Jack et al. show that in cognitively unimpaired individuals, the only independent predictor of high tau PET accumulation rates is amyloidosis. In cognitively impaired individuals, variables that are consistent with an early-onset Alzheimer’s disease phenotype predict higher rates of tau PET accumulation.
Keywords: tau PET, serial tau PET, tau, Alzheimer’s disease, Alzheimer’s disease clinical trials
Abstract
Clinical trials with anti-tau drugs will need to target individuals at risk of accumulating tau. Our objective was to identify variables available in a research setting that predict future rates of tau PET accumulation separately among individuals who were either cognitively unimpaired or cognitively impaired. All 337 participants had: a baseline study visit with MRI, amyloid PET, and tau PET exams, at least one follow-up tau PET exam; and met clinical criteria for membership in one of two clinical diagnostic groups: cognitively unimpaired (n = 203); or cognitively impaired (n = 134, a combined group of participants with either mild cognitive impairment or dementia with Alzheimer’s clinical syndrome). Our primary analyses were in these two clinical groups; however, we also evaluated subgroups dividing the unimpaired group by normal/abnormal amyloid PET and the impaired group by clinical phenotype (mild cognitive impairment, amnestic dementia, and non-amnestic dementia). Linear mixed effects models were used to estimate associations between age, sex, education, APOE genotype, amyloid and tau PET standardized uptake value ratio (SUVR), cognitive performance, cortical thickness, and white matter hyperintensity volume at baseline, and the rate of subsequent tau PET accumulation. Log-transformed tau PET SUVR was used as the response and rates were summarized as annual per cent change. A temporal lobe tau PET meta-region of interest was used. In the cognitively unimpaired group, only higher baseline amyloid PET was a significant independent predictor of higher tau accumulation rates (P < 0.001). Higher rates of tau accumulation were associated with faster rates of cognitive decline in the cognitively unimpaired subgroup with abnormal amyloid PET (P = 0.03), but among the subgroup with normal amyloid PET. In the cognitively impaired group, younger age (P = 0.02), higher baseline amyloid PET (P = 0.05), APOE ε4 (P = 0.05), and better cognitive performance (P = 0.05) were significant independent predictors of higher tau accumulation rates. Among impaired individuals, faster cognitive decline was associated with faster rates of tau accumulation (P = 0.01). While we examined many possible predictor variables, our results indicate that screening of unimpaired individuals for potential inclusion in anti-tau trials may be straightforward because the only independent predictor of high tau rates was amyloidosis. In cognitively impaired individuals, imaging and clinical variables consistent with early onset Alzheimer’s disease phenotype were associated with higher rates of tau PET accumulation suggesting this may be a highly advantageous group in which to conduct proof-of-concept clinical trials that target tau-related mechanisms. The nature of the dementia phenotype (amnestic versus non-amnestic) did not affect this conclusion.
Introduction
Alzheimer’s disease is defined by the presence of two proteinopathies: amyloid-β and 3R/4R tau inclusions (Hyman et al., 2012). Recent disease modifying Alzheimer’s therapeutic trials have targeted amyloid. Many of these trials have enrolled participants who have either preclinical or early Alzheimer’s disease (Sperling et al., 2014; Sevigny et al., 2016; Klein et al., 2019). Eligibility screening is a multi-step process. Participants are initially screened to determine eligibility based on clinical characteristics (i.e. this would happen before any biomarker testing). If clinical criteria are met, potential participants then undergo biomarker testing to determine that they are in the biological Alzheimer’s continuum which in recent trials has required an abnormal amyloid PET scan or CSF test (Cummings et al., 2019b). The early Alzheimer’s group may include individuals who meet clinical criteria for mild cognitive impairment (MCI) or mild dementia and in addition have abnormal biomarkers (Sevigny et al., 2016; Cummings et al., 2019b; Klein et al., 2019). Currently validated tests for amyloidosis are either expensive (PET) or invasive (CSF). To increase efficiency by limiting the pool of potential participants who undergo biomarker screening, investigators have sought to identify less expensive and invasive variables (e.g. cognitive testing, genetics, MRI, etc.) to predict which clinically screened participants are likely to have abnormal amyloid levels (Mielke et al., 2012; Tosun et al., 2016).
While most disease modifying trials to date have targeted amyloid, abundant neuropathological and imaging evidence suggests that tau is the proteinopathy more closely associated, both temporally and topographically, with neurodegeneration and cognitive impairment among individuals in the Alzheimer’s continuum (Arriagada et al., 1992; Gomez-Isla et al., 1997; Bennett et al., 2004; Nelson et al., 2012; Brier et al., 2016; Cho et al., 2016b; Johnson et al., 2016; Ossenkoppele et al., 2016, 2019b; Bejanin et al., 2017; Pontecorvo et al., 2017; Xia et al., 2017; Gordon et al., 2018; Hanseeuw et al., 2019; Jack et al., 2019b; La Joie et al., 2020). Thus tau has emerged as an attractive therapeutic target (Cummings et al., 2019a; Jadhav et al., 2019; Long and Holtzman, 2019).
PET imaging measures at a single point in time reflect lifelong accumulation of pathology (i.e. pathological load), while measures of the rate of change on serial scans indicate current biological activity. Therefore, identifying variables that predict which individuals are likely to have higher rates of tau PET accumulation would be valuable in designing anti-tau clinical trials.
Our objective was to identify variables that are available in a research setting that are predictive of future rates of tau PET accumulation. To be consistent with design of some recent trials, we identified predictors of tau accumulation rates separately in cognitively unimpaired and in cognitively impaired individuals. The latter group was composed of individuals who met clinical criteria for either MCI or mild dementia. While recent disease-modifying Alzheimer’s trials have required evidence of amyloidosis for enrolment, this requirement was not imposed on the two clinical groups in the primary analyses for this study because amyloid is an obvious candidate variable for predicting tau accumulation rates. However, we also performed the analyses in five clinical subgroups: (i) unimpaired participants with normal amyloid PET; (ii) unimpaired participants with abnormal amyloid PET; (iii) MCI; (iv) individuals with an amnestic dementia phenotype; and (v) individuals with a non-amnestic dementia phenotype.
Material and methods
Enrolment and clinical characterization
This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. Written informed consent was obtained from all participants or, in the case of subjects with cognitive impairment sufficient to interfere with capacity, from a legally authorized representative.
All individuals in this study were enrolled in one of the three longitudinal cohort studies at Mayo Clinic that include serial tau PET scanning: (i) The Mayo Clinic Study of Aging (MCSA), which is a longitudinal population-based study of cognitive aging among a stratified random sample of a geographically defined population (Olmsted County, Minnesota, USA) (Roberts et al., 2008; St Sauver et al., 2012); (ii) The Alzheimer’s Disease Research Center (ADRC), which is a longitudinal research study of individuals recruited from the Mayo Clinic neurology practice; and (iii) a neurodegenerative research group (NRG), which recruits participants from the Mayo Clinic neurology practice (Sintini et al., 2019).
For inclusion in the current study, an individual must have been a participant in one of these three observational cohorts, had a study visit with MRI, amyloid PET, and tau PET exams, had at least one follow-up visit with an MRI and tau PET exam, and have met criteria for membership in one of two primary clinical groups at the first visit with tau PET. One primary clinical group was cognitively unimpaired, which was defined as having neither MCI nor dementia. The second primary clinical group was cognitively impaired. The cognitively impaired individuals met criteria for mild dementia [Clinical Dementia Rating (CDR) < 2 (Morris, 1993)] with Alzheimer’s clinical syndrome (Jack et al., 2018a) or MCI (Petersen, 2004). Alzheimer’s clinical syndrome participants with dementia included individuals with a classic amnestic phenotype who would meet clinical criteria for clinically defined probable Alzheimer’s disease (McKhann et al., 2011), as well as established non-amnestic Alzheimer’s phenotypes (i.e. language, visuospatial, and dysexecutive) (Gorno-Tempini et al., 2011; Crutch et al., 2017; Townley et al., 2020). Clinical diagnoses were made blinded to PET results in the MCSA; however, this was not always the case for participants from the ADRC or NRG.
