Abstract
Background
This study evaluates the mortality risk of patients with emergent large vessel occlusion (ELVO) and COVID-19 during the pandemic.
Methods
We performed a retrospective cohort study of two cohorts of consecutive patients with ELVO admitted to a quaternary hospital from March 1 to April 17, 2020. We abstracted data from electronic health records on baseline, biomarker profiles, key time points, quality measures and radiographic data.
Results
Of 179 patients admitted with ischemic stroke, 36 had ELVO. Patients with COVID-19 and ELVO had a higher risk of mortality during the pandemic versus patients without COVID-19 (OR 16.63, p = 0.004). An age-based sub-analysis showed in-hospital mortality in 60% of COVID-19 positive patients between 61-70 years-old, 66.7% in between 51-60 years-old, 50% in between 41-50 years-old and 33.3% in between 31-40 years old. Patients that presented with pulmonary symptoms at time of stroke presentation had 71.4% mortality rate. 27.3% of COVID-19 patients presenting with ELVO had a good outcome at discharge (mRS 0-2). Patients with a history of cigarette smoking (p = 0.003), elevated d-dimer (p = 0.007), failure to recanalize (p = 0.007), and elevated ferritin levels (p = 0.006) had an increased risk of mortality.
Conclusion
Patients with COVID-19 and ELVO had a significantly higher risk for mortality compared to COVID-19 negative patients with ELVO. A small percentage of COVID-19 ELVO patients had good outcomes. Age greater than 60 and pulmonary symptoms at presentation have higher risk for mortality. Other risk factors for mortality were a history of cigarette smoking, elevated, failure to recanalize, elevated d-dimer and ferritin levels.
Keywords: Ischemic stroke, COVID-19, emergent large vessel occlusion, outcomes, mechanical thrombectomy
Introduction
Although the novel coronavirus (COVID-19) is predominantly a disease affecting the lower respiratory tract, recent studies have also reported neurological findings associated with this disease.1,2 Two studies showed that cerebrovascular accidents (mainly ischemic stroke) were more common among severe COVID-19 patients.2,3 A recent report also from New York City described five cases of COVID-19 patients presenting with large vessel occlusion.4 Practicing stroke specialists are familiar with incorporating pre-functional status into clinical decision-making paradigms when considering emergent treatments such as intravenous (IV) tissue-plasminogen activator (tPA) or mechanical thrombectomy (MT).5 It is unclear how COVID-19 status effects mortality in patients with emergent large vessel occlusion (ELVO), and how this should guide clinical treatment decisions.
The aim of this study is to evaluate the impact of COVID-19 on patients with ELVO, as it relates to outcomes and mortality compared to patients without COVID-19 during the same epoch of time during the pandemic. Our hypothesis is that ELVO patients with COVID-19 have a higher rate of mortality compared to ELVO patients without COVID.
Methods
Study design and participants
This study was approved by our institutional review board. Patient informed consent was waived. The data that support the findings of the study are available from the corresponding author upon reasonable request. This retrospective cohort study included two cohorts of patients admitted to one of three major hospitals of our healthcare network including, an academic medical center and comprehensive stroke center, which accepts transfers for complex cases from eight community hospitals, during March 1 to April 17, 2020. All patients diagnosed with ELVO were included. ELVO was defined as acute focal neurological deficits with an associated occlusion of the internal carotid artery, M1 segment middle cerebral artery, M2 segment middle cerebral artery, or basilar artery with radiologic confirmation by computed tomography angiography (CTA). We also included patients with occlusions of anterior cerebral artery, posterior cerebral artery or vertebral artery and labelled them as “other” large vessel occlusion.
Case ascertainment and study variables
Cases were ascertained by primary and secondary ICD10 code for ischemic stroke and ELVO cases were identified through neuroradiology logs. Data abstracted from medical records included demographics, clinical variables, laboratory values, neuroimaging findings (initial Alberta Stroke Program Early CT Score [ASPECTS], location of ELVO, stroke treatment time points and results as they pertain to MT, outcome variables (hemorrhagic transformation, good functional (mRS 0–2), poor functional (mRS 3–5) outcome at discharge, and in-hospital mortality). Pulmonary symptoms at presentation were recorded and defined as fever, hypoxia, or prototypical abnormal CT chest findings.
