Abstract
Phytopesticides are human-friendly beside been easily accessible and bio-degradable, are therefore environmentally friendly compared to the synthetic pesticides which huge adverse effects on human, animals and the ecosystem. Plants are large reservoir of secondary metabolites largely untapped or under-tapped for use as pesticides. One problem associated with this is to identify plants which can be assessed and further exploited for this use. Borreria verticillata belongs to Rubiaceae, it is native to South Americas but gained popularity globally. It is known as a weed, showing resistance to many synthetic pesticides and can be grown on a wide range of soil types. B. verticillata is used traditionally against skin diseases such as eczema, infectious dermatitis and scabies. Its antimicrobial application is large and efficient as revealed by most authors. This article inclines to propose and offer current studies with information on the various application of this plant species against various microorganisms, thereby extending its use against plant parasitic nematodes which cause severe yield losses to numerous agricultural crops. Most search engines, journals and dissertation search engines i.e. Google scholar, pubmed, sciencedirect, scopus, web of science, springer, elsevier, like Open-thesis, OATD, ProQuest and EthOs were queried by employing titles such as B. verticillata, Borreria verticillata and biological activity of B. verticillata. The most synonymous name was queried too i.e. Spermacoce verticillata. This review suggests a main point about this resistant weed i.e. its significant antimicrobial activity. It further emphases the need exploits this useful effect against nematodes since they are microorganisms. Phytochemistry of the B. verticillata was gathered in this study and the compounds isolated from the plant i.e. terpenes, iridoids, flavonoids and alkaloids (29 compounds) further provide a basis for a significant antihelmintic effect. The review concludes on the need to extends its antimicrobial activity to sustainable agriculture. Since it is a very common plant in Nigeria, it is easily accessible to farmer protect their cultivations from plant-parasitic nematode attacks.
Keywords: Antimicrobial, Plant parasitic nematodes, Secondary metabolites, Pesticides, Crop protection, Medicinal crops, Horticulture, Weed control, Biological pest control, Chemical pest control
Antimicrobial; Plant parasitic nematodes; Secondary metabolites; Pesticides; Crop protection; Medicinal crops; Horticulture; Weed control; Biological pest control; Chemical pest control
1. Introduction
Meloidogyne incognita (Root-knot nematode) is one of the main plant-parasitic nematode species threatening the yield i.e. quality and quantity, of agricultural crops both annual and perennial ones. Root-knot nematodes are generally widespread and are responsible for considerable yield losses of a wide range of stable crops. Annual crop yield losses due to plant-parasitic nematodes are valued at about $78 billion worldwide (Sasser and Freckman, 1987). Crops that are affected by root-knot nematodes displayed some unique symptoms, these includes stunting and nutrient deficiency, browning of leaves, early wilting, root galling, suppression in plant growth and reduction of photosynthetic pigments which result into yield losses, poor fruit quality and reduced shelf-life (Oka et al., 2014; Kankam et al., 2015). Numerous ways have been used to manage problems created by nematodes globally. Among control methods against plant parasitic nematodes, synthetic chemical takes the main role. Although it brought instant relative and prove to be efficient, they are actually unaffordable by most rural farmers and not easily accessible for the resource-poor farmers in most developing countries. Over the years, people could notice the adverse effects of these pesticides made from chemicals, they are dangerous on plants, ecosystem, animals and humans. They are very toxic, non-biodegradable and posed hazards to farmers and non-target groups (Chitwood, 2002; Oka et al., 2014; Bello et al., 2019a).
The quest for further environmental and toxicological healthy and more suitable and efficient pesticides has been intensified by various demands. Thus, in plants, an evident solution was tried and found. Secondary metabolites from terrestrial plants have gained much interest in contemporary times as alternative sources for novel biopesticides. The traditional usage of these herbs and species by the indigenes of different regions of the world as antimicrobial and pesticides resources are well known (Dalziel, 1937; Ayensu, 1978; Bello et al., 2017, 2018, 2019b). Possibly, the earliest record shows that tobacco (Nicotiana tabacum) is employed as pesticides. Decoction from the Tobacco leaves are employed to destroy aphids, this leads to the discovery of Nicotine, an alkaloid and a plant from Japan, the plantcRoh-ten with its scientific name referred to as Rhododendron Hortense. Rotenone was isolated as the bioactive compound; it is used from early times as pesticides. Plants are large reservoir of secondary metabolites largely untapped or under-tapped for use as pesticide (Feyisa et al., 2015; Waziri, 2015). This review portrays and abridges the botanical description, folklore uses and the economic impact of B. verticillata but emphases on its biological activity i.e. antimicrobial effects. The review assert that since this plant has a significant antimicrobial activity hence this activity can be drawn against plant-parasitic nematodes. Its significant antimicrobial activity can be channel against root-knot nematodes. Highlight of its phytochemistry was clearly stated because these are the secondary metabolites responsible for the antimicrobial effects hence the proposed nematicidal ability. Its resistance ability attest to the fact that it can be pursue further as a plant-based herbicide.
B. verticillata is a clambering and climbing yearly or perennial plant which is indigenous to the South Americas. This plant is dispersed broadly then irregularly through the Pacific, Africa, Australia and Asia. Stems sprawling, to 110 cm or more, glabrous or nearly so, usually standing straight and simple or thinly branched, often abundantly branched from the base, usually 40 cm high or less, the stems tetragonous. It is highly resistance hence can grow on a number of lands types but frequently needs some intrusion to establish. B. verticillata do form wide and big tufts which can threaten other plants around. B. verticillata is mostly seen as a major weed for most crops and vegetables, for instance in the South America countries, it creates a big challenge against the growth and yield of cassava, carrots, rice, maize, sugarcane and vegetables (Mascarenhas et al., 1999; PIER, 2016).
2. Synonymous scientific names
Other synonyms scientific names of B. verticillata though Spermacoce verticillata is the foremost popular of these names. There are other scientific names by which B. verticillata is known with: Borreria oaxacana M. Martens & Galeotti, Bigelovia verticillata (L.) Spreng. Borreria globularioides Cham. & Schltdl, Borreria graminifolia M. Martens & Galeotti, Borreria laevigata M. Martens & Galeotti, Borreria minima DC., Borreria molleri Gand., Borreria stricta G. Mey, Borreria verticillata (L.) G. Mey., Borreria kohautiana Cham. & Schltdl, Borreria oligodonta Steyerm., Borreria thymocephala Griseb, Borreria podocephala DC., Borreria commutata Spreng., Spermacoce graminifolia (M. Martens & Galeotti) Hemsl, Spermacoce oligodonta (Steyerm.) Govaerts, Spermacoce mucronata Nees, Spermacoce podocephala (DC.) C. Wright, Spermacoce polycephala (DC.) Hemsl., Spermacoce polycephala Bartl. ex DC., Spermacoce oaxacana (M. Martens & Galeotti) Hemsl., Spermacoce thymocephala (Griseb.) C. Wright, Spermacoce globosa Schumach. & Thonn, Tardavel verticillata (L.) Hiern, Spermacoce reclinata Nees, Spermacoce minimai Pohl ex DC., Spermacoce stellata Willd. ex Roem. & Schult., Spermacoce molleri Gand. Govaerts (The Plant List, 2020).
