Abstract
Aims
COVID-19 outbreak has created a public health catastrophe all over the world. Here, we have aimed to conduct a systematic review and meta-analysis on remdesivir use for COVID-19.
Main methods
We searched Pubmed, Scopus, Embase, and preprint sites and identified ten studies for qualitative and four studies for quantitative analysis using PRISMA guidelines. The quantitative synthesis was performed using fixed and random effect models in RevMan 5.4. Heterogeneity was assessed using the I-squared (I2) test.
Key findings
Comparing 10-day remdesivir group with placebo or standard of care (SOC) group, remdesivir reduced 14 days mortality (OR 0.61, CI 0.41–0.91), need for mechanical ventilation (OR 0.73, CI 0.54–0.97), and severe adverse effects (OR 0.69, 95% CI 0.54 to 0.88). Clinical improvement on day 28 (OR 1.59, CI 1.06–2.39), day 14 clinical recovery (OR 1.48, CI 1.19–1.84), and day 14 discharge rate (OR 1.41, CI 1.15–1.73) were better among remdesivir group. Earlier clinical improvement (MD −2.51, CI −4.16 to −0.85); and clinical recovery (MD −4.69, CI −5.11 to −4.28) were seen among the remdesivir group.
Longer course (10 days) of remdesivir showed a higher discharge rate at day 14 (OR 2.11, CI 1.50–2.97), but there were significantly higher rates of serious adverse effects, and drug discontinuation than the 5-day course.
Significance
Remdesivir showed a better 14 days mortality profile, clinical recovery, and discharge rate. Overall clinical improvement and clinical recovery were earlier among the remdesivir group. 10-day remdesivir showed more adverse outcome than 5-day course with no significant benefits.
Keywords: Remdesivir, COVID 19, Therapeutic efficacy, ARDS, Coronavirus-2
Graphical abstract
Highlights
-
•
Remdesivir showed a better 14-day mortality, clinical recovery, and discharge rate comparing with placebo or standard of care in COVID-19.
-
•
Overall clinical improvement and clinical recovery were earlier among 10-day remdesivir group than placebo or standard of care.
-
•
No benefit on 28-day mortality, discharge rate, and overall adverse effect between 10-day remdesivir group and placebo or standard of care.
-
•
Longer course (10-day) of remdesivir is not favored over a shorter course (5-day).
1. Introduction
Corona Virus Disease -19 (COVID-19) outbreak which was first seen in the Hubei province of China in late December of 2019 has become a widespread pandemic. The infection is caused by a new strain of coronavirus which was later named as Severe Acute Respiratory Syndrome – Coronavirus 2 (SARS-CoV 2). The virus has spread all over the globe and created a public health catastrophe meanwhile dragging several countries into economic crises. The symptoms of infection range from mild viral illness symptoms including sore throat, headache, cough, fever to severe symptoms of pneumonia, and ARDS. As of August 28, 2020, 24 million cases have been confirmed and more than 800,000 deaths have been recorded due to COVID-19 [1]. However, a lack of standardized treatment makes the situation even more frightful. Remdesivir is being used as one of the repurposed drugs in combating the illness all around the world.
Remdesivir, a nucleotide analog prodrug that inhibits viral RNA polymerases, has shown in vitro activity against SARS-CoV-2 [2]. Subsequent evaluation by numerous virology laboratories demonstrated the ability of remdesivir to inhibit coronavirus replication, including SARS-CoV-2 [3]. As a nucleoside analog, remdesivir acts as an RNA dependent RNA polymerase (RdRp) inhibitor, targeting the viral genome replication process. The RdRp is the protein complex coronaviruses use to replicate their RNA-based genomes. After the host metabolizes remdesivir into active NTP, the metabolite competes with adenosine triphosphate (ATP: the natural nucleotide normally used in this process) for incorporation into the nascent RNA strand. In addition, the drug is believed to outpace the proofreading property of the virus, thus maintaining the antiviral activity [4,5]. The World Health Organization (WHO) has considered this drug as one of the promising therapeutics against fighting COVID-19. Multiple clinical trials are underway on the use of remdesivir for the treatment of COVID-19 in the United State of America (USA) and all around the world [6].
We aimed to search for clinical evidence to support the use of remdesivir regarding its safety and side effects to find out whether the drug is a real game-changer or just another drug being used in a trial.
2. Objective
2.1. Primary outcome
-
1.
To compare mortality rate, clinical improvement, and discharge among patients receiving 10- day course of remdesivir compared to placebo or standard of care.
-
2.
To compare mortality rate, clinical improvement, and discharge among patients receiving 10- day course of remdesivir compared to a 5-day course of remdesivir therapy
2.2. Secondary outcome
-
•
To compare adverse effects, need for oxygen support, and mean duration of clinical recovery among patients receiving remdesivir compared to standard of care or placebo.
-
•
To compare adverse effects, need for oxygen support, and mean duration of clinical recovery among patients receiving a 10-day course of remdesivir compared to a 5-day course of remdesivir.
3. Materials and methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was used for our systematic review [7].
3.1. Criteria for considering studies for this review
3.1.1. Types of studies
We included the studies focusing on mortality rate, clinical improvement and recovery, discharge rate, adverse effects, mechanical ventilation and respiratory support, mean difference of clinical improvement as well recovery among patients taking remdesivir compared to the patients receiving standard of care alone. Also, we included studies comparing a longer regimen and a shorter regimen of remdesivir comparing the above outcomes.
