Abstract
Background:
Visceral adipose tissue (VAT) has a hazardous influence on systemic inflammation, insulin resistance and an adverse metabolic profile, which increases the risk of developing non-alcoholic fatty liver disease (NAFLD) and chronic complications of diabetes. In our study we aimed to evaluate the association of VAT and the triglyceride glucose (TyG) as a proxy of insulin resistance surrogated with metabolic and liver risk factors among subjects diagnosed with metabolic syndrome (MetS).
Methods:
A cross-sectional study was performed including 326 participants with MetS (55–75 years) from the PREDIMED-Plus study. Liver-status markers, VAT and TyG were assessed. Participants were stratified by tertiles according to VAT (n = 254) and TyG (n = 326). A receiver operating characteristic curve was used to analyse the efficiency of TyG for VAT.
Results:
Subjects with greater visceral fat depots showed worse lipid profile, higher homeostatic model assessment for insulin resistance (HOMA-IR), TyG, alanine transaminase (ALT), fibroblast growth factor-21 (FGF-21), fatty liver index (FLI) and hepatic steatosis index (HSI) compared with participants in the first tertile. The multi-adjusted linear-regression analyses indicated that individuals in the third tertile of TyG (>9.1−10.7) had a positive association with HOMA-IR [β = 3.07 (95% confidence interval (CI) 2.28−3.86; p trend < 0.001)], ALT [β = 7.43 (95% CI 2.23−12.63; p trend = 0.005)], gamma glutamyl transferase (GGT) [β = 14.12 (95% CI 3.64−24.61; p trend = 0.008)], FGF-21 [β = 190.69 (95% CI 93.13−288.25; p trend < 0.001)], FLI [β = 18.65 (95% CI 14.97−22.23; p trend < 0.001)] and HSI [β = 3.46 (95% CI, 2.23−4.68; p trend < 0.001)] versus participants from the first tertile. Interestingly, the TyG showed the largest area under the receiver operating curve (AUC) for women (AUC = 0.713; 95% CI 0.62−0.79) compared with men (AUC = 0.570; 95% CI 0.48−0.66).
Conclusions:
A disrupted VAT enlargement and impairment of TyG are strongly associated with liver status and cardiometabolic risk factors linked with NAFLD in individuals diagnosed with MetS. Moreover, the TyG could be used as a suitable and reliable marker estimator of VAT.
Keywords: insulin resistance, metabolic syndrome, non-alcoholic fatty liver disease, triglyceride glucose index, visceral adipose tissue
Introduction
The metabolic syndrome (MetS) encompasses a cluster of cardiometabolic features like impaired glucose metabolism, dyslipidaemia, abdominal obesity, and elevated blood pressure.1 The strong association between MetS and an increased risk of cardiovascular disease (CVD) as well as all-cause mortality is well documented.2,3 On the other hand, non-alcoholic fatty liver disease (NAFLD) is recognized as the hepatic manifestation of MetS4 that is related with insulin resistance and diabetes type 2 (T2DM).5 NAFLD is a highly prevalent chronic liver illness, whose incidence linearly increases with body mass index (BMI) and adiposity.6 This condition is quite common in obese individuals with central adiposity.7,8 The distribution of adipose tissue is of great importance since abdominal obesity is a key factor in the development of the MetS9 and NAFLD.10 Insulin resistance is considered the primary triggering mechanism for the development of T2DM, NAFLD and MetS when fat accumulates in intra-abdominal depots9,10 Thus, body-fat distribution in older adults is critical for determining how susceptible they are or will be to developing NAFLD and/or other CVD11–14 being partly attributed to sex differences in fat content.12,14 Central obesity is often quantified using waist circumference. But, it can be confounded by varying levels of subcutaneous fat in the waist, and may not accurately reflect visceral fat in all individuals.15 The dual-energy X-ray absorptiometry (DXA) is a practical and valuable tool to assess visceral fat mass.16 Nevertheless, the DXA equipment is expensive and might not be easy to access. In this sense, the identification of non-invasive markers able to discriminate subjects with higher visceral adiposity and higher susceptibility for developing NAFLD would be of great interest, as well as relating to T2DM complications. Indeed, liver biopsy is the gold standard for NAFLD diagnosis,7 but it is an invasive technique not suitable for routine screening and monitoring.7 Several non-invasive markers related to liver status and insulin resistance have been proposed in characterizing NAFLD.7,17–19 A novel potential marker is triglyceride glucose (TyG), which has demonstrated a better predictive value compared with fasting plasma glucose (FPG) for the risk of T2DM in normoglycaemic individuals, as well as being associated with insulin resistance.20 In the present study, the hypothesis was that subjects with a larger amount of VAT and increased TyG levels have higher susceptibility for showing adverse manifestations related to T2DM and development of NAFLD. Therefore, our primary objective was to assess the potential association of TyG with VAT, cardiometabolic risk factors, serum and NAFLD markers in overweight/obese individuals with MetS.
Materials and methods
Study population and design
This research is a cross-sectional study concerning baseline data from participants of the Navarra-Nutrition Centre within the PREDIMED-Plus trial (ISRCTN89898870; http://www.isrctn.com/ISRCTN89898870). PREDIMED-Plus is a multicentre, parallel-group, randomized trial carried out in Spain, aiming to evaluate the effectiveness of an energy-restricted traditional Mediterranean diet, physical activity promotion and behavioural support (intervention group) on the primary prevention of CVD, in comparison with general advised energy-unrestricted Mediterranean diet (control group). Detailed methods and protocols of the study have been published previously.21,22 In brief, 6874 individuals were recruited in 23 Spanish centres. Eligible participants were men (55–75 years) and women (60–75 years) with a BMI ⩾27 kg/m2 and <40 kg/m2 and fulfilling at least three criteria for the MetS: waist circumference (WC) in White people ⩾102 cm for men and ⩾88 cm for women, elevated triglycerides levels ⩾150 mg/dl or drug treatment for hyperlipidemia; reduced high-density lipoprotein cholesterol (HDL-c) <40 mg/dl in men and <50 mg/dl in women or drug treatment; elevated blood pressure systolic ⩾130 mmHg and/or diastolic ⩾85 mmHg or current use of antihypertensive medication; elevated fasting glucose ⩾100 mg/dl or drug treatment, according to guidelines from the International Diabetes Federation/National Heart, Lung and Blood Institute/American Heart Association (2009).23 As described elsewhere, exclusion criteria included a background of alcohol overuse, liver injury, history of previous CVD, gastrointestinal or other disorders, infectious processes, therapy with immunosuppressive drugs, cytotoxic agents or systemic corticosteroids. The protocol and procedures were approved by the Research Ethic Committee for clinical investigations of the University of Navarra (053/2013) according to the Declaration of Helsinki. All participants provided written informed consent. At Navarra-Nutrition Centre, 331 were included in the study, of which 326 participants had available data to calculate TyG, and 254 patients were assessed by DXA.