3. Other names
B. verticillata has many other designations by which it is referred to globally as shown in Table 1. This plant species is popularly called whitehead broom, southern larra flower and shrubby false buttonwood in English language. In West Africa countries, different tribes and ethnic groups have their unique names for this weed (Abdullahi-Gero et al., 2014; Andrioli et al., 2014; Campos et al., 2014).
Table 1.
Other names of B. verticillata.
| Country | Name | Language | References |
|---|---|---|---|
| Spain | botón blanco; botoncito blanco; cardio de frade | Spanish | Abdullahi-Gero et al. (2014); Andrioli et al. (2014) |
| France | borrerie verticillée | French | Andrioli et al. (2014); Campos et al. (2014) |
| Portugal | éribun; poaia; vassourinha-de-botao | Portuguese: | Burger and Taylor (1993) |
| Brazil | coroa-de-frade; poaia miúda; poaia preta; vassourinha; poaia, coroa-defrade, | Portuguese | Moreira et al. (2010); Conserva and Ferreira Júnior (2012) |
| Puerto Rico: | juana la blanca | Spanish | Chiquieri et al. (2004) |
| Saint Lucia: | ti makònèt | Creole | Andrioli et al. (2014); |
| Thailand: | chat sam chan; ya khi kratai | Thai | Campos et al. (2014) |
| Nigeria | Karya garma | Hausa | Biodiversity India, 2016; Ushie and Adamu (2010) |
| Nigeria | Wantiyo kporou | Tiv | Chiquieri et al. (2004); Ushie and Adamu (2010) |
| Nigeria | Irawo-ile | Yoruba | Ushie and Adamu (2010) |
| Nigeria | Abia-ikana | Ibibio | Chiquieri et al. (2004); Ushie and Adamu (2010); |
4. Scientific classification and kingdom
Species: Borreria verticillata/Spermacoce verticillata
Genus: Spermacoce
Family: Rubiaceae
Order: Gentianales
Class: Dicotyledonae
Subphylum: Angiospermae
Phylum: Spermatophyta
Kingdom: Plantae
Traditional Uses
B. verticillata is a common weed in West Africa Countries though it is reputed for its use in traditional medicine in Asia, Africa, Latin America and West Indies. In West Africa region, its decoction (extraction from the upper part) is rub topically on the skin for management of skin related ailments such as Tinea versicolor (eczema), Tinea capitis (ring worm), pityriasis versicolor, skin itches, psoriasis, scabies and various infectious dermatitis (Baldé et al., 1991, 2015). Some authors report that the tea from the roots of B. verticillata is employed in the management of leucorreas and blenorreas (Peixoto Neto and Caetano, 2002). In Brazil, its leaves and flowers' infusion are employed as analgesic and antipyretic (Moreira et al., 2010; Vieira et al., 1999), the blend from the roots is used as emetic and its broad leaves are employed as antidiarrheal, against hemorrhoids and erysipelas (Lorenzi and Matos, 2002). The decoction of the B. verticillata is prepared with Cuscuta and Zebrina Schnizlein employed in the treatment of amenorrhea, also used against diabetes and dysmenorrhea in West India (Ayensu, 1978) while in the Northern Senegal it is employed against leprosy and skin related diseases (Maynart et al., 1980). The endocarp's decoction is prepared with Iresine P. Browne and Desmodium in Jamaica and it is employed against amenorrhea and as a diuretic (Asprey and Thornton, 1955).
It is used as purgative against paralysis, gonorrheal sores, leprosy, biharzia, furuncles, infantile hyperpnexia and ulcers (Bello et al., 2017, 2019; Sofowora, 1982; Ushie et al., 2013). In West Indies, decoction from B. verticillata which is usually called Alpha Marrow or Wild Margaret, it is employed to manage high blood pressure and as an abortifacient (Baldé et al., 2015) (see Figure 1).
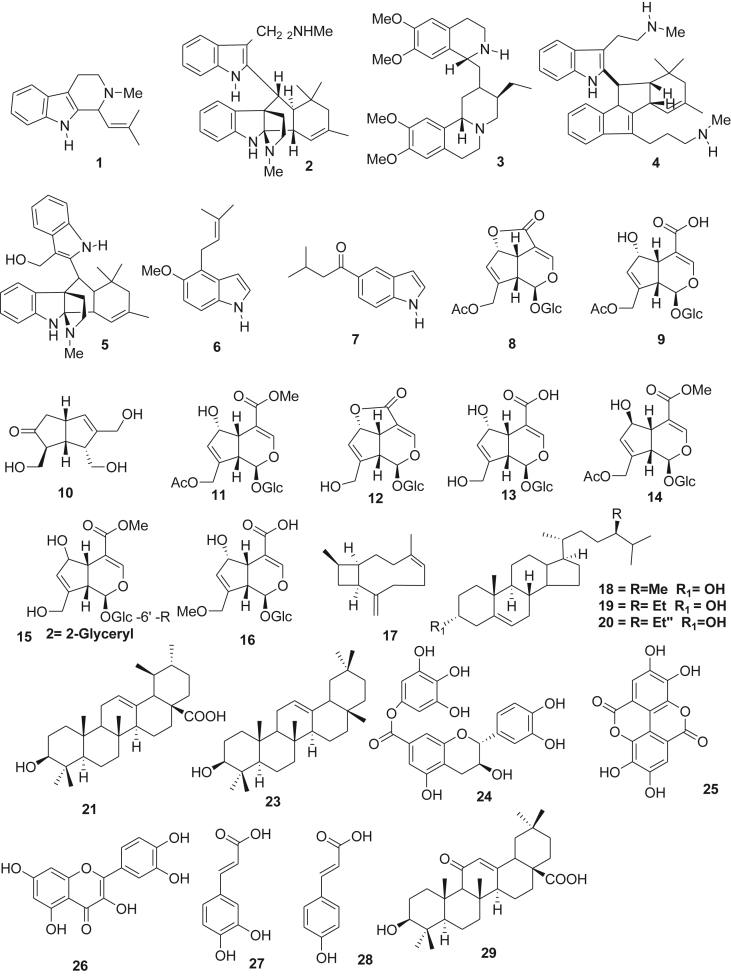
Figure 1.
Isolated compounds from B. verticillata.
