clinical intervention at the late stage of rehabilitation to safely return athletes back to sport.
There are some limitations to consider in this study. We required all athletes to pass a clinical examination before study participation. These criteria included trace or no knee joint effusion, pain-free active and passive knee range of motion, less than 30% knee extensor deficit compared with the uninvolved limb during a 60 deg/s isokinetic test, and consecutive single-leg hop in place without any pain or excessive frontal plane collapse of the knee. This may have selectively biased our cohort within our study, as we chose athletes who were the highest functioning within the ACLR population. However, these athletes still demonstrated a marked improvement in hip biomechanics during the SLD task. It not clear how the NMT program would have affected the ACLR athletes who did not pass the clinical examination, but we hypothesize that the training would have had a much greater effect if the clinical examination did not restrict them from participating.
The role of fatigue on landing biomechanics and ACL injury risk is controversial, and to understand the effect of fatigue was outside the scope of this study. The focus of this study was on SLD, and lack of a countermovement jump after landing does not re-create a change-of-direction task, which may have limited our results. We also did not track the progression of the athletes after their individual training session, which is something we hope to do in future studies. Another limitation is that we did not track reinjuries and return-to-sport rates among this cohort. This study only focused on the effect of the training on SLD biomechanics. The control athletes were also recruited from local high schools and sports teams that were covered by athletic trainers, physical therapists, and physicians from the medical center’s Sports Medicine Institute. These athletes were included in the study because recruitment and implementation of the training program was relatively easier. In addition, we did not present the data that monitored how far in each exercise progression the athletes progressed. The only requisite was the athletes completed the 12 NMT sessions. This suggests that regardless of how far each athlete progressed in the exercise progressions, the NMT program was still effective.
Conclusion
We demonstrated that an NMT program can effectively correct single-leg hip biomechanical and neuromuscular control in a group of ACLR athletes. Importantly, we also demonstrated that normative hip biomechanics during an SLD task can be established after participating in the training program. The results of this study indicate a potential role of a training program to address known hip biomechanical risk factors involved in ACL injury. Further research needs to be conducted to understand if this training program mitigates the risk of second ACL injury and prevents early breakdown of articular cartilage within the joint. The evidence within this study indicates that ACLR athletes returning to sport should seriously consider participating in an NMT program.
Acknowledgment
The authors acknowledge the staff of the Sports Medicine Research Institute at The Ohio State Wexner Medical Center, who helped with recruiting and patient follow-up.
Footnotes
Final revision submitted April 28, 2020; accepted May 12, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding for this study was received from the National Institutes of Health–National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant T32AR56950 to C.N.). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from The Ohio State University Biomedical Sciences Institutional Review Board (study No. 2011H0230).
References
- 1. Allen MM, Pareek A, Krych AJ, et al. Are female soccer players at an increased risk of second anterior cruciate ligament injury compared with their athletic peers? Am J Sports Med. 2016;44(10):2492–2498. [DOI] [PubMed] [Google Scholar]
- 2. Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48(21):1543–1552. [DOI] [PubMed] [Google Scholar]
- 3. Bates NA, Schilaty ND, Nagelli CV, Krych AJ, Hewett TE. Novel mechanical impact simulator designed to generate clinically relevant anterior cruciate ligament ruptures. Clin Biomech (Bristol, Avon). 2017;44:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boden BP, Torg JS, Knowles SB, Hewett TE. Video analysis of anterior cruciate ligament injury: abnormalities in hip and ankle kinematics. Am J Sports Med. 2009;37(2):252–259. [DOI] [PubMed] [Google Scholar]
- 5. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632–644. [DOI] [PubMed] [Google Scholar]
- 6. Delahunt E, Prendiville A, Sweeney L, et al. Hip and knee joint kinematics during a diagonal jump landing in anterior cruciate ligament reconstructed females. J Electromyogr Kinesiol. 2012;22(4):598–606. [DOI] [PubMed] [Google Scholar]
- 7. Delahunt E, Sweeney L, Chawke M, et al. Lower limb kinematic alterations during drop vertical jumps in female athletes who have undergone anterior cruciate ligament reconstruction. J Orthop Res. 2012;30(1):72–78. [DOI] [PubMed] [Google Scholar]
