Abstract
Aim:
To compare the efficacy, safety, and tolerability of abemaciclib plus endocrine therapy (ET) versus ET alone in postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer (ABC) from China, Brazil, India, and South Africa.
Methods:
This randomized, double-blind, phase III study was conducted between 9 December 2016 and 29 March 2019. Postmenopausal women with HR-positive, HER2-negative ABC with no prior systemic therapy in an advanced setting (cohort A) or progression on prior ET (cohort B) received abemaciclib (150 mg twice daily) or placebo plus: anastrozole (1 mg/day) or letrozole (2.5 mg/day) (cohort A) or fulvestrant (500 mg per label) (cohort B). The primary endpoint was progression-free survival (PFS) in cohort A, analyzed using the stratified log-rank test. Secondary endpoints were PFS in cohort B (key secondary endpoint), objective response rate (ORR), and safety. This interim analysis was planned after 119 PFS events in cohort A.
Results:
In cohort A, 207 patients were randomly assigned to the abemaciclib arm and 99 to the placebo arm. Abemaciclib significantly improved PFS versus placebo (median: not reached versus 14.7 months; hazard ratio 0.499; 95% confidence intervals (CI) 0.346–0.719; p = 0.0001). ORR was 65.9% in the abemaciclib arm and 36.1% in the placebo arm (p < 0.0001, measurable disease population). In cohort B, 104 patients were randomly assigned to the abemaciclib arm and 53 to the placebo arm. Abemaciclib significantly improved PFS versus placebo (median: 11.5 versus 5.6 months; hazard ratio 0.376; 95% CI 0.240–0.588; p < 0.0001). ORR was 50.0% in the abemaciclib arm and 10.5% in the placebo arm (p < 0.0001, measurable disease population). The most frequent grade ⩾3 adverse events in the abemaciclib arms were neutropenia, leukopenia, and anemia (both cohorts), and lymphocytopenia (cohort B).
Conclusion:
The addition of abemaciclib to ET demonstrated significant and clinically meaningful improvement in PFS and ORR, without new safety signals observed in this population.
Trial Registration: ClinicalTrials.gov identifier: NCT02763566.
Keywords: abemaciclib, aromatase inhibitors, breast neoplasms, cyclin-dependent kinase 4, cyclin-dependent kinase 6, fulvestrant
Introduction
There is compelling evidence from several phase III studies that cyclin-dependent kinase (CDK) 4 and CDK 6 inhibitors including abemaciclib, palbociclib, and ribociclib in combination with standard endocrine therapy (ET) have significant antitumor activity with tolerable safety profiles in patients with advanced breast cancer with hormone receptor (HR)-positive, human epidermal growth factor 2 (HER2)-negative disease.1–5 However, the lack of data for CDK 4 and CDK 6 inhibitors from China, Brazil, India, and South Africa, which represent approximately 40% of the global population, makes the total evidence for CDK 4 and CDK 6 inhibitors less representative.6
Abemaciclib is a potent and selective inhibitor of CDK 4 and CDK 6.7,8 An efficacy benefit and acceptable safety profile have been shown for abemaciclib in women with HR-positive, HER2-negative advanced breast cancer as: (a) initial ET in combination with a non-steroidal aromatase inhibitor (NSAI), based on a significant improvement in progression-free survival (PFS) in the phase III MONARCH 3 study;4 and (b) as subsequent therapy after progression on ET in combination with fulvestrant in the phase III MONARCH 2 study.5 Besides, as a key secondary end point of the MONARCH 2 study, treatment with abemaciclib plus fulvestrant demonstrated a statistically significant and clinically meaningful median overall survival (OS) improvement.9
The MONARCH plus study presented here is the first study designed to evaluate the efficacy and safety of abemaciclib in combination with ET in patients from China, Brazil, India, and South Africa.
Methods
Study design
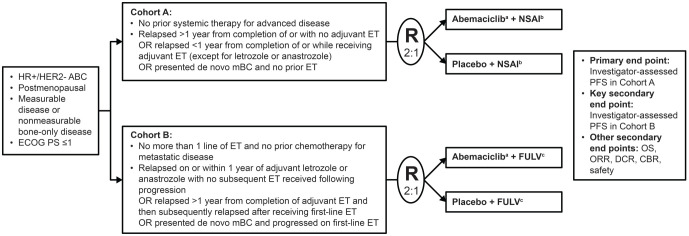
MONARCH plus is a multinational, randomized, placebo controlled, double-blind phase III study conducted at 45 medical institutions in four countries (China, India, Brazil, and South Africa) from 9 December 2016 to the data cut-off of 29 March 2019 (Figure 1). The protocol was approved by the ethics committees of all participating centers (online Supplemental Table 1) and was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation guidelines for Good Clinical Practice, and applicable laws and regulations. All patients provided written informed consent before enrollment. The study is registered at www.ClinicalTrials.gov (NCT02763566) and is ongoing.
Figure 1.
Study design.
aAbemaciclib 150 mg twice daily (continuous schedule).
bAnastrozole 1 mg daily or letrozole 2.5 mg daily per physician’s choice.
cFulvestrant 500 mg on days 1 and 15 of the first 28-day cycle and then every 28 days.
ABC, advanced breast cancer; CBR, clinical benefit rate; DCR, disease control rate; ECOG, Eastern Cooperative Oncology Group; ET, endocrine therapy; FULV, fulvestrant; HER2–, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; mBC, metastatic breast cancer; NSAI, non-steroidal aromatase inhibitor; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PS, performance status; R, randomization.
Patients
Postmenopausal female patients with HR-positive, HER2-negative locoregionally recurrent disease (not amenable to resection or radiation therapy with curative intent) or metastatic disease were eligible for inclusion. Patients in both cohorts were aged ⩾18 years, had measurable disease or non-measurable bone-only disease [based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1], and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients in cohort A (abemaciclib plus NSAI versus placebo plus NSAI) had no prior systemic therapy for metastatic or locoregionally recurrent disease, and patients in cohort B (abemaciclib plus fulvestrant versus placebo plus fulvestrant) had progressed on prior ET and had no prior chemotherapy for metastatic disease. Key inclusion criteria are listed in Figure 1. For both cohorts, the main exclusion criteria were visceral crisis (defined as severe organ dysfunction as assessed by signs and symptoms, laboratory studies, and rapid progression of disease), lymphangitic spread, or leptomeningeal carcinomatosis; inflammatory breast cancer; evidence or history of central nervous system metastasis; current or prior chemotherapy for locoregionally recurrent or metastatic breast cancer; and prior treatment with any CDK 4 or CDK 6 inhibitor. Patients with prior treatment with everolimus or fulvestrant were excluded from cohort B.