3.1.2. Types of participants
We included studies including patients diagnosed with COVID-19 who received remdesivir in addition to the standard of care (SOC) or only receiving standard of care or placebo with comparison made with the remdesivir group.
3.1.3. Types of interventions
Our treatment arm consists of patients taking remdesivir along with the SOC while the control arm consists of patients receiving SOC or placebo.
3.1.4. Types of outcome measures
For our quantitative analysis, mortality, clinical improvement and recovery, discharge rate, mechanical ventilation and respiratory support, adverse effects, mean difference of clinical improvement as well recovery and treatment outcome at day 14 and day 28 among the treatment and control group that occurred during treatment were outcomes of interest.
3.1.5. Outcomes
We compared mortality, clinical improvement, and recovery, discharge rate, mechanical ventilation and respiratory support, adverse effects, mean difference of clinical improvement as well recovery and treatment outcome at day 14, and day 28 between treatment and control arms. Also, we compared the longer regimen and shorter regimen of remdesivir for the above outcomes.
3.2. Search methods for identification of studies
Pubmed, PubMed Central, Embase, Scopus, and preprint servers like medRxiv and bioRxiv were accessed by our reviewers (PB and DBS) who independently searched and evaluated the quality of the studies till August 25, 2020. We filtered the studies using Covidence and extracted data for quantitative and qualitative synthesis. Any potential conflict was solved taking the final opinion of another reviewer (SK). Another reviewer (ER) assessed the risk of bias and cross-checked all the selected studies.
3.2.1. Electronic searches
We have documented the detailed search strategy in Supplementary file no. 1.
3.3. Data collection and analysis
We extracted the data for quantitative synthesis through Covidence and did the analysis using RevMan 5.4. Assessment of heterogeneity was done using the I-squared (I2) test. We used a random/fixed effect for the pooling of selected studies.
3.3.1. Selection of studies
We have included Randomized Control Trials (RCTs), prospective, and retrospective observational studies in which the patients received remdesivir for the treatment of COVID-19 in the qualitative analysis. For the quantitative analysis, we included only RCTs with a treatment arm and a control arm. We excluded studies in which remdesivir was used for treatment among the pediatric age group, pregnant women, patients with Acquired Immuno-deficiency Syndrome (AIDS), end-stage liver disease, and cancer in the entire study population. We excluded meta-analysis, reviews, protocols, in-silico studies, Artificial Intelligence-based simulation studies, and the studies in which the outcome was not properly defined among the patients treated with remdesivir.
3.3.2. Data extraction and management
We evaluated the quality of studies thoroughly and also took into account only the outcomes that were of our interest.
3.3.3. Assessment of risk of bias in included studies
We used the Cochrane Risk of Bias (ROB) 2.0 tool for analysis of our RCTs shown in Fig. 1 [8]. We used the NHLBI (National Heart, Lung, and Blood Institute) quality assessment tools to assess the risk of bias in our prospective and retrospective observational studies (Table 1 ) [9]. Details of NHLBI bias assessment of observational studies are available in supplementary file 2. We used RevMan 5.4 for creating a summary of biases for RCTs using the Cochrane ROB 2.0 tool.
Fig. 1.
Risk of bias assessment of trials.
Table 1.
NHLBI assessment of bias for observational studies.
| Study | Score | Percentage | Quality |
|---|---|---|---|
| Anderson et al. [10] | 8/14 | 57.1% | Fair |
| Antinori et al. [11] | 9/14 | 64.2% | Good |
| Augustin et al. [12] | 10/14 | 71.4% | Good |
| Grein et al. [2] | 8/14 | 57.1% | Fair |
| Olender et al. [13] | 10/14 | 71.4% | Good |
| Pasquini et al. [14] | 10/14 | 71.4% | Good |
Good if they fulfilled 60–100% of the tool items, Fair if 50–59% or Poor if 0–49%.
3.3.4. Assessment of heterogeneity
The I-squared (I2) test was used for the assessment of heterogeneity. We interpreted the I-squared (I2) test done based on the Cochrane Handbook for Systematic Reviews of Interventions as follows [15]:
-
i)
0% to 40%: might not be important.
-
ii)
30% to 60%: may represent moderate heterogeneity.
-
iii)
50% to 90%: may represent substantial heterogeneity.
-
iv)
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on (i) magnitude and direction of effects and (ii) strength of evidence for heterogeneity (e.g. P-value from the chi-squared test, or a confidence interval for I2).
3.3.5. Assessment of reporting biases
Reporting bias was checked by prefixed reporting of the outcome.
3.3.6. Data synthesis
Statistical analysis was performed using RevMan 5.4 software. Risk Ratio (RR)/Odds Ratio (OR) was used for outcome estimation whenever appropriate with 95% Confidence Interval (CI). The fixed/random-effects model was used according to heterogeneities. We analyzed the mean differences among the two groups for the duration of clinical improvement and recovery using the median, sample size, and interquartile range whenever the means and standard deviations were not provided in the study [16].
3.3.7. Subgroup analysis and investigation of heterogeneity
We used the random effect model, in cases of heterogeneity.
