Abstract
Purpose
Neutrophil-to-lymphocyte ratio (NLR) has been suggested as an independent risk factor for progression-free survival (PFS) and overall survival (OS) in small cell lung cancer (SCLC). However, it is still unknown whether there is a linear relationship between the NLR and the risk of death in SCLC. The objective of this study is to provide further results.
Patients and Methods
A retrospective cohort study was performed among a total of 251 participants with SCLC. Smooth curve fitting and piecewise Cox regression model were used to determine the linear relationship between NLR and mortality risk. A multivariable Cox regression model was used to estimate the effects of NLR on OS. Interaction and stratified analyses were conducted according to covariates.
Results
The analysis indicated no significant nonlinear relationship or threshold effect between NLR and hazard of death. Multivariate analysis revealed that every unit increase in NLR was associated with a 10% increase in mortality risk. High NLR (>3.5) at baseline was associated with poor OS (hazard ratio [HR]=1.97, P=0.009). The difference in median OS duration between the high and low NLR groups was statistically significant (9.1 months vs 14.6 months, P=0.0067). Furthermore, interaction analysis identified the chemotherapy regimen to play an interactive role in the association between NLR and hazard of death.
Conclusion
NLR was identified as an independent risk factor for OS in SCLC and the linear correlation was observed between them. Administration of etoposide plus cisplatin (EP) regimen in patients with low NLR resulted in better long-term outcome than that of etoposide plus carboplatin (EC) regimen, while administration of the EC regimen conferred longer OS than that of the EP regimen in patients with high NLR.
Keywords: neutrophil-to-lymphocyte ratio, NLR, overall survival, small cell lung cancer, SCLC, etoposide plus carboplatin/cisplatin, mortality risk
Introduction
Small cell lung cancer (SCLC), which accounts for about 13% of all the primary lung cancer cases,1 is characterized by rapid cell division, poor differentiation, high grade of histological classification, early metastasis, aggressive disease progression, and short survival duration. Most of the patients present with an advanced disease stage at initial diagnosis and lost the opportunity of radical resection, and only a small number of patients at T1-2N0M0 of limited stage (LS) can receive radical surgery.2 Currently, systemic chemotherapy is recommended for patients with extensive stage (ES)-SCLC, while a combination of chemotherapy and radiotherapy is the standard treatment recommended for patients with inoperable LS-SCLC.3 The objective response rate to front-line treatment is very high, although the duration of progression-free survival is relatively short, and disease progression is inevitable along with the emergence of resistance in a majority of cases. Thus, identifying the prognostic predictors of SCLC may change current treatment strategies and improve patients’ long-term prognosis.
One of the clear predictors of prognosis is the stage of SCLC, with a median OS duration of 19.7–27.2 months in inoperable patients with LS and only 10.3–12.3 months in patients with ES.3,4 Furthermore, multiple factors are confirmed to influence the prognosis of SCLC. For instance, the peripheral blood indicators such as lactate dehydrogenase (LDH), C-reactive protein (CRP) and the clinical characteristics such as age, Eastern Cooperative Oncology Group Performance Status (ECOG PS), smoking status, and nutritional status can affect prognosis in SCLC.5–8 Additionally, the TNM staging and the Veterans Administration Lung Study Group of the United States staging are widely used in SCLC, although there are limitations in determining prognosis as these methods cannot provide comprehensive evaluation of the readily available information. Moreover, the staging information may not be available for certain reasons, including the lack of radiographic data. Therefore, it is imperative to explore the factors affecting patient survival to provide better treatment and establish an accurate prognostic model in SCLC.
The occurrence and development of tumors is linked to the dysfunction of the body’s immune system. Moreover, inflammation is a critical aspect of cancer progression.9,10 Previous studies have presented that NLR could serve as a systemic inflammatory response index to provide important information regarding inflammation and immune state. NLR has been identified as a prognostic biomarker in several cancer types, including urinary tract epithelial tumors, prostate cancer, and non-small cell lung cancer (NSCLC),11–14 although the relationship between NLR and long-term outcomes in SCLC remains to be completely understood. Thus, this study was designed to further explore the effects of NLR on the prognosis and identify other factors that modify this association in patients with SCLC.
Patients and Methods
Study Design and Study Population
This was a retrospective cohort study. We consecutively collected data for patients with SCLC between March 2008 and March 2019 from Guangxi Medical University Affiliated Tumor Hospital, Guangxi province, China. The study was approved by the institutional ethics committee of the Guangxi Medical University Cancer Hospital, and conducted in compliance with the Declaration of Helsinki. Patients’ identifiable data were anonymized, and the requirement for informed consent was waived due to the observational nature of the study.
Patients with a histologically or cytologically confirmed diagnosis of SCLC were enrolled for further screening. Peripheral blood examination and clinical records of patients’ sex, age, body mass index (BMI), clinical stage, ECOG PS, smoking status, distant metastases, histological classification, and first-line treatments could be obtained. All patients enrolled in this study received standard first-line treatment regimen in accordance with relevant guidelines and protocols. The exclusion criteria were: a) Early stage patients who can undergo surgery; b) No treatment after diagnosis; c) Second primary tumor; d) Appearance of hematological changes (including autoimmune disease, infection, leukemia, lymphoma, and myelodysplastic syndrome); e) tumors with mixed pathological pattern and other histological types (including adenocarcinoma, squamous carcinoma, adenosquamous carcinoma, alveolar carcinoma, mesohyloma, carcinoid, and large cell carcinoma). Finally, a total of 251 participants were enrolled in the study based on the inclusion and exclusion criteria.
Data Collection
The target-independent variable was NLR, which was obtained during routine blood examination prior to administration of chemotherapy, and the dependent variable was OS duration. The OS duration was calculated as the time from pathological diagnosis till death or the last follow-up, before January 31, 2020.
Covariates involved in the present study can be summarized as follows: 1) We collected information about demographic data and clinical characteristics including sex, age, BMI, clinical stage, ECOG PS, smoking history, sites, and number of distant metastases; and 2) We also collected information about laboratory markers that are reported to affect NLR or overall survival, such as albumin (ALB), LDH, CRP, and first-line treatments.5,15,16
Definitions of clinicopathological characteristics or parameters used in this study included: 1) Patients’ physical status were scored using ECOG PS; 2) Non-smokers were defined as patients who had smoked less than 100 cigarettes in their lifetime. Smokers were individuals who were actively smoking or those who smoked more than 100 cigarettes in their lifetime; 3) Tumor histology was classified according to the fourth edition of WHO classification of lung tumors;17 4) Clinical staging was divided into LS and ES in accordance with the staging methods of the Veterans Administration Lung Study Group of the United States; and 5) OS duration was specified as the time period from pathological diagnosis to death or last follow-up.
