Highlights
-
•
How sacubitril-valsartan (SV) can affect cardiac remodelling is still unclear.
-
•
SV therapy is associated with reduction of left ventricular mass and thickness.
-
•
In our study women had less reverse cardiac remodelling than men.
-
•
SV therapy also induced a decreased need for loop diuretics.
Keywords: Sacubitril valsartan, Entresto, Reverse remodelling, Sex differences, Heart failure, Echocardiography
Abstract
Aim
Sacubitril valsartan (SV) has revolutionized disease history in patients with heart failure and reduced ejection fraction (HFrEF). Our study assessed SV impact on clinical and echocardiographic parameters in HFrEF outpatients previously treated with optimized therapy.
Methods
Forty-nine HFrEF outpatients were retrospectively included in the study. We collected data from transthoracic echocardiography and clinical assessment at baseline and after 3 ± 1 and 12 ± 1 months of treatment with SV. Results were also stratified by sex to analyse possible sex-based differences in reverse remodelling response to SV.
Results
After 3 months of treatment we observed a significative improvement of both systolic and diastolic function with a reduction of left ventricular mass and relative wall thickness (RWT). At 12 months we observed a further improvement of all previous parameters, plus systolic pulmonary artery pressure (PAP) and left atrial (LA) diameter. In women, most of the echocardiographic parameters improved after SV initiation, but did not reach the statistical significance, except for left ventricular ejection fraction (LVEF), PAP and LA diameter. As for clinical parameters, SV improved New York Heart Association (NYHA) Class, systolic blood pressure and loop diuretic dosage with a mild but significative increase in serum creatinine and potassium.
Conclusion
Our study showed significative reverse remodelling properties of SV with an improvement of LV volumes, mass and systo-diastolic function. NYHA Class, systolic blood pressure and loop diuretic dosage also improved with only mild increase in serum creatinine and potassium. Women showed a lesser extent of reverse remodelling compared with men.
1. Introduction
Prevalence of heart failure (HF) in Italy is approximately 1.5%, representing one of the major health problems in the industrialized world. Direct costs of HF in Western Countries vary between 1% and 3% of total health spending [1]. Furthermore, HF is the main cause of hospitalizations in the elderly [2]. About 50% of HF patients shows a reduced ejection fraction, with ischemic cardiomyopathy being the main cause of systolic dysfunction [3]. Keystone therapeutic strategies for heart failure and reduced ejection fraction (HFrEF) involve angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), beta blockers (BBs) and mineralocorticoid receptor antagonists (MRAs). These therapies had largely demonstrated to reduce morbidity and mortality, but the new pharmacological class of angiotensin receptor neprilysin inhibitors (ARNIs) appears to further improve symptoms and prognosis, having been incorporated into the last HF guidelines [4], [5]. Sacubitril-valsartan (SV) is the first example of ARNIs and its safety and efficacy in chronic HFrEF patients were assessed in the PARADIGM-HF trial [5]. However, the property of SV to realize a reverse remodeling has not been extensively investigated so far, even if myocardial remodelling has shown to correlate with HF severity and demonstrated an important prognostic value [6]. Three clinical studies and a metanalysis showed a further improvement of cardiac reverse remodelling with SV compared to ACEi or ARBs [7], [8], [9], [10]. Other clinical studies confirmed long term [11], [12], [13], [14], [15] and short term [16], [17], [18], [19], [20] improvement in left ventricular ejection fraction (LVEF). SV treatment could induce also an improvement in atrial dimensions [7], [10], [13], [14], [15], [19], mitral regurgitation (MR) [9], [16], left ventricular mass (LVM) index [8], [10], [17], diastolic function [7], [15], [16], left ventricle end-systolic volume (ESV) [7], [10], [13], [14], [15], [16], [17], [19], [20] and end-diastolic volume (EDV) [7], [9], [10], [13], [15], [16], [17], [19], [20], and right ventricle function [14], [20]. However, there is a great variability between studies conducted so far and it is still unknown which echocardiographic parameters are more subjected to improve during ARNI. Furthermore, the timing of reverse cardiac remodelling induction after SV initiation has not been fully elucidated. Our study aimed to assess the effects of SV on echocardiographic and clinical parameters both in the short and long time follow up. In addition, we sorted the results by sex in order to analyse possible sex-related differences in echocardiographic outcomes.