3.3.8. Sensitivity analysis
We did not run sensitivity analysis being there were only three RCTs comparing remdesivir with SOC and two only comparing shorter and longer regimens of remdesivir. Also, there is low-moderate heterogeneity in most fields of our analysis so sensitivity analysis was not used.
4. Results
-
A.
Qualitative synthesis
We identified a total of 5573 studies after electronic database searching. We removed 600 duplicates. Screening of the title and abstracts of 4973 studies was done. We excluded 4942 studies and checked 31 articles for full-text eligibility. We excluded 21 studies with definite reasons mentioned in the PRISMA flow diagram in Fig. 2 . At last, 10 studies were selected for qualitative analysis. A discussion of these studies is done in Table 2 .
-
B.
Quantitative synthesis
Fig. 2.
PRISMA diagram.
Table 2.
Qualitative analysis.
| Study | Population | Intervention | Comparator | Outcome |
|---|---|---|---|---|
| Beigel et al. [17], RCT, USA | N: 1059 (T = 538, C = 521) Sex: F = [379; T = 189; C = 190] M = [684; T = 352 C = 332] Mean age(SD) 58.9 (15.0) T = 58.6 (14.6) C = 59.2 (15.4) Inclusion:
|
Remdesivir (200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days) with supportive care | Placebo for 10 days in addition to standard of care | Mortality rate at D14: T = 32/538C = 54/521 Clinical recovery at D15: T = 334/538C = 273/521 Discharge (alive)D15: T = 257/538C = 203/521 Adverse events: T = 156/538; C = 172/521 Serious adverse events T = 114/538; C = 141/521 Events leading to drug discontinuation T = 49/538 C = 53/521 Duration of median recovery, days T = 11(9–12), C = 15 (13–19) Non-invasive mechanical ventilation Baseline: T = 98/541 C = 99/522 D15: T = 16/538C = 14/521 Invasive mechanical ventilation Baseline T = 125/541 C = 147/522 D15 T = 60/538 C = 72/521 |
| Goldman et al. [18], RCT, multi-center study (nine countries) | N: 397 (T = 200, C = 197) Sex: F = 144, M = 253 History: Median age (IQR) yr T5 = 61 (50–69); C10 = 62 (50–71) Inclusion: Oxygen saturation of 94% or less while they were breathing ambient air, and radiologic evidence of pneumonia, age 12 years and above |
Intravenous remdesivir for 5 days 200 mg of remdesivir on day 1 and 100 mg once daily on subsequent days. |
Intravenous remdesivir for 10 days | Mortality rate at D14 T5 = 16/200 C10 = 21/197 Clinical improvement D7: T5 = 71/200 C10 = 54/197 D14 T5 = 129/200 C10 = 107/197 Recovery D7 T5 = 71/200 C10 = 51/197 D14 T5 = 129/200 C10 = 106/197 Discharge (alive) D14 T5 = 16/200 C10 = 68/197 Adverse events T5 = 141/200 C10 = 145/197 Serious adverse events T5 = 42/200, C10 = 68/197 Any grade ≥ 3 adverse event T5 = 61/200; C10 = 84/197 Events leading to drug discontinuation T5 = 2/200 C10 = 8/197 Clinical improvement (median day) T5 = 10 C10 = 11 Clinical improvement D14 T5 = 129/200; C10 = 107/197 Non-invasive mechanical ventilation or high-flow oxygen Baseline T5 = 49/200; C10 = 60/197 At day 14 T5 = 9/200; C10 = 10/197 Invasive mechanical ventilation or ECMO Baseline: T5 = 4/200; C10 = 9/197 At day 14 T5 = 16/200; C10 = 33/197 |
| Spinner et al. [19], RCT, randomized open label multicenter trial, US, Europe, and Asia | N: 596 (T10 = 196, T5 = 199, C = 200) Sex: F = 227, M = 369 History: Median age 57 [interquartile range, 46–66] years Inclusion: Confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and moderate COVID-19 pneumonia (Pulmonary infiltrates and room-air oxygen saturation > 94%) |
Intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions) |
The same volume of placebo infusions for 10 days | Mortality rate D28 T10 3/193 T5 2/191 C 4/200 D14 T10 2/193 T5 1/191 C 4/200 D11 T10 2/193 T5 0/191 C 4/200 Clinical Improvement: (An improvement of at least 2 points from baseline on the 7-point ordinal scale) D28 T10 174/193 T5 171/191 C 166/200 D14 T10 148/193 T5 146/191 C 135/200 D7 T10 92/193 T5 106/191 C 94/200 Recovery (An improvement from a baseline score of 2 to 5 to a score of 6 or 7 or from a baseline score of 6 to a score of 7) D28 T10 178/193 T5 175/191 C 70/200 D14 T10 153/193 T5 153/191 C 145/200 D7 T10 94/193 T5 114/191 C 101/200 Discharge (alive) D28 T10 174/193 T5 170/191 C 166/200 D14 T10 146/193 T5 146/191 C 134/200 D11 T10 125/193 T5 134/191 C 120/200 Adverse events T 10 113/193 T5 98/191 C 93/200 Serious adverse events T10 10/193 T5 9/191 C 18/200 Events leading to drug discontinuation T10 8/193 T5 4/191 C NA/200 Any grade ≥ 3 adverse event T10 24/193 T5 20/191 C 24/200 Undetectable viral RNA (Clearance) D28 T10/193 T5/191C/200 D14 T10/193 T5/191 C/200 D7 T10/193 T5/191 C/200 Duration of median clinical improvement (≥2-pt Improvement), days T10 = 8 (4–14) T5 = 6 (5–14) C = 8 (5–22) Duration of median Recovery, days T10 = 8 (4–13) T5 = 6 (5–10) C = 7 (4–15) Non-invasive mechanical ventilation or high flow oxygen devices Baseline: T10 1/193 T5 2/191 C 2/200 D14 T10 0/193 T5 4/191 C4/200 D28 T10 1/193 T5 1/191 C 0/200 Invasive mechanical ventilation or ECMO Baseline T10 0/193 T5 0/191 C 0/200 D14 T10 1/193 T5 0/191 C 5/200 D28 T10 0/193 T5 0/191 C 4/200 |