Study assessment
Clinical and biochemical measurements
At baseline, participants completed an administered survey, which included questions about socio-demographic characteristics, lifestyle behaviours, disease history and medication. Smoking habits were classified into ‘never’, ‘former’ or ‘current smoker’, as described elsewhere.21 Blood pressure was measured in triplicate using a validated semiautomatic oscillometer (Omron HEM-705CP, Netherlands). T2DM was established as previous diagnosis of diabetes or glycated haemoglobin (HbA1c) ⩾6.5%, use of antidiabetic medication or fasting glucose ⩾126 mg/dl according to the American Diabetes Association guidelines.24 After overnight fasting for at least 12 h, a blood sample was obtained from each participant. Serum and plasma were collected and frozen at −80°C. All biochemical measurements, including plasma glucose, HbA1c, insulin, total cholesterol, HDL-c, triglyceride, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT) were performed using standard laboratory enzymatic methods and following validated protocols.21 The fibroblast growth factor 21 (FGF-21) plasma concentrations were measured using human FGF-21 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA) with an autoanalyzer system (Triturus, Grifols SA, Barcelona, Spain) following the manufacturer’s instructions. Low-density lipoprotein cholesterol (LDL-c) concentration was calculated by Friedewald’s formula and the very-low-density lipoprotein cholesterol (VLDL-c) was calculated as triglycerides / 5.25 Also, homeostatic model assessment for insulin resistance (HOMA-IR) was calculated according to the formula: fasting insulin (mIU/l) × fasting glucose (nmol/l)/22.5.26
Dietary variables
Trained dietitians face-to-face administered a semi-quantitative 143-item food-frequency questionnaire to estimate energy intake and alcohol consumption.27 Also, a 17-item questionnaire was implemented, which is a modified version of the previously validated questionnaire used in the PREDIMED study to assess the participant’s adherence to the Mediterranean diet.28
Physical activity measurement
Physical activity was assessed using the short Registre Gironi del Cor questionnaire that showed high reliability and sensitivity in detecting changes in moderate and vigorous intensity.29,30 This tool was validated in the Spanish adult population, which is a version of the Minnesota Leisure Time.29 This questionnaire evaluated the total energy expenditure in leisure-time physical activity using Metabolic Equivalent Tasks (METs) in minutes/week. Physical activities were classified into light-intensity (<4 METs), moderate intensity (4.0–5.5 METs), and vigorous intensity (⩾6 METs) as detailed in the report.29 Sedentary lifestyles were evaluated using a validated Nurses’ Health Study questionnaire.31 For the present study, physical activity was expressed as MET hours/week.
Anthropometry and body composition measurements
Anthropometric measurements were performed by trained dietitians following standardized PREDIMED-Plus protocols.21 Weight, height and waist circumference (WC), were measured using a calibrated scale, a stadiometer and an anthropometric tape, respectively. BMI was conventionally calculated as weight in kilograms divided by the height in square metres (kg/m2). VAT was estimated using dual-energy X-ray absorptiometry (Lunar iDXA™, software version 6.0, Madison, WI, USA) connected with enCore™ software, which was assessed by trained operators according to standard procedures supplied by the manufacturer.
Non-invasive markers
TyG is a newly described marker reported as a useful screening tool for surrogated insulin resistance,20,32,33 NAFLD,34 and as an early predictor of MetS features.35 This marker was calculated using biochemical data according to the following formula (Equation 1):32,36
| Equation 1. |
The hepatic steatosis index (HSI; Equation 2) was validated in a cohort of patients with NAFLD diagnosed by ultrasonography.37
| Equation 2. |
HSI was also computed to estimate liver status. Another liver marker as an indicator of NAFLD is the fatty liver index (FLI), which was calculated as previously described (Equation 3)39 by:
| Equation 3. |
Statistical analyses
We retrospectively estimated the sample size to find differences between groups with a precision of 0.40 and a standard deviation (SD) of 0.5, and α = 0.05. The statistical power of the study was 90%. Continuous variables are presented as means ± SD and categorical variables as numbers (n) and percentages (%). One-way analysis of variance and Chi-square tests or Fisher’s exact test for categorical variables were used to assess differences between groups, as appropriate. The analysis of covariance test after adjustment was used for the following potential confounders: age (years), physical activity (MET hours/week), energy intake (kcal/d), alcohol intake (g) and smoking status (never, former, current). Bonferroni correction was applied to assess differences in metabolic and liver parameters according to sex-specific VAT tertiles. VAT for men: T1 (1.29 to ⩽2.42), T2 (>2.42 to ⩽3.10), T3 (>3.10 to 5.45); VAT for women: T1 (0.77 to ⩽1.60), T2 (>1.60 to ⩽2.06), T3 (>2.06 to 3.59). Crude and multiple linear regression models adjusted by age (years), sex (male and female), physical activity (MET hours/week), energy intake (kcal/d), alcohol intake (g) and smoking status (never, former, current) were fitted to statistically analyse the association between NAFLD biomarkers and tertiles of TyG. Tests of linear trend were assessed assigning the median value of each tertile of TyG and then using it as a continuous variable and correlation was assessed using the Pearson’s coefficient. The area under the receiver operating characteristics (ROC) curve (AUC) was performed to quantify the value of TyG as a predictor of VAT, considering as reference values the 50th percentile of VAT by sex. All tests were two sided, and cut-off level of significance was defined as 0.05. Statistical analyses were carried out with Stata 12.0 software (StataCorp LP, College Station, TX, USA).
Results
Study sample characteristics
Baseline characteristics of men and women according to VAT sex-specific tertiles are summarized in Table 1. As expected, BMI and WC increased across VAT tertiles. No significant differences were found in the frequency of diabetes, hypertension and smoking habits among tertiles in both sexes. Likewise, blood pressure [systolic blood pressure (SBP) and diastolic blood pressure (DBP)] measurements, energy intake, alcohol consumption, adherence to the Mediterranean diet score and physical activity did not differ statistically.
Table 1.
Clinical and lifestyle characteristics of subjects with MetS according to tertiles of VAT by sex.