2. Methods
2.1. Study population
In this retrospective cohort study, forty-nine consecutive HFrEF outpatients referred to our heart failure clinic were enrolled. Patients were defined eligible for SV therapy in accordance to the PARADIGM-HF [5] clinical and safety criteria. Exclusion criteria included concomitant therapy known to induce reverse remodelling (eg, Cardiac Resynchronization therapy (CRT)), any acute illness requiring hospitalization during study follow-up or in the previous 12 months, any change of home medications in the 2 weeks before SV initiation, and any change of other neurohormonal blockers (e.g. BBs and MRAs) doses during the follow-up. Baseline and follow-up clinical history, physical examination, vital signs, blood tests (haemoglobin, creatinine, uric acid, electrolytes), home medications and standard transthoracic echocardiography (TTE) parameters were collected. SV initial dose was 24/26 mg for most of the participants. A starting dose of 49/51 mg was used for subjects on a previous high dose of ACEi/ARB and blood pressure values ≥140/90 mmHg at the initial visit. Follow-up visits at 3 ± 1 and 12 ± 1 months after SV initiation were scheduled as per regular follow-up. Modifications of SV doses were at discretion of the treating physician. All patients provided informed consent and the study was approved by the local ethical committee (Azienda Ospedaliero – Universitaria di Bologna, Policlinico S.Orsola-Malpighi) in accordance to the declaration of Helsinki.
2.2. Echocardiographic measurements
All the standard TTE exams were performed with a commercially available system [Philips Affiniti 50C]. Images were acquired in the left lateral decubitus position by the same well-trained sonographer. All reported echocardiography measures were averaged from 3 consecutive cycles [or 5 if atrial fibrillation was present] and assessed as recommended by the American Society of Echocardiography and the European Association of Cardiovascular Imaging [21]. All patients underwent baseline and follow-up echocardiography with the same machine and sonographer. Registered parameters were: LVEF, EDV, ESV, LVM, linear LV dimensions [left ventricular posterior wall diastolic diameter (LVPWd), interventricular septum diastolic diameter (IVSd), left ventricular internal diastolic diameter (LVIDd), RWT, E/A, deceleration time (DT), severity of MR, left atrial (LA) diameter, systolic pulmonary artery pressure (PAP), tissue-Doppler Imaging (TDI). EDV and ESV were measured from 4 + 2 chamber apical view. LVEF was calculated using the Simpson biplane method. Mitral flow velocities were recorded using an apical 4-chamber image, placing a pulsed wave Doppler sample velocities and recording their ratio (E/A). DT of the E-wave was recorded as the time interval from peak early mitral filling to an extrapolation of the deceleration to 0 m/s. RWT was calculated as septal wall thickness + posterior wall thickness divided by LV diastolic diameter from parasternal long axis view M-mode. LV mass was assessed with leading edge-to-leading-edge 2D method from parasternal long axis view. Severity of MR was assessed from 4-chamber apical and parasternal long axis views using color Doppler imaging and combining color jet area and vena contracta measures, with a severity grading from 0 [absent] to 3 [severe]. LA diameter was calculated from apical four chambers and long axis view. PAP was estimated combining a continuous wave Doppler regurgitate tricuspid jet signal and inferior vena cava diameter. TDI was also performed to assess alternative left ventricular systolic and diastolic function parameters, such as S peak velocity [cm/s] and E/e’ ratio, with results expressed as mean values of septal and lateral measures.
2.3. Statistical analysis
Continuous variables were presented as the mean ± standard deviation (SD). Categorical variables were expressed as number and percentage. Continuous variables with a normal distribution were compared using Student’s t test for paired data, and with a non-normal distribution using the Wilcoxon‐signed rank test. Statistical significance of differences between categorical variables was tested using the McNemar-Bowker test with Bonferroni correction. A secondary analysis of echocardiographic outcomes stratified by sex was also made. All the analyses were conducted in SPSS version 23 [SPSS Inc., Chicago, IL, USA], Microsoft Windows version, and p ≤ 0.05 from 2-sided tests was considered statistically significant.