COVID-19 screening and diagnosis
Cases were defined as positive by detection of viral RNA using real-time reverse transcriptase–polymerase chain reaction (RT-PCR) assay testing, performed within the hospital system or documented at an outside system prior to transfer. All patients admitted with a stroke diagnosis during the inclusion period were screened for COVID-19.
Statistical analysis
Baseline characteristics between groups, were compared performing parametric and non-parametric analyses with the t test, χ2 test, and Mann-Whitney U test as appropriate. No imputation was made for missing data. Unfavorable outcomes with χ2 test p < 0.05 were analyzed through univariate and multivariate logistic regression controlling for explanatory variables with p < 0.25 on univariate analysis was done if unadjusted model had a p < 0.05. Laboratory values were not considered for multivariate models due to missing data. All data analyses were conducted in IBM SPSS (vs26.0, Armonk, NY: IBM Corp).
Results
We found a total of 36 patients with ELVO during the entire study period, 36% (n = 13) tested positive for COVID-19 (Table 1). Patients with COVID-19 and ELVO had higher rates of in-hospital mortality (63% vs 9%, p = 0.001) (Table 1). They also had significantly higher mortality risk compared to patients without COVID-19 during the pandemic (OR: 16.63 95% CI: 2.47–111.79, p = 0.004) (Table 1), still significant when adjusted for age and comorbidities (OR: 15.13 95% CI: 2.08–110.05, p = 0.007).
Table 1.
COVID-19 positive versus negative for COVID-19 from March to April 2020.
| Variables | COVID-19 negative (n = 23) | COVID-19 positive (n = 13) | P-value |
|---|---|---|---|
| Age-yr., mean (SD) | 68.3 (13.69) | 63 (11.87) | 0.247# |
| Male sex, n (%) | 13 (56.5) | 5 (38.5) | 0.298 |
| Race | |||
| White, n (%) | 7 (30.4) | 1 (7.7) | 0.445 |
| African American, n (%) | 7 (30.4) | 5 (38.5) | |
| Hispanic, n (%) | 7 (30.4) | 6 (46.2) | |
| Other, n (%) | 2 (8.7) | 1 (7.7) | |
| Vascular risk factors | |||
| Hypertension, n (%) | 18 (78.3) | 9 (69.2) | 0.548 |
| Hyperlipidemia, n (%) | 12 (52.2) | 5 (38.5) | 0.429 |
| Diabetes, n (%) | 8 (34.8) | 6 (46.2) | 0.501 |
| Atrial fibrillation, n (%) | 4 (17.4) | 2 (15.4) | 0.877 |
| Congestive heart failure, n (%) | 3 (13) | 0 (0) | 0.174# |
| Smoking, n (%) | 3 (13) | 3 (23.1) | 0.646 |
| Clinical presentation† | |||
| mRS baseline, median (IQR) | 0 (0–2) | 0 (0–1) | 0.494 |
| NIHSS on admission, median (IQR) | 16 (12–20) | 16 (14–20) | 0.871 |
| ASPECTS on admission, median (IQR) | 7 (4–8) | 6 (3–8) | 0.960 |
| Laboratory values on admission‡ | |||
| White blood cell-count, median (IQR) per mm3 | 8.4 (6.4–11.3) | 7.4 (5.2–9.4) | 0.379 |