| Tertiles of visceral adipose tissue (kg) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Men (n = 133) |
Women (n = 121) |
|||||||
| T1 |
T2 |
T3 |
p value* | T1 |
T2 |
T3 |
p value* | |
| (n = 45) |
(n = 44) |
(n = 44) |
(n = 41) |
(n = 40) |
(n = 40) |
|||
| (1.29 to ⩽2.42) | (>2.42 to ⩽3.10) | (>3.10 to 5.45) | (0.77 to ⩽1.60) | (>1.60 to ⩽2.06) | (>2.06 to 3.59) | |||
| Age (years) | 63.9 ± 5.8 | 64.5 ± 5.8 | 64.6 ± 5.1 | 0.818 | 67.7 ± 3.5 | 67.0 ± 4.2 | 67.5 ± 4.4 | 0.751 |
| BMI (kg/m2) | 30.0 ± 2.2 | 31.5 ± 2.2 | 34.0 ± 2.9 | <0.001 | 30.6 ± 2.9 | 32.4 ± 3.6 | 34.4 ± 3.2 | <0.001 |
| WC (cm) | 103.5 ± 5.8 | 109.5 ± 6.2 | 117.3 ± 7.6 | <0.001 | 97.1 ± 6.5 | 102.8 ± 6.9 | 109.5 ± 7.2 | <0.001 |
| VAT (kg) | 2.0 ± 0.3 | 2.8 ± 0.2 | 3.8 ± 0.5 | <0.001 | 1.3 ± 0.2 | 1.8 ± 0.1 | 2.5 ± 0.4 | <0.001 |
| Diabetes, n (%) | 19 (42.2) | 16 (36.4) | 17 (38.6) | 0.849 | 10 (24.4) | 16 (40.0) | 19 (47.5) | 0.089 |
| Hypertension, n (%) | 40 (88.9) | 42 (95.5) | 42 (95.5) | 0.362 | 39 (95.1) | 38 (95.0) | 37 (92.5) | 0.851 |
| SBP (mmHg) | 147.6 ± 18.5 | 142.3 ± 13.8 | 143.4 ± 14.7 | 0.250 | 145.8 ± 16.9 | 143.5 ± 15.8 | 142.0 ± 16.2 | 0.578 |
| DBP (mmHg) | 88.5 ± 9.9 | 88.6 ± 8.5 | 88.0 ± 6.7 | 0.941 | 85.1 ± 8.9 | 87.6 ± 9.9 | 86.7 ± 8.6 | 0.455 |
| Smoking habits, n (%) | 0.092 | 0.677 | ||||||
| Never smoker | 12 (26.7) | 7 (15.9) | 4 (9.1) | 30 (73.2) | 23 (57.5) | 25 (62.5) | ||
| Former smoker | 23 (51.1) | 26 (59.1) | 34 (77.3) | 8 (19.5) | 13 (32.5) | 11 (27.5) | ||
| Current smoker | 10 (22.2) | 11 (25.0) | 6 (13.6) | 3 (7.3) | 4 (10.0) | 4 (10.0) | ||
| Alcohol intake (g/d) | 15.2 ± 14.3 | 18.5 ± 20.3 | 23.3 ± 20.6 | 0.119 | 2.4 ± 6.2 | 5.3 ± 8.3 | 2.6 ± 4.9 | 0.102 |
| Energy intake (kcal/d) | 2655.4 ± 580.2 | 2670.0 ± 439.7 | 2789.0 ± 550.2 | 0.428 | 2451.5 ± 549.5 | 2407.0 ± 511.9 | 2474.2 ± 413.4 | 0.827 |
| Adherence to MedDiet (0–17 points) | 9 ± 2.7 | 8.5 ± 2.2 | 8.8 ± 2.5 | 0.636 | 9.2 ± 2.5 | 9.0 ± 2.8 | 9.4 ± 2.5 | 0.767 |
| Physical activity (MET hours/week) | 59.0 ± 57.6 | 61.2 ± 49.6 | 46.9 ± 41.1 | 0.356 | 43.2 ± 33.6 | 39.4 ± 32.9 | 36.0 ± 26.5 | 0.588 |
p value for differences between tertiles of visceral fat mass by sex was calculated by Chi-square, Fisher’s exact test or ANOVA, as appropriate.
p < 0.05 is considered statistically significant. Data are expressed as mean ± SD.
ANOVA, analysis of variance; BMI, body mass index; DBP, diastolic blood pressure; MedDiet, Mediterranean diet; MET, metabolic equivalent; MetS, metabolic syndrome; SBP, systolic blood pressure; SD, standard deviation; WC, waist circumference; VAT, visceral adipose tissue.
Crosstalk between VAT, TyG and NAFLD risk factors
Anthropometric, metabolic profile and liver status of participants are reported in Table 2. The adjusted analysis revealed that BMI and WC were significantly increased through VAT tertiles specific by sex. Moreover, insulin, TyG and HOMA-IR increased with VAT tertiles reaching statistical differences among them (all p < 0.05). Glucose and HbA1c did not show differences between tertiles. As concerns lipid markers, the T3 group presented significantly higher levels of VLDL-c [mean 32.4 mg/dl (95% CI 29.7−35.2)], triglycerides [mean 162.1 mg/dl (95% CI 148.3−175.9)] and triglyceride (TG)/HDL-c ratio [mean 3.8 mg/dl (95% CI 3.4−4.3)] than T1 participants, while no associations were found regarding total cholesterol, LDL-c and HDL-c serum levels. Participants in the highest VAT tertile showed significantly higher ALT levels, HSI and FLI scores as compared with subjects in the lowest tertile of VAT. No significant differences were found in AST and FGF-21 levels in VAT tertiles.
Table 2.
Anthropometric, body composition, metabolic profile and liver status in subjects with MetS according to VAT sex-specific tertiles.
| Tertiles of visceral adipose tissue (kg) |
p value | |||
|---|---|---|---|---|
| T1 |
T2 |
T3 |
||
| (n = 86) | (n = 84) | (n = 84) | ||
| Men | (1.29 to ⩽2.42) | (>2.42 to ⩽3.10) | (>3.10 to 5.45) | |
| Women | (0.77 to ⩽1.60) | (>1.60 to ⩽2.06) | (>2.06 to 3.59) | |
| Total | (0.77 to 2.42) | (>1.60 to 3.10) | (>2.06 to 5.45) | |
| Anthropometric and body composition | ||||
| BMI (kg/m2) | 30.3 (29.7−30.9)a,b,c | 32.0 (31.4−32.6)b,c | 34.1 (33.5−34.8) | <0.001 |
| WC (cm) | 101.0 (99.5−102.6)a,b,c | 106.3 (104.8−107.8)b,c | 113.1 (111.5−114.6) | <0.001 |
| VAT (kg) | 1.7 (1.6−1.8)a,b,c | 2.3 (2.2−2.4)b,c | 3.1 (3.0−3.2) | <0.001 |
| Glucose profile | ||||
| Glucose (mg/dl) | 115.1 (108.0−122.2) | 118.2 (111.2−125.2) | 123.6 (1116.6−130.6) | 0.247 |
| HbA1c (%) | 6.0 (5.8−6.2) | 6.2 (6.0−6.4) | 6.2 (6.0−6.5) | 0.281 |
| TyG | 8.8 (8.7−8.9)a,b,c | 9.0 (8.9−9.1) | 9.1 (9.0−9.2) | 0.001 |
| Insulin (mU/l) | 10.2 (8.5−11.9)a,b,c | 13.8 (12.1−15.4) | 16.5 (14.9−18.2) | <0.001 |
| HOMA-IR | 2.9 (2.3−3.4)a,b,c | 4.1 (3.5−4.6) | 5.0 (4.5−5.5) | <0.001 |
| Lipid profile | ||||
| Total cholesterol (mg/dl) | 198.0 (190.2−205.8) | 201.2 (193.4−209.0) | 205.6 (197.7−213.5) | 0.411 |
| LDL-c (mg/dl) | 125.5 (118.5−132.5) | 125.8 (118.7−132.9) | 129.1 (121.8−136.4) | 0.747 |
| HDL-c (mg/dl) | 47.8 (45.6−49.9) | 45.5 (43.3−47.6) | 45.9 (43.7−48.1) | 0.315 |
| VLDL-c (mg/dl) | 25.0 (22.2−27.7)a,b,c | 30.2 (27.4−32.9) | 32.4 (29.7−35.2) | <0.001 |
| Triglycerides (mg/dl) | 124.8 (111.0−138.5)a,b,c | 150.9 (137.2−164.5) | 162.1 (148.3−175.9) | <0.001 |
| TG/HDL-c ratio | 2.9 (2.4−3.3)a,c | 3.6 (3.1−4.0) | 3.8 (3.4−4.3) | 0.008 |
| Liver status | ||||
| ALT (U/l) | 23.3 (19.1−27.4)a,c | 29.2 (25.1−33.3) | 32.0 (27.9−36.1) | 0.013 |
| AST (U/l) | 21.8 (19.0−24.6) | 24.2 (21.4−26.9) | 25.0 (22.2−27.7) | 0.268 |
| GGT (U/l) | 37.2 (28.7−45.6) | 46.8 (38.5−55.2) | 40.7 (32.2−49.2) | 0.272 |
| FGF-21 (pg/ml)* | 378.5 (294.5−462.5) | 484.7 (403.8−565.6) | 430.5 (346.7−514.4) | 0.207 |
| FLI (arbitrary units) | 66.6 (63.9−69.3)a,b,c | 79.8 (77.1−82.5)b,c | 86.9 (84.2−89.6) | <0.001 |
| HSI (arbitrary units) | 40.2 (39.4−41.1)a,b,c | 43.2 (42.2−44.1)b,c | 45.9 (45.1−46.8) | <0.001 |
FGF-21 available in 211 patients.
p < 0.05 is considered statistically significant. Data are expressed as mean (95% CI). Variables were adjusted by age (years), physical activity (MET hours/week), energy intake (kcal/d), alcohol intake (g) and smoking status (never, former, current).