3. Results
3.1. Population
A total of 49 patients were recruited in the study. Principal baseline characteristics are outlined in Table 1. Before starting the treatment with SV, 56.8% were on ACE-Is and 43.2% on ARBs. 93.9% of patient’s therapy included BBs, 87.8% loop diuretics, and 57.1% MRAs. Mean dosages of furosemide and canrenone were 82.0 ± 73.6 mg and 35.7 ± 17 mg respectively. SV starting doses were 24/26 mg [38, 77.5%] and 49/51 mg [11, 22.4%]. During the study period, when safety criteria were maintained, the treating physician titrated SV dose until the maximum tolerated dose for each patient. At 12 ± 1 months follow up, 15 [30.6%] patients were on maximum SV dosage [97/103 mg bid], 20 [40.8%] on 49/51 mg and 14 [28.6%] remained on 24/26 mg.
Table 1.
Principal clinical characteristics at baseline and at 3 and 12 months follow‐up.
| Baseline | 3 months | 12 months | |
|---|---|---|---|
| Age [years] | 76 ± 11 years | ||
| Sex [male] | 35 [71.4%] | ||
| Aetiology of HF: Ischemic Valvular Hypertensive Dilatative |
32 [65.3%] 8 [16.3%] 36 [73.5%] 5 [10.2%] |
||
| BMI [kg/mq] | 26.7 ± 4.8 | ||
| SBP [mmHg] | 127 ± 14 | 122 ± 14 | 119 ± 16 * |
| DBP [mmHg] | 73 ± 12 | 72 ± 11 | 73 ± 10 |
| Pulse rate [b.p.m] | 70 ± 15 | 67 ± 11 | 65 ± 11 |
| NYHA Class 1 2 3 |
0 [0%] 36 [36%] 13 [26.5%] |
17 [34.7%] 30 [61.3%] 2 [4%] |
26 [53%] 23 [47%] 0 [0%] |
| Comorbidities: Hypertension Diabetes COPD Dyslipidaemia CKD Anaemia Hyperuricemia |
43 [87.8%] 15 [30.6%] 18 [36.7%] 42 [85.7%] 39 [79.6%] 12 [24.5%] 33 [67.3%] |
||
| AF | 23 [46.9%] | ||
| Smoke History | 15 [30.6%] | ||
| Lab values: Creatinine [mg/dl] Na + [mmol/L] K + [mmol/L] Hb [g/dl] Uricemia [mg/dl] |
1.2 ± 0.4 139 ± 3 4.2 ± 0.4 13.5 ± 1.8 6.8 ± 1.9 |
1.3 ± 0.4 140 ± 3 4.3 ± 0.4 13.2 ± 1.5 6.1 ± 1.9 |
1.4 ± 0.5 * 141 ± 3 4.7 ± 0.5 * 13 ± 1.3 6.3 ± 2.0 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease. Values are expressed as mean ± standard deviation and frequencies. * p ≤ 0.05 vs. baseline.
3.2. 3 ± 1 months follow up
3.2.1. Echocardiography
At 3 ± 1 months follow-up (Table 2), there was a significant increase in LVEF [33.8 ± 6.8 to 47.2 ± 10.6%; p < .001], LVPWd [1.04 ± 0.19 to 1.15 ± 0.18 cm; p .018], and RWT [0.36 ± 0.08 to 0.43 ± 0.09; p .001]. A significant reduction of LVM [273.7 ± 67.2 to 262.6 ± 67.0 g; p .036], LVIDd [6.0 ± 0.7 to 5.6 ± 0.6 cm; p < .001], EDV [169.7 ± 51.4 to 128.2 ± 46.7 mL; p < .001] and ESV [119.6 ± 47.5 to 69.5 ± 34.6 mL; p < .001] was also observed. Despite non-significant, we observed a reduction in LA diameter [5.0 ± 0.8 to 4.8 ± 0.8 cm] and E/A [1.2 ± 0.9 to 0.9 ± 0.5]. Sorting the results by sex, we found that LVM, RWT, LVIDd, EDV, ESV, and E/A significantly improved in men but not in women. Instead, LVEF significantly improved in both sexes (Table 3).
Table 2.