5. Phytochemistry
Though B. verticillata is a weed but its use in traditional medicine is popular and this popularity has resulted into considerable isolation of some secondary metabolites from this plant. Alkaloids, terpenes, iridoids, phenolics and flavonoids are compounds that have been reportedly isolated from the plant. This study reveal that only twenty-nine (29) compounds have been isolated from this plant species so far. Among these compounds, alkaloids and iridoids showed some in vitro or in vivo biological activities. Trease and Evans (1972) identified an alkaloid from aerial parts of B. verticillata, the compound was named emetine (3). Pousset et al. (1973) reported that B. verticillata contains two new tetrahydro- β - carboline alkaloids, borrerine (1) and an apparent dimer, borreverine (2). The structures of these compounds were discovered by combined spectroscopic techniques. Vieira et al. (1999) isolated three iridoidal compounds from Borreria verticillata. A new iridoid aglycone borreriagenin (10), with two known iridoids asperuloside (8) and daphylloside (11). Baldé et al. (1991) isolated a new bis-indole alkaloid, spermacoceine (5) and other known three alkaloids from the aerial parts of Borreria verticillata. These indole alkaloids are borrerine (1), borreverine (2) and isoborreverine (4), these structures were established from spectroscopic data. Cadinene, caryophyllene (17) and guaiene (22), these compounds were identified by the comparison of its IR spectrum from the oil extracted from B. verticillata (Benjamin, 1979). Andre et al. (1976) identified a compound from the leaves of B. verticillata, when all the spectroscopic data were compared with an existing one, the compound was Stigmasterol (20). Silva et al. (2017) identified some secondary metabolites from the hydroalcoholic extract and ethyl acetate fraction from B. verticillata using High-performance liquid chromatography (HPLC-UV) analysis. Some of the compounds discovered are Gallic acid (25), β-sitosterol (19), Glycyrrhetinic acid (29), β-amyrin (23), Caffeic acid (27), Coumaric Acid (28), Quercetin (26), Ursolic acid (21), Ellagic acid (24). Moreira et al. (2010) reportedly isolate three new and two known compounds from Borreria verticillata. These were two novel simple indole alkaloids called verticillatine B (17) and verticillatine A (6), one new iridoid called 6′-O-(2-glyceryl) scandoside methyl ester (15), scandoside methyl ester (14) and asperuloside (8).
6. Biological activities
6.1. Hepatoprotective activity
The effect of aqueous leaf extracts of B. vesticillata on CCl4-induced hepatotoxicity rats was studied. This investigation was carried out at different doses of the extract, the result showed that the test values were statistically lower compared to toxicity control at P < 0.05. The study concluded that the aqueous leaf extract of B. verticillata shows a significant hepatocurative properties of on CCl4-induced hepatotoxicity in rats (Murtala et al., 2015).
6.2. Antinociceptive activity
Both alcoholic and ethyl acetate fractions of B. verticillata were evaluated for antinociceptive activity employing in silico and in vivo studies. The in-vivo studies includes the paw edema test, tail flick test, writhing test, formalin test. The in vitro assessment were carried out on Wistar rats and Swiss mice. Ursolic acid (21) was the compound with best effect among the nine compounds identified. The study concluded that antinociceptive effect of B. verticillata might be because of the secondary actions which include the contribution of anti-inflammatory constituents. Ursolic acid (21) is the major bioactive constituent and may be the favourable basis of COX-2 inhibitors and NMDA receptor antagonists (Silva et al., 2017).
6.3. Lowers blood pressure
Alkaloidal extracts of B. verticillata was separated into two different fraction as primary and secondary alkaloid fraction and quaternary alkaloid fraction. Both were assessed for their pharmacological activities on cardiovascular, uterine and gastrointestinal system. The study concluded that these fractions employed possess these biological activities and validates its traditional use to lower blood pressure and as an abortifacient (Moodie-Henry, 2007).
6.4. Anti-inflammatory and analgesic activity
The anti-inflammatory and analgesic effect of ethanol leaf extract of B. verticillata was investigated for in mice and rats. Significant (p < 0.05) analgesic activity was noticed at 1,000 and 500 mg/kg orally in both phases of formalin induced pain in rats. The extract showed significant anti-inflammatory activity (p < 0.001, p < 0.05) at doses of 200 to 1,000 mg/kg p.o/i.p in the rats and in all models employed (Abdullahi-Gero et al., 2014).
6.5. Antilarvacidal activity
The antilarvacidal effect of solvent-derived extracts of Borreria verticillata (Linnaeus) on Culex quinquefasciatus (Say) was investigated. The bioassay of the extracts on late third larval instars of Culex quinquefasciatus was investigated in the laboratory employing 2, 4, 6 and 8 (mg/l) concentrations. The study recommended that Borreria verticillata may be employed in the management of noxious mosquito species i.e. larvae and function as option in replacement of harmful synthetic insecticides (Kontagora, 2017).
7. Antimicrobial activity
7.1. Antimicrobial activity of extracts of B. verticillata
Peixoto Neto et al. (2002) reported the antimicrobial activity of methanol extract of the roots of B. verticillata. The extract displayed high antimicrobial activity against six different strains of P. aeruginosa displaying inhibition areas between 10 and 18 mm even against resistant strains. Different solvent extracts and alkaloid fraction of different parts of B. verticillata were evaluated for their antimicrobial activity by Baldȇ et al. (2015). Crude alkaloidal fraction was significant against Staphylococcus aureus, moderate against Candida tropicalis, Neisseria gonorrhoeae, Candidas albican, Streptococcus pneumoniae, Mycobacterium fortuitum, Gardnerella vaginalis and weak against Streptococcus viridans. Furthermore, crude fraction of the alkaloids displayed the highest cytotoxicity (6–25 μg/ml) among the tested extracts against herpes, poliomyelitis and semliki forest viruses (Baldé et al., 2015). Different solvent extracts of B. veriticillata leaves were assessed for their antidermatophytic and antidrug resistant pathogens by some authors. The extracts were tested to identify various secondary metabolites present and were evaluated against some gram-positive organisms (B. subtilis, S. aureus), gram-negative organisms (Escherichia. coli, Pseudomonas aeruginosa, Streptococcus typhi, Klebsiella pneumoniae) and dermatophytic infections are caused by some fungi i.e. Epidermophyton flocossum, Microsporum canis, Trichophyton rubrum, T. mentagrophytes. The study shows that the plant possesses activity against dermatophytic and drug resistant pathogens and also significant antimicrobial activity (Aremu et al., 2016).
Ushie and Adamu (2010) reported the antimicrobial effect of ethyl acetate fraction of leaf extract of B. verticillata. The authors noticed that ethyl acetate extracts had the most inhibitory action on pathogenic organisms such as C. albican, E. coli, P. aeriginosa and S. aureus (Ushie and Adamu, 2010). Anti-eczematic nature of the essential oil extracted from the leaves of B. verticillata was investigated by Benjamin (1979). The oil displayed good inhibitory activity against the growth of both gram-positive and gram-negative bacteria.
7.2. Antimicrobial activity of the isolated compounds from B. verticillata
Isoborreverine (4) displayed a significant and broad antimicrobial effect against both gram-positive, gram-negative bacterial and fungal i.e. C. albicans, C. tropicalis, Gardnella, S. aureus, M. fortuitum, Pneumococcus, S. viridans and N. gonorrhoeae but borreverine (2) exhibits a moderate and narrow antimicrobial activity (Baldé et al., 1989). Pousset et al. (1977) beside isolating these compounds borrerine (1), isoborreverine (4), and borreverine (2) from B. verticillata, also reported their antimicrobial activity against S. aureus and several Enterobacteriaceae including Pseudomonas aeruginosa and Vibrio cholera.
