Abstract
Purpose
Long-term outcomes associated with vagus nerve stimulation (VNS) therapy for progressive myoclonic epilepsy (PME) have not been studied. The purpose of this study was to report long-term outcomes of VNS therapy in two patients with PME.
Methods
We performed VNS therapy for two patients with PME. We reviewed the conditions of epileptic seizures, status epilepticus (SE), myoclonus, and Karnofsky performance state (KPS) scale scores at baseline and after 10 years.
Results
A 16-year-old boy with myoclonic epilepsy with ragged-red fibers (MERRF) underwent VNS therapy. Baseline KPS scale score was 50, seizure frequency was weekly, and SE occurred yearly. At 23 years old, KPS scale score was 10. He had remained SE-free and frequency of epileptic seizures had markedly reduced. At 24 years old, he died due to pneumonia. A woman with Gaucher's disease type III underwent VNS therapy at 20 years old. Baseline KPS scale score was 80, seizure frequency was daily, and SE occurred monthly. At 30 years old, KPS scale was 30. She remained SE-free, but still experienced epileptic seizures yearly. Both patients became lethargic during VNS-off periods, with symptoms improving to baseline levels when VNS was resumed.
Conclusion
Long-term outcomes with VNS showed good epileptic seizure control and freedom from SE. VNS might help maintain level of consciousness.
Keywords: Behavioral neuroscience, Cognitive neuroscience, Neuroscience, Neurology, Neurosurgery, Rehabilitation, Pediatrics, Vagus nerve stimulation (VNS), Long-term outcome, Progressive myoclonic epilepsy (PME), Epileptic seizure control, Consciousness
Behavioral neuroscience; Cognitive neuroscience; Neuroscience; Neurology; Neurosurgery; Rehabilitation; Pediatrics; Vagus nerve stimulation (VNS); Long-term outcome; Progressive myoclonic epilepsy (PME); Epileptic seizure control; Consciousness.
1. Introduction
Little is known about the long-term outcomes of vagus nerve stimulation (VNS) therapy for progressive myoclonic epilepsy (PME). We reported on two patients with PME treated by VNS therapy in 2012. Almost 10 years have passed since the first patient with PME underwent VNS therapy in our facility. Smith [1] first reported a patient with Unverricht-Lundborg-type PME treated by VNS therapy, and we then reported two patients with PME due to myoclonic epilepsy with ragged-red fibers (MERRF) and Gaucher's disease type III [2]. Three reports of PME treated by VNS therapy were then published [3, 4, 5]. However, as PME is rare, long-term outcomes associated with VNS have remained unclear. Here, we present two cases followed for 10 years each.
2. Methods
In 2020 and 2011, we performed implantation of a VNS generator (VNS Therapy® System; Cyberonics, Houston, TX or LivaNova PLC, Houston, TX) on two patients with PME for treatment of epilepsy (Table 1).
Table 1.
Long-term outcomes for symptoms in two patients treated with vagus nerve stimulation.
| Age at VNS implantation | Syndrome | Generalized Sz | SE | Myoclonus | Cerebellar symptom | KPS scale | Verbal communication | Follow-up (y) | |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Initial 16 | MERRF | weekly | yearly | yes | wheelchair bound | 50 at age 16 | good | 8 |
| Age at replacement 23, died at 24 | yearly | no | no | bedridden | Replacement 20–10, current 0 | poor | |||
| Patient 2 | Initial 20 | Gaucher III | daily | monthly | yes | ambulatory | 80 at age 20 | good | 10 |
| Age at replacement 27, current age 30 | yearly | no | decreased | bedridden | Replacement 50–40, current 30 | fair |
Sz: seizure; MERRF: mitochondria encephalomyelopathy with ragged-red fibers; SE: status epilepticus; VNS: vagus nerve stimulation; KPS: Karnofsky performance state scale.
2.1. Ethics approval
Written informed consent for publication of case details was obtained from the caregivers of our patients, who were incapable of providing informed consent. The ethics committee at Seirei Hamamatsu General Hospital approved this study.