Echocardiographic parameters at baseline and follow-up.
| Baseline | 3 months | 12 months | |
|---|---|---|---|
| LVEF [%] | 33.8 ± 6.8 | 47.2 ± 10.6 * | 50.3 ± 9.8 * |
| LVM [g] | 273.7 ± 67.2 | 262.6 ± 67 * | 249.2 ± 67 |
| LVIDd [cm] | 6 ± 0.7 | 5.6 ± 0.6 * | 5.45 ± 0.75 * |
| LVPWd [cm] | 1.04 ± 0.19 | 1.15 ± 0.18 * | 1 ± 0.16 |
| IVSd [cm] | 1.11 ± 0.26 | 1.17 ± 0.22 | 1.15 ± 0.21 |
| ESV [ml] | 119.6 ± 47.5 | 69.5 ± 34.6 * | 66.3 ± 32.9 * |
| EDV [ml] | 169.7 ± 51.4 | 128.2 ± 46.7 * | 129.7 ± 49.4 * |
| LA diameter [cm] | 5 ± 0.8 | 4.8 ± 0.8 | 4.4 ± 1 * |
| RWT | 0.36 ± 0.08 | 0.43 ± 0.09 * | 0.41 ± 0.09 * |
| E/A | 1.2 ± 0.9 | 0.79 ± 0.47 | 0.69 ± 0.17 |
| S | 5.39 ± 1.41 | 5.57 ± 1.38 | 5 ± 1.28 |
| E/e’ | 13.5 ± 6.64 | 12.23 ± 5.58 | 10.48 ± 4.39 |
| PAP [mmHg] | 38.8 ± 10.7 | 34.1 ± 12 | 32.4 ± 12.2 * |
| DT [sec] | 0.2 ± 0.06 | 0.21 ± 0.06 | 0.21 ± 0.05 |
A, atrial wave; DT, deceleration time; E, early wave; e’, early’ wave; ESV, end-systolic volume; EDV, end-diastolic volume; IVSd, diastolic interventricular septum; LA, left atrium; LVEF, left ventricular ejection fraction; LVIDd, diastolic left ventricular internal diameter; LVM, left ventricular mass; LVPWd, diastolic left ventricular posterior wall; PAP, systolic pulmonary arterial pressure; RWT, relative wall thickness; S = systolic wave. Values are expressed as mean ± standard deviation. * p ≤ 0.05 vs. baseline.
Table 3.
Men vs. Women echocardiographic parameters at baseline and follow-up.
| Parameter | Men (n = 35) | Women (n = 14) |
|---|---|---|
| LVEF [%] | 34.5 ± 6.4 | 32.0 ± 7.7 |
| LVEF 3 months | 47.1 ± 10.1 * | 47.3 ± 12.6 * |
| LVEF 12 months | 51.0 ± 9.8 * | 48.4 ± 10.3 * |
| LVM [g] | 291.7 ± 59.7 | 219.6 ± 53.9 |
| LVM 3 months | 281.1 ± 66.3 * | 210.4 ± 57.7 |
| LVM 12 months | 272.6 ± 65.9 | 181.9 ± 31.9 |
| RWT | 0.36 ± 0.09 | 0.34 ± 0.08 |
| RWT 3 months | 0.42 ± 0.09 * | 0.35 ± 0.08 |
| RWT 12 months | 0.40 ± 0.08 * | 0.33 ± 0.02 |
| EDV [ml] | 181.5 ± 48.0 | 130.8 ± 45.6 |
| EDV 3 months | 138.9 ± 47.2 * | 108.7 ± 35.4 |
| EDV 12 months | 143.7 ± 48.9 * | 86.5 ± 19.1 |
| ESV [ml] | 115.4 ± 40.8 | 85.4 ± 42.9 |
| ESV 3 months | 76.0 ± 36.8 * | 64.2 ± 30.9 |
| ESV 12 months | 74.0 ± 35.9 * | 46.8 ± 14.7 |
| E/A | 1.15 ± 0.84 | 1.16 ± 1.00 |
| E/A 3 months | 0.59 ± 0.06 | 0.97 ± 0.69 |
| E/A 12 months | 0.64 ± 0.17 | 0.72 ± 0.18 |
| LVIDd [cm] | 6.1 ± 0.7 | 5.7 ± 0.6 |
| LVIDd 3 months | 5.6 ± 0.6 * | 5.5 ± 0.6 |
| LVIDd 12 months | 5.7 ± 0.7 * | 5.1 ± 0.7 |
| PAP [mmHg] | 39.6 ± 10.9 | 37.0 ± 12.3 |
| PAP 3 months | 37.2 ± 13.2 | 32.3 ± 12.0 |
| PAP 12 months | 36.2 ± 13.4 | 24.0 ± 7.4 * |
| LA diameter [cm] | 5.2 ± 0.8 | 4.7 ± 0.8 |
| LA diameter 3 months | 5.0 ± 0.8 | 4.3 ± 0.7 |
| LA diameter 12 months | 4.9 ± 0.9 | 3.8 ± 0.6 * |
A, atrial wave; E, early wave; ESV, end-systolic volume; EDV, end-diastolic volume; LA, left atrium; LVEF, left ventricular ejection fraction; LVIDd, diastolic left ventricular internal diameter; LVM, left ventricular mass; PAP, systolic pulmonary arterial pressure; RWT, relative wall thickness. Values are expressed as mean ± standard deviation. * p ≤ 0.05 vs. baseline.