8. Discussion
Phenolics and flavonoids are major secondary metabolites isolated from B. verticillata, many of these compounds have been associated with antinemic activity. Phenylpropanoids have assessed against broad spectrum of microorganism, their behavior towards was repelling and they were inhibitor of M. incognita's motility especially the simple phenolic compounds i.e. Gallic acid (25), Caffeic acid (27), Coumaric Acid (28), Ellagic acid (24) (Qamar et al., 2005). Nguyen et al. (2013) Aoudia et al. (2012) isolated phenolic compounds from Melia azedarach, these compounds are gallic acid (25), protocatechin, ferulic acid, epicatechin, p-hydroxybenzoic acid and caffeic acid (27). The antinemic activities of these secondary metabolites were also assessed in in vitro and in vivo tests on normal and cancer cells. Some authors investigated various fractions of Viola betonicifolia against M. incognita and M. javanica. A novel methylated flavonoid was isolated with same other compaunds i.e. Kaurene and trachyloban diterpenes from Psidia punctulata of the Compositae family. These compounds were found to exhibit significant nematicidal activities (Simmonds, 2003). The ethyl acetate fraction that is rich in flavonoids and phenolics contents, was discovered to inhibit the growth, mobility and life of M. incognita and M. javanica (Muhammad and Saeed, 2011). These compounds resulted in a significant significant nematicidal activity (Aoudia et al., 2012). Structure-activity relationship (SAR) studies of phenolic compounds reveal that some factors such as the main chemical moiety, the arrangement and position of substituents, and the number of hydroxyl groups in addition to other substituents on the phenolic moiety ring and the esterification of the carboxyl group, all these can affect the antimicrobial activity vis-à-vis the nematicidal effect (Kahkeshani et al., 2019; Dubey et al., 2013). Phenolic compounds like gallic acid (25) and ellagic acid (24) possess more free hydroxyl groups on the phenolic moiety. They have a high measure of hydroxylation in phenol ring and highly methoxylated phenol groups with increased oxidized phenol groups. These helps the compounds to be able prevent motility, adherence and biofilm growth of gram-positive, gram-negative bacteria, fungi and protozoa i.e. Chromobacterium violaceum, Pseudomonas aeruginosa, Listeria monocytogenes, S. aureus, Streptococcus mutans (Kang et al., 2008; Borges et al., 2012; Shao et al., 2015). These compounds can mimic and simulate the antimicrobial effects of known antibiotics i.e. ampicillin, ciprofloxacin, erythromycin, gentamicin, norfloxacin, oxacillin, penicillin via synergism. The antinemic effect of phenolics has been copiously reported by several authors (Simmonds and Stevenson, 2001; Wu et al., 2001; Simmonds, 2003; Carlsen and Fomsgaard, 2008; Popa et al., 2008; Ntalli et al., 2009). The presence of flavonoids i.e. Quercetin (26), Ellagic acid (24) and phenolics i.e. caffeic acid (27) and coumaric acid (28) makes B. verticilatta an effective and alternative candidate as bio-nematicides.
Alkaloids are plants secondary metabolites containing nitrogen atoms and are isolated from some plant families, amongst which these families are Solacaneae, Rubiaceae and Fabaceae. Many authors have reported the nematicidal activity of alkaloids (Rao et al., 1996; Chitwood, 2002; Ntalli and Caboni, 2012; Kahkeshani et al., 2019). Four isoquinoline were isolated from the crude extracts of the aerial parts of Macleaya cordata, this plant has been a source for alkaloids. These compounds i.e. allocryptopine, chelerythrine, protopine and sanguinarine, were evaluated against nematodes i.e. Bursaphelenchus xylophilus, Caenorhabditis elegans and M. incognita. Three of these alkaloids (allocryptopine, chelerytherine and sanguinarine) exerts significant nematicidal activity with a median lethal concentration (LC50) values as 37.45, 28.52 and 34.50 μg/ml, respectively against B. xylophilus; 38.90, 22.78 and 40.25 μg/ml against C. elegans and 76.56, 67.52 and 61.00 μg/ml against M. incognita (Wang et al., 2012). Wen et al. (2013) reported a bioguided assay column chromatography was carried out to identify compounds in Cephalotaxus fortune twigs and leaves with nematicidal activity. The isolated compound called drupacine and the crude alkaloid extract suppress the plant-parasitic nematode hatch, activity of mixed life stages, and population numbers on plant roots. The roots of Waltheria indica exhibited strong nematocidal activity against Meloidogyne incognita hence study was carried out to characterized the constituents responsible for this significant activity (Jang et al., 2019). Three alkaloid compounds, they are 4-quinolone alkaloids i.e. waltherione A, waltherione C and 5′-methoxywaltherione A., were ecofriendly and exhibited good nematicdal activity. Alkaloids contained in plants have been noticed to affect the multiplication of plant-parasitic nematodes and the plants have been confirmed by many authors as nematicidal plants (Perrett and Whitfield, 1995; Kusano et al., 2000; Srivastava et al., 2000; Matsuhashi et al., 2002; Sobkowiak et al., 2011). A common plant in West Africa countries Tithonia diversifolia (Hemsl.) A. Gray is very rich in alkaloids. Authors have assessed its crude alkaloid fraction and they discovered that it suppresses egg hatching of M. incognita by 98 % from the second day after the necessary incubation and displayed 100 % inhibitory effect after 9 days. Tithonia extracts' treatment at the rate of 30 tons/ha on yam (Discoria rotundata) in the greenhouse experiments significantly curbed the growth of M. incognita (5,000 eggs/plant) reproduction, number of eggs, and juveniles, as well as root gall index (Odeyemi and Adewale, 2011). Hence, B. verticillata could display a significant antinemic activity since it is alkaloid containing plant.