Statistical Analysis
The continuous variables NLR, ALB, BMI, CRP, and LDH were divided into low and high groups based on cut-off values determined by time-dependent receiver operating characteristic (ROC) curve and Youden’s index. Categorical variables were expressed as frequency and percentage. We used χ2/Fisher’s exact probability test to identify the differences in variables between low and high NLR. The association between NLR and hazard of death was visually displayed using the smoothing plot after adjustment for potential confounders. Cox proportional hazard model and two-piecewise Cox proportional hazard model was used to examine the linear relationship between NLR and death hazard based on the smoothing plot. Further, Wald test and log likelihood ratio test were applied to examine the saturation effect and determine which model was more suitable for fitting the relationship, respectively. Univariate and multivariate Cox proportional hazard model were employed to evaluate the effect of the independent variable. The subgroup analyses were performed using stratified Cox proportional hazard model. For analysis of continuous variable, we first converted it into a categorical variable based on the cutoff value, and then possible modifications of the association between baseline NLR and hazard of death were evaluated by performing an interaction test. Kaplan Meier curves and Log rank tests were used to assess differences in OS between groups. All the analyses were performed with the statistical software R packages (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA). Two-sided P-values<0.05 were considered statistically significant.
Results
Baseline Characteristics of Selected Patients
Final data analysis was performed with the 251 participants selected based on the stringent screening criteria. According to the ROC curve analysis and Youden’s index, a value of 3.5 was selected as a cut-off value to differentiate the high and low NLR group with a sensitivity and specificity of 0.414 and 0.691, respectively. Using the same method, the cut-off values of ALB, BMI, CRP, and LDH were determined to be 37.2, 20.4, 22, and 223, respectively. Based on the cut-off value of NLR, baseline characteristics of the selected patients are summarized in Table 1. The patients had an average age of 60±8.6 years, and 32 (approximately 13%) were women. Seventy-two (about 28.7%) and 170 (about 67.7%) patients were confirmed with LS and ES, respectively, but staging status could not be confirmed in nine (0.03%) patients. The analysis of survival data indicated that 53 patients survived and 142 patients died, while 56 were lost during follow-up. The median OS duration for patients with LS was 16 months, which was 4.1 months longer than that for patients with ES, but without statistical significance (P=0.18). Further, there were no significant differences between the low and high NLR groups (P>0.05) with respect to the confounders, viz. age, sex, smoking status, ECOG PS, BMI, and data for metastases to the brain, lung, pleura, pericardia, adrenal gland, and bone. However, high NLR was associated with increased CRP and LDH, and decreased ALB (P<0.05) than low NLR. Moreover, patients in the high NLR group had a higher proportion of ES, liver metastases, number of distant metastatic organs≥2, and EC regimen than those in the low NLR group (P<0.05). These potential confounding factors, which were significantly associated with mortality risk in the univariate analysis (P<0.05), were enrolled in the multivariable Cox regression model.
Table 1.
Association Between the NLR and Other Confounders of Study Population
| NLR | NLR≤3.50 (n=159) | NLR>3.50 (n=92) | P-value |
|---|---|---|---|
| Age | 0.380 | ||
| ≤60 | 80 (50.3%) | 41 (44.6%) | |
| >60 | 79 (49.7%) | 51 (55.4%) | |
| Sex | 0.093 | ||
| Male | 143 (89.9%) | 76 (82.6%) | |
| Female | 16 (10.1%) | 16 (17.4%) | |
| Clinical stage | 0.002* | ||
| LS | 57 (35.8%) | 15 (16.3%) | |
| ES | 96 (60.4%) | 74 (80.4%) | |
| Unknown | 6 (3.8%) | 3 (3.3%) | |
| Smoking history | 0.415 | ||
| Yes | 28 (17.6%) | 19 (20.7%) | |
| No | 129 (81.1%) | 70 (76.1%) | |
| Unknown | 2 (1.3%) | 3 (3.3%) | |
| ECOG PS | 0.182 | ||
| <2 | 135 (84.9%) | 72 (78.3%) | |
| ≥2 | 24 (15.1%) | 20 (21.7%) | |
| Brain metastases | 0.653 | ||
| No | 141 (88.7%) | 78 (84.8%) | |
| Yes | 12 (7.5%) | 9 (9.8%) | |
| Unknown | 6 (3.8%) | 5 (5.4%) | |
| Lung metastases | 0.333 | ||
| No | 128 (80.5%) | 67 (72.8%) | |
| Yes | 25 (15.7%) | 20 (21.7%) | |
| Unknown | 6 (3.8%) | 5 (5.4%) | |
| Pleural metastases | 0.194 | ||
| No | 125 (78.6%) | 61 (66.3%) | |
| Yes | 28 (17.6%) | 26 (28.3%) | |
| Unknown | 6 (3.8%) | 5 (5.4%) | |
| Pericardial metastases | 0.207 | ||
| No | 134 (84.3%) | 75 (81.5%) | |
| Yes | 7 (4.4%) | 9 (9.8%) | |
| Unknown | 6 (3.8%) | 5 (5.4%) | |
| Liver metastases | 0.002* | ||
| No | 136 (85.5%) | 62 (67.4%) | |
| Yes | 17 (10.7%) | 25 (27.2%) | |
| Unknown | 6 (3.8%) | 5 (5.4%) | |
| Adrenal gland metastases | 0.674 | ||
| No | 142 (89.3%) | 79 (85.9%) | |
| Yes | 11 (6.9%) | 8 (8.7%) | |
| Unknown | 6 (3.8%) | 5 (5.4%) | |
| Bone metastases | 0.256 | ||
| No | 125 (78.6%) | 64 (69.6%) | |
| Yes | 28 (17.6%) | 23 (25.0%) | |
| Unknown | 6 (3.8%) | 5 (5.4%) | |
| Other metastases | 0.065 | ||
| No | 148 (93.1%) | 78 (84.8%) | |
| Yes | 5 (3.1%) | 9 (9.8%) | |
| Unknown | 6 (3.8%) | 5 (5.4%) | |
| Number of metastatic sites | 0.012* | ||
| <2 | 114 (71.7%) | 49 (53.3%) | |
| ≥2 | 39 (24.5%) | 38 (41.3%) | |
| Unknown | 6 (3.8%) | 5 (5.4%) | |
| Chemotherapy regimen | 0.045* | ||
| EC | 75 (47.2%) | 47 (51.1%) | |
| EP | 64 (40.3%) | 24 (26.1%) | |
| IP | 5 (3.1%) | 3 (3.3%) | |
| Unknown | 15 (9.4%) | 18 (19.6%) | |
| ALB (g/L) | <0.001* | ||
| <37.2 | 71 (46.1%) | 62 (68.1%) | |
| ≥37.2 | 83 (53.9%) | 29 (31.9%) | |
| BMI | 0.213 | ||
| <20.4 | 46 (30.1%) | 33 (37.9%) | |
| ≥20.4 | 107 (69.9%) | 54 (62.1%) | |
| CRP (mg/L) | 0.001* | ||
| ≤22 | 86 (73.5%) | 29 (49.2%) | |
| >22 | 31 (26.5%) | 30 (50.8%) | |
| LDH (U/L) | <0.001* | ||
| ≤223 | 73 (47.4%) | 22 (25.3%) | |
| >223 | 81 (52.6%) | 65 (74.7%) |
Notes: Results in table: N (%). *P-value less than 0.05 is considered as significant difference between groups.