Data is stratified by VAT sex- specific tertiles.
Significant differences between T1 vs T2.
Significant differences between T1 vs T3.
Significant differences between T2 vs T3.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; FGF-21, fibroblast growth factor- 21; FLI, fatty liver index; GGT, gamma-glutamyl transferase; HbA1c, glycated haemoglobin A1c; HDL-c, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment for insulin resistance; HSI, hepatic steatosis index; LDL-c, low-density lipoprotein cholesterol; MET, Metabolic Equivalent Task; MetS, metabolic syndrome; TG/HDL ratio, triglycerides/high-density lipoprotein ratio; TyG, triglyceride glucose; VAT, visceral adipose tissue; VLDL-c, very-low-density lipoprotein cholesterol; WC, waist circumference.
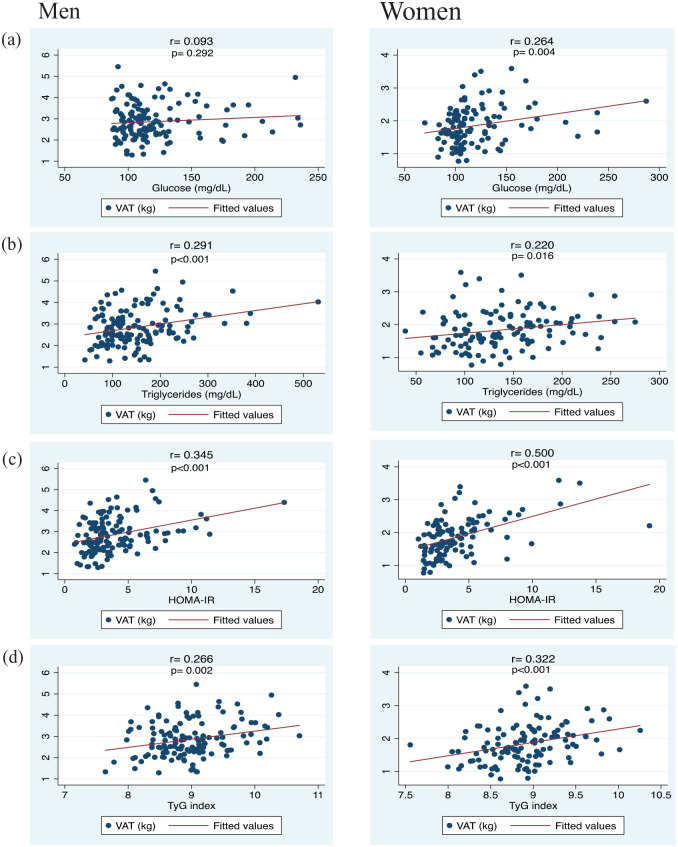
The association of TyG with variables related to liver health was explored (Table 3). Linear regression models were fitted considering NAFLD-related markers as dependent factors and TyG as the independent variable (Table 3). A fully adjusted model revealed that individuals in the third TyG tertile (>9.1−10.7) were significantly associated with higher WC (β = 2.62; 95% CI 0.41–4.84, p for trend = 0.020), HOMA-IR (β = 3.07; 95% CI 2.28–3.86, p for trend < 0.001), ALT (β = 7.43; 95% CI 2.23–12.63, p for trend = 0.005), GGT (β = 14.12; 95% CI 3.64–24.61, p for trend = 0.008), FGF-21 levels (β = 190.69; 95% CI 93.13–288.25, p for trend < 0.001), FLI units (β = 18.65; 95% CI 14.97–22.33, p for trend < 0.001), HSI units (β = 3.46; 95% CI 2.23–4.68, p for trend < 0.001) than participants in the first TyG tertile. Furthermore, variables associated with glucose–insulin homeostasis were significantly correlated with VAT except for men in glucose levels (Figure 1). Glucose (men: r = 0.093, p = 0.292; women: r = 0.264, p = 0.004) [Figure 1(a)], triglycerides (men: r = 0.291, p < 0.001; women: r = 0.220, p = 0.016) [Figure 1(b)], HOMA-IR (men: r = 0.345, p < 0.001; women: r = 0.500, p < 0.001) [Figure 1(c)], and TyG (men: r = 0.266, p = 0.002; women: r = 0.322, p < 0.001) [Figure 1(d)].
Table 3.
Multivariable linear regression analyses evaluating the association between TyG tertiles as independent variable and liver status as dependent variable.
| Tertiles of TyG |
p for trend | |||
|---|---|---|---|---|
| T1 |
T2 |
T3 |
||
| (n = 109) |
(n = 110) |
(n = 107) |
||
| (7.3−8.7) |
(>8.7−9.1) |
(>9.1−10.7) |
||
| β estimates (95% CI) | β estimates (95% CI) | β estimates (95% CI) | ||
| WC (cm) | ||||
| Crude | (0 Ref.) | 1.32 (−1.06 to 3.71) | 3.21 (0.81–5.61) | 0.009 |
| Multivariable adjusted | (0 Ref.) | 1.81 (−0.37 to 3.99) | 2.62 (0.41–4.84) | 0.020 |
| HOMA-IR | ||||
| Crude | (0 Ref.) | 1.21 (0.43–1.99) | 3.09 (2.31–3.88) | <0.001 |
| Multivariable adjusted | (0 Ref.) | 1.25 (0.48–2.01) | 3.07 (2.28–3.86) | <0.001 |
| ALT (U/l) | ||||
| Crude | (0 Ref.) | 3.79 (−1.48 to 9.07) | 8.14 (2.83–13.45) | 0.003 |
| Multivariable adjusted | (0 Ref.) | 4.79 (−0.32 to 9.90) | 7.43 (2.23–12.63) | 0.005 |
| AST (U/l) | ||||
| Crude | (0 Ref.) | 1.84 (−1.51 to 5.18) | 2.56 (−0.80 to 5.93) | 0.137 |
| Multivariable adjusted | (0 Ref.) | 2.29 (−1.00 to 5.57) | 1.98 (−1.36 to 5.33) | 0.246 |
| GGT (U/l) | ||||
| Crude | (0 Ref.) | 3.06 (−7.39 to 13.51) | 16.72 (6.17–27.26) | 0.002 |
| Multivariable adjusted | (0 Ref.) | 4.07 (−6.21 to 14.34) | 14.12 (3.64–24.61) | 0.008 |
| FGF-21 (pg/ml) * | ||||
| Crude | (0 Ref.) | 92.45 (−2.17 to 187.07) | 195.11 (100.49–289.73) | <0.001 |
| Multivariable adjusted | (0 Ref.) | 91.26 (−5.09 to 187.62) | 190.69 (93.13–288.25) | <0.001 |
| FLI (arbitrary units) | ||||
| Crude | (0 Ref.) | 11.18 (7.46–14.90) | 19.60 (15.84–23.36) | <0.001 |
| Multivariable adjusted | (0 Ref.) | 11.78 (8.18–15.39) | 18.65 (14.97–22.33) | <0.001 |
| HSI (arbitrary units) ** | ||||
| Crude | (0 Ref.) | 1.76 (0.52–3.00) | 3.35 (2.11–4.60) | <0.001 |
| Multivariable adjusted | (0 Ref.) | 1.95 (0.75–3.16) | 3.46 (2.23–4.68) | <0.001 |
FGF-21 available in 278 patients.