Randomization and masking
Treatment was determined by a computer-generated random sequence and assigned by study center personnel using an interactive web response system. In cohort A, patients were randomly assigned 2:1 to receive abemaciclib plus NSAI or placebo plus NSAI, stratified by nature of disease (visceral or non-visceral metastases) and prior (neo)adjuvant ET (prior therapy with disease-free interval >12 months from treatment completion, prior therapy with disease-free interval ⩽12 months from treatment completion, or no prior therapy), with a block size of 6. In cohort B, patients were randomly assigned 2:1 to receive abemaciclib plus fulvestrant or placebo plus fulvestrant, stratified by nature of disease (visceral or non-visceral metastases) and sensitivity to ET (primary or secondary resistance).10 Patients, physicians, and investigators were masked to treatment allocation.
Procedures
Study treatments were administered in 28-day cycles. Patients in cohort A received oral abemaciclib (150 mg twice daily) or matching placebo, plus an NSAI (anastrozole 1 mg or letrozole 2.5 mg once daily as determined by the investigator). Patients in cohort B received abemaciclib or matching placebo with the same schedule as cohort A, plus fulvestrant (500 mg) intramuscularly on days 1 and 15 of cycle 1 and then on day 1 of each subsequent cycle (28 days). Treatment continued until disease progression, unacceptable toxicity, death, or patient withdrawal for any reason. Dose interruptions and reductions were allowed for abemaciclib/placebo as defined by prespecified guidelines in the protocol. Dose reduction was not applicable for NSAI per label. Dose reduction for fulvestrant was permitted per local label. Patients were permitted to discontinue either abemaciclib/placebo or NSAI/fulvestrant and continue the other drug.
Tumors were assessed by computed tomography or magnetic resonance imaging according to RECIST version 1.1 within 28 days before randomization (baseline), every second cycle during cycles 2–18, every third cycle thereafter, and within 14 days of clinical progression. All patients underwent bone scintigraphy at baseline and every sixth cycle starting with cycle 6. Hematological and blood chemistry laboratory tests were performed centrally on days 1 and 15 of the first two cycles and day 1 of all remaining cycles. Adverse events (AEs) were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0, and were evaluated at every patient visit from baseline until follow-up.
Outcomes
The primary and key secondary endpoints, investigator-assessed PFS in cohort A and cohort B, respectively, were analyzed from the time of random assignment until objective progressive disease (PD) or death for any reason. Other secondary endpoints included objective response rate [ORR, defined as best overall response of complete response (CR) or partial response (PR)], disease control rate (DCR; CR or PR or stable disease [SD]), clinical benefit rate (CBR; CR, PR, or SD for at least 6 months), duration of response (time from CR or PR until PD or death), overall survival (OS), OS rate at 1 year and 2 years, safety and tolerability, quality of life measures, and pharmacokinetics.
Statistical analysis
The primary PFS analysis tested the superiority of abemaciclib plus NSAI to placebo plus NSAI at a prespecified interim analysis planned after 119 events in cohort A (70% of the planned 170 events at the final analysis) using the log-rank test stratified by randomization strata. Based on the O’Brien Fleming alpha-spending function, the one-sided boundary p-value for the interim analysis was 0.0082. Assuming a hazard ratio of 0.626, the two-look group-sequential design yields power of approximately 80% at a one-sided alpha of 0.025.
Cohort B was not powered for statistical tests; interim analysis of PFS in cohort B was to be performed using the log-rank test stratified by randomization strata if statistical significance was declared at the interim analysis for cohort A.
Stratified Cox proportional hazards models were used to estimate the treatment effect hazard ratios. Stratified Cochran–Mantel–Haenszel tests were performed to compare rates of binary endpoints between treatment arms. Unless otherwise noted, all hypothesis tests were performed at the two-sided 0.05 level, and all confidence intervals (CIs) were 95%. Efficacy analyses were performed on the intent-to-treat (ITT) population, which included all randomly assigned patients. Additional analyses of best overall response were performed in patients with measurable disease. Subgroup analyses of PFS were performed on prespecified prognostic factors and other baseline characteristics. Safety analyses were performed on the safety population, which included all patients who received study treatment. Statistical analyses were performed using SAS (version 9.2 or later; SAS Institute).
Results
Patient disposition and baseline characteristics
Between 9 December 2016 and 21 August 2018, 463 patients were enrolled into cohort A (n = 306, with 207 and 99 patients in the abemaciclib and placebo arms, respectively) or cohort B (n = 157, with 104 and 53 patients, respectively) (online Supplemental Figure 1).
Baseline patient characteristics were well balanced between treatment arms in both cohorts (Tables 1 and 2). Overall, 413 patients (89.2%) in the study were Asian. The majority of patients (317; 60.5%) had visceral disease at baseline. More than half of the patients had received chemotherapy in the adjuvant setting. In cohort A, 183 patients (59.8%) had relapsed on or after completion of adjuvant ET. Among them, 55 patients (30.1% of 183 patients) had relapsed on or within 12 months after completion of adjuvant ET (with ET other than NSAIs). In cohort B, almost all patients (155/157, 98.7%) had received an aromatase inhibitor (AI) as the most recent treatment, 143 patients (91.1%) had been treated with ET in the (neo)adjuvant setting, and 55 patients (35.0%) were reported to have primary resistance to ET.
Table 1.