| Wang et al. [20], randomized controlled trial, China | N: 237 (T = 158, C = 79) Sex: F = 96, M = 140 Inclusion:
|
Intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions) | Same volume of placebo infusions for 10 days | Mortality rate D7 T = 10/158 C = 4/78 D14 T = 15/158 C = 7/78 D28 T = 22/158 C = 10/78 Clinical Improvement (two-point improvement): D28 T 103/158 C 45/78 D14 T 42/158 C 18/78 D7 T 4/158 C 2/78 Discharge (alive) D28 T 92/158 C 45/78 D14 T 39/158 C 18/78 D7 T 4/158 C 2/78 Any Adverse events T = 102/158 C = 50/78 Any Grade 3 or 4 AE T = 13/158 C = 11/78 Serious adverse events T = 28/158; C = 20/78 Serious Grade 3 or 4 AE T = 9/158 C = 10/78 Events leading to drug discontinuation T = 18/158; C = 4/78 Undetectable viral RNA (Clearance) D28 T = 99/131, C = 54/65 D14 T = 93/131 C = 49/65 D7 T = 66/131 C = 32/65 Duration of oxygen support, days T = 19·0 (11·0 to 30·0) C = 21·0 (14·0 to 30·5) Duration of hospital stay, days T = 25·0 (16·0 to 38·0) C = 24·0 (18·0 to 36·0) Duration of median clinical improvement, days T = 21·0 days [IQR 13·0–28·0] C = 23·0 days [15·0–28·0] Non-invasive mechanical ventilation to high-flow nasal cannula Baseline T = 28/158 C = 9/78 D14 T = 13/158 C = 8/78 D28 T = 2/158 C = 2/78 Invasive mechanical ventilation or ECMO Baseline T = 0/158 C = 1/78 D14 T = 4/158 C = 7/78 D28 T = 2/158 C = 3/78 |
| Grein et al. [2], multicenter cohort, ten countries | N = 53 F = 13, M = 40 The age range was 23 to 82 years, and the median age was 64 years (interquartile range, 48 to 71) Inclusion:
|
Patients received a 10-day course of remdesivir, consisting of 200 mg administered intravenously on day 1, followed by 100 mg daily for the remaining 9 days of treatment | Mortality rate T = 7/53 Clinical Improvement T = 36/53 Discharge (alive) T = 25/53 Adverse events T = 32/53 Serious adverse events T = 12/53 Non-invasive mechanical ventilation Baseline T = 7/53 After Treatment T = 3/53 Invasive mechanical ventilation Baseline T = 34/53 After Treatment T = 10/53 |
|
| Anderson et al. [10], Cohort, USA | N = 1643 Median age = 67 (IQR 56–78 years) Majority were Hispanic or Black |
Patients received remdesivir in addition to standard of care. Dose of remdesivir not mentioned. | 586 patients (36%) had a LOS of 1–4 days, 384 patients (23%) had a LOS of 5–8 days, and 673 patients (41%) were hospitalized ≥9 days Median LOS 7 days (3–14 days) In hospital 28-day mortality was 26% 41% of patients both received a 5-day course of remdesivir and have LOS shortened by 4 days or more |
|
| Antiniori [11], prospective open-label study, Italy | N = 35 M = 26, F = 9 ICU = 18, Ward = 17 Inclusion Male or non-pregnant female aged >18 years, had SARS CoV-2 infection confirmed by a positive reverse-transcriptase polymerase chain reaction (RT-PCR) test of a respiratory tract sample and pneumonia confirmed by a chest X-ray or computed tomography (CT) scan, and were mechanically ventilated or had an oxygen saturation (SaO2) level of <94% in room air or a National Early Warning Score (NEWS)2 of ≥4 Exclusion AST and ALT more than 5 times the upper limit Creatinine clearance <30 ml/min |
The 10-day course of remdesivir was completed by 22 patients (63%) and discontinued by 13, of whom eight (22.8%) discontinued because of adverse events An intravenous loading dose of 200 mg on day 1, followed by an intravenous dose of 100 mg/day from day 2 to day 10. |
The median follow-up was 39 days (IQR 25–44). On day 28, Ward patients Discharged: 14 Hospitalized: 2 Mortality: 1 ICU patients Discharged: 6 (33.3%) Mortality: 8 (44.4%) Mechanically ventilated: 3 (16.7%) Improved: 1 (5.6%) Hypertransaminasemia (42.8%) and acute kidney injury (22.8%) are the most frequent side effects |
|
| Augustin et al. [12], prospective open observational study, USA, Europe and Japan | N = 61 (Data of 53 patients because of no follow up data of 7 patients and one patient received incorrect dosage) M = 40, F = 21 Inclusion:
|
