Abstract
Background
Rosette-forming glioneuronal tumors (RGNTs) are rare, low-grade, primary CNS tumors first described in 2002 by Komori et al. RGNTs were initially characterized as a World Health Organization (WHO) grade I tumors typically localized to the fourth ventricle. Although commonly associated with an indolent course, RGNTs have the potential for aggressive behavior.
Methods
A comprehensive search of PubMed and Web of Science was performed through November 2019 using the search term “rosette-forming glioneuronal tumor.” Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed. English, full-text case reports and series with histopathological confirmation were included. Patient demographics, presentations, MRI features, tumor location, treatment, and follow-up of all 130 cases were extracted.
Results
A 19-year-old man with a history of epilepsy and autism presented with acute hydrocephalus. MRI scans from 2013 to 2016 demonstrated unchanged abnormal areas of cortex in the left temporal lobe with extension into the deep gray-white matter. On presentation to our clinic in 2019, the lesion demonstrated significant progression. The patient’s tumor was identified as RGNT, WHO grade I. One hundred thirty patients were identified across 80 studies.
Conclusion
RGNT has potential to transform from an indolent tumor to a tumor with more aggressive behavior. The results of our systematic review provide insight into the natural history and treatment outcomes of these rare tumors.
Keywords: case report, Rosette-forming glioneuronal tumor, systematic review
Key Points.
Rosette-forming glioneuronal tumors have aggressive potential.
While usually stable after resection, RGNT has been shown to occasionally recur.
Importance of the Study.
Because the current literature on RGNT is still so limited, little is definitively understood about the epidemiology, anatomical distribution, and even treatment of these tumors, especially since there is a subset of cases with tumors that recurred. Not only does our case and review of the literature unveil the more aggressive clinical course that is possible with these tumors, but it also highlights other unique characteristics. For instance, our case involves fairly novel locations; there are only 13 other documented cases of the tumor in the third ventricle and only 2 others involving the temporal lobe. We also found that the gender preference, previously documented as at least a 2:1 females:males, was actually closer to 1:1 (68 females and 62 males). Other important findings are detailed in the manuscript.
The rosette-forming glioneuronal tumor (RGNT) is a rare, low-grade, primary CNS tumor that was first described in 2002 in a case series by Komori et al., in which the authors differentiated RGNT as a distinct entity from the dysembryoplastic neuroepithelial tumor.1 In 2007, the RGNT was designated as a World Health Organization (WHO) Grade I tumor and described as a slow-growing tumor typically localized to the fourth ventricle and composed of neurocytes that form neurocytic rosettes and glial components similar to those of the pilocytic astrocytoma.2 In the 2016 WHO classification, the RGNT was again classified as a Grade I tumor and listed under the category of “neuronal and mixed neuronal-glial tumors.” 3 Various other anatomical sites of origin have been reported, including the supratentorial ventricles,4 pineal gland,5 hypothalamus,6 optic chiasm,7,8 and spinal cord.9–11 Due to the rarity of the RGNT, little is known about its epidemiology and natural history. In this study, we present the case of a man with autism spectrum disorder and epilepsy who was diagnosed at 19 years of age with a RGNT. His prior MRI demonstrated stable appearance of nonspecific cortical abnormalities of the left temporal lobe that were thought to be related to his epilepsy. His case highlights the natural history of RGNT and presents a tumor in an uncommon anatomical location spanning from the temporal lobe into the basal ganglia extending to involve the cerebellum. In addition, we report the results of a systematic review of the literature pertaining to this rare neoplasm, analyzing key features of RGNT.
Case Presentation
Consent
Patient consent was obtained for inclusion in this case report. No identifying details have been disclosed.
History and Physical Examination
A 19-year-old man with a history of autism spectrum disorder and simple partial epilepsy manifested by occasional generalized tonic-clonic seizures since childhood presented to our emergency department. He was first diagnosed with infantile spasms around the age of 3 months with up to 8 episodes per day and began antiepileptic therapy at that time. His childhood seizure semiology consisted of episodes of gaze deviation toward his right hand and right arm jerks, lasting 2–3 min. He also had repeated episodes of looking at his right hand, collapsing toward his right side, and heavy breathing. These episodes occurred 2–3 times weekly but were interspersed with asymptomatic periods lasting for several weeks. At the age of 6 years in 2005, the patient underwent an MRI during epilepsy workup, which revealed no pathological findings. Another MRI in 2013 demonstrated left temporal lobe cortical abnormalities with extension into the deep gray-white matter. The initial impression was focal hemimegaloencephaly of the mesial left temporal lobe with some polymicrogyria/pachygyria complex or possibly gliomatosis cerebri as read by neuroradiologists. There was no concern for neoplasm at the time. An MRI in 2016 showed the stable cortical lesions with a slight increase in the T2 signal in the superior cerebellar vermis.