Baseline characteristics of cohort A.a
| Abemaciclib + NSAI (n = 207) | Placebo + NSAI (n = 99) | |
|---|---|---|
| Age, years, median (range) | 54.0 (32.0, 83.0) | 54.0 (27.0, 77.0) |
| Age category | ||
| <65 years | 157 (75.8) | 83 (83.8) |
| ⩾65 years | 50 (24.2) | 16 (16.2) |
| Country | ||
| China | 164 (79.2) | 82 (82.8) |
| Brazil | 21 (10.1) | 8 (8.1) |
| India | 18 (8.7) | 7 (7.1) |
| South Africa | 4 (1.9) | 2 (2.0) |
| Disease setting | ||
| Locoregionally recurrent | 8 (3.9) | 7 (7.1) |
| De novo metastatic | 41 (19.8) | 22 (22.2) |
| Metastatic recurrent | 157 (75.8) | 70 (70.7) |
| Measurable disease | ||
| Yes | 176 (85.0) | 83 (83.8) |
| No (evaluable bone disease only) | 31 (15.0) | 16 (16.2) |
| Nature of disease | ||
| Visceral metastases | 126 (60.9) | 59 (59.6) |
| Non-visceral metastases | 81 (39.1) | 40 (40.4) |
| Prior (neo)adjuvant ET disease-free interval | ||
| >12 months | 87 (42.0) | 41 (41.4) |
| ⩽12 months | 35 (16.9) | 20 (20.2) |
| No prior ET | 83 (40.1) | 37 (37.4) |
| Prior (neo)adjuvant ET | ||
| Aromatase inhibitor containing ET | 20 (9.7) | 13 (13.1) |
| Anti-estrogen therapy onlyb | 101 (48.8) | 48 (48.5) |
| No prior (neo)adjuvant ET | 85 (41.1) | 38 (38.4) |
| Prior (neo)adjuvant chemotherapy | ||
| Yes | 140 (67.6) | 63 (63.6) |
| No | 67 (32.4) | 36 (36.4) |
Data are no. (%), unless otherwise stated.
One patient in abemaciclib arm used ‘unknown endocrine therapy’ as the reported term and was classified into this category.
ET, endocrine therapy.
Table 2.
Baseline characteristics of cohort B.a
| Abemaciclib + fulvestrant (n = 104) | Placebo + fulvestrant (n = 53) | |
|---|---|---|
| Age, years, median (range) | 60.0 (36.0, 80.0) | 60.0 (30.0, 80.0) |
| Age category | ||
| <65 years | 78 (75.0) | 39 (73.6) |
| ⩾65 years | 26 (25.0) | 14 (26.4) |
| Country | ||
| China | 89 (85.6) | 45 (84.9) |
| Brazil | 10 (9.6) | 5 (9.4) |
| India | 5 (4.8) | 2 (3.8) |
| South Africa | 0 | 1 (1.9) |
| Measurable disease | ||
| Yes | 80 (76.9) | 38 (71.7) |
| No (evaluable bone disease only) | 24 (23.1) | 15 (28.3) |
| Nature of disease | ||
| Visceral metastases | 64 (61.5) | 31 (58.5) |
| Non-visceral metastases | 40 (38.5) | 22 (41.5) |
| Prior (neo)adjuvant ET | ||
| Aromatase inhibitor | 86 (82.7) | 41 (77.4) |
| Other | 9 (8.7) | 7 (13.2) |
| No prior (neo)adjuvant ET | 9 (8.7) | 5 (9.4) |
| Prior (neo)adjuvant chemotherapy | ||
| Yes | 84 (80.8) | 45 (84.9) |
| No | 20 (19.2) | 8 (15.1) |
| Sensitivity to ET | ||
| Primary resistance | 36 (34.6) | 19 (35.8) |
| Secondary resistance | 68 (65.4) | 34 (64.2) |
| Prior metastatic ET | ||
| Aromatase inhibitor | 21 (20.2) | 13 (24.5) |
| Anti-estrogen therapy | 2 (1.9) | 0 |
| No prior metastatic ET | 81 (77.9) | 39 (73.6) |
Data are no. (%), unless otherwise stated.
ET, endocrine therapy.
At the interim analysis cut-off (29 March 2019), 148 patients in cohort A and 67 in cohort B were continuing to receive study treatment (online Supplemental Figure 1). In cohort A, 91 patients (44.0%) in the abemaciclib arm and 65 patients (65.7%) in the placebo arm had discontinued treatment. In cohort B, 50 patients (48.1%) in the abemaciclib arm and 40 patients (75.5%) in the placebo arm had discontinued treatment. The majority of patients discontinued treatment due to progressive disease. The comparable median number of cycles received per patient in cohort A (15 for the abemaciclib arm and 13 for the placebo arm) was due to the limited duration of follow-up, while in cohort B, the median number of cycles was 9.5 for the abemaciclib arm and 6.0 for the placebo arm. More patients had dose reductions or omissions in the abemaciclib arm (cohort A: 40.5%/62.4%; cohort B: 39.4%/52.9%) than the placebo arm (cohort A: 2.0%/30.3%; cohort B: 1.9%/17.0%).
Efficacy
The interim analysis occurred after 119 PFS events were observed in the ITT population in cohort A [66 (31.9%) of 207 patients in the abemaciclib arm and 53 (53.5%) of 99 patients in the placebo arm] and the median follow-up was approximately 16 months in both arms. The study met its primary endpoint with an investigator-assessed PFS hazard ratio of 0.499 (95% CI 0.346–0.719; p = 0.0001) in cohort A. Median PFS was not reached in the abemaciclib arm and was 14.7 months in the placebo arm (Figure 2A). PFS rates at 12 months were 72.1% in the abemaciclib arm and 58.0% in the placebo arm (p = 0.0207).
Figure 2.
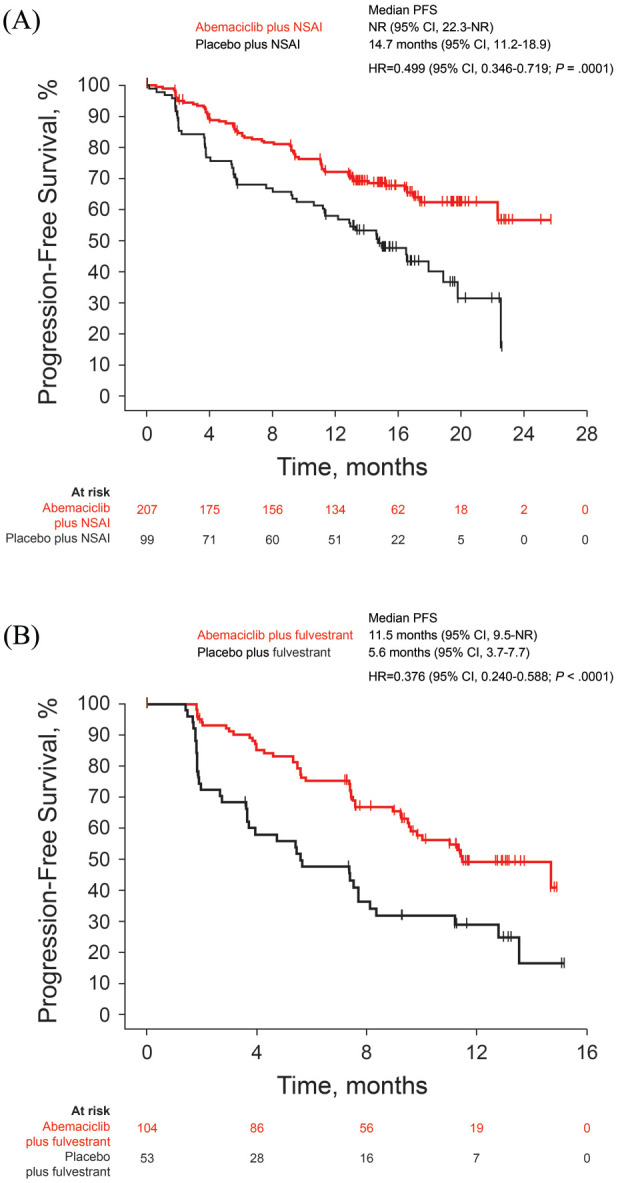
Progression-free survival in the ITT population in (A) cohort A and (B) cohort B.