Terpenes are structurally made when different isoprene units (5-carbon-base; C5) coupled together, they may have oxygen or not i.e. terpenoids and terpenes. The main classes of terpenoid are diterpenes (C20), hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), tetraterpenes (C40), triterpenes (C30). Essential oils are heterogenous mixtures of both terpenes and terpenoids in addition to other aromatic and straight chain constituents. They are mostly found in aromatic plants. Terpenes displayed several biological activities through synergistic and antagonistic ways (Aharoni et al., 2005; Ntalli and Caboni, 2012). Ursolic acid (24), cadinene, caryophyllene (17), Stigmasterol (20), guaiene (22) and β-amyrin (23) are terpenes isolated and identified from B. verticillata. Terpenes or aromatic plant species have been renowned to possess antinemic activity, many authors have reported that nematicidal activity is one of the activity displayed by this type of plant species beside antimicrobial effect (Ntalli et al., 2011). Begum et al. (2008) isolated some pentacyclic triterpenoids from the aerial parts of Lantana camara. They further confirmed that lantanolic acid, lantoic acid and pomolic acid displayed 100% mortality at 1 mg/ml concentration after a day of application and camarin, camarinin, ursolic (24) and lantacin acid exhibited 100% mortality at 1 mg/ml concentration after two days of application. The result was as the discovery of the conventional nematicide used “Furdan” (Begum et al., 2008). Some authors isolated three terpenoids from Curtisia dentata extracts, betulinic acid lupeol and ursolic acid (24). These compounds were evaluated on Haemonchus contortus, Trichostrongylus colubriformis and C. elegans. Betulinic acid and Lupeol were active only on Trichostrongylus colubriformis and Haemonchus contortus but at high concentrations (200 and 1,000 μg/mL). All three compounds were active against C. elegans with an LC50 of 2, 12 and 79 μg/mL, respectively (Shai et al., 2009). Iridoids are classes of organic compounds that belongs to terpenes groups, their antinemic activity is reported by numerous authors (Sultana et al., 2013). Sweroside, an iridoid was isolated from methanol extract of Alstonia scholaris, after the extract gave a significant antinemic activity against M. incognito. This compound exerts good nematicidal activity as reported by the authors (Sultana et al., 2013). This plant is interesting for further research.
8.1. Toxicity and economic impact
Abdullahi-Gero et al. (2014) showed that the oral median lethal dose (LD50) of ethanol leaf extract of B. verticilatta was greater than 5,000 mg/kg body weight in mice and rats, while the intraperitoneal LD50 in mice was 3,807.88 mg/kg and greater than 5,000 mg/kg in rats. This means that B. vesticillata has very low toxicity and well tolerated to rats and mice (Abdullahi-Gero et al., 2014). B. verticillata has a harmful effect on agricultural yield, it greatly reduces the yield. It is an important weed for major crop such root crops, sugarcane and vegetables. B. verticillata is a popular weed in Brazil, Trinidad, Panama, Columbia and most of the West Africa countries. This plant species affect cassava, cocoa, cowpea, rice, maize and beans (Cherigo et al., 2012; Marques et al., 2011). Mixed cropping system, cultivation, frequent grazing and mowing are effective in checking B. verticillata's growth and progress in an area especially beyond the herbaceous stage and creating thick stomps which can impend the growth of other vegetation (ISSG, 2016). Many authors demonstrated the relative resistant nature of B. verticillata to many synthetic herbicides commonly sold. They reported that this weed show great selectivity in its response to these herbicides (Sellers and Ferrell, 2014; FLEPPC, 2016).
Some parts of B. verticillata i.e. flower, draw hence entice Larra bicolor and L. americana, these wasps are employed in Puerto Rico as a biological control tool against crickets (Scapteriscus didactylus) which are responsible for great loss of some crops i.e. turf, coffee, vegetable seedlings, sugarcane and pastures. In Brazil, studies on honey have shown that B. verticillata is the main basis of pollen for Apis mellifera, Melimpona subnitida, and other bee species. B. verticillata is sold as an ornament plant (Souza et al., 2015; de Novais and Absy, 2015; Pinto et al., 2014). Campos et al. (2014) discovered that this plant species has a great tolerance for arsenic polluted land hence proposing that this plant can be useful in detoxifying land mass and areas affected by arsenic.
8.2. Future consideration and conclusion
Alkaloids, terpenes, iridoids, phenolics and flavonoids which are some of the secondary metabolites isolated from B. verticillata, have been reported to possess antinemic property. Phytopesticides are human-friendly beside been easily accessible and bio-degradable, are therefore environmentally friendly compared to the synthetic pesticides which huge adverse effects on human, animals and the ecosystem. Multiple factors have renewed interest in the source of natural products as pesticides for the pesticide industry and the market. Application of these plant extracts to nematodes will be easy because of its accessibility and easy to use, not as the chemical pesticides, costly and dangerous to health. This plant species is widely distributed globally, is mostly regarded as weed. Work is going on, on the extract of B. verticillata on the on egg hatching, mortality, the immobility of second-stage juveniles (J2s), root galling on the Root-Knot Nematode (Meloidogyne incognita). B. verticillata' s antimicrobial activity should be utilized for agricultural purposes. Innovative and knowledge is essential for the discovery of novel nematicidal compounds, and constituents isolated from plants could play a foremost role in the discovery of leading constituents for chemical synthesis. Grasping and knowing the biochemical interaction between metabolites and compounds of B. verticillata and root-knot nematodes is vital in developing novel and environmental friendly bio-pesticides.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdullahi-Gero H.S., Ahmed Abubakar, Zezi Abdulkadir Umar, Hussaini Isa Marte. Preliminary evaluation of ethanol leaf extract of Borreria verticillata Linn (Rubiaceae) for analgesic and anti-inflammatory effects. J. Med. Plants Res. 2014;8(20):736–747. [Google Scholar]