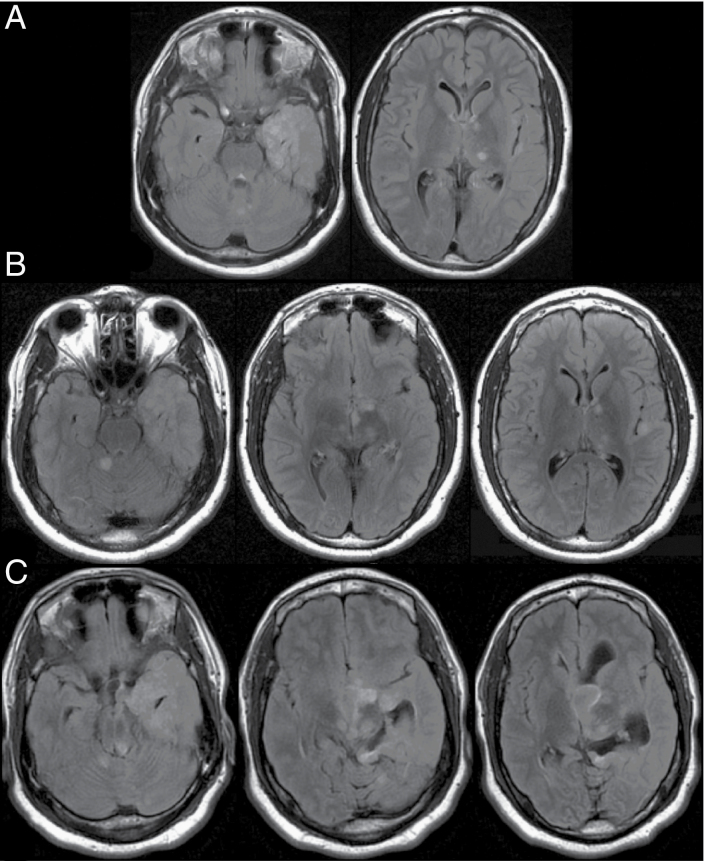
In 2019, at age 19, the patient presented acutely with hydrocephalus manifested by a 2-week history of vomiting and progressive lethargy. Prior to this, he spent his days playing video games and was able to ambulate without difficulties. Upon presentation to the emergency room, he was very lethargic and had difficulties walking, repeatedly leaning, and falling toward his left side. MRI findings at this time showed dramatic expansion of the previously stable left temporal lobe lesion with involvement of the left gangliocapsular region, bilateral thalami, tectum, and cerebellum. In addition, a new nonenhancing cystic component within the left foramen of Monro and third ventricle was identified, resulting in acute obstructive hydrocephalus. The MRI scans from 2013, 2016, and 2019 are shown in Figure 1.
Figure 1.
Axial Flair MRI demonstrating RGNT over a 6-year surveillance period. (A) 2013 MRI showing left temporal lobe cortical abnormalities with extension into the deep gray-white matter. (B) Surveillance MRI taken in 2016 that demonstrated stable cortical lesions with slight increase in the T2 signal in the superior cerebellar vermis. (C) MRI in 2019 at presentation to our institution with dramatic expansion of the previously stable left temporal lobe lesion with involvement of the left gangliocapsular region, bilateral thalami, tectum, and cerebellum. In addition, a new non-enhancing cystic component within the left foramen of Monro and third ventricle was identified, resulting in acute obstructive hydrocephalus.
Operative Course
On the day of presentation, an external ventricular drain (EVD) was placed to alleviate his hydrocephalus, resulting in mental status improvement. Shortly thereafter, an endoscopic biopsy of the third ventricle lesion along with septum pellucidotomy was performed. The initial evaluation of the pathology demonstrated concern for possible RGNT. However, only the rosette-forming neuronal component of the tumor was present within the nonenhancing third ventricular component. Given these results and the extensive nature of the tumor, a second procedure was planned.
The patient underwent both left temporal craniotomy for mesial temporal lobe tumor resection and frontal interhemispheric craniotomy for resection of the intraventricular tumor components. There were no complications intraoperatively, and the patient was transferred to the neurological intensive care unit in stable condition.
Postoperative Course
His final pathology was consistent with RGNT. The postoperative exam was significant for central fever and autonomic dysregulation likely secondary to the tumor and its resection near the hypothalamus. Attempts were made to wean his EVD. However, a follow-up CT scan showed an interval increase in the size of the left lateral ventricle. Symptoms of hydrocephalus continued, and the patient subsequently underwent ventriculoperitoneal shunt placement.
The patient developed a proximal shunt malfunction later that month requiring revision. Gross examination demonstrated tissue buildup within the ventricular catheter fenestrations. Approximately 3 weeks later, the patient again presented with worsening hydrocephalus. Repeat revision of the proximal catheter and shunt valve was performed. At 8 months follow-up, the patient was clinically stable and back to baseline function.
Search Strategy and Collection Criteria
A comprehensive literature review using PubMed and Web of Science was performed through November 2019 following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Articles related to RGNTs were identified using the search term “rosette-forming glioneuronal tumor.” Upon removing duplicates, titles and abstracts were screened for relevant articles. English, full-text case reports and series were included. Non-English, abstract-only text, and review articles were excluded. Studies without mention of histopathological confirmation of the RGNT were further excluded. Ultimately, 80 references meeting the selection criteria were included.1,4–82 Patient demographics, presentations, MRI features, tumor location, treatment, and follow-up of all 130 cases (when available) were extracted and summarized. Patient presentations, genetic characteristics of tumors, recurrence analysis, and surgical follow-up are shown in Tables 1–3 and Supplementary Table 1, respectively.
Table 1.
Presenting Symptoms
| Presenting Symptom | Number of Cases | Percentage |
|---|---|---|
| Headache | 79/112 | 71% |
| Ataxia | 28/112 | 25% |
| Vomiting/nausea | 27/112 | 24% |
| Visual disturbances | 24/112 | 21% |
| Vertigo/dizziness | 18/112 | 16% |
| Loss of consciousness | 4/112 | 4% |
| Dysarthria | 3/112 | 3% |
| Incidental | 14/112 | 13% |
Table 3.