Adjusted for all variables except for sex.
p < 0.05 was considered statistically significant. Data are expressed as mean (95% CI). Models were adjusted by age (years), sex (male and female), physical activity (MET hours/week), energy intake (kcal/d), alcohol intake (g) and smoking status (never, former, current).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; FGF-21, fibroblast growth factor-21; FLI, fatty liver index; GGT, gamma-glutamyl transferase; HOMA-IR, homeostatic model assessment for insulin resistance; HSI, hepatic steatosis index; MET, Metabolic Equivalent Task; Ref., reference; TyG, triglyceride glucose; WC, waist circumference.
Figure 1.
Correlations between VAT and parameters related to glucose and insulin homeostasis in subjects with MetS according to sex.
MetS, metabolic syndrome; VAT, visceral adipose tissue.
Receiver operating characteristic (ROC) analyses for TyG to predict VAT
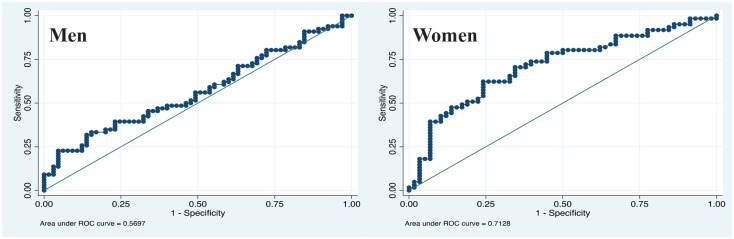
ROC curves were applied to assess the capacity of TyG to identify elevated VAT accumulation in both sexes (Figure 2). The AUCs of the TyG for prediction of VAT was 0.570 (95% CI 0.48–0.66) for men and 0.713 (95% CI 0.62–0.79) for women.
Figure 2.
Receiver operating characteristics (ROC) curve analysis of predictive value of the TyG in subjects with MetS according to sex.
VAT cut-off men: ⩾2.777 kg; VAT cut-off women: ⩾1.748 kg.
MetS, metabolic syndrome; TyG, triglyceride glucose; VAT, visceral adipose tissue.
Discussion
In this translational study, VAT and TyG were associated with relevant liver and cardiometabolic risk factors linked to NAFLD and insulin resistance in subjects with MetS. Moreover, TyG could be a reliable indicator of visceral fat mass. Many metabolic abnormalities related to insulin resistance often occur in obese individuals with higher amount of VAT.9,40 The link between altered VAT triggering with a disorder in glucose and insulin metabolism may appear to be a driving factor in T2DM and NAFLD.4,5
Interestingly, TyG and atherogenic lipid profiles (VLDL-c, triglycerides and TG/HDL-c ratio) were significantly increased across tertiles of sex-specific VAT independently of confounding factors. In line with our results, Lee and colleagues observed VAT and triglycerides being independent risk factors for hepatic steatosis.41 VAT is the main source of free fatty acids (FFAs) and other biological compounds, which enter the portal circulation and contribute to hepatic fat accumulation,40 insulin resistance4,5 and glucose intolerance, promoting a decreased hepatic insulin sensitivity, increasing the risk of developing T2DM and NAFLD.4 Moreover, a statically significant increase of non-invasive hepatic markers (ALT, FLI and HSI) in participants with higher fat-storage capacity in VAT was found. Previously, studies demonstrated that increased VAT was associated with higher ALT levels42 or significant fibrosis in subjects diagnosed with NAFLD.43 Based on these data, central adiposity plays a key role in NAFLD pathogenesis10,14,44 promoting liver damage,43 insulin resistance and disrupted lipid metabolism.45
Currently, NAFLD has become a public health problem with a negative impact over the individual’s health, socioeconomic and healthcare system.6,46 In this context, early screening is crucial in the NAFLD pathogenesis,7 as it is an overlooked T2DM complication.5 Liver biopsy is the gold standard for NAFLD diagnosis.7 However, it has several limitations, such as sampling error, cost, medical complications and technical difficulties.47 In this regard, several methodologies have been used in the detection and featuring of NAFLD shown to be relatively effective, inexpensive and useful in a primary healthcare setting.19,47–49 TyG is a novel marker exhibiting accuracy for recognizing insulin resistance and diabetes-related manifestations.20,50 Furthermore, this marker was found highly sensitive for detecting NAFLD.34 Simental-Mendía et al. suggested that the best TyG level for diagnosis of insulin resistance was Ln 4.65, which showed the highest sensitivity (84.0%) and specificity (45.0%) values.36 Interestingly, the multivariable regression analysis demonstrated that individuals with a higher TyG (>9.1) value were associated with higher levels of HOMA-IR ALT, GGT, FGF-21, FLI and HSI units compared with lower TyG values (⩽8.7), after adjusting for potential confounders, which confirms the relationship with inflammation and T2DM. Evidence supports T2DM is an important risk factor for NAFLD,5,6 which is characterized by a resistance of insulin action in targets tissues and a disruption of the beta cells in the pancreatic islets to secrete enough insulin to overcome this resistance.24 The prevalence of NAFLD and non-alcoholic steatohepatitis (NASH) in individuals diagnosed with T2DM equates to over 60% increased risk of NAFLD pathogenesis and mortality.6 These results suggested that insulin resistance is an important contributor to the development of NAFLD.4 This finding is similar to results from Bonnet et al., who reported that increased levels of ALT and GGT are strongly associated with hepatic insulin resistance and decreased hepatic insulin clearance.51 Another liver marker is the FGF-21, primarily produced in hepatocytes and implicated in the regulation of glucose–lipid metabolism, insulin sensitivity, inflammation and energy homeostasis.52 Several clinical studies and reviews have documented that disrupted adipose tissue and excessive intrahepatic fat accumulation may trigger FGF-21 resistance.52,53 Thus, Shen and colleagues54 found that NAFLD patients showed significantly higher serum FGF-21 levels compared with subjects without NAFLD.54 Furthermore, the present study showed that subjects with higher values of TyG had 3.46 more units of HSI compared with reference (lower values). Taken together, these results can be explained by insulin resistance being a major feature of NAFLD that works by increasing de novo lipogenesis and FFA flux to the liver through decreased inhibition of lipolysis,4 promoting inflammation, oxidative stress55 and hepatocyte injury.56 Thus, individuals with pre-diabetes and T2DM represent an at-high-risk population where early diagnosis of NAFLD is crucial.5
DXA has been considered the gold standard for body composition measurements.16 Nevertheless, this imaging technique for assessing adipose tissue distribution is expensive and not feasible for routine community screening. In our results, we observed a close relationship between insulin resistance and dysfunctional VAT. Interestingly, men had higher amounts of VAT than women. Meanwhile, women and men with ⩾VAT median had similar TyG values (data not shown). Moreover, the ROC curves indicate a moderate predictive ability of TyG to discriminate VAT in women (AUC = 0.713), but it was weak for men (AUC = 0.570).