| Platelet-count, median (IQR) k per mm3 | 212 (188–249) | 249 (178–306) | 0.239 |
| Prothrombin time, median (IQR) sec | 13.9 (13.2–15.1) | 14.3 (13.6–15.6) | 0.345 |
| D-dimer, median (IQR) ng/ml | 1.51 (.50–8.07) | 20 (3.5–20) | 0.055 |
| Fibrinogen, median (IQR) | 449 (388–578.5) | 384 (198–561) | 0.610 |
| Ferritin, median (IQR) | 593.5 (339–679) | 1150 (640–2309) | 0.260 |
| Blood urea nitrogen, median (IQR) | 15 (11–18) | 12 (10–24) | 0.770 |
| Creatinine, median (IQR) | 1.00 (.80–1.2) | .70 (.60–.90) | 0.013 |
| Location of occlusion | |||
| Right MCA, n (%) | 6 (26.1) | 4 (30.8) | 0.947 |
| Left MCA, n (%) | 10 (43.5) | 5 (38.5) | 0.695 |
| Right ICA (with carotid T), n (%) | 1 (4.3) | 1 (7.7) | 0.626 |
| Left ICA (with carotid T), n (%) | 1 (4.3) | 1 (7.7) | 0.626 |
| Right ICA, n (%) | 2 (8.7) | 2 (15.4) | 0.355 |
| Left ICA, n (%) | 4 (17.4) | 1 (7.7) | 0.657 |
| Basilar, n (%) | 1 (4.3) | 0 (0) | 0.366 |
| Other LVO (ACA, PCA, Vertebral) | 3 (13) | 2 (15.4) | 0.606 |
| Treatment information¶ | |||
| Thrombolysis IV, n (%) | 3 (13) | 5 (38.5) | 0.078 |
| Thrombectomy, n (%) | 9 (39.1) | 3 (23.1) | 0.326 |
| Time points | |||
| Emergency patients | |||
| Onset to first hospital, mins, median (IQR) | 342 (149–988) | 870 (161–1560) | 0.621 |
| Onset to our institution, mins, median (IQR) | 604 (303–1160) | 579 (124–1707) | 0.655 |
| >6hs from onset to door, n (%) | 15 (65.2) | 3 (50) | 0.494 |
| Thrombectomy patients | |||
| Door to groin, mins, median (IQR) | 84 (68–110) | 99 (69–126) | 0.797 |
| Groin to recanalization, mins, median (IQR) | 38 (33–56) | 60 (31–68) | 0.727 |
| TICI score ≥2b, n (%) | 8 (88.9) | 2 (66.7) | 0.371 |
| Day 1 NIHSS, median (IQR) | 13 (8–21) | 4 (1–18) | 0.060 |
| Outcomes# | |||
| Hemorrhagic transformation, n (%) | 1 (4.3) | 2 (20) | 0.151 |
| Good outcomes (mRS 0-2), n (%) | 4 (19) | 3 (27.3) | 0.001 |
| Poor outcomes (mRS 3-5), n (%) | 15 (71.4) | 1 (9.1) | |
| In-hospital mortality, n (%) | 2 (9.5) | 7 (63.6) | |
|
Logistic regression |
Poor outcomes at discharge (mRS 3-5) |
In-hospital mortality |
|
| COVID-19 negative (reference) | Unadjusted OR [95% CI] P value | ||
| COVID-19 positive | .089 [.007–1.102] p = 0.060 | 16.63 [2.47-111.79] p = 0.004 | |
|
Multivariate Logistic regression |
In-hospital mortality |
||
| COVID-19 negative (reference) | Adjusted OR [95% CI] P value | ||
| COVID-19 positive | 15.13 [2.08–110.05] p = 0.007 | ||
*Included in multivariate logistic regression.
†Excluded for posterior circulation: ASPECTS 1/36 (2.8%).
‡Missing data: D-dimer 18/36 (50%), Fibrinogen 26/36 (72.2%), Ferritin 23/36 (68.9%), Prothrombin time 2/36 (5.6%).
#Missing data: discharge mRS 4/36 (11.1%).
¶Missing data: Onset to first hospital 5/30 (16.7%), Onset to our institution 1/30 (3.3%).