Recurrence Analysis Table
| Procedure | Total Cases | Number of Recurrences | Time to Recurrence | Mean Time to Recurrence | Relative % of Recurrences | Paper | Adjunctive Treatment |
|---|---|---|---|---|---|---|---|
| GTR | 62 | 6 | 10 years | 6.17 years | 9.70% | Jacques39 | None |
| 9 years | Ellezam28 | None | |||||
| 7 years | Kwon49 | None | |||||
| 4 years | Ramos64 | None | |||||
| 4 years | Ellezam28 | None | |||||
| 3 yearsa | Morris57 | None | |||||
| STR | 32 | 3 | 6 years | 2.92 years | 9.40% | Jayapalan40 | None |
| 2 yearsa | Morris57 | Chemotherapy | |||||
| 9 months | Thurston75 | None | |||||
| PR | 7 | 1 | 4 months | 4 months | 14% | Yamamoto6 | None |
| Biopsy only | 4 | 2 | 6 months | 3.5 months | 50% | Chiba25 | None |
| 1 month | Silveira69 | None |
GTR, gross total resection; PR, partial resection; STR, subtotal resection.
aSame patient. First received STR + chemo, then GTR after initial recurrence.
Discussion
Grading
The RGNT is a rare neoplasm, with 130 patients across 80 studies having been identified in the literature. RGNT is currently classified as a benign tumor,3 although many authors report sudden onset of aggressive behavior after prolonged periods of stability. While the most cases of RGNT have an excellent postresection prognosis without any deterioration, the outcome of our illustrative case and systematic review call this indolent nature into question. Our patient’s 6 years of MRIs demonstrate that RGNT has the potential to become rapidly progressive and infiltrative after years of dormancy. Our patient’s tumor showed significant progression on his scan an additional 3 years later. This MRI also revealed a second tumor in the foramen of Monroe. This type of progression is not unique to our case. Of the 91 cases in our review that reported follow-up, 13 demonstrated either recurrence (with or without malignant transformation) or rapid progression/dissemination, totaling 14% of the cases with reported follow-up.
However, there is much inconsistency with regard to the natural history of these tumors. Chiba et al. described a tumor that was only biopsied at first but demonstrated rapid progression of the tumor as well as a new lesion in only 6 months.25 A case described by Cabezas et al. presented with a secondary intraspinal lesion and leptomeningeal spread of the tumor.21 Silveira et al. also described the dissemination of tumor with drop metastasis in the lumbar spine only 1 month after initial imaging and biopsy of the tumor.69 Other reports have described similarly quick tumor recurrences postoperatively after 4 months6 and 9 months.75 However, recurrences have also been reported as late as 9 years after initial resection.28 Morris et al. described a particularly aggressive RGNT case in a 6-year-old boy.57 In this case, the tumor progressed and extended into the proximal cervical spinal cord 2 years after initial treatment involving both resection and chemotherapy.57 Another round of chemotherapy did not stop the tumor from further progression, so the tumor was totally resected.57 Unfortunately, the tumor recurred yet again 3 years later.57 There are even 2 cases that describe an RGNT with malignant transformation to glioblastoma after several years.40,49 As a greater number of cases are reported, it appears that some RGNTs may not be as indolent as previously thought.
Epidemiology
RGNT most commonly arises in young adults, as in the case of our 19-year-old patient. However, the youngest reported patient was 4 years old, and the oldest was 81 years old.25,26 The mean age of diagnosis is 29.8 years, which is very similar to earlier findings.1,11,25 However, we noted a much less dramatic gender preference with a female:male ratio of 1.1:1 (68 females and 62 males) in contrast to previous reports describing a female predominance closer to 2:1.1,5,42,76
Presenting Symptoms
Common presenting symptoms of RGNT from our review include headache (71%), ataxia/gait disturbance (25%), vomiting/nausea (24%), visual disturbances (21%), and vertigo/dizziness (16%). Several cases were also found incidentally (13%). Presenting symptoms are displayed in Table 1.
Imaging Characteristics
The location of our patient’s tumor makes this case unique from other reports. The original lesion remained stable in the patient’s temporal lobe before progressing to involve the gangliocapsular region, bilateral thalami, tectum, and cerebellum. To our knowledge, only 2 other cases report any involvement of the temporal lobe with RGNT,56,80 and only 6 others had involvement of the cerebellum.42,43,66,74,76 The appearance of the second mass is also notable, especially with its growth into the third ventricle and foramen of Monro. While there have been 13 other reports of RGNT invasion of the third ventricle, to our knowledge, this is the first case to demonstrate invasion and blockage of the foramen of Monro.
Genetics/Molecular Markers
The origin of RGNT has yet to be elucidated due to the rarity of the tumor and its indolent course. It has been hypothesized that the neoplasm originates from pluripotent cells of the subependymal plate,60 and Chakraborti et al. showed an evidence of differentiation of stem cells in their case series on RGNT.83 Many genetic mutations have also been found in association with RGNT. The most common genetic marker associated with RGNT is the PIK3CA mutation.28,29,51,74,84,85 FGFR1 mutations have also been implicated in tumor pathogenesis51,84–86 as well as IDH1,40 KIAA1549/BRAF gene fusion,20 PPP1R1A,51 and RNF21.51
Most cases presented in the literature did not test any molecular markers, and among those that did, most did not test for the now-known genetic associations such as PIK3CA and FGFR1. However, a recent retrospective cohort study was able to obtain tissue samples of 18 different patients with RGNT for comprehensive genomic testing.84 Sequencing of those samples revealed that all 18 contained an FGFR1 mutation with 13 carrying a comorbid PIK3CA mutation. The findings of this study confirm that constitutive activation of the FGFR signaling likely plays a role in a large portion of these tumors. Because constitutive PI3K pathway activation (caused by PIK3CA mutation) has also been implicated in several of the cases in our review as well as in the previously mentioned cohort study, RGNT may commonly be a multiple-pathway disease, which is typically more characteristic of high-grade tumors.84 The findings of this study were not included in our genetics summary table (Table 2) due to publication after our initial database search.