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; LS, limited stage; ES, extensive stage; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EC, etoposide plus carboplatin; EP, etoposide plus cisplatin; IP, irinotecan plus platinum; ALB, albumin; BMI, body mass index; LDH, lactate dehydrogenase; CRP, C-reactive protein.
Univariates Analysis
Next, we used the univariate Cox proportional hazard model to perform the univariate analysis, and the results are as summarized in Table 2. We found pleural metastases (HR=1.68), liver metastases (HR=2.19), other metastases (HR=2.40), metastatic sites≥2 (HR=1.71), male sex (HR=1.78), elevated NLR (HR=1.58), CRP (HR=1.69), LDH (HR=1.70), decreased ALB (HR=1.93), and BMI (HR=1.74) to be positively associated with the mortality risk (P<0.05). Furthermore, better prognosis was observed in patients who received etoposide plus cisplatin (EP) than in those who received etoposide plus carboplatin (EC) or irinotecan plus platinum (IP) chemotherapy regimen, but without significant differences between the outcomes, consistent with available literature.18 Additionally, advanced age, ES, high ECOG PS score, smoker status, and metastases were also associated with higher mortality risk, but without statistical significance (P>0.05). Variables significantly or potentially associated with mortality risk were included in multivariate Cox regression analysis, except pleural, liver and other metastases due to their overlap with the number of metastatic organs.
Table 2.
Univariate Analysis of Factors Potentially Associated with Overall Survival
| Variables | N (%) | HR (95% CI) P-value |
|---|---|---|
| Sex | ||
| Female | 32 (12.75%) | 1.0 |
| Male | 219 (87.25%) | 1.78 (1.02–3.09) 0.0421* |
| Age | ||
| ≤60 | 121 (48.21%) | 1.0 |
| >60 | 130 (51.79%) | 1.09 (0.78–1.51) 0.6121 |
| Clinical stage | ||
| LS | 72 (28.69%) | 1.0 |
| ES | 170 (67.73%) | 1.29 (0.89–1.88) 0.1797 |
| Unknown | 9 (3.59%) | 1.92 (0.75–4.90) 0.1721 |
| ECOG PS | ||
| <2 | 207 (82.47%) | 1.0 |
| ≥2 | 44 (17.53%) | 1.26 (0.83–1.91) 0.2724 |
| Smoking history | ||
| No | 47 (18.73%) | 1.0 |
| Yes | 199 (79.28%) | 1.43 (0.93–2.22) 0.1057 |
| Unknown | 5 (1.99%) | 2.48 (0.95–6.51) 0.0640 |
| Brain metastases | ||
| No | 219 (87.25%) | 1.0 |
| Yes | 21 (8.37%) | 1.24 (0.72–2.12) 0.4332 |
| Unknown | 11 (4.38%) | 0.62 (0.27–1.42) 0.2594 |
| Lung metastases | ||
| No | 195 (77.69%) | 1.0 |
| Yes | 45 (17.93%) | 1.34 (0.87–2.07) 0.1906 |
| Unknown | 11 (4.38%) | 0.64 (0.28–1.46) 0.2861 |
| Pleural metastases | ||
| No | 186 (74.10%) | 1.0 |
| Yes | 54 (21.51%) | 1.76 (1.19–2.61) 0.0047* |
| Unknown | 11 (4.38%) | 0.67 (0.29–1.54) 0.3495 |
| Pericardial metastases | ||
| No | 224 (89.24%) | 1.0 |
| Yes | 16 (6.37%) | 1.71 (0.89–3.26) 0.1058 |
| Unknown | 11 (4.38%) | 0.63 (0.27–1.43) 0.2645 |
| Liver metastases | ||
| No | 198 (78.88%) | 1.0 |
| Yes | 42 (16.73%) | 2.14 (1.39–3.30) 0.0005* |
| Unknown | 11 (4.38%) | 0.67 (0.29–1.53) 0.3381 |
| Adrenal gland metastases | ||
| No | 221 (88.05%) | 1.0 |
| Yes | 19 (7.57%) | 1.56 (0.86–2.82) 0.1429 |
| Unknown | 11 (4.38%) | 0.63 (0.28–1.43) 0.2700 |
| Bone metastases | ||
| No | 189 (75.30%) | 1.0 |
| Yes | 51 (20.32%) | 1.13 (0.76–1.67) 0.5413 |
| Unknown | 11 (4.38%) | 0.63 (0.27–1.44) 0.2718 |
| Other metastases | ||
| No | 226 (90.04%) | 1.0 |
| Yes | 14 (5.58%) | 2.40 (1.26–4.59) 0.0080* |
| Unknown | 11 (4.38%) | 0.63 (0.28–1.44) 0.2781 |
| Number of metastatic sites | ||
| <2 | 163 (64.94%) | 1.0 |
| ≥2 | 77 (30.68%) | 1.72 (1.20–2.46) 0.0033* |
| Unknown | 11 (4.38%) | 0.70 (0.31–1.61) 0.4031 |
| Chemotherapy regimen | ||
| EC | 122 (48.61%) | 1.0 |
| EP | 88 (35.06%) | 0.82 (0.57–1.17) 0.2702 |
| IP | 8 (3.19%) | 1.01 (0.41–2.50) 0.9899 |
| Unknown | 33 (13.15%) | 0.99 (0.56–1.73) 0.9583 |
| NLR | ||
| ≤3.5 | 159 (63.35%) | 1 |
| >3.5 | 92 (36.65%) | 1.58 (1.13–2.21) 0.0073* |
| ALB (g/L) | ||
| ≥37.2 | 112 (45.71%) | 1.0 |
| <37.2 | 133 (54.29%) | 1.93 (1.37–2.73) 0.0002* |
| BMI | ||
| ≥20.4 | 161 (67.08%) | 1.0 |
| <20.4 | 79 (32.92%) | 1.74 (1.22–2.48) 0.0022* |
| CRP (mg/L) | ||
| ≤22 | 115 (65.34%) | 1.0 |
| >22 | 61 (34.66%) | 1.69 (1.13–2.54) 0.0111* |
| LDH (U/L) | ||
| ≤223 | 95 (39.42%) | 1.0 |
| >223 | 146 (60.58%) | 1.70 (1.20–2.41) 0.0029* |
Notes: Figures in the table: N (%) or HR (95% CI) P-value. *P-values less than 0.05.