Patients received 200 mg remdesivir iv on the first day, followed by 9 days of 100 mg remdesivir therapy iv. | Follow up time of 18 days Improvement: 36/53 Extubation: 17/30 of invasive ventilated patients Termination of ECMO: ¾ Mortality: 7/53 Side effects: 32/53 Liver abnormalities, diarrhea, rash, renal impairment and hypotension |
|
| Olender et al. [13], phase 3, randomized, open-label trial and retrospective cohort study, multicenter study | N = 1130 T:312 C:818 Inclusion
|
Standard of care treatment (subject to clinical practice stipulated by individual sites) plus remdesivir 200 mg on day 1, followed by remdesivir 100 mg daily on days 2–5 Standard-of-care plus remdesivir 200 mg on day 1, followed by remdesivir 100 mg daily on days 2–10 (remdesivir-cohort) Remdesivir cohort obtained from Phase 3 randomized trial Non remdesivir cohort obtained from retrospective cohort study |
At Day 14 Remdesivir cohort 74.4% recovered, OR 2.03, 95% CI 1.34–3.08 Non Remdesivir cohort 59% recovered Mortality at day 14 OR 0.38, 95% CI 0.22–0.68 7.6% in remdesivir cohort vs 12.5% in the non-remdesivir cohort |
|
| Pasquini et al. [14], retrospective observational study, Italy | N = 51 T = 25C = 26 M = 47 F = 4 Median age = 47 Inclusion criteria
|
First dose of 200 mg IV on Day 1, plus 100 mg daily from Day 2 to Day 10 in the treatment group Concomitant therapies include hydroxychloroquine, tocilizumab and lopinavir/ritonavir |
Better survival with remdesivir using Charlson Comorbidity Index OR 3.506 (95% CI 1.768–6.954) Median follow up 52 days (46–57) |
|
AE: Adverse Effect; ALT: Alanine Transaminase; AST: Aspartate Transaminase; C: Control; CI: Confidence Interval; CT: Computed Tomography; D7: Day7; D14: Day 14; D28: Day 28; ECMO: Extra Corporeal Membrane Oxygenation; F: Female; FiO2: Fraction of Inspired Oxygen; h: hours; ICU: Intensive Care Unit; IV: Intravenous; IQR: Interquartile Range; LOS: Length of Stay; M: Male; N: Number; OR: Odd's Ratio; PaO2: Partial pressure of oxygen; RT-PCR: Reverse Transcription Polymerase Chain Reaction; T: Treatment; T5: 5 days treatment group; T10: 10 days treatment group; USA: United States of America.
Overall four RCTs are included in the quantitative synthesis.
4.1. Treatment outcome
We have compared outcomes of randomized studies with 10 days of remdesivir versus placebo or standard of care (SOC) and also longer course (10 days) of remdesivir with shorter one (5 days). Mortality rate, clinical improvement (≥2-point improvement in the ordinal score), clinical recovery (an improvement from a baseline score of 2 to 5 to a score of 6 or 7 or from a baseline score of 6 to a score of 7 in the ordinal score), and discharge rate were our primary outcome variables. Development of adverse effects (severe, overall, and grade ≥ 3 adverse events), invasive and non-invasive mechanical ventilation requirement, and mean duration of clinical recovery and improvement were our secondary outcome variables between remdesivir versus placebo or standard of care (SOC) and shorter (5 days) versus longer (10 days) use of remdesivir. Among the included studies meta-analysis, we found there is low-high heterogeneity, may be due to different study design, biological variability among studies, and risk of bias among studies that could not be omitted fully.
4.1.1. Remdesivir (10 days) versus placebo or standard of care: mortality rate
The meta-analysis of odds ratios (OR) for remdesivir compared with placebo or SOC using fixed effect model among three randomized studies showed that remdesivir reduces 14 days mortality (OR 0.61, 95% CI 0.41 to 0.91; participants = 1688; studies = 3; I2 = 0%). Meanwhile, there is no significant difference between two groups for 28 days mortality (OR 1.02, 95% CI 0.50 to 2.06; participants = 629; studies = 2; I2 = 0%) (Fig. 3 ).
Fig. 3.
Forest plot for mortality comparing remdesivir versus placebo or standard of care.
4.1.2. Remdesivir (10 days) versus placebo or standard of care: clinical improvement and recovery
Meta-analysis for clinical improvement (≥2-point improvement in ordinal score) showed slight improvement with statistical significance only in day 28. (Day 14; OR 1.45, 95% CI 1.00 to 2.08; participants = 629; studies = 2; I2 = 0%; Day 28; OR 1.59, 95% CI 1.06 to 2.39; participants = 629; studies = 2; I2 = 0%) (Fig. 4 ). Similarly, there is clinically significant clinical recovery in day 14 among remdesivir groups compared to placebo or SOC (OR 1.48, 95% CI 1.19 to 1.84; participants = 1452; studies = 2; I2 = 0%) (Fig. 5 ).