Table 2.
Genetics Table
| Cachia 201422 | Ellezam 201228 | Ellezam 201228 | Ellezam 201228 | Eye 201729 | Kitamura 201847 | Kitamura 201847 | Kitamura 201847 | Lin 201651 | Thommen 201374 | |
|---|---|---|---|---|---|---|---|---|---|---|
| PIK3CA | Exon 9: G>A E545K |
Exon 9: A>G E542K |
Exon 20: >G H1047R |
Exon 20: A>G H1047R |
Exon 9: G>A E545K |
Wild type | Wild type | Exon 20: A>G H1047R |
Exon 20: A>G H1047R |
Exon 20: A>G H1047R |
| FGFR1 | NT | NT | NT | NT | NT | Exon 14: A>G K656E and A>G D652G |
Exon 14: A>G K656E and A>G D652G |
Exon 12: C>A N546K [Glial component only] |
Exon 12: C>A N546K |
NT |
NT, not tested.
Additionally, a few cases have been found in association with neurofibromatosis type 1,7,45,68 as well as 2 with Noonan syndrome44,51 and 1 with multiple sclerosis.70 Interestingly, there was one case that presented 4 months after a grade II diffuse astrocytoma.22 Future research in this area may provide further answers about the pathogenesis of RGNT as well as direction for future drug therapy. A summary of the PIK3CA and FGFR1 genetic results from the literature review is given in Table 2.
Treatment
Currently, surgery is the mainstay of treatment. Because RGNT has historically been thought of as indolent, gross total resection (GTR) is typically only attempted if there is very low risk of damaging surrounding structures. Subtotal resection (STR), partial resection (PR), and simple biopsy with observation have all been employed for these tumors due to the risk of doing more harm with unnecessary aggressive removal.23,37,67 Among the cases that reported treatment (116 cases), 62 underwent GTR (53%), 32 underwent STR (28%), 7 underwent a partial resection (6%), and 4 underwent a biopsy only (3%). Other adjunctive treatments included radiotherapy (9 cases, 8%) and chemotherapy (4 cases, 3%). Though there is not a large enough sample size of recurrences to draw significant conclusions regarding the most effective treatment plan, Morris et al. postulate a pattern of delayed recurrence of tumor for patients that receive GTR as opposed to earlier recurrence for those that receive STR.57 Although the results of our analysis are not quite as striking, the average time to tumor recurrence for patients that underwent GTR was longer at 6.17 years compared to 2.92 years to recurrence for patients that underwent STR. However, even with this small sample size, the probability of recurrence approximates 10% with either GTR or STR. Thus, while RGNT might have a more aggressive nature than previously thought, aggressive resection in all cases may not be warranted. Based on the potential for a rapidly progressive clinical course, it is our opinion that gross total resection should be pursued when feasible. Surgical follow-up data for each case, including location and treatment, are displayed in Supplemental Table 1. Recurrence data are summarized in Table 3.
The effectiveness of chemotherapy and radiotherapy as primary or adjunctive therapy remains unclear. These therapies have very rarely been applied to RGNT treatment and typically only in cases of recurrent or disseminated tumors. We identified only one case, described by Morris et al., in which chemotherapy was not effective at limiting the rapid spread of RGNT.57 In this case, 4 cycles of vincristine, etoposide, and carboplatin were given after the initial resection, which resulted in stable tumor remnant for 2 years. When the tumor recurred and extended into the spinal cord, another chemotherapy regimen was attempted using weekly vinblastine for 6 months, which did not halt the progression of tumor growth.57 However, all other reported uses of chemotherapy (3) and all reported uses of radiotherapy (9) in cases of RGNT have yielded stable tumor remnant and no recurrence for varying lengths of follow-up.
Conclusion
RGNT is a rare low-grade central nervous tumor usually localized within the fourth ventricle that is typically managed with surgical resection and often remains stable at follow-up. We present a case with stable tumor size and character on imaging for 3 years and sudden progression with a development of a secondary lesion 3 years later. Upon review of the literature, there are many examples of rapid progression, recurrences, and even malignant transformation, calling RGNT’s indolent reputation into question. There is insufficient evidence to support adjunctive treatment such as chemotherapy and radiotherapy in prevention of recurrence, but GTR and STR seem to have similar effectiveness as measured by patient survival and tumor recurrence. Further research is necessary to uncover the pathogenesis and genetic basis of RGNT so that better targeted therapies for aggressive cases that may be developed.
Supplementary Material
Acknowledgments
Author contributions: Project administration: C.A.G.; Writing—original draft: C.P.W. and A.R.C.; Writing—review and editing: C.P.W., A.R.C., P.E.P., H.H.S., C.K.M., and C.A.G.; Resources: P.E.P., H.H.S., S.S., T.M., and J.E.P.
Conflict of interest statement. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. There are no competing interests with regards to this project.