Participants from all three cohorts underwent CDR (Morris, 1993) and Short Test of Mental Status (STMS) (Kokmen et al., 1991) or Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) testing. MoCA total scores were used if available; STMS total score was mapped to MoCA total score otherwise (Townley et al., 2019). MCSA participants also underwent formal psychometric testing which included the Wechsler Memory Scale-Revised (WMS-R) Logical Memory-II (delayed recall), WMS-R Visual Reproduction-II (delayed recall), and Auditory Verbal Learning Test (delayed recall). A memory domain z-score was calculated as the mean of these three component tests (Jack et al., 2019a).
Imaging
Amyloid PET imaging was performed with Pittsburgh compound B (Klunk et al., 2004) and tau PET with flortaucipir(Xia et al., 2013). The PET data processing pipeline was described in detail in previous work (Schwarz et al., 2019). Amyloid and tau PET standardized uptake value ratios (SUVRs) were calculated by dividing the voxel-number weighted average of median uptake across a set of regions of interest by uptake in the cerebellar crus grey matter (Jack et al., 2017). The amyloid PET meta-region of interest included the prefrontal, orbitofrontal, parietal, temporal, anterior and posterior cingulate, and the precuneus (Jack et al., 2017). We used a cut-off point of SUVR ≥ 1.48 (Centiloid 22; Klunk et al., 2015) to denote abnormal amyloid PET scans (Jack et al., 2017). A tau PET temporal meta-region of interest was used in this analysis which included the amygdala, entorhinal cortex, fusiform, parahippocampal, and inferior temporal and middle temporal gyri (Jack et al., 2017) (Supplementary Fig. 1). PET data were not partial volume corrected in the primary analyses; however, we performed a sensitivity analysis with partial volume correction using the two-compartment Meltzer method (Meltzer et al., 1999) as described in Schwarz et al. (2019).
MRI was performed at 3 T. An Alzheimer's disease composite cortical thickness meta-region of interest defined as the surface area weighted average of mean thickness across the entorhinal cortex, fusiform, inferior temporal and middle temporal gyri was derived using FreeSurfer (v5.3) (Schwarz et al., 2016; Jack et al., 2017). Global cerebral white matter hyperintensity volumes were measured from FLAIR images using a semi-automated segmentation algorithm (Graff-Radford et al., 2019) and were scaled by total intracranial volume.
Statistical analysis
Our primary analyses of predictors of longitudinal change in tau were performed separately in the two primary clinical groups, cognitively unimpaired and impaired. Separate subgroup analyses were also performed in which the unimpaired group was divided into those who had normal amyloid PET (A−) and those with abnormal amyloid PET (A+). Subgroup analyses in the impaired group included only MCI, only amnestic dementia, and only non-amnestic dementia.
For descriptive figures, we computed the annual per cent change in tau PET within each individual via a least squares fit with log-transformed tau PET SUVR as the outcome. To summarize the mean slopes by age in these figures, we used overlapping age bins, which is a well-established graphical method often referred to as ‘shingling’ (Cleveland, 1993). This has been found to aid visual perception of any patterns in the data, but is not used in the underlying models.
To assess predictors of tau accumulation rates, we fit linear mixed effects models with tau PET SUVR as the response and included the following predictor variables as terms at baseline that could affect the rate of change in tau PET (i.e. time × covariate product terms): age, sex, education, APOE genotype, cognitive test performance, amyloid PET SUVR, cortical thickness, white matter hyperintensity as a fraction of total intracranial volume, and tau PET SUVR. Time was defined as the number of years since a participant’s first tau PET study. Since any difference between participants in their initial tau PET SUVR, or predictors thereof, was not the focus, the model included a random slope effect for each person and an unconstrained person-specific intercept. Amyloid and tau PET SUVR measurements have an approximately constant coefficient of variation across their range which implies that models of log-transformed tau PET and/or log-transformed amyloid PET will be statistically most efficient in regression, i.e. have an approximately constant standard deviation (SD) across the range. Therefore, amyloid PET, tau PET, and WHM as a percentage of total intracranial volume were log-transformed. Models were fit separately within cognitively unimpaired and cognitively impaired groups and within the five subgroups described above. Differences in effect sizes among any of the three cognitively impaired subgroups were tested using two degree of freedom joint Wald tests on a model including all time × covariate × subgroup interactions. Differences in effect sizes between two specific subgroups (e.g. cognitively unimpaired A+ versus A− or amnestic versus non-amnestic dementia) were directly tested using estimates and standard errors from their respective models. Residual plots and plots of fitted versus observed data were inspected to evaluate model adequacy.
The generic interpretation of the coefficients for time × covariate product terms included in our models would be that β represents the difference in the mean annual per cent change in tau PET for a 1-unit difference in the predictor X. However, since a 1-unit difference is not necessarily meaningful (or standardized) given the scales of our predictors, we report effects for clinically meaningful contrasts. We show effect sizes for a 20% increase in baseline amyloid or tau PET, a 0.2 mm decrease in cortical thickness, and a 0.5% versus 1.5% comparison for white matter hyperintensity volume as a percentage of total intracranial volume. We used a 10-year increase for age and a 4-year decrease in years of education. Choosing the contrasts in this way facilitates comparing the size of the coefficients even though the actual scales of these predictor variables are not the same.
We used a memory domain composite z-score as the cognitive performance predictor in the cognitively unimpaired group and used the calculated MoCA total score in the cognitively impaired groups. By using separate tests we avoided floor and ceiling effects in the two different clinical groups.
Finally, we computed the annual change in cognitive performance within each individual via a least squares fit with memory z-score or calculated MoCA as the outcome and summarized the association between the contemporaneous rate of change in cognitive performance and the rate of change in tau PET using Spearman rank correlations separately in the unimpaired and impaired groups.
All analyses were done using the R language and environment for statistical computing version 3.6.2 with mixed models fit using the nlme package version 3.1-145.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Study participants
There were 337 individuals who met inclusion criteria for this study. Of these, 203 were cognitively unimpaired and all were participants in the MCSA by design (Table 1). Seventy (34%) cognitively unimpaired participants had abnormal amyloid PET studies. The cognitively impaired participants (n = 134) were distributed across the three cohort studies. Seventy-one (53%) participants in the impaired group had a diagnosis of MCI and 63 (47%) a diagnosis of dementia with Alzheimer’s clinical syndrome. Syndromic diagnoses within the Alzheimer’s clinical syndrome group were amnestic in 24 individuals, visuospatial in 16, aphasic in 15, and dysexecutive in eight (Gorno-Tempini et al., 2011; Wolk et al., 2012; Crutch et al., 2017; Townley et al., 2020). Amyloid PET scans were abnormal at baseline in 44 (62%) of MCI participants and in all 24 amnestic dementia and all 39 non-amnestic dementia participants (Supplementary Table 1).
Table 1.
Participant characteristics by primary clinical group
| Characteristic | Cognitively unimpaired n = 203 | Cognitively impaired n = 134 | |
|---|---|---|---|
| Cohort, n (%) | |||
| Mayo Clinic Study of Aging | 203 (100) | 37 (28) | |
| Mayo Alzheimer's Disease Research Center | 0 (0) | 73 (54) | |
| Mayo Neurodegenerative Research Group | 0 (0) | 24 (18) | |
| Clinical diagnosis, n (%) | |||
| Cognitively unimpaired | 203 (100) | 0 (0) | |
| MCI | 0 (0) | 71 (53) | |
| Dementia with Alzheimer's clinical syndrome | 0 (0) | 63 (47) | |
| Age, years | |||
| Median (IQR) | 70 (63, 79) | 71 (64, 78) | |
| Min, Max | 52, 94 | 52, 94 | |
| Male sex, n (%) | 110 (54) | 74 (55) | |
| Education, years, median (IQR) | 16 (13, 16) | 16 (13, 17) | |
| APOE ε4, n (%) | 67 (33) | 66 (49) | |
| Clinical Dementia Rating Scale-Sum of Boxes, median (IQR) | 0 (0, 0) | 2 (1, 3) | |
| Montreal Cognitive Assessment, median (IQR) | 26 (24, 27) | 20 (17, 23) | |
| Memory z-score, median (IQR) | 0.6 (−0.2, 1.3) | – | |
| Amyloid PET SUVR | |||
| Median (IQR) | 1.40 (1.34, 1.56) | 2.25 (1.60, 2.59) | |
| Abnormala, n (%) | 70 (34) | 107 (80) | |
| Tau PET SUVR, median (IQR) | 1.19 (1.13, 1.24) | 1.50 (1.21, 2.00) | |
| Cortical thickness, mm, median (IQR) | 2.71 (2.61, 2.80) | 2.54 (2.40, 2.65) | |
| White matter hyperintensity, % of TIV, median (IQR) | 0.58 (0.29, 1.16) | 0.79 (0.49, 1.39) | |
| Total tau PET scans, n (%) | |||
| 2 | 177 (87) | 87 (65) | |
| 3 | 26 (13) | 37 (28) | |
| 4 | 0 (0) | 10 (7) | |
| Tau PET scan interval (first to last), years | |||
| Median (IQR) | 2.4 (1.3, 2.6) | 1.3 (1.1, 2.1) | |
| Min, Max | 1.0, 4.0 | 0.8, 4.1 |
aAbnormal amyloid PET was defined as SUVR ≥ 1.48 (centiloid ≥ 22).