3. Results
3.1. Patient 1
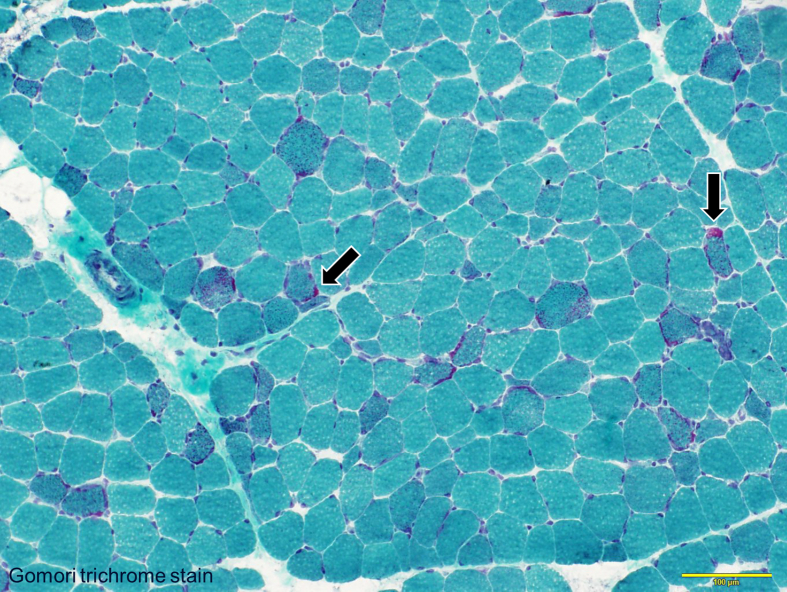
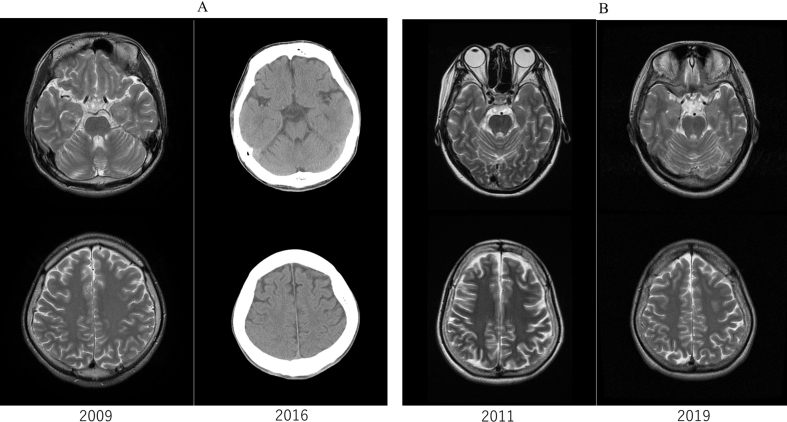
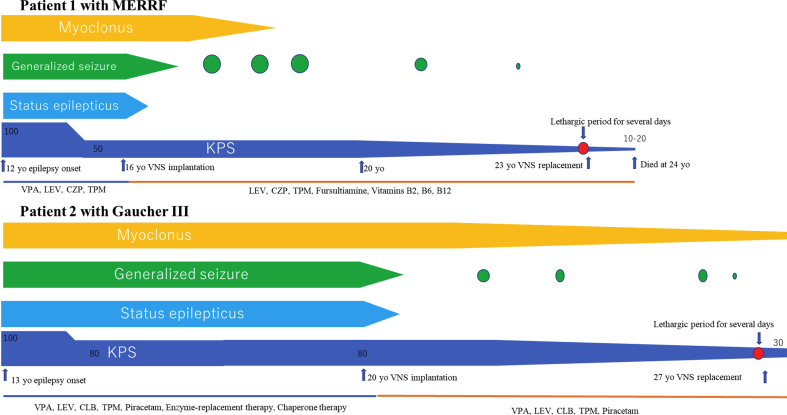
A 16-year-old, right hand-dominant boy with MERRF confirmed from muscle biopsy (Figure 1) underwent VNS therapy. At the time, Karnofsky performance state (KPS) scale score was 50 (“requires considerable assistance and frequent medical care”). Seizures consisted of generalized tonic-clonic seizures with or without proceeding myoclonus, lasting 1–2 min. This seizure pattern repeated several times an hour, on rare occasions lasting up to 4–5 h as status epilepticus (SE). Seizure frequency was weekly, with SE occurring yearly. With the introduction of VNS, the patient achieved freedom from SE and generalized tonic-clonic seizures improved from weekly to yearly. Eventually, generalized tonic-clonic seizures occurred once every few years and no myoclonus had been seen for more than 7 years. At 23 years old, a depletion warning was received for the VNS battery, indicating that the battery should be changed within 1 month. We suggested that the battery be replaced, but as KPS scale score had decreased to 20 (very sick)-10 (moribund) as battery life decreased, his family members had not originally wanted him to undergo battery replacement. Three months later, his caregiver noted that he had been lethargic without any medication changes. Blood sampling test during the lethargic state showed normal concentrations of electrolytes and blood sugar. Even though he was at KPS score 20–10, he managed to respond to his family when the VNS was working. He had remained on the same anti-seizure medication (levetiracetam, topiramate, clonazepam) since VNS implantation, and had also been receiving fursultiamine and vitamins B2, B6 and B12 since implantation of the VNS. He had been using valproic acid until he had experienced repeated episodes of pancreatitis, after which time use of valproic acid was avoided (Table 2). As the only apparent change during the period of lethargy was the end of the battery life for the VNS, we replaced the battery. After several