The connection between body fat distribution and adipose-tissue biology with insulin resistance varies by sex, age and other factors.11 In general, women have more total body fat mass and men present with higher abdominal/visceral fat mass.11 However, decreased levels of oestrogen and adipose tissue redistribution by increased depots of VAT are characterized in postmenopausal women.11,12 A disbalance of hormonal levels promotes insulin resistance and an atherogenic lipid profile, which increases the risk of CVD in older women.11 Interestingly, some studies have suggested that obese women are more insulin sensitive than men despite a higher amount of VAT;12 however, the mechanism is still unclear. Recently, the Netherlands Epidemiology of Obesity Study showed that in obese women, VAT was differently associated with cardiometabolic risk factors as compared with obese men.57 However, this outcome was in contrast with Ferrara et al., who reported that older obese men are more insulin resistant compared with older women, even adjusted for differences in abdominal fat distribution measured by DXA.13 One possible explanation for our results could be that women exhibit a greater amount of FFA delivery derived from VAT lipolysis.58 Moreover, Serra et al. showed that postmenopausal women (overweight or obese) diagnosed with MetS had lower adipose-tissue lipoprotein-lipase activity and limited capacity for lipid accumulation in subcutaneous abdominal adipose tissue, leading to higher levels of lipids, accumulation of VAT and insulin resistance.59
Our results reinforce that a VAT dysfunction and higher TyG values increase risk of developing NAFLD and suggest a role for glucose intolerance. Moreover, the ROC analysis reflected that TyG could be a suitable predictor of VAT. Chronic diseases are the leading causes of death and disability worldwide. MetS comprises several clinical and metabolic risk factors that increase the risk of developing T2DM and other comorbidities.1 Individuals with T2DM and NAFLD exhibit more severe insulin resistance and liver damage. Also, in T2DM the presence of fatty liver is associated with poor glycaemic control, resulting in the need for higher insulin doses.5 Meanwhile, ageing and biological differences between men and women play an important role in body fat distribution and health status. Our findings suggest a strong association between excessive accumulation of VAT, insulin resistance, cardiometabolic risk factors and poor liver status in subjects with MetS. Moreover, the TyG, a novel marker of insulin resistance could be used as an easy and reliable marker for dysfunctional VAT, which could constitute a new proxy for healthcare professionals in the screening of individuals diagnosed with MetS. In this context, the improvement of knowledge of these inter-relationships in subjects with MetS should be useful in easily identifying individuals with a high risk of NAFLD, which may allow early intervention and prevention of NAFLD complications.
The strengths of this study are that VAT was objectively measured with a validated imaging technique. Also, the novelty of this study comes from the use of TyG as a suitable marker of VAT in subjects at high cardiovascular risk diagnosed with MetS. However, some limitations require consideration. First, there is the relatively small sample size. Despite this, the achieved statistical power for VAT and TyG variables was higher than 90%. Second, the cross-sectional design cannot imply a causal relationship. Third, there is lack of NAFLD diagnosis by liver biopsy or imaging techniques, but important to note that liver biopsy is not available or feasible in large epidemiological studies. On the other hand, we used validated non-invasive markers to estimate hepatic fat accumulation.38
Conclusion
VAT and the TyG were associated with liver and cardiometabolic risk factors linked to NAFLD in individuals with overweight/obesity and MetS. Moreover, we demonstrated that in addition to anthropometric measurements or the DXA approach, TyG could be a useful simple marker to identify dysfunctional VAT phenotype in patients with diabetic profiles and MetS manifestations.
Acknowledgments
We thank all the volunteers for their participation and the personnel for their contribution in the PREDIMED-Plus trial.
Footnotes
Author contribution(s): Vanessa Bullón-Vela: Conceptualization; Formal analysis; Investigation; Writing-original draft; Writing-review & editing.
Itziar Abete: Conceptualization; Formal analysis; Investigation; Writing-original draft; Writing-review & editing.
Josep A. Tur: Investigation; Writing-review & editing.
Jadwiga Konieczna: Investigation; Writing-review & editing.
Dora Romaguera: Investigation; Writing-review & editing.
Xavier Pintó: Investigation; Writing-review & editing.
Emili Corbella: Investigation; Writing-review & editing.
Miguel Martinez-Gonzalez: Investigation; Writing-review & editing.
Carmen Sayón-Orea: Investigation; Writing-review & editing.
Estefanía Toledo: Investigation; Writing-review & editing.
Dolores Corella: Investigation; Writing-review & editing.
Manuel Macías-Gonzalez: Investigation; Writing-review & editing.
Francisco Tinahones: Investigation; Writing- review & editing.
Montserrat Fitó: Investigation; Writing-review & editing.
Ramon Estruch: Formal analysis; Writing-review & editing.
Emilio Ros: Investigation; Writing-review & editing.
Jordi Sala-Salvado: Investigation; Writing-review & editing.
Lidia Daimiel: Investigation; Writing-review & editing.
Catalina Mascaró: Investigation; Writing-review & editing.
Maria Angeles Zulet: Conceptualization; Formal analysis; Investigation; Writing-original draft; Writing-review & editing.
José Alfredo Martínez: Conceptualization; Formal analysis; Investigation; Writing-original draft; Writing-review & editing.
Conflict of interest statement: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: the PREDIMED-Plus trial was supported by the European Research Council (Advanced Research grant 2014–2019; agreement #340918; granted to Dr Martínez-González); the official Spanish institutions for funding scientific biomedical research, CIBER Fisiopatología de la Obesidad y Nutrición (CIBERobn) and Instituto de Salud Carlos III (ISCIII) through the Fondo de Investigación para la Salud (FIS) that is co-funded by the European Regional Development Fund (coordinated FIS projects led by Drs Salas-Salvadó and Vidal, including the following projects: PI13/00673, PI13/00492, PI13/00272, PI13/01123, PI13/00462, PI13/00233, PI13/02184, PI13/00728, PI13/01090, PI13/01056, PI14/01722, PI14/00636, PI14/00618, PI14/00696, PI14/01206, PI14/01919, PI14/00853, PI14/01374, PI14/00972, PI14/00728, PI14/01471, PI16/00473, PI16/00662, PI16/01873, PI16/01094, PI16/00501, PI16/00533, PI16/00381, PI16/00366, PI16/01522, PI16/01120, PI17/00764, PI17/01183, PI17/00855, PI17/01347, PI17/00525, PI17/01827, PI17/00532, PI17/00215, PI17/01441, PI17/00508, PI17/01732, PI17/00926, PI19/00957, PI19/00386, PI19/00309, PI19/01032, PI19/00576, PI19/00017, PI19/01226, PI19/00781, PI19/01560, PI19/01332) and the Especial Action Project ‘Implementación y evaluación de una intervención intensiva sobre la actividad física Cohorte PREDIMED-Plus’ (Dr Salas-Salvadó); the Recercaixa (grant number 2013ACUP00194) (Dr Salas-Salvadó); J. Salas-Salvadó, gratefully acknowledges the financial suuport by ICREA under the ICREA Academia program; the SEMERGEN grant; International Nut and Dried Fruit Council–FESNAD (Long-term effects of an energy-restricted Mediterranean diet on mortality and cardiovascular disease 2014–015; no. 201302; Dr Martínez-González); the AstraZeneca Young Investigators Award in Category of Obesity and T2D 2017 (Dr Romaguera); grants from the Consejería de Salud de la Junta de Andalucía (PI0458/2013; PS0358/2016; PI0137/2018), the PROMETEO/2017/017 grant from the Generalitat Valenciana, the SEMERGEN grant; grant of support to research groups 35/2011 (Balearic Islands Gov; FEDER funds; Drs Tur and Bouz). JK is contracted for the ‘FOLIUM’ programme within the FUTURMed project. Talent for the medicine within the future from the Fundación Instituto de Investigación Sanitaria Illes Balears (financed by 2017 annual plan of the sustainable tourism tax and at 50% with charge to the ESF Operational Programme 2014–2020 of the Balearic Islands). CMM received an FPU grant from the Ministry of Education, Spain. VB-V received a grant from the Centre for Nutrition Research of the University of Navarra.