- 8. Di Stasi S, Myer GD, Hewett TE. Neuromuscular training to target deficits associated with second anterior cruciate ligament injury. J Orthop Sports Phys Ther. 2013;43(11):777–792 , A771-A711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gokeler A, Benjaminse A, van Eck CF, Webster KE, Schot L, Otten E. Return of normal gait as an outcome measurement in ACL reconstructed patients. A systematic review. Int J Sports Phys Ther. 2013;8(4):441–451. [PMC free article] [PubMed] [Google Scholar]
- 10. Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: Part 2. A meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34(3):490–498. [DOI] [PubMed] [Google Scholar]
- 11. Hewett TE, Myer GD. The mechanistic connection between the trunk, hip, knee, and anterior cruciate ligament injury. Exerc Sport Sci Rev. 2011;39(4):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765–773. [DOI] [PubMed] [Google Scholar]
- 13. Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600–613. [DOI] [PubMed] [Google Scholar]
- 15. Johnston PT, McClelland JA, Webster KE. Lower limb biomechanics during single-leg landings following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Sports Med. 2018;48(9):2103–2126. [DOI] [PubMed] [Google Scholar]
- 16. Kaur M, Ribeiro DC, Theis JC, Webster KE, Sole G. Movement patterns of the knee during gait following ACL reconstruction: a systematic review and meta-analysis. Sports Med. 2016;46(12):1869–1895. [DOI] [PubMed] [Google Scholar]
- 17. Khayambashi K, Ghoddosi N, Straub RK, Powers CM. Hip muscle strength predicts noncontact anterior cruciate ligament injury in male and female athletes: a prospective study. Am J Sports Med. 2016;44(2):355–361. [DOI] [PubMed] [Google Scholar]
- 18. Krosshaug T, Nakamae A, Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35(3):359–367. [DOI] [PubMed] [Google Scholar]
- 19. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551–1557. [DOI] [PubMed] [Google Scholar]
- 20. Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. [DOI] [PubMed] [Google Scholar]
- 21. Lopes TJA, Simic M, Myer GD, Ford KR, Hewett TE, Pappas E. The effects of injury prevention programs on the biomechanics of landing tasks: a systematic review with meta-analysis. Am J Sports Med. 2018;46(6):1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Myer GD, Chu DA, Brent JL, Hewett TE. Trunk and hip control neuromuscular training for the prevention of knee joint injury. Clin Sports Med. 2008;27(3):425–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Myer GD, Ford KR, Palumbo JP, Hewett TE. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J Strength Cond Res. 2005;19(1):51–60. [DOI] [PubMed] [Google Scholar]
- 24. Myklebust G, Holm I, Maehlum S, Engebretsen L, Bahr R. Clinical, functional, and radiologic outcome in team handball players 6 to 11 years after anterior cruciate ligament injury: a follow-up study. Am J Sports Med. 2003;31(6):981–989. [DOI] [PubMed] [Google Scholar]
- 25. Noyes FR, Barber Westin SD. Anterior cruciate ligament injury prevention training in female athletes: a systematic review of injury reduction and results of athletic performance tests. Sports Health. 2012;4(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. [DOI] [PubMed] [Google Scholar]
- 27. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42(7):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42–51. [DOI] [PubMed] [Google Scholar]
- 31. Sadoghi P, von Keudell A, Vavken P. Effectiveness of anterior cruciate ligament injury prevention training programs. J Bone Joint Surg Am. 2012;94(9):769–776. [DOI] [PubMed] [Google Scholar]
- 32. Sturgill LP, Snyder-Mackler L, Manal TJ, Axe MJ. Interrater reliability of a clinical scale to assess knee joint effusion. J Orthop Sports Phys Ther. 2009;39(12):845–849. [DOI] [PubMed] [Google Scholar]
- 33. Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):641–647. [DOI] [PubMed] [Google Scholar]
- 34. Webster KE, Hewett TE. Meta-analysis of meta-analyses of anterior cruciate ligament injury reduction training programs. J Orthop Res. 2018;36(10):2696–2708. [DOI] [PubMed] [Google Scholar]
- 35. Wellsandt E, Failla MJ, Snyder-Mackler L. Limb symmetry indexes can overestimate knee function after anterior cruciate ligament injury. J Orthop Sports Phys Ther. 2017;47(5):334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD. Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med. 2016;44(7):1861–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wren TAL, Mueske NM, Brophy CH, et al. Hop distance symmetry does not indicate normal landing biomechanics in adolescent athletes with recent anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2018;48(8):622–629. [DOI] [PubMed] [Google Scholar]
- 38. Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. Deficits in neuromuscular control of the trunk predict knee injury risk: a prospective biomechanical-epidemiologic study. Am J Sports Med. 2007;35(7):1123–1130. [DOI] [PubMed] [Google Scholar]
- 39. Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. The effects of core proprioception on knee injury: a prospective biomechanical-epidemiological study. Am J Sports Med. 2007;35(3):368–373. [DOI] [PubMed] [Google Scholar]