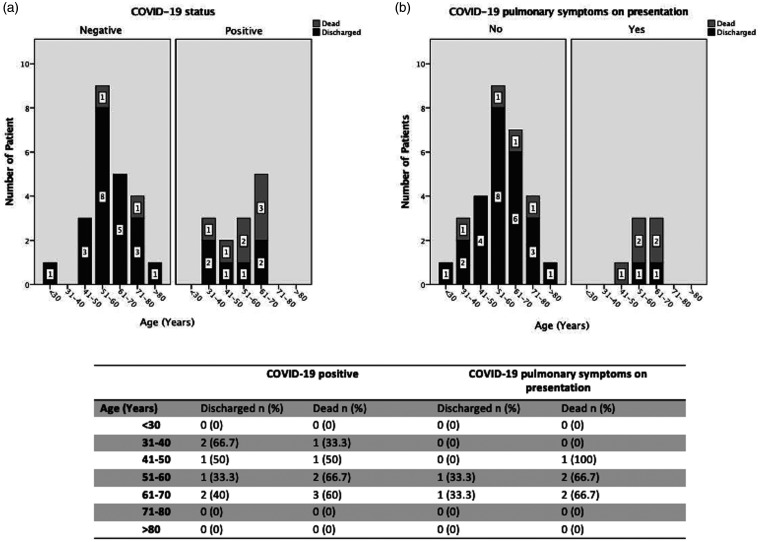
The age breakdown sub-analysis showed in-hospital mortality in 60% of COVID-19 positive patients between 61–70 years-old, 66.7% in between 51–60 years-old, 50% in between 41–50 years-old and 33.3% in between 31–40 years old (Figure 1(a)). 53.8% of the COVID-19 patients presented with pulmonary symptoms (Figure 1(b)). Within this group 71.4% of these patients expired (Figure 1(b).
Figure 1.
(a) Age breakdown for in-hospital mortality in patients tested positive for COVID-19 and (b) in patients with COVID-19 pulmonary symptoms on presentation.
Table 2.
Discharge and deaths breakdown by age in patients tested positive for COVID-19 and patients with pulmonary symptoms on presentations (Supplement to Figure 1(a) and (b)).
| COVID-19 positive |
COVID-19 pulmonary symptoms on presentation |
|||
|---|---|---|---|---|
| Age (years) | Discharged n (%) | Dead n (%) | Discharged n (%) | Dead n (%) |
| <30 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 31–40 | 2 (66.7) | 1 (33.3) | 0 (0) | 0 (0) |
| 41–50 | 1 (50) | 1 (50) | 0 (0) | 1 (100) |
| 51–60 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) |
| 61–70 | 2 (40) | 3 (60) | 1 (33.3) | 2 (66.7) |
| 71–80 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| >80 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
On univariate analysis for predictors for mortality, we also found that elevated d-dimer (median 20 ng/ml vs. 1.51 ng/ml, p = 0.007), a history of cigarette smoking (n = 5, 55.6% vs. n = 1, 3.7%, p = 0.003), increased ferritin (median 2426 ng/ml vs. 628 ng/ml, p = 0.006), failure to recanalize (TICI >2 b n = 1, 33.3% vs. n = 9, 100%) were all significantly associated with mortality (Table 2). On multivariate analysis pulmonary symptoms (OR 20.81 [3.26–133.03]), p = 0.001), and d-dimer (OR 1.21 [1.04–1.39], p = 0.012) were significant predictors of mortality (Table 3).
Table 2.
Univariate analysis of predictors of in-hospital mortality for patients with ELVO.