3.2.2. Clinical parameters
NYHA Class improved, with most patients in NYHA 1 and 2 and only 2 patients (from 13 at baseline) in NYHA Class 3 [p .000 and p .048 for classes 1–2 and 2–3 respectively]. Also furosemide dosage [81.9 ± 73.6 mg to 70.5 ± 69.7 mg, p .001] and systolic blood pressure [127 ± 14 to 122 ± 14, p .005] decreased significantly. As for blood tests no significative changes were found.
3.3. 12 ± 1 months follow up
3.3.1. Echocardiography
The global comparison between basal and 12 ± 1 months TTE evaluation (Table 2) showed a significant improvement of LVEF [33.8 ± 6.8 to ± 50.3 ± 9.8%; p < .001], a significant increase of RWT [0.36 ± 0.08 to 0.41 ± 0.09; p .005], and a significant reduction in LV diameters [LVIDd 6.0 ± 0.7 to 5.4 ± 0.7 cm; p .012] and volumes [EDV 169.7 ± 51.4 to 129.7 ± 49.4 mL, p < .001; ESV 119.6 ± 47.5 to 66.3 ± 32.9 mL, p < .001]. Notably, there was also a significant reduction of LA diameter [5.0 ± 0.8 to 4.4 ± 1.0 cm; p .02] and PAP [38.8 ± 10.7 to 32.4 ± 12.2 mmHg; p .014]. In addition, we showed a further improvement of LVM, E/A and E/e’ compared to baseline and 3 months follow up, despite non-significant. Even if a further tendency towards myocardial remodelling was observed, all echocardiographic parameters did not reach the statistical significance compared to 3 months follow-up. Sorting the results by sex, we observed that LVM, LV volumes, RWT and LVIDd did not significantly improve in women. However, LA diameter and PAP improved only in women. As for men, the improvement in LVEF reached the statistical significance also in the 12 months vs. 3 months comparison (p .017).
3.3.2. Clinical parameters
NYHA Class further improved, with most patients in NYHA 1 and 2 and no patients in NYHA Class 3. Furosemide dosage [81.9 ± 73.6 to 75.4 ± 86 mg, p .004] and systolic blood pressure [127 ± 14 to 119 ± 16 mg, p .023] improved compared to baseline. Instead, serum potassium [4.2 ± 0.4 to 4.7 ± 0.5, p .025] and creatinine [1.2 ± 0.4 to 1.4 ± 0.5, p .030] increased. However, differences between 3 and 12 months follow up did not reach the statistical significance.