Abbreviations: HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EC, etoposide plus carboplatin; EP, etoposide plus cisplatin; IP, irinotecan plus platinum; NLR, neutrophil-to-lymphocyte ratio; ALB, albumin; BMI, body mass index; CRP, C-reactive protein; LDH, lactate dehydrogenase.
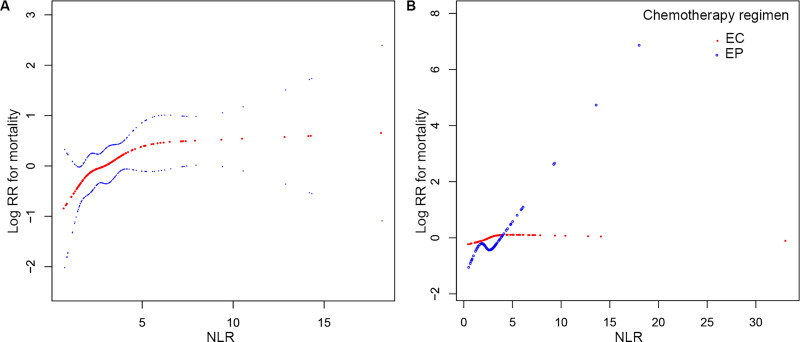
Linearity of NLR and Mortality Risk
We analyzed the linear relationship between NLR and overall survival (Figure 1A). Spline smoothing using a generalized additive model showed that NLR tended to have a saturation effect on mortality risk after adjusting for sex, tumor stage, smoking status, number of metastatic sites, ALB, BMI, CRP, and LDH. We used both adjusted Cox proportional hazard model and adjusted two-piecewise Cox proportional hazard model (Table 3) to fit the association and select the best fit model based on P-value in the log likelihood ratio test. Wald test indicated an insignificant nonlinear relationship and saturation effect between NLR and death risk, meanwhile the Cox proportional hazard model was more suitable to fit the association between NLR and mortality risk according to log likelihood ratio test.
Figure 1.
Smooth curve fitting for the relationship between baseline NLR and log (RR) for risk of mortality in SCLC patients. Red dotted lines represent the spline plots of log (RR) for mortality, and blue dotted lines represent the 95% CIs of the spline plots. (A) The relationship between NLR and log (RR) for mortality. Adjusted for sex, tumor stage, smoking status, number of metastatic sites, ALB, BMI, CRP, and LDH. (B) The relationship between NLR and log (RR) for mortality stratified by first-line chemotherapy regimen without adjustment.
Table 3.
Analysis of Threshold Effect of NLR (per Unit Increase) on Death Risk
| Outcome | HR (95% CI) P-value |
|---|---|
| Model I | 1.10 (1.01–1.19) 0.0283* |
| Model II | |
| Inflection point (K=4.5) | |
| <K | 1.32 (1.03–1.69) 0.0258* |
| >K | 1.02 (0.89–1.16) 0.8079 |
| Wald test | 0.1110 |
| Log likelihood ratio test | 0.107 |
Notes: Figures in the table: HR (95% CI) P value. Model I: Cox proportional hazard model; Model II: two-piecewise Cox proportional hazard model; both models adjusted for sex, tumor stage, smoking status, number of metastatic sites, ALB, BMI, CRP, and LDH. The Wald test was performed to determine whether HR (<K) was equal to HR (>K); Log likelihood ratio test was performed to determine any differences between model I and model II. *P-values less than 0.05.
Results of Unadjusted and Adjusted Cox Proportional Hazard Model
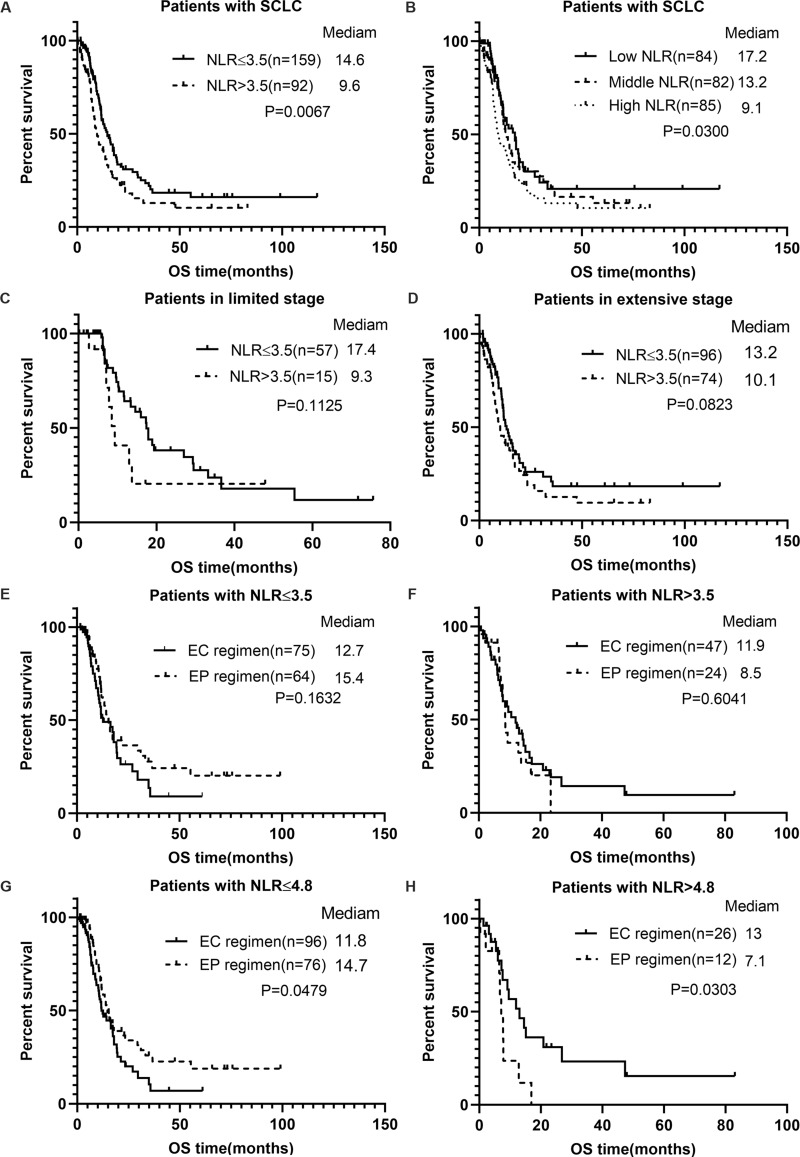
Independent effects of NLR on overall survival were analyzed by two models – univariate and multivariate Cox proportional hazard model – in this study. The HR and 95% confidence intervals are listed in Table 4. The results of both models showed a statistically significant correlation between NLR and the risk of death (P<0.05). In the unadjusted model, the effect value was 1.04, indicating that every unit increase in NLR would contribute to an extra 4% risk of death when other variables remained unadjusted. After the NLR was divided into low and high groups by cut-off point 3.5, the risk of death increased by 58% in the high group than that in low group. Kaplan-Meier survival analysis indicates that patients in