Fig. 4.
Forest plot for clinical improvement comparing remdesivir versus placebo or standard of care.
Fig. 5.
Forest plot for clinical recovery comparing remdesivir versus placebo or standard of care.
4.1.3. Remdesivir (10 days) versus placebo or standard of care: discharge rate
Result on discharge rate showed increased discharge rate among remdesivir group both in 14 days and 28 days but it is statistically significant for 14 days only (day 14; OR 1.41, 95% CI 1.15 to 1.73; participants = 1688; studies = 3; I2 = 0%; day 28; OR 1.35, 95% CI 0.91 to 2.02; participants = 629; studies = 2; I2 = 53%) (Fig. 6 ).
Fig. 6.
Forest plot for discharge rate comparing remdesivir versus placebo or standard of care.
4.2. Remdesivir (10 days) versus placebo or standard of care: adverse effects
The meta-analysis of three randomized controlled trials showed that the odds of having severe adverse effects is less among remdesivir group (OR 0.69, 95% CI 0.54 to 0.88; participants = 1688; studies = 3; I2 = 0%) though odds for the development of overall adverse effect (OR 1.10, 95% CI 0.70 to 1.72; participants = 1688; studies = 3; I2 = 74%) and grade ≥ 3 adverse event (OR 0.85, 95% CI 0.52 to 1.38; participants = 629; studies = 2; I2 = 32%) among two groups is statistically insignificant (Fig. 7 ). It is also important to note that the discontinuation of the drug (due to adverse effects in patients) is not significant among the groups. (OR 1.26, 95% CI 0.50 to 3.19) (Supplementary file 3. Fig. 1).
Fig. 7.
Forest plot for adverse events comparing remdesivir versus placebo or standard of care.
4.3. Remdesivir (10 days) versus placebo or standard of care: mechanical ventilation and respiratory support
Meta-analysis of baseline mechanical ventilation and respiratory support among included studies showed no statistical differences between two arms (OR 0.87, 95% CI 0.71 to 1.06; participants = 2991; studies = 5; I2 = 16%). Also there is no difference for both Non-invasive Mechanical Ventilation (NIMV) and high-flow oxygen (OR 1.01, 95% CI 0.76 to 1.34; participants = 1692; studies = 3; I2 = 0%) and Invasive Mechanical Ventilation (IMV) or Extra-corporeal Membrane Oxygenation (ECMO) (OR 0.76, 95% CI 0.57 to 1.00; participants = 1299; studies = 2; I2 = 0%) (Supplementary file 3. Fig. 2).
But, at 14 days, the need for mechanical ventilation and respiratory support is significantly lower among remdesivir groups (OR 0.73, 95% CI 0.54 to 0.97; participants = 3377; studies = 6; I2 = 27%). On subgroup analysis, the result shows decreased need for IMV or ECMO requirement among remdesivir groups at day 14(OR 0.69, 95% CI 0.49 to 0.97; participants = 1689; studies = 3; I2 = 49%). While for NIMV and high-flow oxygen it is of no statistical significance (OR 0.84, 95% CI 0.49 to 1.45; participants = 1688; studies = 3; I2 = 16%) (Fig. 8 ).
Fig. 8.
Forest plot for day 14 ventilation and respiratory support comparing remdesivir versus placebo or standard of care.
4.4. Remdesivir (10 days) versus placebo or standard of care: mean differences of duration of clinical improvement and recovery
Meta-analysis on clinical improvement (≥2-point improvement in ordinal score) showed clinical improvement among remdesivir group (MD −2.51, 95% CI −4.16 to −0.85; participants = 629; studies = 2; I2 = 10%) approximately 2.5 days earlier. While clinical recovery (an improvement from a baseline score of 2–5 to a score of 6 or 7 or from a baseline score of 6 to a score of 7 in the ordinal score) was seen about 4.5 days earlier among the remdesivir group (MD −4.69, 95% CI −5.11 to −4.28; participants = 1452; studies = 2; I2 = 97%) (Fig. 9 ).
Fig. 9.
Forest plot of mean differences of the duration of clinical improvement and recovery among remdesivir group versus placebo or SOC.
4.5. Treatment outcome at day 14: remdesivir shorter (5 days) versus longer (10 days) course of treatment
Meta-analysis comparing the longer 10-day course of remdesivir with shorter 5-day course showed higher discharge rate at day 14 (OR 2.11, 95% CI 1.50 to 2.97; participants = 781; studies = 2; I2 = 96%). But there is significantly higher rates of serious adverse effects (OR 1.77, 95% CI 1.19 to 2.65; participants = 781; studies = 2; I2 = 20%), grade ≥ 3 adverse event (OR 1.53, 95% CI 1.09 to 2.16; participants = 781; studies = 2; I2 = 0%), and drug discontinuation due to medication intolerance (OR 2.74, 95% CI 1.06 to 7.07, participants = 781, studies = 2, I2 = 0%) among longer (10 days) course than shorter (5 days) treatment group. Also IMV or ECMO requirement is higher among longer (10 days) course group (OR 2.34, 95% CI 1.26 to 4.35, participants = 781, studies = 2, I2 = 0%) than shorter (5 days) remdesivir group.