4. Discussion
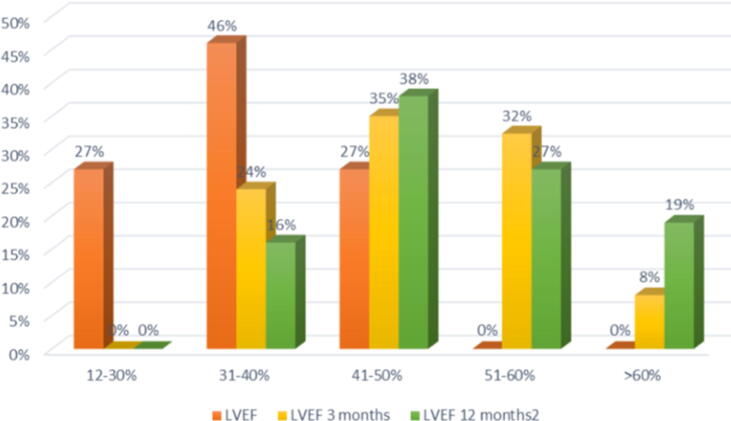
Given the impact on hard outcomes showed in the PARADIGM-HF [5] trial, and on the basis of other numerous real-life studies still confirming efficacy and safety performances, the introduction of SV among the treatment options of HFrEF has completely overturned the pharmacological approach to this condition. However, the studies conducted so far did not fully clarify the effects of SV on cardiac function and myocardium reverse remodelling, with results sometimes discordant [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]. Our study analysed the impact of SV therapy on echocardiographic and clinic parameters of HFrEF outpatients previously treated with an optimal individualized pharmacological therapy which included ACEIs or ARBs, BBs and in most patients MRAs. Our main findings indicate that switching to SV induces incremental reverse remodelling, affecting both systolic and diastolic function, which could lead to a great impact on the progression and prognosis of HFrEF [6]. From baseline to 12 months follow-up we observed a progressive improvement of LVEF (Fig. 1). LVEF improves in 93.8% (n.46) patients as early as 3 months of treatment. Of the 3 non-responder patients (6.1%), 2 (4%) showed LVEF improvement in the next follow-up assessment and in only 1 patient (2%) LVEF did not improve at all. The non-responder patient had the highest baseline LVEF compared with the rest of the population (LVEF 47%) and started ARNI because of severe clinical symptoms unless maximal optimized therapy. No others pre-treatment characteristics were able to predict delay or absence of reverse myocardial remodelling.
Fig. 1.
Distribution of LVEF frequencies of patients at basal (orange), 3 ± 1 months follow-up (yellow) and 12 ± 1 months follow up (green) echocardiography. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The positive effect on LV diameters, volumes and systo-diastolic performance seems to occur early after treatment initiation, in line with previous data on short term effect of ARNI [16], [17], [18], [19], [20]. In fact, at 3 months follow-up we observed a global myocardial remodelling with a significative improvement of cardiac function. Prior studies on SV remodelling properties showed an improvement of LV volumes [16], [17], [19] and mass [17]. Our results confirmed these findings and demonstrated a significant improvement of RWT after SV initiation. In addition, as previously observed [18], we demonstrated an increase of E/A, which could represent an expression of diastolic function improvement.
As for long time follow-up, we found a significative improvement of LVEF, LV diameters and volumes, PAP, and LA diameter, compared to baseline. Of note, PAP and LA diameter improvement did not reach the statistical significance in the 3 months follow-up vs. baseline comparison. This finding could suggest a latency of SV in reducing the LV filling pressure. Even if not significant, we also observed a further reduction of LVM, E/A and E/e’, compared to baseline and 3 months follow-up. Interestingly, from our best knowledge, a diastolic improvement in long term follow-up has been only found by Januzzi and colleagues [15]. It must be noted that the improvement of all echocardiographic parameters did not reach the statistical significance in the 12 vs. 3 months follow-up comparison.
Since women were underrepresented in SV clinical trials, we repeated the statistical analyses on the echocardiographic parameters after stratification by sex (Table 3). Our data showed significative sex-related differences in SV capacity to induce myocardial reverse remodelling. In the 3 months follow-up LV volumes and diameters, LVM, RWT, and E/A significantly improved in men but not in women. Of note, E/A improvement was seen only in men and not in the entire population analysis. In the last follow-up we still observed less improvement in women compared with men. However, LA diameter and PAP improvements reached the statistical significance only in women. Altogether these findings suggested the presence of sex-related differences in the myocardial response to SV. To our best knowledge this study is the first to investigate sex-related differences in echocardiographic parameters during SV therapy. However, it must be taken into account that stratifying the population by sex significantly reduced the sample size and increased the risk for biases in the statistical analyses. This is even more significant considering the different sample size of men vs. women population. There is a need for further studies to unravel the role of sex in myocardial response to SV since sex-related differences could have great implications in the management and therapy of HFrEF patients.