the high NLR group showed significantly shorter OS duration than those in the low NLR group (9.6 months vs 14.6 months, P=0.0067) (Figure 2A). Moreover, mortality risk was found to be significantly increased in patients in the high tertile than those in middle and low tertiles (P for trend=0.0107). Patients with middle NLR had shorter OS duration than those with low NLR (13.2 months vs 17.2 months), while they had longer OS duration than those with high NLR (13.2 months vs 9.1 months), and the difference was statistically significant (Figure 2B). Further, in the full-adjusted mode, the results remained significant and robust after adjusting for sex, tumor stage, smoking status, number of metastatic sites, ALB, BMI, CRP, and LDH. Every unit increase in NLR was found to be associated with a 10% increase in mortality risk, and the mortality risk in patients with high NLR (>3.5) was about twice that of patients with low NLR (≤3.5). Thus, the two models suggested a stable correlation between NLR and long-term prognosis. Furthermore, multivariate analysis also identified male sex, number of metastatic sites≥2, and elevated LDH as independent risk factors for the death of SCLC patients.
Table 4.
Multivariate Cox Regression Model for Death Risk in SCLC
| Exposure | Non-Adjusted | Adjusted |
|---|---|---|
| NLR, per unit increase | 1.04 (1.00–1.08) 0.0389* | 1.10 (1.01–1.19) 0.0283* |
| NLR | ||
| ≤3.50 | 1.0 | 1.0 |
| >3.50 | 1.58 (1.13–2.21) 0.0073* | 1.97 (1.18–3.29) 0.0090* |
| NLR tertile | ||
| Low | 1.0 | 1.0 |
| Middle | 1.20 (0.78–1.83) 0.4059 | 1.47 (0.83–2.61) 0.1861 |
| High | 1.68 (1.13–2.50) 0.0109* | 2.35 (1.29–4.31) 0.0055* |
| P for trend | 0.0107* | 0.0057* |
| Sex | ||
| Female | 1.0 | 1.0 |
| Male | 1.78 (1.02–3.09) 0.0421* | 7.32 (2.24–23.95) 0.0010* |
| Number of metastatic sites | ||
| <2 | 1.0 | 1.0 |
| ≥2 | 1.72 (1.20–2.46) 0.0033* | 1.81 (1.10–3.00) 0.0208* |
| ALB (g/L) | ||
| ≥37.2 | 1.0 | 1.0 |
| <37.2 | 1.93 (1.37–2.73) 0.0002* | 1.40 (0.86–2.28) 0.1718 |
| BMI | ||
| ≥20.4 | 1.0 | 1.0 |
| <20.4 | 1.74 (1.22–2.48) 0.0022* | 1.19 (0.73–1.92) 0.4894 |
| CRP (mg/L) | ||
| ≤22 | 1.0 | 1.0 |
| >22 | 1.69 (1.13–2.54) 0.0111* | 1.06 (0.66–1.71) 0.8151 |
| LDH (U/L) | ||
| ≤223 | 1.0 | 1.0 |
| >223 | 1.70 (1.20–2.41) 0.0029* | 1.81 (1.11–2.95) 0.0177* |
Notes: Figures in the table: HR (95% CI) P-value. *P-values less than 0.05. Non-adjusted model adjusted for: None; Adjusted model adjusted for sex, stage, smoking status, number of metastatic sites, ALB, BMI, NLR, CRP, and LDH other than itself.
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; ALB, albumin; BMI, body mass index; CRP, C-reactive protein; LDH, lactate dehydrogenase.
Figure 2.
Survival curve of each index. (A) Survival curves of SCLC patients stratified by NLR=3.5; (B) survival curves of SCLC patients stratified by NLR tertile; (C) survival curves of LS-SCLC patients stratified by NLR=3.5; (D) survival curves of ES-SCLC patients stratified by NLR=3.5; (E) survival curves of SCLC patients with NLR≤3.5 stratified by chemotherapy regimen; (F) survival curves of SCLC patients with NLR>3.5 stratified by chemotherapy regimen; (G) survival curves of SCLC patients with NLR≤4.8 stratified by chemotherapy regimen; (H) survival curves of SCLC patients with NLR>4.8 stratified by chemotherapy regimen.