- Aharoni A., Jongsma M.A., Bouwmeester H.J. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 2005;10:594–602. doi: 10.1016/j.tplants.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Andre R., Delaveau P., Jacquemin H. Phytochemical research on several Madagascan Rubiaceae plant. Med Phytother. 1976;10:233–242. [Google Scholar]
- Andrioli W.J., Conti R., Araújo M.J., Zanasi R., Cavalcanti B.C., Manfrim V., Toledo J.S., Tedesco D., de Moraes M.O., Pessoa C., Cruz A.K., Bertucci C., Sabino J., Nanayakkara D.N.P., Pupo M.T., Bastos J.K. Mycoleptones A-C and polyketides from the endophyte Mycoleptodiscus indicus. J. Nat. Prod. 2014;77(1):70–78. doi: 10.1021/np4006822. [DOI] [PubMed] [Google Scholar]
- Aoudia H., Ntalli N., Aissani N., Yahiaoui-Zaidi R., Caboni P. Nematotoxic phenolic compounds from Melia azedarach against Meloidogyne incognita. J. Agric. Food Chem. 2012;60:11675–11680. doi: 10.1021/jf3038874. [DOI] [PubMed] [Google Scholar]
- Aremu S.O., Iheukwumere C.C., Umeh E.U. Evaluation of leafy part of Borreria verticillata (L) (Rubiaceae) crude extracts for anti-dermatophytic properties and anti-drug resistant pathogens. FUW Trends Sci. Technol. J. 2016;1(2):602–607. [Google Scholar]
- Asprey G.F., Thornton P. Medicinal plants of Jamaica. IV. West Indian Med. J. 1955;4:145–165. [PubMed] [Google Scholar]
- Ayensu E.S. Reference Publications Inc.; Algonac Michigan, U.S.A: 1978. Medicinal Plants of West Africa; p. 110. [Google Scholar]
- Baldé A.M., Gergely A., Pieters L.A., Claeys M., Van den Berghe D.A., Vlietinck A.J. Antimicrobial alkaloids from Borreria verticillata. Planta Med. 1989;55:652. [Google Scholar]
- Baldé A.M., Pieters L.A., Gergely A., Wray V., Claeys M., Vlietinck A.J. Spermacoceine, a bis-indole alkaloid from Borreria verticillata. Phytochemistry. 1991;30:997–1000. [Google Scholar]
- Baldé A.M., Pieters L.A., Traore M.S., Camara A., Balde M.A., Oulare K., Barry M.S., Diallo M.S.T., Balde E.S., Diane S., Vlietinck A.J. Chemotherapeutical evaluation of Borreria verticillata extracts. J. Plant Sci. Spec. Issue Ethnopharmacological Invest. Med. Plants. 2015;3(1-2):28–31. [Google Scholar]
- Begum S., Zehra Syeda Qamar, Siddiqui Bina Shaheen, Fayyaz Shahina, Ramzan Musarrat. Pentacyclic triterpenoids from the aerial parts of Lantana camara and their nematicidal activity. Chem. Biodivers. 2008;5:1856–1865. doi: 10.1002/cbdv.200890173. [DOI] [PubMed] [Google Scholar]
- Bello Oluwasesan M., Zaki Ahmed A., Khan Shabana, Fasinu Pius S., Ali Zulfiqar, Khan Ikhlas A., Ajao Usman L., Olubunmi Oguntoye S. Assessment of selected medicinal plants indigenous to West Africa for antiprotozoal activity. South Afr. J. Bot. 2017;113:200–211. [Google Scholar]
- Bello M. Oluwasesan, Ogbesejana Abiodun B., Oguntoye Stephen O. Insight into Launaea taraxacifolia (Willd) amin ex C. Jeffrey; an underutilized vegetable from Nigeria as functional food: a review” the annals of the university Dunarea de Jos of Galati. Fascicle VI-Food Technol. 2018;42(2):137–152. [Google Scholar]
- Bello Oluwasesan Micheal, Jagaba Safiya Mohammed, Bello Oluwatoyin Eunice. A wild edible vegetable Anchomanes difformis (Blume) Engl.: its ethnomedicinal, phytochemistry, nutritional importance and other uses. Eur Asia J. BioSci. 2019;13:1137–1147. [Google Scholar]
- Bello Oluwasesan M., Ogbesejana Abiodun B., Adetunji Charles Oluwaseun, Oguntoye Stephen O. Flavonoids isolated from Vitex grandifolia: an underutilized vegetable, exert Monoamine A & B inhibitory and anti-inflammatory effects and structure activity relationship (SAR) Turk. J. Pharm. Sci. 2019;16(4):437–443. doi: 10.4274/tjps.galenos.2018.46036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello Oluwasesan Micheal, Jagaba Safiya Muhammad, Bello Oluwatoyin Eunice, Ogbesejana Abiodun Busuyi, Dada Oluwasogo A., Adetunji Charles Oluwaseun, Abubakar Saudat Muhammad. Phytochemistry, pharmacology and perceived health uses of non-cultivated vegetable Cyphostemma adenocaule (Steud. ex A. Rich.) Desc. ex Wild and R.B. Drumm: a review. Sci. Afr. 2019;2 [Google Scholar]
- Benjamin T.V. Investigation of Borreria verticillata, an antieczematic plant of Nigeria quart. J. Crude Drug Res. 1979;1(3-4):135–136. [Google Scholar]
- Biodiversity India . 2016. India Biodiversity portal.http://indiabiodiversity.org/http://indiabiodiversity.org/ Bangalore, India. [Google Scholar]
- Borges A., Saavedra M.J., Simoes M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling. 2012;28:755–767. doi: 10.1080/08927014.2012.706751. [DOI] [PubMed] [Google Scholar]
- Burger W., Taylor C.M. Flora costaricensis. Fieldiana. 1993;33:1–333. [Google Scholar]
- Campos N.V., Loureiro M.E., Azevedo A.A. Differences in phosphorus translocation contributes to differential arsenic tolerance between plants of Borreria verticillata (Rubiaceae) from mine and non-mine sites. Environ. Sci. Pollut. Control Ser. 2014;21(8):5586–5596. doi: 10.1007/s11356-013-2444-9. [DOI] [PubMed] [Google Scholar]
- Carlsen S.C.K., Fomsgaard I.S. Biologically active secondary metabolites in white clover (Trifolium repens L.): a review focusing on contents in the plant, plant−pest interactions and transformation. Chemoecology. 2008;18:129–170. [Google Scholar]
- Cherigo L., Lezcano J., Spadafora C., Martínez-Luis S. Evaluation of phytotoxic, cytotoxic and antiparasitic in vitro activities of Borreria verticillata, a weed of Panamanian coffee crops. Biosci. Res. 2012;9(2):82–86. [Google Scholar]
- Chiquieri A., Di Maio F.R., Peixoto A.L. A distribuição geográfica da família Rubiaceae Juss. na Flora Brasiliensis de Martius. Rodriguésia. 2004;55:47–57. [Google Scholar]
- Chitwood D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002;40:221–249. doi: 10.1146/annurev.phyto.40.032602.130045. [DOI] [PubMed] [Google Scholar]
- Conserva L.M., Ferreira Júnior J.C. Borreria and Spermacoce species (Rubiaceae): a review of their ethnomedicinal properties, chemical constituents, and biological activities. Pharmacogn. Rev. 2012;6:46–55. doi: 10.4103/0973-7847.95866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel J.M. Crown Agents for Overseas Governments; London: 1937. The Useful Plants of West Tropical Africa. [Google Scholar]