Regarding clinical parameters, NYHA Class, systolic blood pressure and furosemide dosage decreased significantly in both 3 and 12 months follow-up. Instead, creatinine and potassium increased in the 12 months follow-up vs. baseline assessment. NYHA Class and furosemide dosage improvements demonstrated the clinical efficacy of ARNI. In addition, furosemide dosage decrease may also suggest that treatment with SV could reduce the requirement for loop diuretics in patients with HFrEF. This finding is in line with a recent sub-analysis of the PARADIGM-HF trial, where patients in the SV arm were more likely to reduce diuretic dose compared to those randomized to enalapril [22]. As for the antihypertensive effect of ARNI, the superiority of SV over enalapril and olmesartan in reducing systolic blood pressure has already been demonstrated [7], [23]. Our study corroborated these results, suggesting that in the future ARNI could represent a further therapeutic strategy for hypertensive patients, with a possible central role in preventing hypertensive myocardial remodelling. Finally, in our opinion, creatinine and potassium increases are intrinsically related to ARNI pharmacodynamics and do not have to be considered a safety problem unless there is evidence of acute kidney injury and/or severe hyperkalaemia. Supporting these considerations, in our study no patients developed these conditions.
The biological basis of SV reverse remodelling properties could involve the regulation of the expression of different proteins contributing to hypertrophy, cardiomyocyte cell death and LV extracellular matrix composition. As demonstrated by Iborra-Egea and colleagues transcriptome data analysis, valsartan could reverse the cardiac remodelling by inhibiting guanine nucleotide-binding protein family, while sacubitril could act on cardiomyocyte cell death, hypertrophy and impaired myocyte contractility through the inhibition of phosphatase and tensin homolog [24].
Despite all the encouraging results, there are several limitations to the present study. First of all the observational retrospective study design and the lack of a control group. The latter limitation could be partially overcome considering that our patients were on maximum optimized therapy before SV initiation and did not change any other neurohormonal therapy before and during the study. Another important limitation is the small sample size. This is even more significant if we consider sex comparison because the sample size was further reduced. Finally, we did not evaluate atrial volume but diameters with a possible inaccuracy of our findings.
In conclusion, our study showed significative reverse remodelling properties of SV with an improvement of LV volumes, mass, RWT, systo-diastolic function, LA diameter and PAP after ARNI initiation. Furthermore, we observed sex-related differences on myocardial response to SV, with women having less echocardiographic improvements except for LA diameter and PAP. Finally, SV was able to significantly reduce NYHA Class, systolic blood pressure and loop diuretic dosage at expenses of mild creatinine and potassium increase. Large controlled studies are needed to better understand SV effects on myocardial function and reverse remodelling in both sexes.
Funding
This work was supported by Novartis.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
Acknowledgement
We would like to thank Arianna Tonelli for the support for the initial assessment.
Author contributions
Matteo Landolfo and Federica Piani equally contributed to the article and both are considered as first authors.
References
- 1.Corrao G., Ghirardi A., Ibrahim B. Burden of new hospitalization for heart failure: a population-based investigation from Italy. Eur. J. Heart Fail. 2014;16:729–736. doi: 10.1002/ejhf.105. [DOI] [PubMed] [Google Scholar]
- 2.Maggioni A., Orso F., Calabria S. The real-world evidence of heart failure: findings from 41413 patients of the ARNO database. Eur. J. Heart Fail. 2016;18:402–410. doi: 10.1002/ejhf.471. [DOI] [PubMed] [Google Scholar]
- 3.Tavazzi L., Senni M., Metra M. Multicenter prospective observational study on acute and chronic heart failure. One-year follow-up results of IN-HF [Italian Network on Heart Failure] Outcome Registry. Circ. Heart Fail. 2013;6:473–481. doi: 10.1161/CIRCHEARTFAILURE.112.000161. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P., Voors A., Anker S. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology [ESC]. Developed with the special contribution of the Heart Failure Association [HFA] of the ESC. Eur. J. Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 5.McMurray J., Packer M., Desai A. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 6.Sekaran N.K., Crowley A.L., de Souza F.R. The Role for cardiovascular remodelling in cardiovascular outcomes. Curr. Atheroscler. Rep. 2017;19(5):23. doi: 10.1007/s11883-017-0656-z. [DOI] [PubMed] [Google Scholar]