While comparison of the cognitively unimpaired and cognitively impaired clinical groups was not an objective of this study, as would be expected at baseline the impaired group had a greater proportion of APOE ε4 carriers, worse cognitive performance, greater amyloid and tau PET load, reduced cortical thickness, greater white matter hyperintensity, and a higher rate of tau accumulation. Most individuals in both clinical groups had only two tau PET scans (87% of cognitively unimpaired, 65% of cognitively impaired). However, 13% of cognitively unimpaired individuals had three tau PET scans and among cognitively impaired, 28% had three tau PET scans and 7% had four tau PET scans. The median interval from baseline to last tau PET scan was 2.4 years in the cognitively unimpaired and 1.3 years in the cognitively impaired group.
Primary analyses: cognitively unimpaired and impaired groups
Descriptive data: tau PET trajectories by age
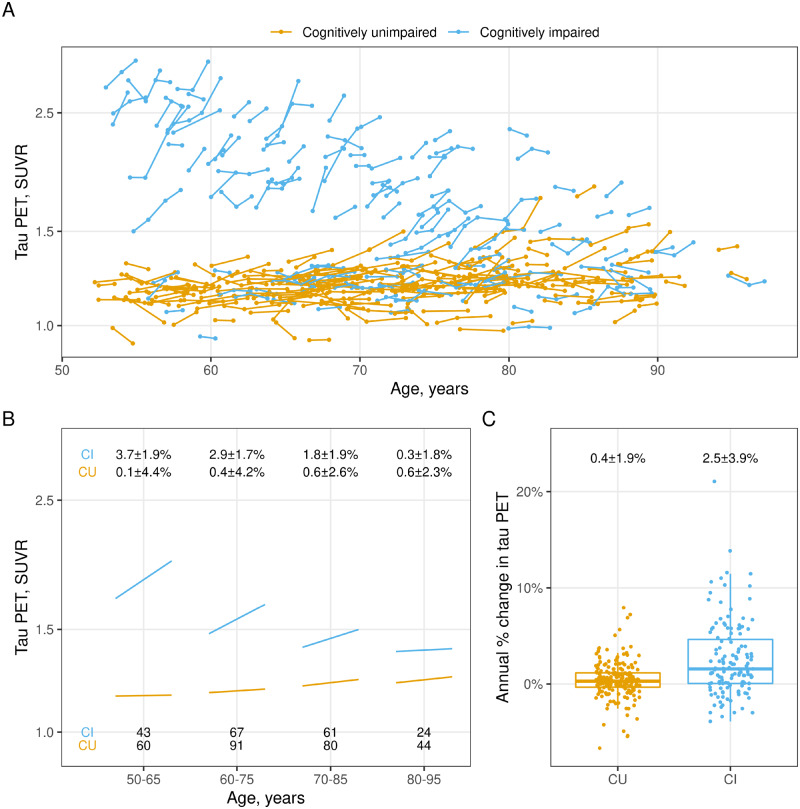
Tau PET participant trajectories are illustrated in Fig. 1A showing the 337 individuals by age, colour-coded by cognitively unimpaired and cognitively impaired groups. Group-wise tau accumulation rates by interval are shown in Fig. 1B. We use overlapping age intervals as a smoothing technique to reduce random variation. Most cognitively unimpaired individuals had low baseline tau PET SUVR values and flat trajectories over time; however, there were small increases in mean baseline SUVR and rate of tau PET accumulation with age. Rates of accumulation averaged 0.4% ± 1.9% per year in the unimpaired group and 2.5% ± 3.9% in the impaired group with considerable overlap between the groups (Fig. 1C). However, a pronounced age effect was present in the impaired group where both baseline tau PET SUVR and the rate of accumulation were less in older compared to younger individuals (Fig. 1A and B).
Figure 1.
Tau PET trajectories by age and primary clinical group. (A) Tau PET trajectories within each individual over age (time) coloured by cognitive status: cognitively unimpaired (CU) or cognitively impaired (CI). (B) Mean tau PET trajectory by age within each diagnosis group. Mean baseline tau PET SUVR and annual per cent change in tau PET were calculated within age groups of 50–65, 60–75, 70–85, and 80–95. Overlapping age groups were used to reduce noise and number of individuals in each age and cognitive group are shown at the bottom of the figure. Mean ± SD annual per cent change for each age and cognitive group are shown at the top. (C) Distribution of annual per cent change in tau PET by clinical diagnosis with mean ± SD annual per cent change for each cognitive group at the top. Box plots indicate median and interquartile range (IQR) of the distributions with whiskers extending from the quartiles to the farthest point within 1.5 times the IQR.
Prediction of tau PET accumulation rates
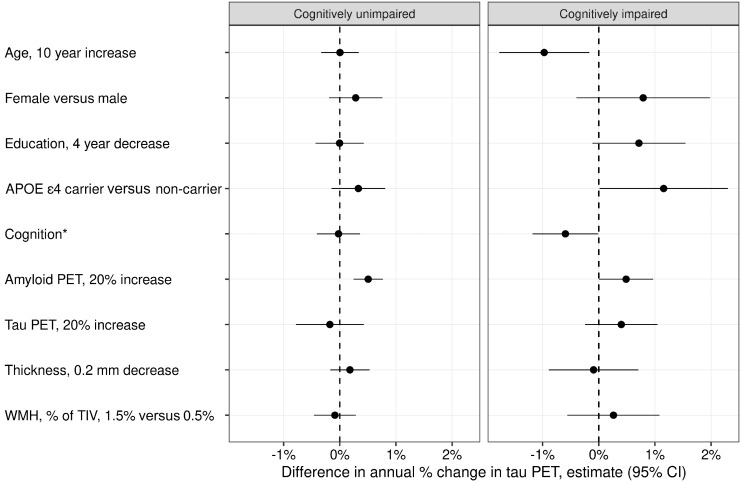
In the unimpaired group, only higher baseline amyloid PET was a significant independent predictor of higher tau accumulation rates (P < 0.001) such that a 20% increase in amyloid SUVR was associated with a 0.5% faster annual rate of tau accumulation (95% CI: 0.2%–0.8%) (Fig. 2 and Supplementary Table 2).
Figure 2.
Multivariable associations with annual per cent change in tau PET among cognitively unimpaired and cognitively impaired. The estimated mean (95%) difference in annual per cent change in tau PET from a linear mixed effects model fit separately within each group is shown for specified differences in predictors. For cognition, the difference in annual per cent change in tau PET is shown for a 1.5 unit decrease in memory z-score among cognitively unimpaired individuals and for a 5-point decrease in the MoCA for cognitively impaired individuals.