- de Novais J.S., Absy M.L. Melissopalynological records of honeys from Tetragonisca angustula (Latreille, 1811) in the Lower Amazon, Brazil: pollen spectra and concentration. J. Apicult. Res. 2015;54(1):11–29. [Google Scholar]
- Dubey S., Ganeshpurkar Aditya, Bansal Divya, Dubey Nazneen. Experimental studies on bioactive potential of rutin. Chron Young Sci. 2013;4(2):153–157. [Google Scholar]
- Feyisa B., Lencho A., Selvaraj T., Getaneh G. Evaluation of some botanicals and Trichoderma harzianum for the management of tomato root-knot nematode (Meloidogyne incognita (Kofoid and White) Chit Wood) Adv. Crop Sci. Technol. 2015;1:1–10. [Google Scholar]
- FLEPPC . Florida Exotic Pest Plant Council; USA: 2016. Spermacoce vertillata L. Florida. [Google Scholar]
- ISSG . 2016. Global Invasive Species Database (GISD). Invasive Species Specialist Group of the IUCN Species Survival Commission. [Google Scholar]
- Jang Ja Yeong, Le Dang Quang, Choi Gyung Ja, Park HaeWoong, Kim Jin-Cheol. Control of root-knot nematodes using Waltheria indica producing 4-quinolone alkaloids. Pest Manag. Sci. 2019;75:2264–2270. doi: 10.1002/ps.5363. [DOI] [PubMed] [Google Scholar]
- Kahkeshani N., Farzaei F., Fotouhi M., Alavi S.S.H., Bahramsoltani R., Naseri R., Momtaz S., Abbasabadi Z., Rahimi R., Farzaei M.H., Bishayee A. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225–237. doi: 10.22038/ijbms.2019.32806.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Oh J., Kang I., Hong S., Choi C. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008;46:744–750. doi: 10.1007/s12275-008-0235-7. [DOI] [PubMed] [Google Scholar]
- Kankam F., Sowley Elias Nortaa Kunedeb, Mohammed Alhassan. Management of root-knot nematode (Meloidogyne spp.) on okra (Abelmoschus esculuntus (L.) Moench) with aqueous sesame seed extract. Int. J. Appl. Agric. Res. 2015;6(4):24–31. [Google Scholar]
- Kontagora Fatima Garba. Department of Biology, Faculty of Life Sciences, Ahmadu Bello University; Zaria, Nigeria: 2017. Phytochemical Screening and Larvicidal Effects of Borreria verticillata (Linnaeus) and Striga asiatica (Linnaeus) against Culex quinquefasciatus (Say) M. Sc. Thesis. [Google Scholar]
- Kusano M., Koshino H., Uzawa J., Fujioka S., Kawano T., Kimura Y. Nematicidal alkaloids and related compounds produced by the fungus Penicillium cf. simplicissimum. Biosci. Biotechnol. Biochem. 2000;64:2559–2568. doi: 10.1271/bbb.64.2559. [DOI] [PubMed] [Google Scholar]
- Lorenzi H., Matos F.J. Nova Odessa; São Paulo: 2002. Plantas Medicinais Do Brasil. [Google Scholar]
- Marques L.J.P., Silva M.R.M., Lopes G.S., Corrêa M.J.P., Araujo M.S., Costa E.A., Muniz F.H. Phytosociology of weeds in cowpea and cassava crops under the h-and-burn with plow. (Dinâmica de populações e fitossociologia de plantas daninhas no cultivo do feijão-caupi e mandioca no sistema corte e queima com o uso de arado) Planta Daninha. 2011;29(Especial):981–989. [Google Scholar]
- Mascarenhas R.E.B., Modesto Júnior Mde S., Dutra S., Souza Filho APda S., Teixeira Neto J.F. Weeds of a low yield pasture area in the northeast of Pará State. (Plantas daninhas de uma pastagem cultivada de baixa produtividade no nordeste Paraense. Planta Daninha. 1999;17(3):399–418. [Google Scholar]
- Matsuhashi R., Satou T., Koike K., Yokosuka A., Mimaki Y., Sashida Y. Nematocidal activity of isoquinoline alkaloids against a species of Diplogastridae. Planta Med. 2002;68:168–171. doi: 10.1055/s-2002-20249. [DOI] [PubMed] [Google Scholar]
- Maynart G., Pousset J.L., Mboup S., Denis F. Antibacterial activity of borreverine, an alkaloid isolated from Borreria verticillata (Rubiaceae) CR Seances Soc. Biol. Fil. 1980;174:925–928. [PubMed] [Google Scholar]
- Moodie-Henry B.J. Department of Basic Medical Sciences, University of West Indies; 2007. Chemical and Pharmacological Investigation of Alkaloidal Extracts of Borreria verticillata. M.Sc Thesis. [Google Scholar]
- Moreira V.F., Oliveira R.R., Mathias L., Braz-Filho R., Vieira I.J. New chemical constituents from Borreria verticillata (Rubiaceae) Helv. Chim. Acta. 2010;93:1751–1757. [Google Scholar]
- Muhammad N., Saeed M. Biological screening of Viola betonicifolia Smith whole plant. Afr. J. Pharm. Pharmacol. 2011;5:2323–2329. [Google Scholar]
- Murtala Y., Babandi A., Sunusi M.M., Shehu D., Babagana K., Alhassan A.J. Effect of aqueous leaf extract of Borreria verticillata species of Sudano-Sahelian Savanna on CCl4 induced hepatotoxicity. J. Nat. Sci. Res. 2015;5(24):73–78. [Google Scholar]
- Nguyen Dang-Minh-Chanh, Seo Dong-Jun, Kim Kil-Yong, Park Ro-Dong, Kim Dong-Hyun, Han Yeon-Soo, Kim Tae-Hwan, Jung Woo-Jin. Nematicidal activity of 3,4-dihydroxybenzoic acid purified from Terminalia nigrovenulosa bark against Meloidogyne incognita. Microb. Pathog. 2013;59(60):52–59. doi: 10.1016/j.micpath.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Ntalli N.G., Caboni Pierluigi. Botanical nematicides: a review. J. Agric. Food Chem. 2012;60:9929–9940. doi: 10.1021/jf303107j. [DOI] [PubMed] [Google Scholar]
- Ntalli N.G., Menksissoglu-Spiroudi U., Giannakou I.O., Prophetou-Athanasiadou D.A. Efficacy evaluation of a neem (Azadirachta indica A. Juss) formulation against root-knot nematodes Meloidogyne incognita. Crop Protect. 2009;28:489–494. [Google Scholar]
- Ntalli N.G., Ferrari Federico, Giannakou Ioannis, Menkissoglu-Spiroudi Urania. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag. Sci. 2011;67:341–351. doi: 10.1002/ps.2070. [DOI] [PubMed] [Google Scholar]
- Odeyemi I.S., Adewale K.A. Phythonematoxic properties and nematicidal potential of Tithonia diversifolia extract and residue on Meloidogyne incognita infecting yam (Discoria rotundata) Arch. Phytopathol. Plant Protect. 2011;44:1745–1753. [Google Scholar]
- Oka Y., Shuker S., Tkachi N., Trabelcy B., Gerchman Y. Nematicidal activity of Ochradenus baccatus against the root-knot nematode, Meloidogyne javanica. Plant Pathol. 2014;63(1):221–231. [Google Scholar]
- Peixoto Neto P.A., Caetano L.C. EDUFAL; Maceio: 2002. Plantas Medicinais Do Popular Ao Cient´ıfico. [Google Scholar]