| Variables | Discharged (n = 27) | Dead (n = 9) | P value |
|---|---|---|---|
| Age-yr., mean (SD) | 65.89 (13.52) | 68 (12.6) | 0.683 |
| Male sex, n (%) | 14 (51.9) | 4 (44.4) | 0.700 |
| Pulmonary symptoms, n (%) | 3 (11.1) | 6 (66.7) | 0.001 |
| Race | |||
| White, n (%) | 6 (22.2) | 2 (22.2) | 0.118 |
| African American, n (%) | 11 (40.7) | 1 (11.1) | |
| Hispanic, n (%) | 7 (25.9) | 6 (66.7) | |
| Other, n (%) | 3 (11.1) | 0 (0) | |
| Vascular risk factors | |||
| Hypertension, n (%) | 23 (85.2) | 4 (44.4) | 0.015 |
| Hyperlipidemia, n (%) | 13 (48.1) | 4 (44.4) | 0.847 |
| Diabetes, n (%) | 9 (33.3) | 5 (55.6) | 0.236 |
| Atrial fibrillation, n (%) | 5 (18.5) | 1 (11.1) | 0.606 |
| Congestive heart failure, n (%) | 3 (11.1) | 0 (0) | 0.296 |
| Smoking, n (%) | 1 (3.7) | 5 (55.6) | 0.003 |
| Clinical presentation | |||
| mRS baseline, median (IQR) | 0 (0–1) | 0 (0–2) | 0.747 |
| NIHSS on admission, median (IQR) | 15 (10–20) | 19 (17–20) | 0.117 |
| ASPECTS on admission, median (IQR) | 7 (4–8) | 6 (2–8) | 0.446 |
| Laboratory values on admission | |||
| White blood cell-count, median (IQR) per mm3 | 8.4 (5.7–10.7) | 8.7 (5.2–10.5) | 0.943 |
| Platelet-count, median (IQR) k per mm3 | 217 (188–256) | 220 (178–250) | 0.858 |
| Prothrombin time, median (IQR) sec | 13.9 (13.5–15.1) | 13.8 (13.5–15.6) | 0.827 |
| D-dimer, median (IQR) ng/ml | 1.51 (.50–8.86) | 20 (19.3–20) | 0.007 |
| Fibrinogen, median (IQR) | 420 (362–485) | 379.5 (137–696.5) | 0.914 |
| Ferritin, median (IQR) | 628 (119–691) | 2426 (1729–3075) | 0.006 |
| Blood urea nitrogen, median (IQR) | 14 (10–18) | 23 (11–26) | 0.312 |
| Creatinine, median (IQR) | 1.00 (.70–1.2) | .80 (.60–.90) | 0.032 |
| Location of occlusion | |||
| Right MCA, n (%) | 8 (29.6) | 2 (22.2) | 0.667 |
| Left MCA, n (%) | 11 (40.7) | 4 (44.4) | 0.845 |
| Right ICA (with carotid T), n (%) | 2 (7.4) | 0 (0) | 0.401 |
| Left ICA (with carotid T), n (%) | 0 (0) | 2 (22.2) | 0.012 |
| Right ICA, n (%) | 3 (11.1) | 1 (11.1) | 1.0 |
| Left ICA, n (%) | 3 (11.1) | 2 (22.2) | 0.404 |
| Basilar, n (%) | 1 (3.7) | 0 (0) | 0.558 |
| Other LVO (ACA, PCA, Vertebral) | 3 (11.1) | 2 (22.2) | 0.151 |
| Treatment information | |||
| Thrombolysis i.v, n (%) | 6 (22.2) | 2 (22.2) | 1.0 |
| Thrombectomy, n (%) | 9 (33.3) | 3 (33.3) | 1.0 |
| Time points | |||
| Emergency patients | |||
| Onset to first hospital, mins, median (IQR) | 342.5 (149–988) | 870 (161–1312) | 0.767 |
| Onset to our institution, mins, median (IQR) | 582 (245–1136) | 998 (235–1577) | 0.716 |
| >6hs from onset to door, n (%) | 15 (62.5) | 3 (60) | 0.917 |
| Thrombectomy patients | |||
| Door to groin, mins, median (IQR) | 84 (68–99) | 126 (69–134) | 0.373 |
| Groin to recanalization, mins, median (IQR) | 38 (31–56) | 60 (36–68) | 0.482 |
| TICI score ≥2b, n (%) | 9 (100) | 1 (33.3) | 0.007 |
| Day 1 NIHSS, median (IQR) | 11 (6–12) | 19 (1–23) | 0.482 |
Table 3.