Subgroup Analysis
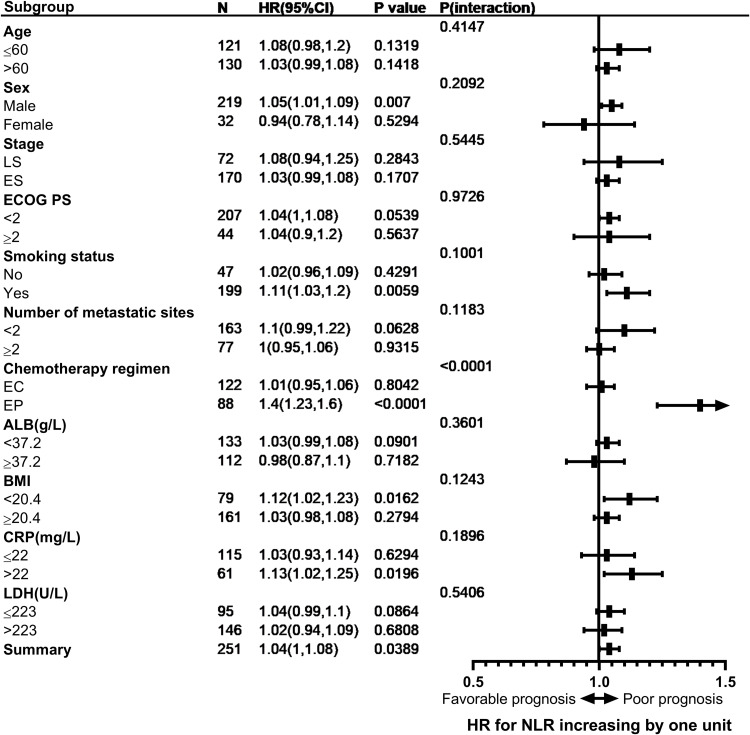
A series of potential confounders were regarded as the stratification variables to analyze the association between NLR and prognosis in patients with SCLC. (Figure 3). In the stratified Cox proportional hazard model, none of the stratification variables significantly modified the association between NLR and hazard of death, except for chemotherapy regimen. NLR had no significant correlation with prognosis in patients receiving EC chemotherapy (per unit increase: HR=1.01; 95% CI=0.91–1.12; P=0.8695) after adjustment, but had a significant correlation with prognosis in patients receiving EP chemotherapy (per unit increase: HR=1.51; 95% CI=1.20–1.89; P=0.0003). Moreover, the analysis indicated a significant interaction between NLR and chemotherapy regimen with risk of death (P for interaction=0.0006).
Figure 3.
The association between baseline NLR (per unit increase) and mortality risk in various subgroups without adjustment.
In the hierarchical survival curve, the median OS duration of the high NLR group was shorter than that of the low NLR group in both LS and ES patients (Figure 2C and D), although it was statistically insignificant (LS: 9.3 months vs 17.4 months, P=0.1125; ES: 10.1 months vs 13.2 months, P=0.0823). Additionally, in patients separated by a NLR cut-off point of 3.5, survival analysis indicated that administration of the EP regimen led to increased survival over that of EC in the low NLR group, but without a statistically significant difference (15.4 months vs 12.7 months, P=0.1632) (Figures 2E and F). In contrast, administration of EC conferred a better survival outcome than that of EP in the high NLR group, but with no statistical difference (11.9 vs 8.5 months, P=0.6041). Further, unadjusted smooth curve fitting (Figure 1B) indicated that curves of EP and EC intersected at about NLR=4.8; and the mortality risk of patients receiving an EP regimen was lower than those receiving an EC regimen with NLR<4.8, while the risk was inverse with NLR>4.8. Correspondingly, for a cut-off value of 4.8 in patients in the low NLR group, treatment with EP conferred a longer total survival duration than that with EC, and the difference was statistically significant (14.7 months vs 11.8 months, P=0.0479) (Figure 2G and H). However, in patients with NLR>4.8, treatment with the EC regimen conferred better OS outcome than that with the EP regimen, and the difference was statistically significant (13 months vs 7.1 months, P=0.0303).
Disussion
In this study, we identified high NLR to be associated with high levels of CRP and LDH as well as low levels of ALB. Patients in the high NLR group tended to have a high proportion of extensive stage, liver metastases and distant metastatic organs≥2 than those in the low NLR group. However, the underlying biological mechanisms involved in these associations remain to be completely understood. A literature review suggested ALB and BMI to be indicators of nutritional status, and reports by Kaya et al19,20 and Baldwin indicated high NLR to be associated with malnutrition in elder patients and a poor prognosis. On the other hand, neutrophils are known to secret interleukin-8 (IL-8), vascular endothelial growth factor (VEGF), and elastase, which are essential for tumor growth, infiltration, and metastasis.21 Thus, we speculated that NLR was associated with high tumor burden, which may explain the reason for high tumor stage, increased metastatic sites, and elevated levels of LDH and CRP in patients with high NLR compared to those with low NLR.
Moreover, the univariate analysis indicated that sex, pleural metastases, liver metastases, other metastases, numbers of metastatic sites, ALB, BMI, CRP, and LDH were additional factors affecting patients’ prognosis. As an imbalance of confounding factors between low and high NLR could nullify the results of this study, we used the multivariate Cox regression model adjusted for the variables significantly or potentially associated with risk of death to evaluate the independent effect of NLR on the prognosis. Furthermore, the effect of NLR on mortality risk in the non-adjusted univariate analysis was lower than that in adjusted multivariate analysis, indicating that the confounders had reduced the impact of NLR on mortality risk and that conclusions of this should be reliable.
Previous studies have suggested NLR to be an independent predictor of PFS and OS in SCLC.5,15,22,23 The present study extends this conclusion by confirming a linear positive correlation between NLR and risk of death, and the association between them can be significantly modified by chemotherapy regimen.
Chronic inflammation can dysregulate the secretion of various cytokines and chemokines, which leads to uncontrolled cell repair and obstructed death processes, thus inducing tumors.24 Studies have shown that tumor-associated macrophages (TAMs) and tumor-associated neutrophils (TANs) can release inflammatory mediators in the tumor microenvironment, and contribute to tissue remodeling, angiogenesis, and distant metastases in several tumor types.25–27 As NLR represents the ratio of neutrophil count to lymphocyte count, it could reflect the systemic inflammatory properties as a peripheral hematological index. Recent studies have found NLR to be related to inferior prognosis in various solid tumors.5,11,12,16,28 A higher NLR value often indicates a higher neutrophil or lower lymphocyte count. Neutrophils can secrete essential factors, including IL-8, VEGF, and elastase for tumor growth and metastases.21 Shaul et al29 suggested that the peripheral blood in patients with advanced lung cancer was the preferred site for accumulation of low-density neutrophils that are associated with poor prognosis. Moreover, proinflammatory neutrophils can release neutrophil extracellular traps (NETs) to capture pathogens and function as an antibacterial barrier in the body, although these neutrophils are also associated with the incidence of chronic inflammation and cancer.27 In the study by Yang et al,27 neutrophils were found to release NETs to capture and increase the immortalization and metastasis potential of hepatocellular carcinoma cells via activation of toll-like receptor 4/9-cyclooxygenase-2 (TLR4/9-COX2) signaling. Therefore, a large neutrophil count indicates a high level of low-density neutrophils and NETs, and affects the prognosis of patients.