In the impaired group, individuals who were 10 years younger had a 1.0% faster annual rate of tau accumulation (95% CI: 0.2%–1.8%; P = 0.02). APOE ε4 carriers had 1.2% faster annual rate of tau accumulation on average (95% CI: 0.0%–2.3%; P = 0.05). A 5-point higher MoCA score was associated with a 0.6% faster annual rate of tau accumulation (95% CI: 0.0%–1.2%; P = 0.05). A 20% higher amyloid PET SUVR value was associated with a 0.5% faster annual increase in tau (95% CI: 0.0%–1.0%; P = 0.05). A 20% higher baseline tau PET SUVR was associated with a 0.4% faster annual increase in tau (95% CI: −0.2% to 1%). This effect size was similar to that of amyloid PET but with a wider confidence interval it did not meet statistical significance (P = 0.22). Female sex (P = 0.19) and four fewer years of education (P = 0.09) were not statistically significant, but had effect sizes of 0.8% faster and 0.7% faster annual rates of tau accumulation.
Partial volume correction had no appreciable effect on the estimates reported above for either clinical group (Supplementary Fig. 2).
Secondary analyses: clinical subgroups
Descriptive data for clinical subgroup analyses: tau PET trajectories by age
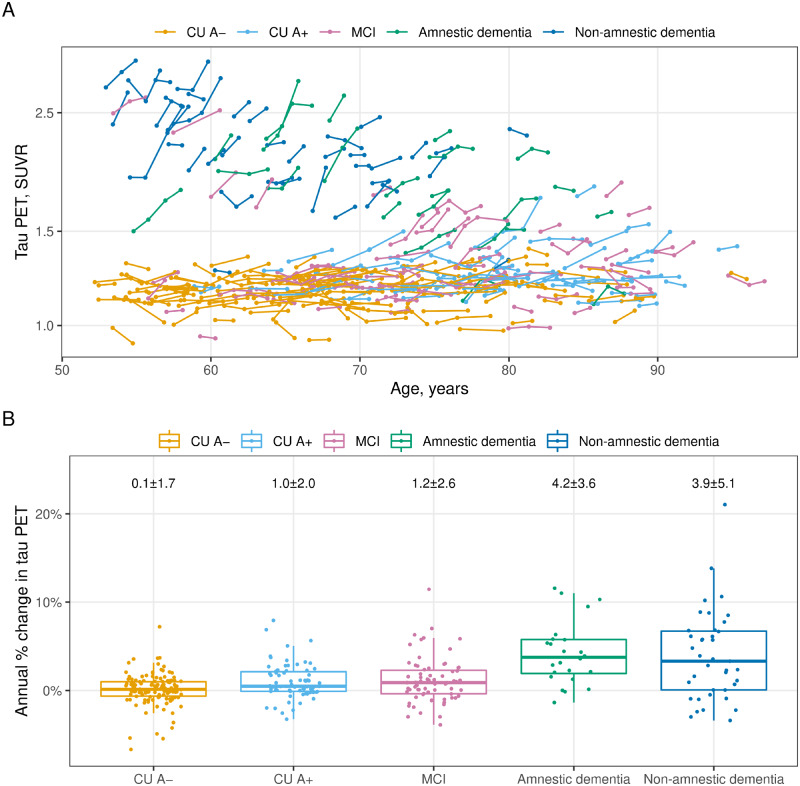
Tau PET participant trajectories are illustrated in Fig. 3A showing the 337 individuals by age, colour-coded by clinical subgroup. Rates of accumulation averaged 0.1% ± 1.7% per year in A– cognitively unimpaired, 1.0% ± 2.0% per year in A+ cognitively unimpaired, 1.2% ± 2.6% per year in MCI, 4.2% ± 3.6% per year in amnestic dementia, and 3.9% ± 5.1% per year in non-amnestic dementia participants (Fig. 3B).
Figure 3.
Tau PET trajectories by age and clinical subgroups. The cognitively unimpaired (CU) individuals are split by normal/abnormal amyloid PET (A−/A+) and the cognitively impaired individuals are split by clinical syndrome: MCI, amnestic dementia, and non-amnestic dementia. (A) Tau PET trajectories within each individual over age (time) coloured by subgroup. (B) The distribution of annual per cent change in tau PET by subgroup with mean ± SD annual per cent change for each subgroup at the top. Box plots indicate median and IQR of the distributions with whiskers extending from the quartiles to the farthest point within 1.5 times the IQR.
Prediction of rates of tau PET accumulation
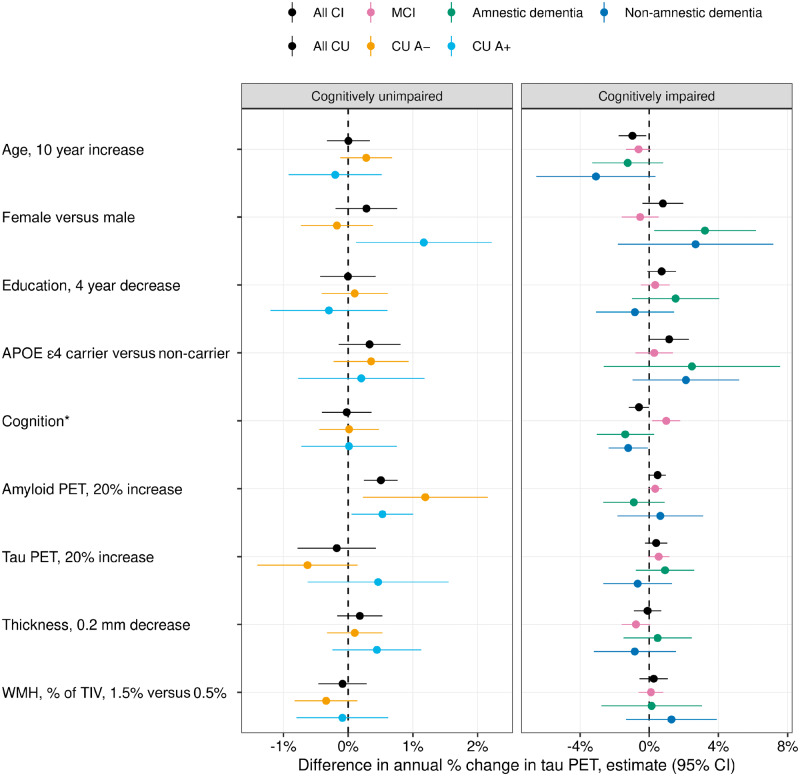
Subgroup analyses are challenging and can invite misinterpretations such as assuming a difference in significance corresponds to a meaningful difference in the clinical subgroups (Gelman and Stern, 2006). Nevertheless, with a cautious interpretation the estimates can be informative and address important questions. Among both A− and A+ cognitively unimpaired individuals, higher amyloid PET was a significant independent predictor of tau accumulation rates (Fig. 4 and Supplementary Table 3). For A− individuals, a 20% increase in amyloid PET SUVR was associated with a 1.2% faster annual increase in tau (95% CI: 0.2%–2.2%; P = 0.02). For A+ individuals the effect size was less at 0.5% (95% CI: 0.1%–1.0%; P = 0.03) although not significantly different (P = 0.22). There was a difference in the sex association between A+ and A− subgroups (P = 0.02) whereby among A+ individuals, females increased at an annual rate that was 1.2% faster than males (95% CI: 0.1%–2.2%). In contrast, among A− individuals this sex differential was absent (estimate: −0.2%; 95% CI: −0.7% to 0.4%).
Figure 4.
Multivariable associations with annual per cent change in tau PET among cognitively unimpaired (CU) and cognitively impaired (CI) subgroups. The cognitively unimpaired individuals were split by normal/abnormal amyloid PET (A−/A+) and the cognitively impaired individuals were split by clinical syndrome: MCI, amnestic dementia, and non-amnestic dementia. The estimated mean (95%) difference in annual per cent change in tau PET from a linear mixed effects model fit separately within each group is shown for specified differences in predictors. For cognition, the difference in annual per cent change in tau PET is shown for a 1.5 unit decrease in memory z-score among cognitively unimpaired subgroups and for a 5-point decrease in the MoCA for cognitively impaired subgroups. Note that because of the wide confidence intervals in the cognitively unimpaired subgroups, the x-axis ranges for the cognitively unimpaired and cognitively impaired panels are different.