- Peixoto Neto P.A., Silva M.V., Campos N.V., Porfirio Z., Caetano L.C. Antibacterial activity of Borreria verticillata roots. Fitoterapia. 2002;73:529–531. doi: 10.1016/s0367-326x(02)00166-1. [DOI] [PubMed] [Google Scholar]
- Perrett S., Whitfield P.J. Atanine (3-dimethylallyl-4-methoxy-2- quinolone), an alkaloid with anthelmintic activity from the Chinese medicinal plant, Evodia rutaecarpa. Planta Med. 1995;61:276–278. doi: 10.1055/s-2006-958073. [DOI] [PubMed] [Google Scholar]
- PIER . HEAR, University of Hawaii; Honolulu, USA: 2016. Pacific Island Ecosystems at Risk. [Google Scholar]
- Pinto R.S., Albuquerque P.M.C., Rêgo M.M.C. Pollen analysis of food pots stored by Melipona subnitida Ducke (Hymenoptera: Apidae) in a restinga area. Sociobiology. 2014;61(4):461–469. [Google Scholar]
- Popa V.I., Dumitru M., Volf I., Anghel N. Review. Lignin and polyphenols as allelochemicals. Ind. Crop. Prod. 2008;27:144–149. [Google Scholar]
- Pousset Jean-Louis, Joseph Kerharo, Guy Maynart, Monseur Xavier, Cav Andre, Et, Goutarel Robert. La borrerine: Nouvel alcaloyde Isole Du Borreria verticillata. Phytochemistry. 1973;12:2308–2310. [Google Scholar]
- Pousset J.L., Cave A., Chiaroni A., Riche C. A novel bis-indole alkaloid. X-ray crystal structure determination of borreverine and its rearrangement product on diacetylation. J. Chem. Soc. Chem. Commun. 1977;8:261–262. [Google Scholar]
- Qamar F., Begum S., Raza S.M., Wahab A., Siddiqui B.S. Nematicidal natural products from the aerial parts of Lantana camara Linn. Nat. Prod. Res. 2005;19:609–613. doi: 10.1080/14786410512331330594. [DOI] [PubMed] [Google Scholar]
- Rao M.S., Reddy P.P., Mittal A., Chandravadana M.V., Nagesh M. Effect of some secondary plant metabolites as seed treatment agents against Meloidogyne incognita on tomato. Nematol. Medit. 1996;24:49–51. [Google Scholar]
- Sasser J.N., Freckman D.W. A world perspective on nematology: the role of the Society. In: Veech J.A., Dickson D.W., editors. Vistas on Nematology. Society of Nematologists Inc.; Hyattsville, Maryland, USA: 1987. pp. 7–14. [Google Scholar]
- Sellers B., Ferrell J. Whitehead broom: a useful plant with invasive properties. Fla. Cattlem. Livest. J. 2014 [Google Scholar]
- Shai L.J., Bizimenyera E.S., Bagla V., McGaw L.J., Elo J.N. Curtisia dentata (Cornaceae) leaf extracts and isolated compounds inhibit motility of parasitic and free-living nematodes. Onderstepoort J. Vet. Res. 2009;76:249–256. doi: 10.4102/ojvr.v76i2.49. [DOI] [PubMed] [Google Scholar]
- Shao D., Li J., Li J., Tang R., Liu L., Shi J. Inhibition of gallic acid on the growth and biofilm formation of Escherichia coli and Streptococcus mutans. J. Food Sci. 2015;80:1299–1305. doi: 10.1111/1750-3841.12902. [DOI] [PubMed] [Google Scholar]
- Silva R.H.M., de Lima Nathália Fátima M., Lopes Alberto J.O., Vasconcelos Cleydlenne C., de Mesquita José W.C., de Mesquita Ludmilla S.S., Lima Fernando C.V. M., de Ribeiro Maria N.S., Ramos Ricardo M., de Cartágenes Maria do Socorro S., Garcia João B.S. Antinociceptive activity of Borreria verticillata: In vivo and In silico studies. Front. Pharmacol. 2017;8(283):1–8. doi: 10.3389/fphar.2017.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds M.S. Flavonoid-insect interactions: recent advances in our knowledge. Phytochemistry. 2003;64:21–30. doi: 10.1016/s0031-9422(03)00293-0. [DOI] [PubMed] [Google Scholar]
- Simmonds M.S.J., Stevenson P.C. Effects of isoflavonoids from Cicer on larvae of Heliocoverpa armigera. J. Chem. Ecol. 2001;27:965–977. doi: 10.1023/a:1010339104206. [DOI] [PubMed] [Google Scholar]
- Sobkowiak R., Kowalski M., Lesicki A. Concentration- and time-dependent behavioral changes in Caenorhabditis elegans after exposure to nicotine. Pharmacol. Biochem. Behav. 2011;99:365–370. doi: 10.1016/j.pbb.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Sofowora A. first ed. Vol. 1. Spectrum Books; Ibadan, Africa: 1982. pp. 14–20. (Medicinal Plants and Traditional Medicines). [Google Scholar]
- Souza HRde, Corrêa AMda S., Cruz-Barros MAVda, Albuquerque PMCde. Pollen spectrum of Scaptotrigona aff. Postica (Hymenoptera, Apidae, Meliponini) propolis from Barra do Corda, MA, Brazil. (Espectro polínico da própolis de Scaptotrigona aff. postica (Hymenoptera, Apidae, Meliponini) em Barra do Corda, MA, Brasil. Acta Amazonica. 2015;45(3):307–315. [Google Scholar]
- Srivastava S., Prasad D., Singh R.P. Antinemic studies of the chemical constituents of the leaves of Murraya koenigii (L.) Spreng. Ann. Plant Protect. Sci. 2000;8:183–186. [Google Scholar]
- Sultana N., Akhter Musarrat, Saify Zafar Saeed, Khatoon Zakia, Mahmood-Ul-Hasan, Qazi Muhammed Saleem, Ali Yousaf. Isolation and structure determination of nematicidal iridoid sweroside from Alstonia scholaris. J. Entomol. Nematol. 2013;5(2):19–23. [Google Scholar]
- The Plant List . 2020. Version 1. Published on the Internet.http://www.theplantlist.org/ Retrieved on 27 April 2020 from. [Google Scholar]
- Trease G.E., Evans W.C. lth ed. Bailliere Tindall); 1972. Pharmacognosy 1; p. 750. [Google Scholar]
- Ushie O.A., Adamu H.M. Phytochemical screening of Borreria verticillata leaves. J. Agric. Biotechnol. Ecol. 2010;3(1):108–117. [Google Scholar]
- Ushie O.A., Adamu H.M., Ogar D.A., Gunda H.J. Phytochemistry of Borreria verticillata stem bark. Int. J. Tradit. Nat. Med. 2013;2(2):97–103. [Google Scholar]
- Vieira I.J.C., Mathias L., Braz-Filho R., Schripsema J. Iridoids from Borreria verticillata. Org. Lett. 1999;1(8):1169–1171. [Google Scholar]
- Wang Kui, Luo Chao, Liu Hao, Xu Jianmei, Sun Weibo, Zhou Ligang. Nematicidal activity of the alkaloids from Macleaya cordata against certain nematodes. Afr. J. Agric. Res. 2012;7(44):5925–5929. [Google Scholar]
- Waziri H.M.A. Plants as antiviral agents. J. Plant Pathol. Microbiol. 2015;2:1–5. [Google Scholar]
- Wen Yanhua, Meyer Susan L.F., Masler Edward P., Zhang Fengxian, Liao Jinling, Wei Xiaoyi, Chitwood David J. Nematotoxicity of drupacine and a Cephalotaxus alkaloid preparation against the plant-parasitic nematodes Meloidogyne incognita and Bursaphelenchus xylophilus. Pest Manag. Sci. 2013;69:1026–1033. doi: 10.1002/ps.3548. [DOI] [PubMed] [Google Scholar]
- Wu H.J., Pratley D.L., Haig T. Allelopathy in wheat (Triticum aestivum) Ann. Appl. Biol. 2001;139:1–9. [Google Scholar]