CI, confidence interval; HR, hazard ratio; ITT, intent-to-treat; NSAI, non-steroidal aromatase inhibitor; PFS, progression-free survival.
In cohort B, at the time of the interim analysis cut-off, 82 PFS events [46 (44.2%) of 104 patients in the abemaciclib arm and 36 (67.9%) of 53 patients in the placebo arm] were observed and median follow-up was 12.2 and 11.1 months, respectively. Median PFS was 11.5 months in the abemaciclib arm and 5.6 months in the placebo arm (Figure 2B); the hazard ratio was 0.376 (95% CI 0.240–0.588; p < 0.0001). PFS rates at 12 months were 49.1% in the abemaciclib arm and 28.9% in the placebo arm (p = 0.0229).
In both cohorts A and B, ORR was significantly improved by the addition of abemaciclib to NSAI or fulvestrant (Table 3) with the difference being greater in patients with measurable disease (online Supplemental Table 2). Consistent improvement was also observed in DCR and CBR (Table 3, online Supplemental Table 2). In cohort A, median duration of response (DoR) was 20.6 months in the abemaciclib arm and 14.3 months in the placebo arm, while in cohort B, median DoR was 9.3 months in the abemaciclib arm and was not reached in the placebo arm due to only one event being observed among four responders (7.5%) at data cut-off.
Table 3.
Best overall response in the ITT population.a
| Cohort A | Cohort B | |||
|---|---|---|---|---|
| Abemaciclib + NSAI (n = 207) | Placebo + NSAI (n = 99) | Abemaciclib + fulvestrant (n = 104) | Placebo + fulvestrant (n = 53) | |
| Best overall response | ||||
| Complete response | 1.0 (0.0–2.3) | 0 | 0 | 1.9 (0.0–5.5) |
| Partial response | 55.1 (48.3–61.8) | 30.3 (21.3–39.4) | 38.5 (29.1–47.8) | 5.7 (0.0–11.9) |
| Stable disease | 35.3 (28.8–41.8) | 52.5 (42.7–62.4) | 53.8 (44.3–63.4) | 62.3 (49.2–75.3) |
| ⩾6 months | 26.6 (20.6–32.6) | 32.3 (23.1–41.5) | 39.4 (30.0–48.8) | 37.7 (24.7–50.8) |
| Progressive disease | 4.8 (1.9–7.8) | 14.1 (7.3–21.0) | 6.7 (1.9–11.5) | 26.4 (14.5–38.3) |
| Not evaluable | 3.9 (1.2–6.5) | 3.0 (0.0–6.4) | 1.0 (0.0–2.8) | 3.8 (0.0–8.9) |
| Objective response rate | 56.0 (49.3–62.8) | 30.3 (21.3–39.4) | 38.5 (29.1–47.8) | 7.5 (0.4–14.7) |
| Stratified p-value | <0.0001 | <0.0001 | ||
| Disease control rate | 91.3 (87.5–95.1) | 82.8 (75.4–90.3) | 92.3 (87.2–97.4) | 69.8 (57.5–82.2) |
| Stratified p-value | 0.0456 | 0.0004 | ||
| Clinical benefit rate | 82.6 (77.4–87.8) | 62.6 (53.1–72.2) | 77.9 (69.9–85.9) | 45.3 (31.9–58.7) |
| Stratified p-value | 0.0003 | <0.0001 | ||
Data are % (95% confidence interval), unless otherwise stated.
ITT, intent-to-treat; NSAI, nonsteroidal aromatase inhibitor.
The best overall change in tumor size in patients with measurable disease is shown in online Supplemental Figure 2. In both cohorts, the depth of tumor reduction was significantly greater in the abemaciclib arm than in the placebo arm.
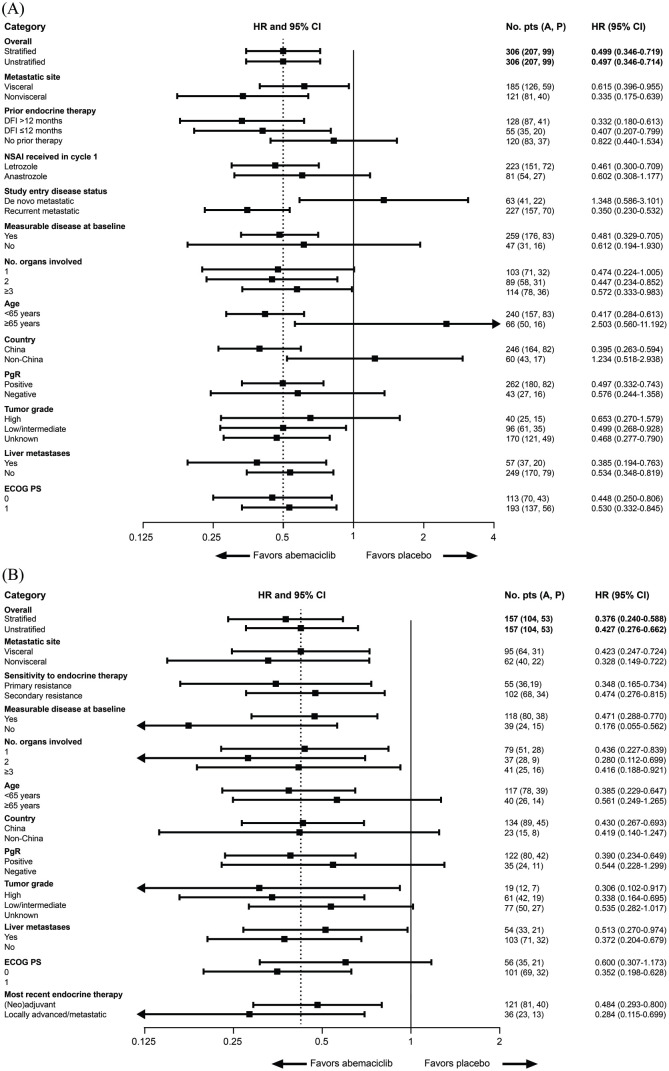
The treatment effect of abemaciclib on PFS was consistent overall across patient subgroups in both cohorts (Figure 3). Although benefit was not observed in subgroups of patients from non-China sites, aged ⩾65 years, or with de novo metastatic disease in cohort A, caution should be used in interpreting the observation due to the limited sample size and number of events at the time of the preplanned interim analysis of PFS. OS data were immature at the time of data cut-off.