- 7.Desai A.S., Solomon S.D., Shah A.M. Effect of Sacubitril-Valsartan vs Enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019 doi: 10.1001/jama.2019.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmieder R.E., Wagner F., Mayr M. The effect of SV compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: the results of a randomized, double-blind, active-controlled study. Eur. Heart J. 2017;38(44):3308–3317. doi: 10.1093/eurheartj/ehx525. [DOI] [PubMed] [Google Scholar]
- 9.Kang D.H., Park S.J., Shin S.H., Hong G.R., Lee S., Kim M.S., Yun S.C., Song J.M., Park S.W., Kim J.J. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139(11):1354–1365. doi: 10.1161/CIRCULATIONAHA.118.037077. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Zhou R., Lu C. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodelling: meta-analysis. J. Am. Heart Assoc. 2019;8(13) doi: 10.1161/JAHA.119.012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morillas-Climent H., Seller-Moya J., Vicedo-López Á. Evolution of functional class, biochemical and echocardiographic parameters and clinical outcomes after SV initiation in daily practice. J. Comp. Eff. Res. 2019;8(9):685–697. doi: 10.2217/cer-2019-0014. [DOI] [PubMed] [Google Scholar]
- 12.De Vecchis R., Paccone A., Di Maio M. SV improves left ventricular longitudinal deformation in heart failure patients with reduced ejection fraction. MinervaCardioangiol. 2019;67(6):456–463. doi: 10.23736/S0026-4725.19.04971-5. [DOI] [PubMed] [Google Scholar]
- 13.Castrichini M., Manca P., Nuzzi V. SV induces global cardiac reverse remodelling in long-lasting heart failure with reduced ejection fraction: standard and advanced echocardiographic evidences. J. Clin. Med. 2020;9(4) doi: 10.3390/jcm9040906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correale M., Mallardi A., Mazzeo P. SV improves right ventricular function in a real-life population of patients with chronic heart failure: the Daunia Heart Failure Registry. Int. J. Cardiol. Heart Vasc. 2020;25(27) doi: 10.1016/j.ijcha.2020.100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Januzzi J.L., Jr, Prescott M.F., Butler J., Felker G.M., Maisel A.S. Association of change in N-Terminal Pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019:1–11. doi: 10.1001/jama.2019.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martens P., Beliën H., Dupont M. The reverse remodelling response to SV therapy in heart failure with reduced ejection fraction. Cardiovasc. Ther. 2018;36(4) doi: 10.1111/1755-5922.12435. [DOI] [PubMed] [Google Scholar]
- 17.Almufleh A., Marbach J., Chih S., Stadnick E., Davies R., Liu P., Mielniczuk L. Ejection fraction improvement and reverse remodelling achieved with SV in heart failure with reduced ejection fraction patients. Am. J. Cardiovasc. Dis. 2017;7(6):108–113. [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzetti S., Scifo C., Abete R. Short-term echocardiographic evaluation by global longitudinal strain in patients with heart failure treated with SV. ESC. Heart Fail. 2020 doi: 10.1002/ehf2.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L.W., Wu P.C., Chiu M.Y., Tu P.F., Fang C.C. SV improves left ventricular ejection fraction and reverses cardiac remodelling in taiwanese patients with heart failure and reduced ejection fraction. Acta Cardiol. Sin. 2020;36(2):125–132. doi: 10.6515/ACS.202003_36(2).20190812A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayard G., Da Costa A., Pierrard R., Roméyer-Bouchard C., Guichard J.B., Isaaz K. Impact of SV on echo parameters in heart failure patients with reduced ejection fraction a prospective evaluation. Int. J. Cardiol. Heart Vasc. 2019;3(25) doi: 10.1016/j.ijcha.2019.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 22.Vardeny O., Claggett B., Kachadourian J., Desai A.S. Reduced loop diuretic use in patients taking SV compared with enalapril: the PARADIGM-HF trial. Eur. J. Heart Fail. 2019;21(3):337–341. doi: 10.1002/ejhf.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams B., Cockcroft J.R., Kario K., Zappe D.H., Brunel P.C., Wang Q., Guo W. Effects of SV versus olmesartan on central hemodynamics in the elderly with systolic typertension: the PARAMETER Study. Hypertension. 2017;69:411–420. doi: 10.1161/HYPERTENSIONAHA.116.08556. [DOI] [PubMed] [Google Scholar]
- 24.Iborra-Egea O., Gálvez-Montón C., Roura S. Mechanisms of action of sacubitril/valsartan on cardiac remodeling: a systems biology approach. NPJ Syst. Biol. Appl. 2017;18(3):12. doi: 10.1038/s41540-017-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]