References
- 1. Komori T, Scheithauer BW, Hirose T. A rosette-forming glioneuronal tumor of the fourth ventricle: infratentorial form of dysembryoplastic neuroepithelial tumor? Am J Surg Pathol. 2002;26(5):582–591. [DOI] [PubMed] [Google Scholar]
- 2. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wen PY, Huse JT. 2016 World Health Organization classification of central nervous system tumors. Continuum (Minneap Minn). 2017;23(6, Neuro-oncology):1531–1547. [DOI] [PubMed] [Google Scholar]
- 4. Xiong J, Liu Y, Chu SG, et al. Rosette-forming glioneuronal tumor of the septum pellucidum with extension to the supratentorial ventricles: rare case with genetic analysis. Neuropathology. 2012;32(3):301–305. [DOI] [PubMed] [Google Scholar]
- 5. Solis OE, Mehta RI, Lai A, et al. Rosette-forming glioneuronal tumor: a pineal region case with IDH1 and IDH2 mutation analyses and literature review of 43 cases. J Neurooncol. 2011;102(3):477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamamoto T, Matsubara T, Satomi K, et al. Rosette-forming glioneuronal tumor originating in the hypothalamus. Brain Tumor Pathol. 2015;32(4):291–296. [DOI] [PubMed] [Google Scholar]
- 7. Scheithauer BW, Silva AI, Ketterling RP, Pula JH, Lininger JF, Krinock MJ. Rosette-forming glioneuronal tumor: report of a chiasmal-optic nerve example in neurofibromatosis type 1: special pathology report. Neurosurgery. 2009;64(4):E771–2; discussion E772. [DOI] [PubMed] [Google Scholar]
- 8. Bharadwaj R, Chickabasaviah YT, Rao S, et al. Rosette-forming glioneuronal tumor in the optic pathway of a child. J Pediatr Hematol Oncol. 2019;42(7):655–658. [DOI] [PubMed] [Google Scholar]
- 9. Anan M, Inoue R, Ishii K, et al. A rosette-forming glioneuronal tumor of the spinal cord: the first case of a rosette-forming glioneuronal tumor originating from the spinal cord. Hum Pathol. 2009;40(6):898–901. [DOI] [PubMed] [Google Scholar]
- 10. Collin A, Adle-Biassette H, Lecler A. Rosette-forming glioneuronal tumor of spinal cord. World Neurosurg. 2018;119:242–243. [DOI] [PubMed] [Google Scholar]
- 11. Duan L, Zhang Y, Fu W, Geng S. Rosette-forming glioneuronal tumor originating from the spinal cord: report of 2 cases and literature review. World Neurosurg. 2017;98:875.e1–875.e7. [DOI] [PubMed] [Google Scholar]
- 12. Adachi J, Nishikawa R, Hirose T, Matsutani M. Mixed neuronal-glial tumor of the fourth ventricle and successful treatment of postoperative mutism with bromocriptine: case report. Surg Neurol. 2005;63(4):375–379. [DOI] [PubMed] [Google Scholar]
- 13. Albanese A, Mangiola A, Pompucci A, et al. Rosette-forming glioneuronal tumour of the fourth ventricle: report of a case with clinical and surgical implications. J Neurooncol. 2005;71(2):195–197. [DOI] [PubMed] [Google Scholar]
- 14. Allinson KS, O’Donovan DG, Jena R, Cross JJ, Santarius TS. Rosette-forming glioneuronal tumor with dissemination throughout the ventricular system: a case report. Clin Neuropathol. 2015;34(2):64–69. [DOI] [PubMed] [Google Scholar]
- 15. Alnaami I, Aronyk K, Lu JQ, Johnson ES, O’Kelly C. Rosette-forming glioneuronal tumors in the posterior third ventricle. Can J Neurol Sci. 2013;40(6):885–888. [DOI] [PubMed] [Google Scholar]
- 16. Alturkustani M, Ang LC. Rosette-forming glioneuronal tumour of the 4th ventricle in a NF1 patient. Can J Neurol Sci. 2012;39(1):95–96. [DOI] [PubMed] [Google Scholar]
- 17. Arai A, Sasayama T, Tamaki M, et al. Rosette-forming glioneuronal tumor of the fourth ventricle–case report. Neurol Med Chir (Tokyo). 2010;50(3):224–228. [DOI] [PubMed] [Google Scholar]
- 18. Bera G, Das A, Chatterjee S, Chatterjee U. Rosette-forming glioneuronal tumor: a rare posterior fossa tumor in an adolescent. J Pediatr Neurosci. 2017;12(2):168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beuriat PA, Tauziede-Espariat A, Pages M, Varlet P, Di Rocco F. Rosette-forming glioneuronal tumor outside the fourth ventricle: a case-based update. Childs Nerv Syst. 2016;32(1):65–68. [DOI] [PubMed] [Google Scholar]
- 20. Bidinotto LT, Scapulatempo-Neto C, Mackay A, et al. Molecular profiling of a rare rosette-forming glioneuronal tumor arising in the spinal cord. PLoS One. 2015;10(9):e0137690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cabezas SG, Blanch RS, Sanchez-Sanchez R, Eito AP. Rosette-forming glioneuronal tumour (RGNT) of the fourth ventricle: a highly aggressive case. Brain Tumor Pathol. 2015;32(2):124–130. [DOI] [PubMed] [Google Scholar]
- 22. Cachia D, Prado MP, Theeler B, Hamilton J, McCutcheon I, Fuller GN. Synchronous rosette-forming glioneuronal tumor and diffuse astrocytoma with molecular characterization: a case report. Clin Neuropathol. 2014;33(6):407–411. [DOI] [PubMed] [Google Scholar]