ORCID iDs: Itziar Abete  https://orcid.org/0000-0002-6475-5387
https://orcid.org/0000-0002-6475-5387
José Alfredo Martínez  https://orcid.org/0000-0001-5218-6941
https://orcid.org/0000-0001-5218-6941
Contributor Information
Vanessa Bullón-Vela, Department of Nutrition, Food Science and Physiology, Center for Nutrition Research, University of Navarra, Pamplona, Spain.
Itziar Abete, Department of Nutrition, Food Science and Physiology, Centre for Nutrition Research, University of Navarra, Irunlarrea 1, 31008 Pamplona, Spain; Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Navarra Institute for Health Research (IdiSNA), Pamplona, Spain; CIBER Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III, Madrid, Spain.
Josep A. Tur, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain Research Group on Community Nutrition and Oxidative Stress, University of the Balearic Islands, Palma de Mallorca, Spain; Health Research Institute of the Balearic Islands (IdISBa), Palma de Mallorca, Spain.
Jadwiga Konieczna, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Research Group on Nutritional Epidemiology & Cardiovascular Physiopathology (NUTRECOR), Health Research Institute of the Balearic Islands (IdIsBa), University Hospital of the Balearic Islands, Palma de Mallorca, Spain.
Dora Romaguera, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Research Group on Nutritional Epidemiology & Cardiovascular Physiopathology (NUTRECOR), Health Research Institute of the Balearic Islands (IdISBa), Palma de Mallorca, Spain.
Xavier Pintó, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Lipids and Vascular Risk Unit, Internal Medicine, Hospital Universitario de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain.
Emili Corbella, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Lipids and Vascular Risk Unit, Internal Medicine, Hospital Universitario de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain.
Miguel A. Martínez-González, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain Department of Preventive Medicine and Public Health, University of Navarra, Pamplona, Spain; Navarra Institute for Health Research (IdisNA), Pamplona, Spain.
Carmen Sayón-Orea, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Department of Preventive Medicine and Public Health, University of Navarra, Pamplona, Spain; Navarra Institute for Health Research (IdisNA), Pamplona, Spain.
Estefanía Toledo, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Department of Preventive Medicine and Public Health, University of Navarra, Pamplona, Spain; Navarra Institute for Health Research (IdisNA), Pamplona, Spain.
Dolores Corella, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Department of Preventive Medicine, University of Valencia, Valencia, Spain.
Manuel Macías-Gonzalez, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Department of Endocrinology, Virgen de la Victoria Hospital, Institute of Biomedical Research in Málaga (IBIMA), University of Málaga, Málaga, Spain.
Francisco J. Tinahones, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain Department of Endocrinology, Virgen de la Victoria Hospital, Institute of Biomedical Research in Málaga (IBIMA), University of Málaga, Málaga, Spain.
Montserrat Fitó, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Cardiovascular Risk and Nutrition Research Group (CARIN), Hospital del Mar Research Institute (IMIM), Barcelona, Spain.
Ramon Estruch, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Department of Internal Medicine, University of Barcelona, Barcelona, Spain.
Emilio Ros, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Department of Endocrinology and Nutrition, Institut d’Investigacions Biomèdiques August Pi Sunyer (IDIBAPS), Barcelona, Spain.
Jordi Salas-Salvadó, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Unversitat Rovira i Virgili, Department de Bioquímica i Biotecnologia, Unitat de Nutrició Humana, Reus, Spain; Institut d’Investigació Pere Virgili (IISPV), Hospital Universitari Sant Joan de Reus, Reus, Spain.
Lidia Daimiel, Precision Nutrition Programme, IMDEA Food, CEI UAM + CSIC, Madrid, Spain.
Catalina M. Mascaró, Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain Research Group on Community Nutrition and Oxidative Stress, University of the Balearic Islands, Palma de Mallorca, Spain; Health Research Institute of the Balearic Islands (IdISBa), Palma de Mallorca, Spain.
Maria Angeles Zulet, Department of Nutrition, Food Science and Physiology, Center for Nutrition Research, University of Navarra, Pamplona, Spain; Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Navarra Institute for Health Research (IdiSNA), Pamplona, Spain.
José Alfredo Martínez, Department of Nutrition, Food Science and Physiology, Center for Nutrition Research, University of Navarra, Pamplona, Spain; Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERobn), Institute of Health Carlos III (ISCIII), Madrid, Spain; Navarra Institute for Health Research (IdiSNA), Pamplona, Spain; Precision Nutrition Programme, IMDEA Food, CEI UAM + CSIC, Madrid, Spain.