Because of small sample sizes, there was more uncertainty in the effect size estimates for the cognitively impaired syndromic subgroups. In general, the pattern of associations seen among the subgroups was similar to those seen in the overall cognitively impaired group (Fig. 4 and Supplementary Table 3). When testing differences in covariate effects on the rate of tau accumulation across MCI, amnestic dementia, and non-amnestic dementia subgroups, we found differences only for sex (P = 0.01) and cognition (P < 0.001) but these results were driven by differences in the MCI subgroup relative to the dementia subgroups. We observed no clear differences in effect sizes between the amnestic and non-amnestic dementia subgroups.
Cognitive trajectories
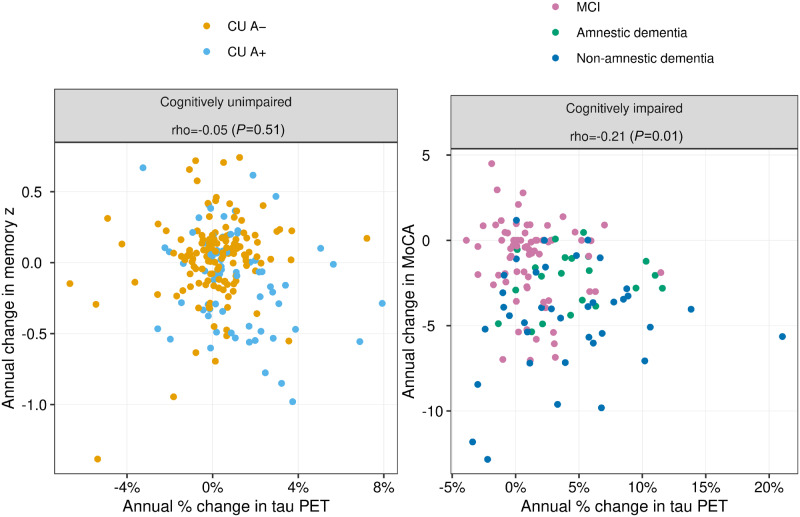
Higher rates of tau accumulation were not associated with faster rates of decline on the memory composite in the cognitively unimpaired group as a whole (r = −0.05, P = 0.51; Fig 5). However, in the A+ cognitively unimpaired subgroup faster decline on memory performance was associated with faster rates of tau accumulation (r = −0.26, P = 0.03). Among impaired individuals, faster decline on MoCA was associated with faster rates of tau accumulation (r = −0.21, P = 0.01).
Figure 5.
Scatter plots of annual change in cognition versus annual change in tau PET among cognitively unimpaired (CU) and cognitively impaired individuals. Points are coloured by normal/abnormal amyloid PET (A−/A+) within the cognitively unimpaired group and by clinical syndrome (MCI, amnestic dementia, and non-amnestic dementia) in the cognitively impaired group. Spearman correlation coefficients and P-values are shown at the top of each plot. Memory z-score was used for the cognition measure in the cognitively impaired group and the MoCA was used in the cognitively impaired group.
Discussion
While recent disease-modifying trials have mostly targeted amyloid-β (Cummings et al., 2019b), interventions in individuals on the Alzheimer’s continuum that seek to slow or arrest the rate of accumulation of tau are increasingly recognized as a rational therapeutic strategy (Jack et al., 2018b; McDade and Bateman, 2018; Ossenkoppele et al., 2018; Cummings et al., 2019a; Jadhav et al., 2019; Leuzy et al., 2019; Long and Holtzman, 2019). Approaches may include therapies to inhibit tau aggregation, seeding, and spreading, to stabilize micro tubules, to inhibit abnormal tau phosphorylation, to enhance clearance, or to reduce tau expression (Cummings et al., 2019a; Jadhav et al., 2019; Long and Holtzman, 2019). Enrolling individuals with higher expected rates of tau accumulation translates into greater statistical power over the limited timeframe of a trial aimed at therapeutic reduction in the rate of tau accumulation. Thus variables that independently predict high tau accumulation rates would be useful to create effective screening and enrolment criteria.
Among all cognitively unimpaired individuals, only higher baseline amyloid SUVR was a significant independent predictor of higher tau accumulation rates (Fig. 2 and Supplementary Table 2). This association was anticipated based on prior cross-sectional studies demonstrating that greater amyloid load is associated with greater tau load cross-sectionally (Johnson et al., 2016; Pontecorvo et al., 2017; Lowe et al., 2018; Ossenkoppele et al., 2018; Jack et al., 2019b; Koscik et al., 2019; Sperling et al., 2019). Harrison et al. (2019) reported no difference in tau accumulation rates between amyloid PET positive versus negative healthy older adults. Our findings differ and this may be due to several factors including differing sample sizes and cohort characteristics.
Based on prior cross-sectional and longitudinal literature we expected that higher baseline tau PET, worse baseline cognitive performance, older age, APOE ε4, greater white matter hyperintensity, and lower cortical thickness might independently predict higher tau accumulation rates in unimpaired individuals (Lim et al., 2014; Cho et al., 2016a, 2019; Scholl et al., 2016; Donohue et al., 2017; Maass et al., 2017; Mishra et al., 2017; Mormino et al., 2017; Chiotis et al., 2018; Gordon et al., 2019; Knopman et al., 2019; Pontecorvo et al., 2019). The fact that these were not independent predictors in this analysis (Fig. 2 and Supplementary Table 2) is likely explained by their shared variance with beta-amyloidosis, which has been demonstrated previously (Becker et al., 2011; Fleisher et al., 2013; Sperling et al., 2013; Villemagne et al., 2013; Jansen et al., 2015, 2018; Mattsson et al., 2015; Burnham et al., 2016; Knopman et al., 2019).
The fact that of the variables examined, only higher baseline amyloid PET SUVR was a significant independent predictor of tau accumulation rates suggests that a straightforward screening approach to identifying cognitively unimpaired persons who are more likely to have high tau accumulation rates might be possible in that only one variable needs to be screened. An obvious challenge though is that amyloid PET is expensive and the CSF analogue, CSF amyloid-β42, requires an invasive procedure. However, if plasma amyloid-β assays (Ovod et al., 2017; Nakamura et al., 2018) are shown to reliably indicate amyloidosis then our data suggest that a blood test alone could be effective for screening for inclusion in trials seeking to reduce tau accumulation rates among cognitively unimpaired individuals. More recently plasma phosphorylated tau assays have been shown to correlate well with tau PET in vivo (Mielke et al., 2018; Barthelemy et al., 2020; Karikari et al., 2020; Thijssen et al., 2020) and with Alzheimer’s disease pathology at autopsy (Thijssen et al., 2020). These studies suggest, but do not prove, that plasma measures could be prognostic of tau accumulation rates. Longitudinal studies are needed to evaluate this possibility.
As was true in the unimpaired group as a whole, baseline amyloid PET was a significant independent predictor of tau accumulation rates in both the A+ and A− cognitively unimpaired subgroups. In addition, female sex was also an independent predictor of tau accumulation rates in only the A+ cognitively unimpaired subgroup. The literature on the relationship between sex and risk of incident dementia usually with an Alzheimer’s clinical syndrome phenotype is complicated (Brookmeyer et al., 2011; Fiest et al., 2016). Some studies indicate greater risk for females (Andersen et al., 1999) but others do not (Edland et al., 2002), and sex-associated risk is age dependent in some studies (Andersen et al., 1999; Fiest et al., 2016). Furthermore, a sex × APOE ε4 interaction has been reported with female APOE ε4 carriers being at higher risk of dementia (Farrer et al., 1997; Altmann et al., 2014) and an age dependence in sex-specific risk associated with APOE ε4 has also been reported (Neu et al., 2017). We found a difference in the sex effect on rates of tau accumulation between A+ and A− cognitively unimpaired individuals which is consistent with the literature indicating greater risk in females.
Among all cognitively impaired individuals, younger age, APOE ε4, better cognitive performance, and higher baseline amyloid PET were significant predictors of higher tau accumulation rates (Fig. 2 and Supplementary Table 2). This set of variables is characteristic of early onset Alzheimer’s disease (Koss et al., 1996; Ho et al., 2002; Cho et al., 2017; Scholl et al., 2017; Ossenkoppele et al., 2019a). While our main focus was on tau rates, Fig. 1 also demonstrates that the highest baseline tau levels were found in younger impaired individuals. Very high cross-sectional tau PET SUVR values are characteristic of early onset Alzheimer’s disease (Cho et al., 2017; Scholl et al., 2017; Ossenkoppele et al., 2019a). This set of predictors of high tau accumulation rates coupled with generally lower burden of non-Alzheimer’s disease pathologies at younger age (Nelson et al., 2011) leads to the conclusion that early onset Alzheimer’s disease might be a highly advantageous group in which to conduct clinical trials that examine the feasibility of targeting tau-related mechanisms. This is particularly the case for proof-of-concept trials seeking to establish evidence of target engagement.