Multivariate analysis for predictors of in-hospital mortality.
| Multivariate analysis | OR [95% CI] P values |
|---|---|
| Pulmonary symptoms | 20.81 [3.26–133.03] p = 0.001 |
| Hypertension | .471 [.11–2.05] p = 0.316 |
| Current smoker | 1.71 [.27–10.97] p = 0.570 |
| Elevated d-dimer | 1.21 [1.04–1.39] p = 0.012 |
| Elevated Ferritin | 1.004 [.99–1.01] p = 0.143 |
| Elevated Creatinine | .622 [.027–14.39] p = 0.767 |
Discussion
We aimed to evaluate the effect of the COVID-19 on ELVO patients on patient outcomes with focus on mortality risk. We evaluated sociodemographic data, comorbidities, established treatment time parameters, established imaging parameters, neurologic outcomes, and mortality between the two groups.
The mortality for patients with ELVO and COVID-19 was extremely high (63.6%), however a minority did survive with good outcome (27.3%) (Table 1). Poor outcomes were significantly less in the COVID-19 positive group however it appears this is likely secondary to the fact that these patients who would otherwise survive with poor outcomes expired secondary to COVID-19. COVID-19 patients that presented with pulmonary symptoms had the highest risk of death (71.4%) (Figure 1). The mortality rate in this series was quite similar to a group in Paris.6 They concluded that COVID-19 was not primarily responsible for stroke during the pandemic. They also reported that 70% of stroke patients had mild or no respiratory symptoms. D-dimer levels were much higher in the COVID-19 positive group (20 ng/ml) versus the COVID-19 negative group (1.51 ng/ml), which did not reach clinical significance however given the small sample size is likely the reason as other studies have shown this value to be elevated in stroke patients with COVID-19. Elevated d-dimer along with ferritin was significantly associated with increase mortality on univariate analysis however only d-dimer remained significant on multivariate analysis. A history of cigarette smoking was also a predictor of mortality on univariate analysis however was not significant on multivariate analysis. This may be due to the small sample size as these findings are similar to previously reports in the literature.7 The presence or absence of gastrointestinal symptoms was not addressed in this cohort and is still an area of future research.
It is unclear how to incorporate COVID-19 status into the clinical decision making for evaluation of ELVO patients. Careful consideration is warranted in deciding to move forward with thrombectomy in patients with ELVO that also are presenting with pulmonary findings of COVID-19, and patients beyond the age of 60 as these patients have particularly high mortality rates (Figure 1). COVID-19 status alone should not preclude a patient from thrombectomy or thrombolysis, as we had ELVO patients survive from ages 30–70 with COVID-19, however careful counselling with family regarding the prognosis is warranted. The limitations of this study are its retrospective and single-center design, lack of long-term outcome follow up, and the sample size is small. Future clinical outcomes research is needed with larger sample sizes to help guide treatment paradigms.
Acknowledgements
In memorium to James T. Goodrich, MD PhD, beloved faculty member of Montefiore Medical Center, who passed away from COVID-19 during the pandemic.
Authorship
All authors made valuable contributions to the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclosures
The manuscript submitted does not contain information about medical device(s)/drug(s).
Ethical statement
The content of this study was approved by the Montefiore-Einstein IRB #2020-11430.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
David J Altschul https://orcid.org/0000-0002-5130-1378
Santiago R Unda https://orcid.org/0000-0001-7319-6794
Joseph Dardick https://orcid.org/0000-0003-4460-8731
References
- 1.Needham EJ, Chou SH, Coles AJ, et al. Neurological implications of COVID-19 infections. Neurocrit Care 2020; 32: 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry 2020; 91: 889–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med 2020; 382: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 6.Escalard S, Maïer B, Redjem H, et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19: experience from Paris. Stroke 2020; 51: 2540–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan YK, Goh C, Leow AST, et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis Epub ahead of print 13 July 2020. DOI: 10.1007/s11239-020-02228-y. [DOI] [PMC free article] [PubMed]