Figure 3.
Progression-free survival subgroup analyses in the ITT population in (A) cohort A and (B) cohort B.Progression-free survival hazard ratios are indicated by squares and 95% CIs are indicated by the crossing horizontal lines. Hazard ratios are unstratified and estimated with the adjustment of treatment arm by subgroup interaction, with the exception of the PFS hazard ratio for the overall study population, which is also presented as the stratified hazard ratio. Groups with <10% of randomly assigned patients were omitted [patients with locoregionally recurrent disease (n = 15) in the ‘study entry disease status’ category (panel A)]. In panel A, the error bar for the subgroup of patients aged ⩾65 years is clipped at the upper limit. In panel B, the error bars for the subgroups of patients with no measurable disease at baseline, two organs involved in metastasis, high tumor grade, and most recent endocrine therapy in the locally advanced/metastatic setting are clipped at the lower limit. A, abemaciclib; AI, aromatase inhibitor; CI, confidence interval; DFI, disease-free interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; ITT, intent-to-treat; NSAI, non-steroidal aromatase inhibitor; P, placebo; PgR, progesterone receptor; PS, performance status.
Safety and tolerability measures
The most frequently reported treatment-emergent adverse events (TEAEs) in the abemaciclib arms of both cohorts were neutropenia, diarrhea, leukopenia, and anemia (Table 4). The majority of reported AEs of neutropenia were grade 1 and 2 in severity (Table 4). On the basis of central laboratory analysis, most laboratory abnormalities of grade ⩾3 neutropenia in the abemaciclib arms (cohorts A and B) occurred during the first two cycles. For laboratory abnormalities, see online Supplemental Table 4. Only one (0.5%) patient, in the abemaciclib plus NSAI arm, reported febrile neutropenia (grade 3) and recovered after supportive treatment. Four (2.0%) patients in the abemaciclib plus NSAI arm and no patients in the abemaciclib plus fulvestrant arm discontinued study treatment due to neutropenia.
Table 4.
Treatment-emergent adverse events.a
| Cohort A | ||||||
|---|---|---|---|---|---|---|
| Abemaciclib + NSAI (n = 205) | Placebo + NSAI (n = 99) | |||||
| All | Grade 3 | Grade 4 | All | Grade 3 | Grade 4 | |
| Any adverse event | 204 (99.5) | 111 (54.1) | 10 (4.9) | 88 (88.9) | 20 (20.2) | 3 (3.0) |
| Neutropeniab | 164 (80.0) | 53 (25.9) | 1 (0.5) | 20 (20.2) | 5 (5.1) | 1 (1.0) |
| Diarrhea | 164 (80.0) | 8 (3.9) | 0 | 16 (16.2) | 1 (1.0) | 0 |
| Leukopeniac | 156 (76.1) | 26 (12.7) | 1 (0.5) | 27 (27.3) | 2 (2.0) | 0 |
| Anemia | 127 (62.0) | 23 (11.2) | 0 | 20 (20.2) | 3 (3.0) | 0 |
| Thrombocytopeniad | 91 (44.4) | 11 (5.4) | 0 | 7 (7.1) | 2 (2.0) | 0 |
| ALT increased | 71 (34.6) | 11 (5.4) | 1 (0.5) | 23 (23.2) | 1 (1.0) | 0 |
| AST increased | 71 (34.6) | 8 (3.9) | 1 (0.5) | 21 (21.2) | 1 (1.0) | 1 (1.0) |
| Fatigue | 60 (29.3) | 1 (0.5) | 0 | 25 (25.3) | 1 (1.0) | 0 |
| Nausea | 55 (26.8) | 0 | 1 (0.5) | 19 (19.2) | 0 | 0 |
| Decreased appetite | 48 (23.4) | 0 | 0 | 11 (11.1) | 1 (1.0) | 0 |
| Weight decreased | 38 (18.5) | 0 | 0 | 4 (4.0) | 0 | 0 |
| Abdominal pain | 36 (17.6) | 2 (1.0) | 0 | 9 (9.1) | 1 (1.0) | 0 |
| Lymphocytopeniae | 34 (16.6) | 12 (5.9) | 0 | 4 (4.0) | 1 (1.0) | 0 |
| Vomiting | 32 (15.6) | 3 (1.5) | 1 (0.5) | 13 (13.1) | 0 | 0 |
| Upper respiratory tract infection | 31 (15.1) | 0 | 0 | 22 (22.2) | 1 (1.0) | 0 |
| Cough | 31 (15.1) | 0 | 0 | 9 (9.1) | 0 | 0 |
| Insomnia | 25 (12.2) | 0 | 0 | 18 (18.2) | 0 | 0 |
| Blood creatinine increased | 24 (11.7) | 0 | 0 | 2 (2.0) | 1 (1.0) | 1 (1.0) |
| Pain | 21 (10.2) | 0 | 0 | 7 (7.1) | 0 | 0 |
| Cohort B | ||||||
| Abemaciclib + fulvestrant (n = 104) | Placebo + fulvestrant (n = 53) | |||||
| All | Grade 3 | Grade 4 | All | Grade 3 | Grade 4 | |
| Any adverse event | 103 (99.0) | 49 (47.1) | 5 (4.8) | 42 (79.2) | 8 (15.1) | 0 |
| Leukopeniac | 86 (82.7) | 23 (22.1) | 0 | 12 (22.6) | 2 (3.8) | 0 |
| Neutropeniab | 84 (80.8) | 30 (28.8) | 1 (1.0) | 10 (18.9) | 2 (3.8) | 0 |
| Diarrhea | 82 (78.8) | 2 (1.9) | 0 | 5 (9.4) | 0 | 0 |
| Anemia | 73 (70.2) | 11 (10.6) | 0 | 8 (15.1) | 1 (1.9) | 0 |
| Thrombocytopeniad | 43 (41.3) | 3 (2.9) | 0 | 5 (9.4) | 1 (1.9) | 0 |
| ALT increased | 36 (34.6) | 6 (5.8) | 0 | 12 (22.6) | 0 | 0 |
| AST increased | 32 (30.8) | 2 (1.9) | 1 (1.0) | 14 (26.4) | 0 | 0 |
| Fatigue | 24 (23.1) | 0 | 0 | 8 (15.1) | 0 | 0 |
| Decreased appetite | 23 (22.1) | 0 | 0 | 6 (11.3) | 0 | 0 |
| Blood creatinine increased | 22 (21.2) | 1 (1.0) | 0 | 1 (1.9) | 0 | 0 |