The observation that younger age was associated with higher rates of tau accumulation in the impaired group as a whole (Fig. 2 and Supplementary Table 2) is consistent with the longitudinal tau PET study of Pontecorvo et al. (2019). It is also conceptually consistent with clinical observations that younger onset Alzheimer’s disease has a faster, more aggressive clinical course (Koss et al., 1996; Cho et al., 2017; Ossenkoppele et al., 2019a; La Joie et al., 2020). The rate of tau accumulation among the oldest impaired individuals (i.e. over 80) in our study was quite low (Fig. 1B). There are several possible explanations for this ‘inverse’ age effect. First, the cognitively impaired cohort is based on a referral sample of symptomatic individuals and older impaired participants do not indicate the future course for symptomatic individuals who enter the study at a younger age. Most of the impaired individuals over 80 years of age in this study had MCI; therefore, those with less severe clinical disease were oldest. Second, Alzheimer’s disease is a less dominate pathologic substrate of dementia among the very old (whose impairment is more likely due to a combination of pathologies) compared with younger ages (Nelson et al., 2011; Kawas et al., 2015). A final potential explanation is simply selection bias. Individuals who have highly aggressive Alzheimer's disease either do not survive to old age or if they do survive are too impaired to participate in longitudinal observational studies because of the combination of an aggressive Alzheimer’s disease phenotype plus non-Alzheimer’s brain pathologies common in old age (Nelson et al., 2011; Kawas et al., 2015; Wisse et al., 2015; Nelson et al., 2019). All these factors are relevant to the design of anti-tau clinical trials in impaired individuals.
The fact that better cognitive performance at baseline predicted higher tau accumulation rates in the impaired group as a whole (Fig. 2 and Supplementary Table 2) may seem counter-intuitive, but older individuals are much more likely than younger subjects to have comorbid brain pathologies in addition to Alzheimer’s disease (Nelson et al., 2011; Wisse et al., 2015; Botha et al., 2018a, b; Nelson et al., 2019) as well as less cognitive reserve (Scholl et al., 2017). This could explain better cognitive performance in younger impaired individuals despite higher tau accumulation rates.
Based on prior longitudinal flortaucipir studies (Jack et al., 2018b; Cho et al., 2019; Pontecorvo et al., 2019; Sintini et al., 2019; Franzmeier et al., 2020) we expected that both baseline tau and amyloid PET would be independent predictors of tau accumulation rates in the impaired group as a whole. While the point estimates of effect sizes of these variables were very similar, only amyloid-β was significant (Fig. 2 and Supplementary Table 2). Tau is closely linked with beta-amyloidosis; with rare exceptions high baseline neocortical tau is only seen in the presence of high amyloid (Johnson et al., 2016; Pontecorvo et al., 2017; Lowe et al., 2018; Ossenkoppele et al., 2018; Jack et al., 2019b; Koscik et al., 2019; Sperling et al., 2019). The predictive effects of both baseline amyloid and baseline tau for tau accumulation rates were likely attenuated by their shared variance in the model. As a cautionary note, these results should not be interpreted as offering support for the notion that baseline amyloid was a better or stronger predictor than baseline tau in the impaired group since the effect sizes for these two PET measures were similar.
Our ability to detect subgroup differences among impaired individuals was limited by small sample sizes, particularly in the two dementia subgroups (Fig. 4 and Supplementary Table 3). With that limitation in mind, it was interesting that we found no significant differences in effect sizes between the amnestic and non-amnestic dementia subgroups. Thus despite different clinical phenotypic presentations, relationships between tau accumulation rates and many underlying biological features (age, sex, APOE, cognitive performance) were similar in these dementia subgroups. There were differences in the relationships between predictor variables and tau accumulation rates in MCI versus the two dementia subgroups. Female sex was associated with higher rates of tau accumulation in the two dementia subgroups but not among MCI. In addition, higher/worse MoCA scores were associated with greater rates of tau accumulation among MCI but with lower rates of tau accumulation among the dementia subgroups. Possible explanations for these differences between the MCI and the dementia subgroups include: the MCI group had a less severe clinical presentation, lower rates of tau accumulation, and 38% had normal amyloid levels indicating that they were not in the Alzheimer’s disease pathway. In addition, half the MCI group was from the MCSA (a population-based cohort) whereas most dementia participants were enrolled from the clinical practice (with the associated referral biases).
A strong motivating factor underlying the growing interest in anti-tau interventions (Cummings et al., 2019a; Jadhav et al., 2019; Long and Holtzman, 2019) is abundant evidence suggesting that tau (rather than amyloid-β) is the proteinopathy more closely associated with cognitive impairment among individuals in the Alzheimer’s continuum (Arriagada et al., 1992; Gomez-Isla et al., 1997; Bennett et al., 2004; Nelson et al., 2012; Brier et al., 2016; Cho et al., 2016b; Johnson et al., 2016; Ossenkoppele et al., 2016, 2019b; Bejanin et al., 2017; Pontecorvo et al., 2017; Xia et al., 2017; Gordon et al., 2018; Hanseeuw et al., 2019; Jack et al., 2019b; La Joie et al., 2020). We therefore assessed the correlation between change in cognitive performance and contemporaneous tau accumulation rates. This analysis was done separately for the unimpaired versus the impaired group. Floor/ceiling effects differ for these two groups; therefore, we used a memory composite as the cognitive test in the unimpaired group and the MoCA in the impaired group. Higher rates of tau accumulation were associated with faster rates of decline on MoCA in the impaired group, but were not associated with rates of memory decline in the unimpaired group as a whole (Fig. 5). The null finding in the unimpaired group as a whole is likely due to the fact that cognitively unimpaired individuals exhibit little change in memory performance over relatively short intervals and that a majority of the unimpaired group were A− and had little change in tau PET. Among A+ cognitively unimpaired individuals, higher rates of tau accumulation were associated with faster rates of decline on memory z-score.
A limitation of this study was that the cognitively impaired sample was composed from one population-based cohort and two referral-based clinic cohorts. Consequently, the study sample as a whole does not represent a cohesive group of individuals that was identified by a common mechanism. An advantage of this approach though is that the study sample includes the full spectrum of participants that are found in different types of research studies. Another limitation is that participants included in this study from the two clinical cohorts (the ADRC and NRG) came from samples that are biased by referral practices.
Supplementary Material
Acknowledgements
We thank AVID Radiopharmaceuticals, Inc., for their support in supplying flortaucipir (Tauvid) precursor, chemistry production advice and oversight, and FDA regulatory cross-filing permission and documentation needed for this work.
Funding
National Institutes of Health (R01-AG50603, R37 AG011378, RO1 AG041851, R01 AG056366, R01 NS097495, U01 AG06786, R01 AG034676, P50 AG016574), Alexander Family Professorship of Alzheimer’s Disease Research, the Robert Wood Johnson Foundation, The Elsie and Marvin Dekelboum Family Foundation, The Liston Family Foundation, GHR Foundation, Foundation Dr. Corinne Schuler, Alzheimer’s Association and the Mayo Foundation.