The systemic immune status plays an important role in the occurrence and evolution of tumor and affects the efficacy and prognosis of the immunotherapy.30 Lymphocytes are the main effector cells of anti-tumor immunity. High circulating lymphocyte count represents a better immune state of the body, and increased tumor infiltrating lymphocytes confer strong anti-tumor cell activity and better prognosis. Studies suggest that neutrophils could inhibit the number and function of lymphocytes and natural killer cells, in vitro, leading to immunosuppression.31–33 Thus, these studies support the prognostic effects of NLR as observed in our study.
Disease management strategies and survival data of SCLC have remained unchanged in the recent two decades after platinum-etoposide and platinum-irinotecan were established as first-line chemotherapy.34,35 After the use of immunotherapy became popular in NSCLC, atezolizumab, a drug with anti-PD-L1 property, was recently approved as a first-line treatment regimen in combination with platinum-etoposide in ES-SCLC, although the total survival duration of patients was extended by only 2 months in the IMpower133 trial.4 Therefore, the prognosis remains relatively poor, and newer treatment methods and strategies are urgently required. In the stratified analysis, we observed an interactive effect of baseline NLR and chemotherapy regimens with mortality risk in SCLC. NLR was found to affect prognosis in patients receiving EP regimen, but not in those receiving EC regimen. These results were in contrast to those of Pan et al,23 although they used a smaller sample size and performed stratified survival analysis only, which affected the reliability and evidence in their study.
Carboplatin has overall lower toxicity than cisplatin in all the organs, except the bone marrow. The main dose-limiting toxicity of carboplatin is myelosuppression. When treated with carboplatin alone, the leucopenia was reported in 27–38% of the patients. Ferry et al36 observed that treatment with carboplatin induced grade 3 or higher neutrophil cytopenia, which was about 3-times higher than that with cisplatin (37.1% vs 13.6%) in a Phase III clinical trial in advanced NSCLC. Therefore, we hypothesized that the neutrophil cytopenia in patients receiving EC regimen progressed to a higher degree due to hematological toxicity than that in patients using EP regimen, and the NLR decreased with EC chemotherapy cycles, thus affecting the prognosis in patients. We believe that checking NLR before treatment should aid in determining first-line chemotherapy regimen in SCLC, and be of clinical significance after validation by future studies.
There are few limitations in the present study. First, the research subjects were patients with SCLC who could not undergo radical resection; thus, the universality is somehow not ideal and the research requires further extrapolation. Second, this is a retrospective study with limited sample size, and needs to be further validated in a prospective multicenter study with larger sample size. Third, and finally, the cellular and molecular mechanism remains to be explored to better comprehend the results.
Conclusion
In conclusion, elevated pretreated peripheral blood NLR independently predicts poor OS duration in SCLC patients. Furthermore, the chemotherapy regimen significantly modified the association between NLR and mortality risk in patients with SCLC. Peripheral blood indexes and other clinicopathological features would provide alternatives to establish better predictive models which can be more beneficial to evaluate the prognosis of patients, which would mainly be the future work of our group.
Acknowledgments
The authors thank all the staff members of the Guangxi Medical University Affiliated Tumor Hospital for their support in the work. We would like to thank for English language editing.
Funding Statement
This study was supported by a “139 Talent Planning” granted by Guangxi Health Commission (grant number: 201903030) and the Natural Science Foundation of Guangxi Zhuang Autonomous Zone (grant number: 2015GXNSFAA139162), China.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Govindan R, Page N, Morgensztern D. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. doi: 10.1200/JCO.2005.04.4859 [DOI] [PubMed] [Google Scholar]
- 2.Yang CJ, Chan DY, Shah SA, et al. Long-term survival after surgery compared with concurrent chemoradiation for node-negative small cell lung cancer. Ann Surg 2018;268(6):1105–1112. doi: 10.1097/SLA.0000000000002287 [DOI] [PubMed] [Google Scholar]
- 3.Takada M, Fukuoka M, Kawahara M. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20(14):3054–3060. doi: 10.1200/JCO.2002.12.071 [DOI] [PubMed] [Google Scholar]
- 4.Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 5.Xie D, Marks R, Zhang M, et al. Nomograms predict overall survival for patients with small-cell lung cancer incorporating pretreatment peripheral blood markers. J Thorac Oncol. 2015;10(8):1213–1220. PubMed: 26200277. doi: 10.1097/JTO.0000000000000585 [DOI] [PubMed] [Google Scholar]
- 6.Hong S, Kang YA, Cho BC, Kim DJ. Elevated serum C-reactive protein as a prognostic marker in small cell lung cancer. Yonsei Med J. 2012;53(1):111–117. doi: 10.3349/ymj.2012.53.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong X, Xu Q, Yang Z. The value of prognostic factors in Chinese patients with small cell lung cancer: a retrospective study of 999 patients. Clin Respir J. 2018;12(2):433–447. doi: 10.1111/crj.12534 [DOI] [PubMed] [Google Scholar]
- 8.Lee G-W, Go S-I, Kim D-W. Geriatric nutritional risk index as a prognostic marker in patients with extensive-stage disease small cell lung cancer: results from a randomized controlled trial. Thorac Cancer. 2020;11(1):62–71. doi: 10.1111/1759-7714.13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura Y, Nagai N, Tsunekawa N. IL-17A-producing CD30 + Vδ1 T cells drive inflammation-induced cancer progression. Cancer Sci. 2016;107(9):1206–1214. doi: 10.1111/cas.13005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T-J, Jiang Y-M, Hu Y-F. Interleukin-17-producing neutrophils link inflammatory stimuli to disease progression by promoting angiogenesis in gastric cancer. Clin Cancer Res. 2017;23(6):1575–1585. doi: 10.1158/1078-0432.CCR-16-0617 [DOI] [PubMed] [Google Scholar]