| Lymphocytopeniae | 22 (21.2) | 11 (10.6) | 1 (1.0) | 1 (1.9) | 0 | 0 |
| Vomiting | 20 (19.2) | 1 (1.0) | 0 | 5 (9.4) | 0 | 0 |
| Nausea | 19 (18.3) | 1 (1.0) | 0 | 9 (17.0) | 0 | 0 |
| Abdominal pain | 15 (14.4) | 1 (1.0) | 0 | 3 (5.7) | 0 | 0 |
| Weight decreased | 15 (14.4) | 0 | 0 | 1 (1.9) | 0 | 0 |
| Upper respiratory tract infection | 14 (13.5) | 0 | 0 | 4 (7.5) | 1 (1.9) | 0 |
| Insomnia | 13 (12.5) | 0 | 0 | 1 (1.9) | 0 | 0 |
| Cough | 12 (11.5) | 0 | 0 | 4 (7.5) | 0 | 0 |
| Pain | 6 (5.8) | 2 (1.9) | 0 | 8 (15.1) | 1 (1.9) | 0 |
The table shows treatment-emergent adverse events (all causality) occurring in at least 15% of patients in either treatment group of cohort A or cohort B.
Data are no. (%).
CTCAE term neutrophil count decreased.
CTCAE term white blood cell count decreased.
CTCAE term platelet count decreased.
CTCAE term lymphocyte count decreased.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, common terminology criteria for adverse events; NSAI, non-steroidal aromatase inhibitor.
No grade 4 diarrhea was reported in the study (Table 4). Diarrhea of grade 3 was reported for 3.9% and 1.9% of patients in the abemaciclib arms of cohorts A and B, respectively. Only one abemaciclib-treated patient (cohort A) reported a serious adverse event (SAE) of diarrhea. Diarrhea could be well managed by antidiarrheal therapy and dose modification. No patients discontinued study treatment due to diarrhea.
In cohort A, pneumonitis was reported by 13 (6.3%) patients in the abemaciclib arm and three (3.0%) patients in the placebo arm; one patient in each arm reported grade 3 pneumonitis. Three patients (1.5%) in the abemaciclib arm and one patient (1.0%) in the placebo arm discontinued study treatment due to pneumonitis. In Cohort B, pneumonitis was reported by only one patient in each arm (1.0% and 1.9%, respectively), being grade 2 in the abemaciclib arm and grade 1 in the placebo arm. None of the patients discontinued study treatment due to pneumonitis. No deaths due to pneumonitis were reported.
In cohort A, four patients (2.0%) reported venous thromboembolic events (VTEs) [grade 1: one (0.5%) patient; grade 2: three (1.5%) patients] in the abemaciclib arm. In cohort B, four patients (3.8%) reported VTEs in the abemaciclib arm [grade 2: three (2.9%) patients; grade 3: one (1.0%) patient]. No placebo-treated patients reported VTEs. These events all resolved after anticoagulant treatment. Only one patient (0.5%), in the abemaciclib plus NSAI arm, discontinued study treatment due to embolism. No deaths due to VTE were reported.
SAEs were reported in 19.5% and 15.4% of patients in the abemaciclib arms of cohorts A and B, respectively, and 9.1% and 7.5% in the corresponding placebo arms, respectively. Lung infection was the most frequently reported SAE (2.9% versus 1.0% in the abemaciclib and placebo arms, respectively, of cohort A; 1.9% versus 1.9% in cohort B). AEs leading to discontinuation in the abemaciclib arms were reported in 10.7% of patients in cohort A and 3.8% in cohort B. Two deaths (1.0%; one patient with lung infection and one with dyspnea) in the abemaciclib plus NSAI arm and one death (1.0%; lung infection) in the abemaciclib plus fulvestrant arm that occurred during study treatment or within 30 days of treatment discontinuation were considered treatment related.
Discussion
MONARCH plus is the first randomized controlled trial to demonstrate the efficacy and safety profile of abemaciclib in a population of postmenopausal women with HR-positive, HER2-negative advanced breast cancer from China, Brazil, India, and South Africa.
Abemaciclib in combination with either an NSAI or fulvestrant showed significant and clinically meaningful improvements in PFS in this population, with an early and sustained separation of the curves between treatment arms apparent beginning at approximately 8 weeks. ORRs were also significantly improved in the abemaciclib arms, and the depth of tumor reduction was greater in the abemaciclib arm than the placebo arm in both cohorts. The efficacy benefit in MONARCH plus is consistent with the MONARCH 2 and 3 trials.4,5 An overall consistent efficacy benefit of abemaciclib was seen across the predefined subgroups, although the limited sample size and number of events within these subgroups at the time of the interim analysis should be kept in mind when interpreting these results. AEs were generally monitorable, manageable, and reversible. These results support abemaciclib plus ET as a recommended treatment option for this population.