Competing interests
C.R.J. has served as a consultant for Eisai and serves on an independent data monitoring board for Roche but he receives no personal compensation from any commercial entity; receives funding from the NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. D.S.K. serves on a Data Safety Monitoring Board for the DIAN study; is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California; and receives research support from the NIH/NIA. J.G-R. receives funding from the NIH. D.T.J. receives funding from the NIH and the Minnesota Partnership for Biotechnology and Medical Genomics. T.J.F. receives support from the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program and NIH. B.B.B. has served as an investigator for a clinical trial sponsored by Biogen. He receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from the NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program, and the Little Family Foundation. K.K. serves on the data safety monitoring board for Takeda Global Research and Development Center, Inc.; receives research support from Avid Radioparmaceuticals and Eli Lilly, and receives funding from NIH and Alzheimer’s Drug Discovery Foundation. V.J.L. consults for Bayer Schering Pharma, Piramal Life Sciences, and Merck Research, and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and the NIH (NIA, NCI). P.V. receives funding from the NIH. M.M.M. receives research support from the NIH and unrestricted research grants from Biogen and Lundbeck. J.W. receives funding from the NIH. K.A.J. receives funding from the NIH. C.G.S. receives funding from the NIH. M.L.S. at the time of manuscript submission, owned shares of the following medical related stocks, unrelated to the current work: Align Technology, Inc., LHC Group, Inc., Mesa Laboratories, Inc., Natus Medical Incorporated, Varex Imaging Corporation. R.C.P. consults for Roche, Inc.; Merck, Inc.; Genentech, Inc.; Biogen, Inc.; Eisai, Inc. and GE Healthcare and receives royalties from Oxford University Press for Mild Cognitive Impairment. All other authors report no competing interests.
Glossary
- MCI =
mild cognitive impairment
- MoCA =
Montreal Cognitive Assessment
- SUVR =
standardized uptake value ratio
References
- Altmann A, Tian L, Henderson VW, Greicius MD.. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol 2014; 75: 563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, et al. Gender differences in the incidence of AD and vascular dementia: the EURODEM Studies. EURODEM Incidence Research Group. Neurology 1999; 53: 1992–7. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT.. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 1992; 42: 631–9. [DOI] [PubMed] [Google Scholar]
- Barthelemy NR, Li Y, Joseph-Mathurin N, Gordon BA, Hassenstab J, Benzinger TLS, et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer's disease. Nat Med 2020; 26: 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, et al. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol 2011; 69: 1032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejanin A, Schonhaut DR, La Joie R, Kramer JH, Baker SL, Sosa N, et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer's disease. Brain 2017; 140: 3286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE.. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol 2004; 61: 378–84. [DOI] [PubMed] [Google Scholar]
- Botha H, Mantyh WG, Graff-Radford J, Machulda MM, Przybelski SA, Wiste HJ, et al. Tau-negative amnestic dementia masquerading as Alzheimer disease dementia. Neurology 2018. a; 90: e940. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H, Mantyh WG, Murray ME, Knopman DS, Przybelski SA, Wiste HJ, et al. FDG-PET in tau-negative amnestic dementia resembles that of autopsy-proven hippocampal sclerosis. Brain 2018. b; 141: 1201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med 2016; 8: 338ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Evans DA, Hebert L, Langa KM, Heeringa SG, Plassman BL, et al. National estimates of the prevalence of Alzheimer's disease in the United States. Alzheimers Dement 2011; 7: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham SC, Bourgeat P, Dore V, Savage G, Brown B, Laws S, et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer's disease pathophysiology (SNAP) or Alzheimer's disease pathology: a longitudinal study. Lancet Neurol 2016; 15: 1044–53. [DOI] [PubMed] [Google Scholar]
- Chiotis K, Saint-Aubert L, Rodriguez-Vieitez E, Leuzy A, Almkvist O, Savitcheva I, et al. Longitudinal changes of tau PET imaging in relation to hypometabolism in prodromal and Alzheimer's disease dementia. Mol Psychiatry 2018; 23: 1666–73. [DOI] [PubMed] [Google Scholar]
- Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol 2016. a; 80: 247–58. [DOI] [PubMed] [Google Scholar]
- Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM, et al. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology 2016. b; 87: 375–83. [DOI] [PubMed] [Google Scholar]
- Cho H, Choi JY, Lee SH, Lee JH, Choi Y-C, Ryu YH, et al. Excessive tau accumulation in the parieto-occipital cortex characterizes early-onset Alzheimer's disease. Neurobiol Aging 2017; 53: 103–11. [DOI] [PubMed] [Google Scholar]
- Cho H, Choi JY, Lee HS, Lee JH, Ryu YH, Lee MS, et al. Progressive Tau Accumulation in Alzheimer Disease: 2-Year Follow-up Study. J Nucl Med 2019; 60: 1611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland WS. Visualizing data. New Jersey: At&T Bell Laboratories; Hobart Press; 1993. [Google Scholar]
- Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, van der Flier WM, et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement 2017; 13: 870–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Blennow K, Johnson K, Keeley M, Bateman RJ, Molinuevo JL, et al. Anti-Tau trials for Alzheimer's disease: a report from the EU/US/CTAD Task Force. J Prev Alzheimers Dis 2019. a; 6: 157–63. [DOI] [PubMed] [Google Scholar]
- Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K.. Alzheimer's disease drug development pipeline: 2019. Alzheimer's Dement 2019. b; 5: 272–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS, et al. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 2017; 317: 2305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edland SD, Rocca WA, Petersen RC, Cha RH, Kokmen E.. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol 2002; 59: 1589–93. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997; 278: 1349–56. [PubMed] [Google Scholar]
- Fiest KM, Roberts JI, Maxwell CJ, Hogan DB, Smith EE, Frolkis A, et al. The prevalence and incidence of dementia due to Alzheimer's disease: a systematic review and meta-analysis. Can J Neurol Sci 2016; 43: S51–82. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Liu X, Ayutyanont N, Roontiva A, Thiyyagura P, et al. Apolipoprotein E epsilon4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging 2013; 34: 1–12. [DOI] [PubMed] [Google Scholar]
- Franzmeier N, Neitzel J, Rubinski A, Smith R, Strandberg O, Ossenkoppele R, et al. Functional brain architecture is associated with the rate of tau accumulation in Alzheimer's disease. Nat Commun 2020; 11: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Stern H.. The difference between “significant” and “not significant” is not itself statistically significant. Am Stat 2006; 60: 328–31. [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol 1997; 41: 17–24. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Blazey TM, Christensen J, Dincer A, Flores S, Keefe S, et al. Tau PET in autosomal dominant Alzheimer's disease: relationship with cognition, dementia and other biomarkers. Brain 2019; 142: 1063–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BA, McCullough A, Mishra S, Blazey TM, Su Y, Christensen J, et al. Cross-sectional and longitudinal atrophy is preferentially associated with tau rather than amyloid beta positron emission tomography pathology. Alzheimers Dement (Amst) 2018; 10: 245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, Schwarz CG, Brown RD, Rabinstein AA, et al. White matter hyperintensities: relationship to amyloid and tau burden. Brain 2019; 142: 2483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, et al. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol 2019; 76: 915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, La Joie R, Maass A, Baker SL, Swinnerton K, Fenton L, et al. Longitudinal tau accumulation and atrophy in aging and alzheimer disease. Ann Neurol 2019; 85: 229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho GJ, Hansen LA, Alford MF, Foster K, Salmon DP, Galasko D, et al. Age at onset is associated with disease severity in Lewy body variant and Alzheimer's disease. Neuroreport 2002; 13: 1825–8. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012; 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018. a; 14: 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Botha H, Weigand SD, Therneau TM, Knopman DS, et al. The bivariate distribution of amyloid-beta and tau: relationship with established neurocognitive clinical syndromes. Brain 2019. b; 142: 3230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Wiste HJ, Schwarz CG, Lowe VJ, Senjem ML, Vemuri P, et al. Longitudinal tau PET in ageing and Alzheimer's disease. Brain 2018. b; 141: 1517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Wiste HJ, Therneau TM, Weigand SD, Knopman DS, Mielke MM, et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA 2019. a; 321: 2316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement 2017; 13: 205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav S, Avila J, Scholl M, Kovacs GG, Kovari E, Skrabana R, et al. A walk through tau therapeutic strategies. Acta Neuropathol Commun 2019; 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015; 313: 1924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Tijms BM, Fagan AM, Hansson O, Klunk WE, et al. Association of cerebral amyloid-beta aggregation with cognitive functioning in persons without dementia. JAMA Psychiatry 2018; 75: 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Shultz A, Betensky RA, Becker JA, Sepulcre J, Rentz DM, et al. Tau positron emission tomographic imaging in aging and early Alzheimer's disease. Ann Neurol 2016; 79: 110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020; 19: 422–33. [DOI] [PubMed] [Google Scholar]
- Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM.. Multiple pathologies are common and related to dementia in the oldest-old: the 90+ Study. Neurology 2015; 85: 535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G, Delmar P, Voyle N, Rehal S, Hofmann C, Abi-Saab D, et al. Gantenerumab reduces amyloid-beta plaques in patients with prodromal to moderate Alzheimer's disease: a PET substudy interim analysis. Alzheimers Res Ther 2019; 11: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 2004; 55: 306–19. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Koeppe RA, Price JC, Benzinger T, Devous M, Jagust W, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimer's Dementia 2015; 11: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Lundt ES, Therneau TM, Vemuri P, Lowe VJ, Kantarci K, et al. Entorhinal cortex tau, amyloid-beta, cortical thickness and memory performance in non-demented subjects. Brain 2019; 142: 1148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC.. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol 1991; 48: 725–8. [DOI] [PubMed] [Google Scholar]
- Koscik RL, Betthauser TJ, Jonaitis EM, Allison SL, Clark LR, Hermann BP, et al. Amyloid duration is associated with preclinical cognitive decline and tau PET. bioRxiv 2019; 12: e12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss E, Edland S, Fillenbaum G, Mohs R, Clark C, Galasko D, et al. Clinical and neuropsychological differences between patients with earlier and later onset of Alzheimer's disease: a CERAD analysis, Part XII. Neurology 1996; 46: 136– 41. [DOI] [PubMed] [Google Scholar]
- La Joie R, Visani AV, Baker SL, Brown JA, Bourakova V, Cha J, et al. Prospective longitudinal atrophy in Alzheimer's disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med 2020; 12: eaau5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzy A, Chiotis K, Lemoine L, Gillberg PG, Almkvist O, Rodriguez-Vieitez E, et al. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol Psychiatry 2019; 24: 1112–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer's disease. Brain 2014; 137: 221–31. [DOI] [PubMed] [Google Scholar]
- Long JM, Holtzman DM.. Alzheimer disease: an update on pathobiology and treatment strategies. Cell 2019; 179: 312–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe VJ, Wiste HJ, Senjem ML, Weigand SD, Therneau TM, Boeve BF, et al. Widespread brain tau and its association with ageing, Braak stage and Alzheimer's dementia. Brain 2018; 141: 271–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, et al. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer's disease. Neuroimage 2017; 157: 448–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Insel PS, Aisen PS, Jagust W, Mackin S, Weiner M.. Brain structure and function as mediators of the effects of amyloid on memory. Neurology 2015; 84: 1136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade E, Bateman RJ.. Tau Positron Emission Tomography in Autosomal Dominant Alzheimer Disease: small Windows, Big Picture. JAMA Neurol 2018; 75: 536–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Assocation Workgroup. Alzheimers Dement 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Kinahan PE, Greer PJ, Nichols TE, Comtat C, Cantwell MN, et al. Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med 1999; 40: 2053–65. [PubMed] [Google Scholar]
- Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer's disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 2018; 14: 989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Wiste HJ, Weigand SD, Knopman DS, Lowe VJ, Roberts RO, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology 2012; 79: 1570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Gordon BA, Su Y, Christensen J, Friedrichsen K, Jackson K, et al. AV-1451 PET imaging of tau pathology in preclinical Alzheimer disease: defining a summary measure. Neuroimage 2017; 161: 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Papp KV, Rentz DM, Donohue MC, Amariglio R, Quiroz YT, et al. Early and late change on the preclinical Alzheimer's cognitive composite in clinically normal older individuals with elevated amyloid beta. Alzheimers Dement 2017; 13: 1004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–4. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018; 554: 249–54. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53: 695–9. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 2012; 71: 362–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 2019; 142: 1503–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, et al. Alzheimer's disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol 2011; 121: 571–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, et al. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol 2017; 74: 1178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Lyoo CH, Sudre CH, van Westen D, Cho H, Ryu YH, et al. Distinct tau PET patterns in atrophy-defined subtypes of Alzheimer's disease. Alzheimers Dement 2019. a; 16: 335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Rabinovici GD, Smith R, Cho H, Scholl M, Strandberg O, et al. Discriminative Accuracy of [18F]flortaucipir Positron Emission Tomography for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 2018; 320: 1151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Schonhaut DR, Scholl M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain 2016; 139: 1551–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Smith R, Ohlsson T, Strandberg O, Mattsson N, Insel PS, et al. Associations between tau, Abeta, and cortical thickness with cognition in Alzheimer disease. Neurology 2019. b; 92: e601. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement 2017; 13: 841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256: 183–94. [DOI] [PubMed] [Google Scholar]
- Pontecorvo MJ, Devous MD, Kennedy I, Navitsky M, Lu M, Galante N, et al. A multicentre longitudinal study of flortaucipir (18F) in normal ageing, mild cognitive impairment and Alzheimer's disease dementia. Brain 2019; 142: 1723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontecorvo MJ, Devous MD Sr., Navitsky M, Lu M, Salloway S, Schaerf FW, et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain 2017; 140: 748–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30: 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl M, Lockhart SN, Schonhaut DR, O'Neil JP, Janabi M, Ossenkoppele R, et al. PET Imaging of tau deposition in the aging human brain. Neuron 2016; 89: 971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl M, Ossenkoppele R, Strandberg O, Palmqvist S, Jogi J, Ohlsson T, et al. Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer's disease. Brain 2017; 140: 2286–94. [DOI] [PubMed] [Google Scholar]
- Schwarz CG, Gunter JL, Lowe VJ, Weigand S, Vemuri P, Senjem ML, et al. A comparison of partial volume correction techniques for measuring change in serial amyloid PET SUVR. Jad 2019; 67: 181–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CG, Gunter JL, Wiste HJ, Przybelski SA, Weigand SD, Ward CP, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. NeuroImage: Clin 2016; 11: 802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature 2016; 537: 50–6. [DOI] [PubMed] [Google Scholar]
- Sintini I, Martin PR, Graff-Radford J, Senjem ML, Schwarz CG, Machulda MM, et al. Longitudinal tau-PET uptake and atrophy in atypical Alzheimer's disease. Neuroimage Clin 2019; 23: 101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, et al. Amyloid deposition detected with florbetapir F 18 [(18)F-AV-45] is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging 2013; 34: 822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Mormino EC, Schultz AP, Betensky RA, Papp KV, Amariglio RE, et al. The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann Neurol 2019; 85: 181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med 2014; 6: 228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012; 41: 1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med 2020; 26: 387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D, Chen YF, Yu P, Sundell KL, Suhy J, Siemers E, et al. Amyloid status imputed from a multimodal classifier including structural MRI distinguishes progressors from nonprogressors in a mild Alzheimer's disease clinical trial cohort. Alzheimers Dement 2016; 12: 977–86. [DOI] [PubMed] [Google Scholar]
- Townley RA, Graff-Radford J, Mantyh WG, Botha H, Polsinelli A, Przybelski SA, et al. Progressive dysexecutive syndrome due to alzheimer’s disease: a description of 55 cases and comparison to other phenotypes. Brain Commun 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townley RA, Syrjanen JA, Botha H, Kremers WK, Aakre JA, Fields JA, et al. Comparison of the short test of mental status and the montreal cognitive assessment across the cognitive spectrum. Mayo Clin Proc 2019; 94: 1516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 2013; 12: 357–67. [DOI] [PubMed] [Google Scholar]
- Wisse LE, Butala N, Das SR, Davatzikos C, Dickerson BC, Vaishnavi SN, et al. Suspected non-AD pathology in mild cognitive impairment. Neurobiol Aging 2015; 36: 3152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Price JC, Madeira C, Saxton JA, Snitz BE, Lopez OL, et al. Amyloid imaging in dementias with atypical presentation. Alzheimers Dement 2012; 8: 389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, et al. 18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimers Dement 2013; 9: 666–76. [DOI] [PubMed] [Google Scholar]
- Xia C, Makaretz SJ, Caso C, McGinnis S, Gomperts SN, Sepulcre J, et al. Association of In Vivo [18F]AV-1451 Tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol 2017; 74: 427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.