- 23. Cebula H, Chibbaro S, Santin MN, Kremer S, Chaussemy D, Proust F. Thalamic rosette-forming a glioneuronal tumor in an elderly patient: Case report and literature review. Neurochirurgie. 2016;62(1):60–63. [DOI] [PubMed] [Google Scholar]
- 24. Chen SY, Wang W, Wang LM, et al. Glioneuronal tumours with features of rosette-forming glioneuronal tumours of the fourth ventricle and dysembryoplastic neuroepithelial tumours: a report of three cases. Histopathology. 2016;68(3):378–387. [DOI] [PubMed] [Google Scholar]
- 25. Chiba K, Aihara Y, Eguchi S, Tanaka M, Komori T, Okada Y. Rosette-forming glioneuronal tumor of the fourth ventricle with neurocytoma component. Childs Nerv Syst. 2014;30(2):351–356. [DOI] [PubMed] [Google Scholar]
- 26. Damodaran O, Robbins P, Shivapathasundram G, Bynevelt M, Lee GYF. Rosette-forming glioneural tumor of the fourth ventricle: surgery complicated by cerebellar mutism in an elderly patient. Neurosurg Q. 2013;23(2):122–126. [Google Scholar]
- 27. Eastin M, Shah KJ, Newell KL, Chamoun R. Rosette-forming glioneuronal tumor of the thalamus. Clin Neuropathol. 2016;35(5):326–328. [DOI] [PubMed] [Google Scholar]
- 28. Ellezam B, Theeler BJ, Luthra R, Adesina AM, Aldape KD, Gilbert MR. Recurrent PIK3CA mutations in rosette-forming glioneuronal tumor. Acta Neuropathol. 2012;123(2):285–287. [DOI] [PubMed] [Google Scholar]
- 29. Eye PG, Davidson L, Malafronte PJ, Cantrell S, Theeler BJ. PIK3CA mutation in a mixed dysembryoplastic neuroepithelial tumor and rosette forming glioneuronal tumor, a case report and literature review. J Neurol Sci. 2017;373:280–284. [DOI] [PubMed] [Google Scholar]
- 30. Frydenberg E, Laherty R, Rodriguez M, Ow-Yang M, Steel T. A rosette-forming glioneuronal tumour of the pineal gland. J Clin Neurosci. 2010;17(10):1326–1328. [DOI] [PubMed] [Google Scholar]
- 31. Fushimi Y, Miyasaki A, Taki H, et al. Rosette-forming glioneuronal tumor of the fourth ventricle with bilateral olivary degeneration. Jpn J radiol. 2011;29(6):445–448. [DOI] [PubMed] [Google Scholar]
- 32. Gao C, Xie R, Cao XY, Huang FP. Rosette-forming glioneuronal tumor of the fourth ventricle. a rare neoplasm of the central nervous system. Neurosciences (Riyadh). 2009;14(3):292–293. [PubMed] [Google Scholar]
- 33. Gessi M, Lambert SR, Lauriola L, Waha A, Collins VP, Pietsch T. Absence of KIAA1549-BRAF fusion in rosette-forming glioneuronal tumors of the fourth ventricle (RGNT). J Neurooncol. 2012;110(1):21–25. [DOI] [PubMed] [Google Scholar]
- 34. Gessi M, Waha A, Setty P, Waha A, Pietsch T. Analysis of KIAA1549-BRAF fusion status in a case of rosette-forming glioneuronal tumor of the fourth ventricle (RGNT). Neuropathology. 2011;31(6): 654–657. [DOI] [PubMed] [Google Scholar]
- 35. Ghosal N, Furtado SV, Hegde AS. Rosette forming glioneuronal tumor pineal gland and tectum: an intraoperative diagnosis on smear preparation. Diagn Cytopathol. 2010;38(8):590–593. [DOI] [PubMed] [Google Scholar]
- 36. Hakan T, Aker FV. Rosette-forming glioneuronal tumour of the fourth ventricle: case report and review of the literature. Folia Neuropathol. 2016;54(1):80–87. [DOI] [PubMed] [Google Scholar]
- 37. Haryu S, Saito R, Kanamori M, et al. Rosette-forming glioneuronal tumor: rare case presented with spontaneous disappearance of contrast enhancement. NMC Case Rep J. 2015;2(2):65–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hsu C, Kwan G, Lau Q, Bhuta S. Rosette-forming glioneuronal tumour: imaging features, histopathological correlation and a comprehensive review of literature. Br J Neurosurg. 2012;26(5):668–673. [DOI] [PubMed] [Google Scholar]
- 39. Jacques TS, Eldridge C, Patel A, et al. Mixed glioneuronal tumour of the fourth ventricle with prominent rosette formation. Neuropathol Appl Neurobiol. 2006;32(2):217–220. [DOI] [PubMed] [Google Scholar]
- 40. Jayapalan RR, Mun KS, Wong KT, Sia SF. Malignant transformation of a rosette-forming glioneuronal tumor with IDH1 mutation: a case report and literature review. World Neurosurg X. 2019;2:100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiménez-Heffernan JA, Romero J, Bárcena C, Cañizal JM. Cytological features of rosette-forming glioneuronal tumor of the fourth ventricle. Diagn Cytopathol. 2019;47(10):1082–1085. [DOI] [PubMed] [Google Scholar]
- 42. Johnson M, Pace J, Burroughs JF. Fourth ventricle rosette-forming glioneuronal tumor. Case report. J Neurosurg. 2006;105(1):129–131. [DOI] [PubMed] [Google Scholar]
- 43. Joseph V, Wells A, Kuo YH, et al. The ‘rosette-forming glioneuronal tumor’ of the fourth ventricle. Neuropathology. 2009;29(3):309–314. [DOI] [PubMed] [Google Scholar]