Although, in general, the patient eligibility criteria for inclusion in cohorts A and B are adopted from the global MONARCH 3 and MONARCH 2 studies, respectively, there were still some adjustments in inclusion criteria that could help to answer unaddressed questions in MONARCH 2 and 3. The most important question relates to patients who relapsed within 12 months of completion of adjuvant ET. These patients were excluded from MONARCH 3 and only included in MONARCH 2. Considering that the breast cancer onset age in China is approximately 5–10 years earlier than in western countries,11 a significant proportion of patients will relapse on adjuvant anti-estrogen treatment who are still eligible for NSAIs. Whether they could benefit from abemaciclib plus NSAI treatment has not been answered by MONARCH 3. In this MONARCH plus study, we defined cohort A to include all NSAI-eligible patients, that is, patients who were never exposed to or were still considered sensitive to NSAI treatment. Patients who relapsed within 12 months of completion of adjuvant ET other than NSAIs were included in cohort A, whereas patients with NSAIs as adjuvant ET were included in cohort B. Subgroup analysis showed that abemaciclib in combination with fulvestrant (cohort B) or NSAIs (cohort A) could provide significant benefit in patients who relapsed within 12 months of completion of adjuvant ET with NSAIs or in patients receiving non-NSAI ET (hazard ratio 0.484 and 0.407, respectively). The results of this study also provide evidence for the update of the most influential China national breast cancer treatment guidelines issued by the Chinese Society of Clinical Oncology (CSCO) which, for the first time, recommend stratified treatment considering previous ET.12
The median PFS in cohort B of this study was shorter than in the MONARCH 2 study for both the abemaciclib and placebo arms (11.5 versus 5.6 months in cohort B and 16.4 versus 9.3 months in MONARCH 2).5 A possible reason is the difference in baseline characteristics between the two populations, as more patients with poor prognostic factors were included in cohort B compared with MONARCH 2. In cohort B, more patients had an ECOG performance status of 1 (64.3% versus 39.3%) and had primary resistance (35.0% versus 25.3%) than in MONARCH 2. Moreover, a previous study showed that the median PFS for fulvestrant was shorter in patients who relapsed from AI than anti-estrogen treatment (5.8 versus 8.1 months).13 In cohort B, almost all patients received prior AI treatment compared with two-thirds of patients in MONARCH 2. This difference may also have led to the shorter median PFS in cohort B for both arms. The results from cohort B demonstrate that in this population with relatively poorer prognoses, the addition of abemaciclib to fulvestrant provided an even larger PFS benefit in cohort B compared with MONARCH 2 (hazard ratio 0.376 versus 0.553).
No new safety signals were identified, and the most commonly reported AEs were similar between this MONARCH plus study and MONARCH 2 and 3,4,5 demonstrating a consistent safety profile across the study populations. Diarrhea was of low grade in the majority of patients and the incidence of grade ⩾3 events was relatively lower in this study compared with MONARCH 2 and 3, which might be due to raised awareness and improved management. Overall, diarrhea was manageable with antidiarrheal therapy, dose reductions, or dose omission in this population; no patients discontinued study treatment because of diarrhea. The incidence of TEAEs of neutropenia in the abemaciclib arms of this study (80.0% in cohort A; 80.8% in cohort B) was higher than in MONARCH 2 and 3 (46.0% and 41.3%, respectively), while the incidence of laboratory-based abnormalities was comparable.4,5 One possible reason for this discrepancy might be differences in AE reporting patterns among countries. Furthermore, the incidence of AEs and laboratory-based abnormalities of grade ⩾3 in this study was comparable with MONARCH 2 and 3, the incidence of study treatment discontinuations due to neutropenia was low, and only one event of febrile neutropenia was reported.
A strength of the current study is that it is the first phase III study designed to assess prospectively the efficacy and safety profile of abemaciclib in patient populations from China, Brazil, India, and South Africa, where clinical research resources are limited.14 The innovative study design allowed efficient assessment of two treatment settings in a single study, while ensuring sufficient power for the primary endpoint. A possible limitation of this study is that cohort B was designed with a sample size to show consistency (defined as 80% probability to retain at least 50% of the effective size in PFS) with MONARCH 2 and had no alpha reserved or gated for formal statistical testing. Although the sample size of cohort B was small, the results should be sufficiently robust when interpreted in conjunction with the reliable results of the large global MONARCH 2 study. Meanwhile, the OS data are immature at the data cut-off date.
In conclusion, abemaciclib in combination with ET showed significant and clinically meaningful improvements in PFS in a population of postmenopausal women with advanced breast cancer from China, Brazil, India, and South Africa. The safety profile was tolerable in this patient population and no new safety signals were observed. The data add to the totality of evidence showing the benefit of abemaciclib in populations from these countries, which were not included in most CDK 4 and CKD 6 inhibitor trials yet represent nearly 40% of the global population.
Supplemental Material
Supplemental material, MONARCH_plus_supplement_27Jul20 for MONARCH plus: abemaciclib plus endocrine therapy in women with HR+/HER2– advanced breast cancer: the multinational randomized phase III study by Qing Yuan Zhang, Tao Sun, Yong Mei Yin, Hui Ping Li, Min Yan, Zhong Sheng Tong, Christina P. Oppermann, Yun Peng Liu, Romulo Costa, Man Li, Ying Cheng, Qu Chang Ouyang, Xi Chen, Ning Liao, Xin Hong Wu, Xiao Jia Wang, Ji Feng Feng, Roberto Hegg, G.B. Kanakasetty, Maria A. Coccia-Portugal, Ru Bing Han, Yi Lu, Hai Dong Chi, Ze Fei Jiang and Xi Chun Hu in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors thank Cong Xu for his contribution during the study design and execution. They also thank Shu Wang (Eli Lilly and Company), for her contribution to the editing the first draft and the final revision and review and critical suggestions for improvement. The authors sincerely thank the patients, their families, and the study personnel across all sites for participating in this study. Medical writing assistance was provided by Linda Donnini and Justine Southby of ProScribe – Envision Pharma Group, and was funded by Eli Lilly and Company. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Footnotes
Author contributions: All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. QYZ, TS, YMY, HPL, MY, ZST, YPL, ML, YC, QCO, XC, NL, XHW, XJW, JFF, RH, HDC, ZJ and XCH were involved in the study design. ZJ and XCH were investigators in the study, and QYZ, TS, YMY, HPL, MY, ZST, CPO, YPL, RC, ML, YC, QCO, XC, NL, XHW, XJW, JFF, RH, GBK, MAC-P, ZJ and XCH were involved in data collection. YL conducted the statistical analysis, and ZJ, XCH, RBH, YL and HDC were involved in data interpretation.