There is no statistical significance among two groups for overall 14 days mortality rate (OR 1.41, 95% CI 0.73 to 2.72, participants = 781, studies = 2, I2 = 0%), clinical improvement at 14 days (OR 0.79, 95% CI 0.58 to 1.07, participants = 781, studies = 2, I2 = 48%), clinical recovery (OR 0.75, 95% CI 0.55 to 1.02, participants = 781, studies = 2, I2 = 31%), overall adverse effects (OR 1.26, 95% CI 0.93 to 1.69, participants = 781, studies = 2, I2 = 0%), and NIMV or high flow oxygen requirement (OR 0.78, 95% CI 0.34 to 1.77, participants = 781, studies = 2, I2 = 58%) (Fig. 10 ).
-
C.
Clinical trials
Fig. 10.
Forest plot of treatment outcome at day 14: remdesivir shorter (5 days) vs longer (10 days) course of treatment.
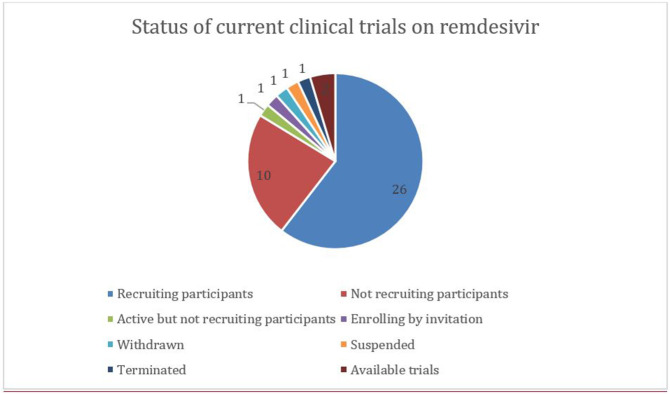
A total of 48 trials for the assessment of Remdesivir on COVID-19 has been registered until now in ClinicalTrials.gov (ClinicalTrials.gov) (Supplementary file 4). In most cases, these trials are being conducted with the primary outcome as a time to recovery, mortality, clinical improvement, and need for mechanical ventilation. Including the United States (23 trials), France (4 trials), a total of 15 countries (among the locations provided) are managing such trials around the globe. One of them in France has an enrollment of 6 hundred thousand participants and it is the largest trial. A total of 38 trials are clinical trials while the rest of the trials are of observational types or expanded access (see Fig. 11 ).
Fig. 11.
Pie-chart showing the status of clinical trials on remdesivir used to treat COVID-19 around the world.
5. Discussion
Although multiple studies are conducted around the world there is no specific treatment that proves to be efficacious with minimal adverse effects. To obtain precise evidence to date, this meta-analysis is conducted with available four RCTs to gauge the effectiveness of remdesivir in comparison to placebo or standard of care.
Our meta-analysis of odds ratio showed reduced 14-day mortality (OR 0.61, 95% CI 0.41 to 0.91) in patients taking remdesivir for ten days; whereas no significant difference in 28 days mortality between remdesivir groups and SOC or placebo (OR 1.02, 95% CI 0.50 to 2.06). In contrast, Piscayo et al. showed no reduction of all-cause mortality in 14 days (RR 0.71, 95% CI 0.39 to 1.28) [21]. This may be due to inclusion of data from Spinner CD et al. in the present meta-analysis, which was not there in Piscayo et al. Meta-analysis of clinical recovery on day 14 and 28 were clinically and statistically significant in favor of patients taking remdesivir in comparison to placebo or standard of care (day 14: OR 1.48, 95% CI 1.19 to 1.84; day 28: OR 2.09, 95% CI 1.09 to 4.03). The findings are concurrent with Piscoya et al. [21]. Findings on discharge rate on day 14 and 28 were higher among the remdesivir group, but it is statistically insignificant on day 28. Analysis of clinical improvement showed approximately 2.5 days earlier improvement among the Remdesivir group than SOC. Zhu et al. also showed similar findings with the discharge rate that was reflective of the patient's recovery and clinical outcome [22]. A higher discharge rate was seen with 10 days use of remdesivir in comparison to 5 days. However, the difference in clinical improvement was not significant with 5 days and 10 days use of drugs.
Severe adverse effects (acute respiratory failure, respiratory failure, septic shock, hypoxia, viral pneumonia) occurrence was less with remdesivir use in comparison with placebo or SOC, but its use for 10 days showed increased severe adverse effects, grade ≥ 3 adverse effect, and drug discontinuation in comparison with 5 days use. Grade 3 adverse effects include decrease in creatinine clearance, ALT elevation, AST elevation, and increased bilirubin. The overall adverse effect was less with placebo but it was statistically insignificant. Alexander et al. study also showed less severe adverse effect with remdesivir use with fixed-effect modeling but this result appeared statistically insignificant with random-effect modeling in their study [23].
Based on our meta-analysis, the need for mechanical ventilation and respiratory support at day 14 was significantly less with remdesivir, which was also supported with subgroup analysis among IMV and ECMO receiving patients, whereas among NIMV and high-flow oxygen receiving patients it was of no significance. Also, the 10 days course of the drug showed the increased requirement of ECMO and IMV in contrast to the 5 days drug course. Piscoya et al. study showed no significant decrease in the requirement of invasive ventilation with remdesivir (RR 0.57, 95% CI 0.23 to 1.42) [21].