- 44. Karafin M, Jallo GI, Ayars M, Eberhart CG, Rodriguez FJ. Rosette forming glioneuronal tumor in association with Noonan syndrome: pathobiological implications. Clin Neuropathol. 2011;30(6):297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kemp S, Achan A, Ng T, Dexter MA. Rosette-forming glioneuronal tumour of the lateral ventricle in a patient with neurofibromatosis 1. J Clin Neurosci. 2012;19(8):1180–1181. [DOI] [PubMed] [Google Scholar]
- 46. Kinno M, Ishizawa K, Shimada S, et al. Cytology is a useful tool for the diagnosis of rosette-forming glioneuronal tumour of the fourth ventricle: a report of two cases. Cytopathology. 2010;21(3):194–197. [DOI] [PubMed] [Google Scholar]
- 47. Kitamura Y, Komori T, Shibuya M, et al. Comprehensive genetic characterization of rosette-forming glioneuronal tumors: independent component analysis by tissue microdissection. Brain Pathol. 2018;28(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kumar M, Samant R, Ramakrishnaiah R, et al. Rosette-forming glioneuronal tumor of the fourth ventricle. Radiol Case Rep. 2013;8(1):740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kwon SM, Kim JH, Byun J, et al. Malignant transformation of a rosette-forming glioneuronal tumor to glioblastoma. World Neurosurg. 2019;130:271–275. [DOI] [PubMed] [Google Scholar]
- 50. Li YM, Li WQ, Pan Y, et al. Rosette-forming glioneuronal tumour of the fourth ventricle with previous intratumoural haemorrhage: case report and review of the literature. J Int Med Res. 2009;37(3):958–966. [DOI] [PubMed] [Google Scholar]
- 51. Lin FY, Bergstrom K, Person R, et al. Integrated tumor and germline whole-exome sequencing identifies mutations in MAPK and PI3K pathway genes in an adolescent with rosette-forming glioneuronal tumor of the fourth ventricle. Cold Spring Harb Mol Case Stud. 2016;2(5):a001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luan S, Zhuang D, Sun L, Huang FP. Rosette-forming glioneuronal tumor (RGNT) of the fourth ventricle: case report and review of literature. Clin Neurol Neurosurg. 2010;112(4):362–364. [DOI] [PubMed] [Google Scholar]
- 53. Maiti TK, Arimappamagan A, Mahadevan A, Yasha TC, Pandey P, Santosh V. Rare pathologies in the posterior third ventricular region in children: case series and review. Pediatr Neurosurg. 2015;50(1):42–47. [DOI] [PubMed] [Google Scholar]
- 54. Makita K, Ohta T, Yamamuro S, et al. Gene alteration of rosette-forming glioneuronal tumor in a supurasellar lesion. Int J Clin Exp Med. 2016;9(3):6873–6881. [Google Scholar]
- 55. Marhold F, Preusser M, Dietrich W, Prayer D, Czech T. Clinicoradiological features of rosette-forming glioneuronal tumor (RGNT) of the fourth ventricle: report of four cases and literature review. J Neurooncol. 2008;90(3):301–308. [DOI] [PubMed] [Google Scholar]
- 56. Matyja E, Grajkowska W, Kunert P, Marchel A. A peculiar histopathological form of dysembryoplastic neuroepithelial tumor with separated pilocytic astrocytoma and rosette-forming glioneuronal tumor components. Neuropathology. 2014;34(5):491–498. [DOI] [PubMed] [Google Scholar]
- 57. Morris C, Prudowsky ZD, Shetty V, et al. Rosette-forming glioneuronal tumor of the fourth ventricle in children: case report and literature review. World Neurosurg. 2017;107:1045.e9–1045.e16. [DOI] [PubMed] [Google Scholar]
- 58. Nair AR, Gopalakrishnan CV, Kapilamoorthy TR, Radhakrishnan N. Rosette forming glioneuronal tumor of the fourth ventricle in squash cytology smear. J Cytol. 2014;31(4):215–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ogut B, Memis L, Emmez OH, Oner AY, Uluoglu O. Rosette forming glioneuronal tumour of fourth ventricle: a case report. Virchows Arch. 2015;467:S210. [Google Scholar]
- 60. Pimentel J, Resende M, Vaz A, et al. Rosette-forming glioneuronal tumor: pathology case report. Neurosurgery. 2008;62(5):E1162–3; discussion E1163. [DOI] [PubMed] [Google Scholar]
- 61. Podlesek D, Geiger K, Hendry DJ, Schackert G, Krex D. Rosette-forming glioneuronal tumor of the fourth ventricle in an elderly patient. J Neurooncol. 2011;103(3):727–731. [DOI] [PubMed] [Google Scholar]
- 62. Pradhan R, Mondal S, Pal S, Chatterjee S, Banerjee A, Bhattacharyya D. Rosette-forming glioneuronal tumor of the fourth ventricle. Neurol India. 2017;65(5):1176–1177. [DOI] [PubMed] [Google Scholar]
- 63. Rainov NG, Wagner T, Heidecke V. Rosette-forming glioneuronal tumor of the fourth ventricle. Cent Eur Neurosurg. 2010;71(4):219–221. [DOI] [PubMed] [Google Scholar]