- 11.Suh J, Jung JH, Jeong CW, Kwak C, Kim HH, Ku JH. Clinical significance of pre-treated neutrophil-lymphocyte ratio in the management of urothelial carcinoma: a systemic review and meta-analysis. Front Oncol. 2019;9:1365. doi: 10.3389/fonc.2019.01365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan Y, Xiong H, Feng Y, et al. Revealing the prognostic landscape of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with abiraterone or enzalutamide: a meta-analysis. Prostate Cancer P D. 2020. doi: 10.1038/s41391-020-0209-3 [DOI] [PubMed] [Google Scholar]
- 13.Harris B, Phan V, Perera V, et al. Inability of current dosing to achieve carboplatin therapeutic targets in people with advanced non-small cell lung cancer: impact of systemic inflammation on carboplatin exposure and clinical outcomes. Clin Pharmacokinet. 2020;59(8):1013–1026. PubMed: 32034726. doi: 10.1007/s40262-020-00870-6. [DOI] [PubMed] [Google Scholar]
- 14.Sayan M, Kankoc A, Ozkan ND, et al. Simple peripheral blood cell parameters to predict prognosis in non-small cell lung cancer. Indian J Surg. 2020. doi: 10.1007/s12262-020-02237-4 [DOI] [Google Scholar]
- 15.Suzuki R, Wei X, Allen PK, Cox JD, Komaki R, Lin SH. Prognostic significance of total lymphocyte count, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio in limited-stage small-cell lung cancer. Clin Lung Cancer. 2019;20(2):117–123. PubMed: 30611672. doi: 10.1016/j.cllc.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 16.Mandaliya H, Jones M, Oldmeadow C, Nordman IIC. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8(6):886–894. doi: 10.21037/tlcr.2019.11.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson AG. The WHO 2015 classification of lung tumours: evolution of tumour classification in lung cancer. Pathology. 2016;48:S7. doi: 10.1016/j.pathol.2015.12.019 [DOI] [Google Scholar]
- 18.Karam I, Jiang SY, Khaira M, Lee CW, Schellenberg D. Outcomes of small cell lung cancer patients treated with cisplatin-etoposide versus carboplatin-etoposide. Am J Clin Oncol. 2015;38(1):51–54. PubMed: 23563211. doi: 10.1097/COC.0b013e31828aab2a [DOI] [PubMed] [Google Scholar]
- 19.Kaya T, Açıkgöz SB, Yıldırım M, Nalbant A, Altaş AE, Cinemre H. Association between neutrophil-to-lymphocyte ratio and nutritional status in geriatric patients. J Clin Lab Anal. 2019;33(1):e22636. doi: 10.1002/jcla.22636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldwin C. The effectiveness of nutritional interventions in malnutrition and cachexia. Proc Nutr Soc. 2015;74(4):397–404. doi: 10.1017/S0029665115002311 [DOI] [PubMed] [Google Scholar]
- 21.Marino F, Tozzi M, Schembri L. Production of IL-8, VEGF and elastase by circulating and intraplaque neutrophils in patients with carotid atherosclerosis. PLoS One. 2015;10(4):e0124565. doi: 10.1371/journal.pone.0124565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang MH, Go S-I, Song H-N. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Brit J Cancer. 2014;111(3):452–460. doi: 10.1038/bjc.2014.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan Z, Zhang L, Liu C, et al. Cisplatin or carboplatin? Neutrophil to lymphocyte ratio may serve as a useful factor in small cell lung cancer therapy selection. Oncol Lett. 2019;18(2):1513–1520. PubMed: 31423218. doi: 10.3892/ol.2019.10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Sarireh B, Eremin O. Tumour-associated macrophages (TAMS): disordered function, immune suppression and progressive tumour growth. J R Coll Surg Edinb. 2000;45(1):1–16. PubMed:10815374. [PubMed] [Google Scholar]
- 25.Erroi A, Sironi M, Chiaffarino F, Zhen-Guo C, Mengozzi M, Mantovani A. IL-1 and IL-6 release by tumor-associated macrophages from human ovarian carcinoma. Int J Cancer. 1989;44(5):795–801. PubMed: 2583859. doi: 10.1002/ijc.2910440508 [DOI] [PubMed] [Google Scholar]
- 26.Nagahashi M, Abe M, Sakimura K, Takabe K, Wakai T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci. 2018;109(12):3671–3678. doi: 10.1111/cas.13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang LY, Luo Q, Lu L, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13(1):3. doi: 10.1186/s13045-019-0836-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris BDW, Phan V, Perera V, et al. Inability of current dosing to achieve carboplatin therapeutic targets in people with advanced non-small cell lung cancer: impact of systemic inflammation on carboplatin exposure and clinical outcomes. Clin pharmacokinet. 2020. doi: 10.1007/s40262-020-00870-6 [DOI] [PubMed] [Google Scholar]
- 29.Shaul ME, Eyal O, Guglietta S, et al. Circulating neutrophil subsets in advanced lung cancer patients exhibit unique immune signature and relate to prognosis. FASEB J. 2020;34(3):4204–4218. PubMed: 31957112. doi: 10.1096/fj.201902467R. [DOI] [PubMed] [Google Scholar]
- 30.Peng L, Wang Y, Liu F, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. CancerImmunol Immunother. 2020. doi: 10.1007/s00262-020-02585-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone PCW, Lally F, Rahman M. Transmigrated neutrophils down-regulate the expression of VCAM-1 on endothelial cells and inhibit the adhesion of flowing lymphocytes. J Leukocyte Biol. 2005;77(1):44–51. doi: 10.1189/jlb.0504304 [DOI] [PubMed] [Google Scholar]
- 32.Spiegel A, Brooks MW, Houshyar S. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6(6):630–649. doi: 10.1158/2159-8290.CD-15-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rotondo R, Bertolotto M, Barisione G. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J Leukocyte Biol. 2011;89(5):721–727. doi: 10.1189/jlb.1109737 [DOI] [PubMed] [Google Scholar]
- 34.Oze I, Ito H, Nishino Y. Trends in small-cell lung cancer survival in 1993–2006 based on population-based cancer registry data in Japan. J Epidemiol. 2019;29(9):347–353. doi: 10.2188/jea.JE20180112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim D-W, Kim H-G, Kim J-H. Randomized Phase III trial of irinotecan plus cisplatin versus etoposide plus cisplatin in chemotherapy-naïve Korean patients with extensive-disease small cell lung cancer. Cancer Res Treat. 2019;51(1):119–127. doi: 10.4143/crt.2018.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferry D, Billingham L, Jarrett H, et al. Carboplatin versus two doses of cisplatin in combination with gemcitabine in the treatment of advanced non-small-cell lung cancer: results from a British Thoracic Oncology Group randomised phase III trial. Eur J Cancer. 2017;83:302–312. PubMed: 28780466. doi: 10.1016/j.ejca.2017.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]