days, the patient returned to the baseline level of consciousness seen when the VNS was on. While the battery was depleted, the patient had not shown any deterioration of epileptic seizures. A year after battery replacement, at 24 years old, the patient died of pneumonia. Throughout this process, no exacerbation of brain atrophy had been identified (Figure 2). A summary of the clinical course is shown in Figure 3.
Figure 1.
Ragged red fibers in Patient 1 (Gomori trichrome stain). Ragged red fibers (arrows) appear as red-stained structures in the myofiber membrane.
Table 2.
Parameters of vagus nerve stimulation and pharmacotherapy.
| Parameters of VNS | Anti-seizure medication | Medication for PME | |
|---|---|---|---|
| Patient 1 | output current, 1.75 mA; on/off time, 30 s/5 min; signal frequency, 30 Hz; pulse width, 500 ms. | LEV, TPM, CZP | fursultiamine, vitamins B2, B6, B12 |
| Patient 2 | output current, 1.75 mA; on/off time, 30 s/5 min; signal frequency, 30 Hz; pulse width, 500 ms. | LEV, TPM, CZP, VPA | piracetam (on enzyme-replacement therapy and chaperone therapy until 21 yo) |
VNS: vagus nerve stimulation; LEV: levetiracetam; TPM: topiramate; CZP; clonazepam; VPA: valproic acid.
Figure 2.
Follow-up neuroimaging. A) Brain of Patient 1 in 2009 and 2016. B) Brain of Patient 2 in 2011 and 2019. No obvious progression of atrophy is seen in either patient. Computed tomography was performed in Patient 1 as the generator for vagus nerve stimulation had not received approval for magnetic resonance imaging in Japan at the time of imaging.
Figure 3.
Clinical courses. Epileptic seizures started at 12 years old in Patient 1 and at 13 years old in Patient 2. After vagus nerve stimulation therapy (VNS), both remained free from status epilepticus. Frequencies and intensities of generalized seizures improved after VNS. While VNS was off, patients became more lethargic, but returned to baseline level of consciousness when VNS was restarted. VNS: vagus nerve stimulation; KPS: Karnofsky performance state scale; LEV: levetiracetam; TPM: topiramate; CZP: clonazepam; VPA: valproic acid.