Conflict of interest: RBH, YL and HDC are employees and minor shareholders of Eli Lilly and Company. XHW has received research funding from Eli Lilly and Company. QYZ, TS, YMY, HPL, MY, ZST, CPO, YPL, RC, ML, YC, QCO, XC, NL, XJW, JFF, RH, GBK, MAC-P, ZJ and XCH have no conflicts of interest to declare.
Data availability: Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data access should comply with local laws and regulations. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Eli Lilly and Company, manufacturer/licensee of abemaciclib.
Role of the sponsor: The funder of the study (Eli Lilly and Company) had a role in study design, data collection, data analysis, data interpretation (in collaboration with all authors), and writing of the report. The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ORCID iD: Yi Lu  https://orcid.org/0000-0003-2252-9813
https://orcid.org/0000-0003-2252-9813
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Qing Yuan Zhang, Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China.
Tao Sun, Department of Medical Oncology, Cancer Hospital of China Medical University/ Liaoning Cancer Hospital, Shenyang, China.
Yong Mei Yin, Department of Oncology, Jiangsu Province Hospital, Nanjing, China.
Hui Ping Li, Department of Breast Oncology, Peking University Cancer Hospital & Institute, Beijing, China.
Min Yan, Department of Breast Disease, Henan Breast Cancer Center, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China.
Zhong Sheng Tong, Department of Breast Oncology, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China.
Christina P. Oppermann, Centro de Pesquisa Clínica de Oncologia e Hematologia, Hospital Mãe de Deus/AESC, Porto Alegre, Brazil
Yun Peng Liu, Department of Medical Oncology, First Affiliated Hospital of China Medical University,Shenyang, China.
Romulo Costa, Oncologia Clínica, Instituto do Cancer do Estado de São Paulo – ICESP, São Paulo, Brazil.
Man Li, Department of Oncology, Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Ying Cheng, Department of Medical Thoracic Oncology, Jilin Provincial Cancer Hospital, Changchun, China.
Qu Chang Ouyang, Department of Breast Cancer Medical Oncology, Hunan Cancer Hospital, Changsha, China.
Xi Chen, Department of Medical Oncology, Fuzhou General Hospital of Nanjing Military Command, Fuzhou, China.
Ning Liao, Department of Breast Cancer, Guangdong General Hospital, Guangzhou, China.
Xin Hong Wu, Department of Breast Cancer, Hubei Cancer Hospital, Wuhan, China.
Xiao Jia Wang, Department of Breast Medical Oncology, Zhejiang Cancer Hospital, Hangzhou, China.
Ji Feng Feng, Department of Medical Oncology, Jiangsu Province Cancer Hospital, Nanjing, China.
Roberto Hegg, Department of Gynecology and Obstetrics, Hospital Pérola Byington and FMUSP, São Paulo-SP, Brazil.
G.B. Kanakasetty, Department of Medical Oncology, HCG Hospitals and Kidwai Memorial Institute of Oncology, Bangalore, India
Maria A. Coccia-Portugal, Clinical Trial Department, Eastleigh Breast Care Center, Pretoria, South Africa
Ru Bing Han, Eli Lilly and Company, Shanghai, China.
Yi Lu, Eli Lilly and Company, Indianapolis, IN, United States of America.
Hai Dong Chi, Eli Lilly and Company, Shanghai, China.
Ze Fei Jiang, Department of Breast Cancer, Fifth Medical Center of Chinese PLA General Hospital, 100 West Fourth Ring Middle Road, Beijing 100071, China.
Xi Chun Hu, Department of Medical Oncology, Fudan University Shanghai Cancer Center, 270 Dong’an Road, Shanghai 200032, China.
References
- 1. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17: 425–439. [DOI] [PubMed] [Google Scholar]
- 2. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 3. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 4. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638–3646. [DOI] [PubMed] [Google Scholar]
- 5. Sledge GW, Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2– advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35: 2875–2884. [DOI] [PubMed] [Google Scholar]
- 6. Population Reference Bureau. Population mid-2019. https://www.prb.org/international/indicator/population/table (2019, accessed 28 November 2019).
- 7. Corona SP, Generali D. Abemaciclib: a CDK4/6 inhibitor for the treatment of HR+/HER2– advanced breast cancer. Drug Des Devel Ther 2018; 12: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Brien N, Conklin D, Beckmann R, et al. Preclinical activity of abemaciclib alone or in combination with antimitotic and targeted therapies in breast cancer. Mol Cancer Ther 2018; 17: 897–907. [DOI] [PubMed] [Google Scholar]
- 9. Sledge GW, Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy – MONARCH 2. JAMA Oncol 2020; 6: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014; 23: 489–502. [DOI] [PubMed] [Google Scholar]
- 11. Breast cancer screening guideline for Chinese women. Cancer Biol Med 2019; 16: 822–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu F, Jiang Z. CSCO BC guideline: updates for hormone receptor-positive breast cancer in 2020. Transl Breast Cancer Res 2020; 1: 3. [Google Scholar]
- 13. Zhang Q, Shao Z, Shen K, et al. Fulvestrant 500 mg vs 250 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer: a randomized, double-blind registrational trial in China. Oncotarget 2016; 7: 57301–57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramaswami R, Paulino E, Barrichello A, et al. Disparities in breast, lung, and cervical cancer trials worldwide. J Glob Oncol 2018; 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MONARCH_plus_supplement_27Jul20 for MONARCH plus: abemaciclib plus endocrine therapy in women with HR+/HER2– advanced breast cancer: the multinational randomized phase III study by Qing Yuan Zhang, Tao Sun, Yong Mei Yin, Hui Ping Li, Min Yan, Zhong Sheng Tong, Christina P. Oppermann, Yun Peng Liu, Romulo Costa, Man Li, Ying Cheng, Qu Chang Ouyang, Xi Chen, Ning Liao, Xin Hong Wu, Xiao Jia Wang, Ji Feng Feng, Roberto Hegg, G.B. Kanakasetty, Maria A. Coccia-Portugal, Ru Bing Han, Yi Lu, Hai Dong Chi, Ze Fei Jiang and Xi Chun Hu in Therapeutic Advances in Medical Oncology