In present meta-analysis, remdesivir showed a statistically significant reduction in 14-day mortality, speedy clinical improvement/recovery leading to discharge, less requirement of the ventilator with a lesser degree of severe adverse events compared with placebo or SOC. Many other drugs were repurposed like remdesivir as candidate treatment options for COVID-19. A study was done by Shrestha et al. on favipiravir - another potential candidate drug that showed significant results on clinical improvement at day 14 (RR 1.41, 95% CI 1.10 to 1.80) [24]. Many other potential treatment options like corticosteroids, convalescent plasma therapy, and hydroxy-chloroquine showed mixed results in individual studies.
There is no uniformity in findings of the various randomized controlled with conflicting outcomes in terms of clinical improvement following treatment with remdesivir. Although we have included four RCTs, they have several limitations, and also, the presence of heterogeneity among studies. Also, non-uniform treatment options lead to some difficulties comparing them. The biases among the included studies in our meta-analysis were due to lack of complete follow-up, early reporting, and additional use of several therapeutic agents as supportive care. These all lead to some discrepancy in the study results and uncertainty on findings regarding the result of remdesivir use. Safety and efficacy of remdesivir in COVID-19 patients is still dubious. Henceforth, we believe that these findings should be supported by undergoing large scale double-blinded RCTs to increase the confidence of remdesivir use in COVID-19.
6. Conclusion
The preliminary evidence shows patients receiving remdesivir had lesser 14 days mortality, improved clinical recovery and discharge rates. The clinical improvement and recovery were reported to be earlier in patients receiving remdesivir. Shorter course is preferred over longer because 10-day remdesivir showed more adverse outcome than 5-day course with no significant benefits. However, the current evidences are drawn from limited studies and mostly open-label studies with small sample size impose some limitation. Despite early promise, further ongoing large-scale trials results should be awaited to make a full decision.
Acknowledgment
We want to acknowledge Mr. Aavash Budhathoki for his contribution in language editing and proof-reading the manuscript.
Footnotes
Research question: Does remdesivir safely improve the clinical outcome in patients suffering from COVID-19?
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lfs.2020.118663.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4
Supplementary material 5
References
- 1.Worldometer Coronavirus cases. Worldometer. 2020:1–22. [Google Scholar]
- 2.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eastman R.T., Roth J.S., Brimacombe K.R. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Central Science. 2020;6(5):672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNAdependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020;295(15):4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Search of: Remedesvir | COVID 19 - list results - ClinicalTrials.gov. Accessed August 29, 2020. https://clinicaltrials.gov/ct2/results?cond=COVID+19&term=Remedesvir&cntry=&state=&city=&dist=.
- 7.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed) 2009:339. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RoB 2 A revised Cochrane risk-of-bias tool for randomized trials|Cochrane Bias. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials [DOI] [PubMed]
- 9.Study Quality Assessment Tools NHLBI, NIH. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 10.Anderson M.R., Bach P.B., Baldwin M.R. August 2020. Hospital Length of Stay for Severe COVID-19 Patients: Implications for Remdesivir’s Value. medRxiv. 2020.08.10.20171637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antinori S., Cossu M.V., Ridolfo A.L. Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and non-ICU patients: clinical outcome and differences in post-treatment hospitalisation status. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Augustin M., Hallek M., Nitschmann S. Remdesivir for patients with severe COVID-19. Internist. 2020;61(6):644–645. doi: 10.1007/s00108-020-00800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olender S.A., Perez K.K., Go A.S. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin. Infect. Dis. July 2020 doi: 10.1093/cid/ciaa1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquini Z., Montalti R., Temperoni C. Effectiveness of remdesivir in patients with COVID-19 under mechanical ventilation in an Italian ICU. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa321. Published online August 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochrane handbook for systematic reviews of interventions|Cochrane training. https://training.cochrane.org/handbook
- 16.Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. https://web.archive.org/web/20181224162602/http:/www.comp.hkbu.edu.hk/~xwan/median2mean.html [DOI] [PMC free article] [PubMed]
- 17.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 — preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2007764. May. [DOI] [PubMed] [Google Scholar]
- 18.Goldman J.D., Lye D.C.B., Hui D.S. Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2015301. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spinner C.D., Gottlieb R.L., Criner G.J. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020 doi: 10.1001/jama.2020.16349. August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piscoya A., Ng-Sueng L.F., Parra del Riego A. May 2020. Efficacy and Harms of Remdesivir for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. medRxiv. 2020.05.26.20109595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y., Teng Z., Yang L. June 2020. Efficacy and Safety of Remdesivir for COVID-19 Treatment: An Analysis of Randomized, Double-Blind, Placebo-Controlled Trials. medRxiv. 2020.06.22.20136531. [DOI] [Google Scholar]
- 23.Alexander P.E., Piticaru J., Lewis K. May 2020. Remdesivir Use in Patients with Coronavirus COVID-19 Disease: A Systematic Review and Meta-Analysis. medRxiv. 2020.05.23.20110932. [DOI] [Google Scholar]
- 24.Shrestha D.B., Budhathoki P., Khadka S., Shah P.B., Pokharel N., Rashmi P. Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol. J. 2020;17(1):141. doi: 10.1186/s12985-020-01412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4
Supplementary material 5