3.2. Patient 2
A 20-year-old, right hand-dominant woman with Gaucher's disease type III underwent VNS therapy. She did not show splenohepatomegaly. Blood sampling showed deficient enzyme activity for glucosylceramidase. Her sibling had also been diagnosed with the same disease. At the time, KPS scale score was 80 (normal activity with effort; some signs or symptoms of disease). Seizures consisted of long-lasting (>1 h) myoclonus of bilateral hands in an awake state. During this myoclonus, muscle tonus of the extremities increased, finally leading to generalized tonic-clonic seizures. Seizure frequency was daily, with SE occurring monthly. She had been on the same anti-seizure medication (levetiracetam, topiramate, clobazam, valproic acid) and had been on enzyme-replacement therapy and chaperone therapy until 21 years old (Table 2). She remained free from SE and showed improvement of generalized tonic-clonic seizures from daily to yearly. At 27 years old, a VNS-battery depletion warning indicated that the battery should be changed within 3 months, and we thus suggested battery replacement. However, seizures had been well controlled except for myoclonus. She and her caregiver therefore elected to undergo removal of the VNS device instead of changing the battery. Before removing the device, we turned it off and monitored the patient for seizure recurrences for 3 days. While the VNS generator was off, epileptic seizures showed no exacerbation. However, she also gradually became increasingly lethargic when the VNS was off. Within 3 days, she became unable to eat independently due to her lethargic state. We therefore turned the VNS back on, and level of consciousness returned to baseline levels within a day. As we believed the VNS was contributing positively to her level of consciousness, we replaced the battery when she was 27 years old. The patient is currently 30 years old. She has remained SE-free, but still experienced yearly epileptic seizures and myoclonus. KPS scale score as of the most recent follow-up, in May 2020, was 30. No brain atrophy was seen during the clinical course of this patient (Figure 2). A summary of the clinical course is shown in Figure 3.
The SE in this study was repetitive or long-lasting (>5 min) generalized tonic-clonic seizures without recovery of baseline level of consciousness. As both patients underwent long-term video electroencephalography (EEG) for 7 days and the findings showed no subclinical seizures, we regarded the witnessed clinical repetitive or long-lasting generalized tonic-clonic seizures as SE in this study. Seizure frequency was determined based on diaries kept by the parents and caregivers of the patients.
4. Discussion
VNS therapy appeared effective for both patients in terms of epilepsy treatment over the long term. In particular, VNS therapy appeared to contribute to freedom from SE. However, the conditions of patients had deteriorated, and one patient died, the other was bedridden. In addition, one patient was free from myoclonus and the other one showed a reduction in myoclonus frequency after VNS therapy was initiated.
Another positive aspect of VNS therapy was that the level of consciousness for both patients was able to be sustained with this treatment. Both patients experienced a temporary lethargic state when the VNS generators were off. This meant that the VNS seemed to be related to sustaining the level of consciousness. As neither patient showed subclinical seizures during long-term video-EEG, and long-lasting alterations in level of consciousness had not been observed during follow-up, we considered these lethargic states as differing from a state of non-convulsive SE.
The VNS might modulate brain activities and alleviate disorders of consciousness [6, 7], although the mechanisms of action underlying VNS remained unknown. However, some studies have suggested that VNS therapy stimulates activation of the brainstem and increases levels of monoamines [8]. Coma caused by severe head trauma has also been reported to be improved [9, 10], and day-time sleeps reduced [11] using VNS. The mechanisms underlying improvement of conscious in this study might be explained by this mechanism. As both patients showed almost no progression of brain atrophy, the VNS might at least have contributed positively to patients with PME.
During the period of VNS depletion or turning off, neither patient showed exacerbation of epileptic seizures. The efficacy of VNS reportedly comes from cumulative stimulation, which might have established a neuropeptide system [9, 10]. One case report [12] showed re-implantation of a VNS generator 1.5 years after removal of the generator. A certain period might be needed to before seizures recur.
Even though freedom from SE is known to increase quality of life [13, 14, 15], SE-freedom was not fully assessed in either patient with PME in this study. We therefore cannot definitively state that VNS therapy contributed to improvement in quality of life.
As little is known about the long-term outcomes of PME treated by VNS, we hope these case summaries provide valuable data. In the future, we will use VNS therapy in more patients and analyze data for patients from other countries and multiple facilities.