References
- 1. Dominguez LJ, Barbagallo M. The biology of the metabolic syndrome and aging. Curr Opin Clin Nutr 2016; 19: 5–11. [DOI] [PubMed] [Google Scholar]
- 2. Rani V, Deep G, Singh RK, et al. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci 2016; 148: 183–193. [DOI] [PubMed] [Google Scholar]
- 3. Mansourian M, Babahajiani M, Jafari-Koshki T, et al. Metabolic syndrome components and long-term incidence of cardiovascular disease in Eastern Mediterranean Region: a 13-year population-based Cohort Study. Metab Syndr Relat D 2019; 17: 362–366. [DOI] [PubMed] [Google Scholar]
- 4. Kitade H, Chen G, Ni Y, et al. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients 2017; 9: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lomonaco R, Chen J, Cusi K. An endocrine perspective of nonalcoholic fatty liver disease (NAFLD). Ther Adv Endocrinol Metab 2011; 2: 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Younossi ZM. Non-alcoholic fatty liver disease – a global public health perspective. J Hepatol 2019; 70: 531–544. [DOI] [PubMed] [Google Scholar]
- 7. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67: 328–357. [DOI] [PubMed] [Google Scholar]
- 8. Rinella ME. Nonalcoholic fatty liver disease. J Am Med Assoc 2015; 313: 2263. [DOI] [PubMed] [Google Scholar]
- 9. González-Muniesa P, Mártinez-González M-A, Hu FB, et al. Obesity. Nat Rev Dis Primers 2017; 3: 17034. [DOI] [PubMed] [Google Scholar]
- 10. Bosy-Westphal A, Braun W, Albrecht V, et al. Determinants of ectopic liver fat in metabolic disease. Eur J Clin Nutr 2019; 73: 209–214. [DOI] [PubMed] [Google Scholar]
- 11. Karastergiou K, Smith SR, Greenberg AS, et al. Sex differences in human adipose tissues – the biology of pear shape. Biol Sex Differ 2012; 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frank AP, De Souza Santos R, Palmer BF, et al. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res 2019; 60: 1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrara CM, Goldberg AP, Nicklas BJ, et al. Sex differences in insulin action and body fat distribution in overweight and obese middle-aged and older men and women. Appl Physiol Nutr Metab 2008; 33: 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki A, Abdelmalek MF. Nonalcoholic fatty liver disease in women. Women Health 2009; 5: 191–203. [DOI] [PubMed] [Google Scholar]
- 15. Kuk JL, Saunders TJ, Davidson LE, et al. Age-related changes in total and regional fat distribution. Ageing Res Rev 2009; 8: 339–348. [DOI] [PubMed] [Google Scholar]
- 16. Micklesfield LK, Goedecke JH, Punyanitya M, et al. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity 2012; 20: 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu P, Chen Q, Chen L, et al. Dose-response relationship between alanine aminotransferase levels within the reference interval and metabolic syndrome in Chinese adults. Yonsei Med J 2017; 58: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheung C-L, Lam KS, Wong IC, et al. Non-invasive score identifies ultrasonography-diagnosed non-alcoholic fatty liver disease and predicts mortality in the USA. BMC Med 2014; 12: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cantero I, Elorz M, Abete I, et al. Ultrasound/elastography techniques, lipidomic and blood markers compared to magnetic resonance imaging in non-alcoholic fatty liver disease adults. Int J Med Sci 2019; 16: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, et al. Triglyceride–glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the Vascular-Metabolic CUN cohort. Prev Med 2016; 86: 99–105. [DOI] [PubMed] [Google Scholar]
- 21. Martínez-González MA, Buil-Cosiales P, Corella D, et al. Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial. Int J Epidemiol 2019; 48: 387–388o. [DOI] [PubMed] [Google Scholar]
- 22. Sayón-Orea C, Razquin C, Bulló M, et al. Effect of a nutritional and behavioral intervention on energy-reduced Mediterranean diet adherence among patients with metabolic syndrome. J Am Med Assoc 2019; 322: 1486–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome. Circulation 2009; 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 24. Atroshenko SA, Korolyov IA, Didenko N. Standards of medical care in diabetes—2014. Diabetes Care 2014; 37: S14–S80. [DOI] [PubMed] [Google Scholar]
- 25. Bairaktari ET, Seferiadis KI, Elisaf MS. Evaluation of methods for the measurement of low-density lipoprotein cholesterol. J Cardiovasc Pharmacol Ther 2005; 10: 45–54. [DOI] [PubMed] [Google Scholar]
- 26. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 27. Fernández-Ballart JD, Piñol JL, Zazpe I, et al. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr 2010; 103: 1808–1816. [DOI] [PubMed] [Google Scholar]
- 28. Schröder H, Fitó M, Estruch R, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr 2011; 141: 1140–1145. [DOI] [PubMed] [Google Scholar]
- 29. Molina L, Sarmiento M, Peñafiel J, et al. Validation of the regicor short physical activity questionnaire for the adult population. PLoS ONE 2017; 12: e0168148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosique-Esteban N, Babio N, Díaz-López A, et al. Leisure-time physical activity at moderate and high intensity is associated with parameters of body composition, muscle strength and sarcopenia in aged adults with obesity and metabolic syndrome from the PREDIMED-Plus study. Clin Nutr 2019; 38: 1324–1331. [DOI] [PubMed] [Google Scholar]
- 31. Martínez-González MA, López-Fontana C, Varo JJ, et al. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr 2005; 8: 920–927. [DOI] [PubMed] [Google Scholar]
- 32. Fedchuk L, Nascimbeni F, Pais R, et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2014; 40: 1209–1222. [DOI] [PubMed] [Google Scholar]
- 33. Zheng R, Du Z, Wang M, et al. A longitudinal epidemiological study on the triglyceride and glucose index and the incident nonalcoholic fatty liver disease. Lipids Health Dis 2018; 17: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang S, Du T, Zhang J, et al. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis 2017; 16: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moon S, Park JS, Ahn Y. The cut-off values of triglycerides and glucose index for metabolic syndrome in American and Korean adolescents. J Korean Med Sci 2017; 32: 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 2008; 6: 299–304. [DOI] [PubMed] [Google Scholar]
- 37. Lee J-H, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010; 42: 503–508. [DOI] [PubMed] [Google Scholar]
- 38. Bugianesi E, Rosso C, Cortez-Pinto H. How to diagnose NAFLD in 2016. J Hepatol 2016; 65: 643–644. [DOI] [PubMed] [Google Scholar]
- 39. Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006; 6: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parker R. The role of adipose tissue in fatty liver diseases. Liver Res 2018; 2: 35–42. [Google Scholar]
- 41. Lee HW, Kim KJ, Jung KS, et al. The relationship between visceral obesity and hepatic steatosis measured by controlled attenuation parameter. PLoS ONE 2017; 12: e0187066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chung GE, Kim D, Kwark MS, et al. Visceral adipose tissue area as an independent risk factor for elevated liver enzyme in nonalcoholic fatty liver disease. Medicine 2015; 94: e573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu SJ, Kim W, Kim D, et al. Visceral obesity predicts significant fibrosis in patients with nonalcoholic fatty liver disease. Medicine 2015; 94: e2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 2007; 13: 3540–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khan RS, Bril F, Cusi K, et al. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology 2019; 70: 711–724. [DOI] [PubMed] [Google Scholar]
- 46. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 47. Zhou JH, She ZG, Li HL, et al. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J Gastroenterol 2019; 25: 1307–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martínez-González MA, Bastarrika G. Mediterranean diet as the ideal model for preventing non-alcoholic fatty liver disease (NAFLD). Hepatobiliary Surg Nutr 2020; 9: 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bullón-Vela V, Abete I, Tur JA, et al. Influence of lifestyle factors and staple foods from the Mediterranean diet on non-alcoholic fatty liver disease among older individuals with metabolic syndrome features. Nutrition 2020; 71: 110620. [DOI] [PubMed] [Google Scholar]
- 50. Du T, Yuan G, Zhang M, et al. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol 2014; 13: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bonnet F, Ducluzeau PH, Gastaldelli A, et al. Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes 2011; 60: 1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu J, Xu Y, Hu Y, et al. The role of fibroblast growth factor 21 in the pathogenesis of non-alcoholic fatty liver disease and implications for therapy. Metabolism 2015; 64: 380–390. [DOI] [PubMed] [Google Scholar]
- 53. Inagaki T. Research perspectives on the regulation and physiological functions of FGF21 and its association with NAFLD. Front Endocrinol 2015; 6: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shen Y, Ma X, Zhou J, et al. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc Diabetol 2013; 12: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Koliaki C, Szendroedi J, Kaul K, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 2015; 21: 739–746. [DOI] [PubMed] [Google Scholar]
- 56. Bullón-Vela V, Abete I, Martínez J, et al. Obesity and nonalcoholic fatty liver disease: role of oxidative stress. In: Marti del Moral A, García CMA. (eds) Obesity: oxidative stress and dietary antioxidants. London: Elsevier, 2018, pp.111–133. [Google Scholar]
- 57. Elffers TW, de Mutsert R, Lamb HJ, et al. Body fat distribution, in particular visceral fat, is associated with cardiometabolic risk factors in obese women. PLoS ONE 2017; 12: e0185403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nielsen S, Guo Z, Johnson CM, et al. Splanchnic lipolysis in human obesity. J Clin Invest 2004; 113: 1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Serra MC, Ryan AS, Goldberg AP. Reduced LPL and subcutaneous lipid storage capacity are associated with metabolic syndrome in postmenopausal women with obesity. Obes Sci Pract 2017; 3: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]