Declarations
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Smith B., Shatz R., Elisevich K., Bespalova I.N., Burmeister M. Effects of vagus nerve stimulation on progressive myoclonus epilepsy of Unverricht-Lundborg type. Epilepsia. 2000;41(8):1046–1048. doi: 10.1111/j.1528-1157.2000.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto A., Yamazoe T., Yokota T., Enoki H., Sasaki Y., Nishimura M., Yamamoto T. Clinical utility of vagus nerve stimulation for progressive myoclonic epilepsy. Seizure. 2012;21(10):810–812. doi: 10.1016/j.seizure.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Hajnsek S., Petelin Gadze Z., Borovecki F., Nankovic S., Mrak G., Gotovac K., Sulentic V., Kovacevic I., Bujan Kovac A. Vagus nerve stimulation in Lafora body disease. Epilepsy Behav. Case Rep. 2013;1:150–152. doi: 10.1016/j.ebcr.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikati M.A., Tabbara F. Managing Lafora body disease with vagal nerve stimulation. Epileptic Disord. Int. Epil. J. Videotape. 2017;19(1):82–86. doi: 10.1684/epd.2017.0892. [DOI] [PubMed] [Google Scholar]
- 5.Serino D., Davico C., Specchio N., Marras C.E., Fioretto F. Berardinelli-Seip syndrome and progressive myoclonus epilepsy. Epileptic Disord. Int. Epil. J. Videotape. 2019;21(1):117–121. doi: 10.1684/epd.2019.1038. [DOI] [PubMed] [Google Scholar]
- 6.Corazzol M., Lio G., Lefevre A., Deiana G., Tell L., André-Obadia N., Bourdillon P., Guenot M., Desmurget M., Luauté J., Sirigu A. Restoring consciousness with vagus nerve stimulation. Curr. Biol. 2017;27(18):R994–R996. doi: 10.1016/j.cub.2017.07.060. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y-t, Yang Y., Wang L-b, Fang J-l, Chen Y-y, He J-h, Rong P-j. Transcutaneous auricular vagus nerve stimulation in disorders of consciousness monitored by fMRI: the first case report. Brain Stimul. 2017;10(2):328–330. doi: 10.1016/j.brs.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham J.T., Mifflin S.W., Gould G.G., Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by Vagal nerve stimulation. Neuropsychopharmacology. 2008;33(8):1884–1895. doi: 10.1038/sj.npp.1301570. [DOI] [PubMed] [Google Scholar]
- 9.Dong X.Y., Feng Z. Wake-promoting effects of vagus nerve stimulation after traumatic brain injury: upregulation of orexin-A and orexin receptor type 1 expression in the prefrontal cortex. Neural Regen. Res. 2018;13(2):244–251. doi: 10.4103/1673-5374.226395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi C., Flanagan S.R., Samadani U. Vagus nerve stimulation to augment recovery from severe traumatic brain injury impeding consciousness: a prospective pilot clinical trial. Neurol. Res. 2013;35(3):263–276. doi: 10.1179/1743132813Y.0000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malow B.A., Edwards J., Marzec M., Sagher O., Ross D., Fromes G. Vagus nerve stimulation reduces daytime sleepiness in epilepsy patients. Neurology. 2001;57(5):879–884. doi: 10.1212/wnl.57.5.879. [DOI] [PubMed] [Google Scholar]
- 12.Braakman H.M., Creemers J., Hilkman D.M., Klinkenberg S., Koudijs S.M., Debeij-van Hall M., Cornips E.M. Improved seizure control and regaining cognitive milestones after vagus nerve stimulation revision surgery in Lennox-Gastaut syndrome. Epilepsy Behav. Case Rep. 2018;10:111–113. doi: 10.1016/j.ebcr.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sajobi T.T., Jette N., Fiest K.M., Patten S.B., Engbers J.D., Lowerison M.W., Wiebe S. Correlates of disability related to seizures in persons with epilepsy. Epilepsia. 2015;56(9):1463–1469. doi: 10.1111/epi.13102. [DOI] [PubMed] [Google Scholar]
- 14.Puka K., Speechley K.N., Ferro M.A. Convulsive status epilepticus in children recently diagnosed with epilepsy and long-term health-related quality of life. Seizure. 2020;80:49–52. doi: 10.1016/j.seizure.2020.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Ferro M.A., Chin R.F., Camfield C.S., Wiebe S., Levin S.D., Speechley K.N. Convulsive status epilepticus and health-related quality of life in children with epilepsy. Neurology. 2014;83(8):752–757. doi: 10.1212/WNL.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]