- 64. Ramos AA, Vega IF, Batista KP, Fernandez VM, Sanchez CR, Vega MAA. Rosette-forming glioneuronal tumour of the fourth ventricle. Not always a foreseeable development. Contemp Oncol (pozn). 2018;22(4):270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sekar A, Rudrappa S, Gopal S, Ghosal N, Rai A. Rosette-forming glioneuronal tumor in opticochiasmatic region-novel entity in new location. World Neurosurg. 2019;125:253–256. [DOI] [PubMed] [Google Scholar]
- 66. Shah MN, Leonard JR, Perry A. Rosette-forming glioneuronal tumors of the posterior fossa. J Neurosurg Pediatr. 2010;5(1):98–103. [DOI] [PubMed] [Google Scholar]
- 67. Sharma P, Swain M, Padua MD, Ranjan A, Lath R. Rosette-forming glioneuronal tumors: a report of two cases. Neurol India. 2011;59(2):276–280. [DOI] [PubMed] [Google Scholar]
- 68. Sieg EP, Payne R, Langan S, Specht CS. Case report: a rosette-forming glioneuronal tumor in the tectal plate in a patient with neurofibromatosis type I. Cureus. 2016;8(11):e857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Silveira L, DeWitt J, Thomas A, Tranmer B. Disseminated rosette-forming glioneuronal tumor with spinal drop metastasis, a uniquely aggressive presentation of rare tumor. World Neurosurg. 2019;132:7–11. [DOI] [PubMed] [Google Scholar]
- 70. Simmons DB, Clark ME, Mathis DA, Sladky JH. Rosette-forming glioneuronal tumour in a patient with multiple sclerosis. Histopathology. 2015;67(5):751–753. [DOI] [PubMed] [Google Scholar]
- 71. Sumitomo N, Ishiyama A, Shibuya M, et al. Intractable epilepsy due to a rosette-forming glioneuronal tumor with a dysembryoplastic neuroepithelial background. Neuropathology. 2018;38(3):300–304. [DOI] [PubMed] [Google Scholar]
- 72. Tan CC, Gonzales M, Veitch A. Clinical implications of the infratentorial rosette-forming glioneuronal tumor: case report. Neurosurgery. 2008;63(1):E175–6; discussion E176. [DOI] [PubMed] [Google Scholar]
- 73. Tanaka F, Matsukawa M, Kogue R, et al. A case of a rosette-forming glioneuronal tumor arising from the pons with disappearance of contrast enhancement. Radiol Case Rep. 2019;14(8):899–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thommen F, Hewer E, Schäfer SC, Vassella E, Kappeler A, Vajtai I. Rosette-forming glioneuronal tumor of the cerebellum in statu nascendi: an incidentally detected diminutive example indicates derivation from the internal granule cell layer. Clin Neuropathol. 2013;32(5): 370–376. [DOI] [PubMed] [Google Scholar]
- 75. Thurston B, Gunny R, Anderson G, et al. Fourth ventricle rosette-forming glioneuronal tumour in children: an unusual presentation in an 8-year-old patient, discussion and review of the literature. Childs Nerv Syst. 2013;29(5):839–847. [DOI] [PubMed] [Google Scholar]
- 76. Vajtai I, Arnold M, Kappeler A, et al. Rosette-forming glioneuronal tumor of the fourth ventricle: report of two cases with a differential diagnostic overview. Pathol Res Pract. 2007;203(8):613–619. [DOI] [PubMed] [Google Scholar]
- 77. Wang Y, Xiong J, Chu SG, et al. Rosette-forming glioneuronal tumor: report of an unusual case with intraventricular dissemination. Acta Neuropathol. 2009;118(6):813–819. [DOI] [PubMed] [Google Scholar]
- 78. Xiong J, Ding L, Chen H, Chen H, Wang Y. Mixed glioneuronal tumor: a dysembryoplastic neuroepithelial tumor with rosette-forming glioneuronal tumor component. Neuropathology. 2013;33(4):431–435. [DOI] [PubMed] [Google Scholar]
- 79. Xu J, Yang Y, Liu Y, et al. Rosette-forming glioneuronal tumor in the pineal gland and the third ventricle: a case with radiological and clinical implications. Quant Imaging Med Surg. 2012;2(3):227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yapıcıer Ö, Demir MK, Jaafar E, Bozbuğa M. Mesial temporal lobe rosette-forming glioneuronal tumor: an unusual location for a rare tumor. Acta Neurol Belg. 2020;120(2):465–467. [DOI] [PubMed] [Google Scholar]
- 81. Yin B, Liu L, Chen XR, Li K, Geng DY. Rosette-forming glioneuronal tumor of the fourth ventricle. J Neuroradiol. 2012;39(2):129–130. [DOI] [PubMed] [Google Scholar]
- 82. Zhang J, Babu R, McLendon RE, Friedman AH, Adamson C. A comprehensive analysis of 41 patients with rosette-forming glioneuronal tumors of the fourth ventricle. J Clin Neurosci. 2013;20(3):335–341. [DOI] [PubMed] [Google Scholar]
- 83. Chakraborti S, Mahadevan A, Govindan A, et al. Rosette-forming glioneuronal tumor – evidence of stem cell origin with biphenotypic differentiation. Virchows Arch. 2012;461(5):581–588. [DOI] [PubMed] [Google Scholar]
- 84. Sievers P, Appay R, Schrimpf D, et al. Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol. 2019;138(3):497–504. [DOI] [PubMed] [Google Scholar]
- 85. Yamada S, Nobusawa S, Yamazaki T, et al. An epilepsy-associated glioneuronal tumor with mixed morphology harboring FGFR1 mutation. Pathol Int. 2019;69(6):372–377. [DOI] [PubMed] [Google Scholar]
- 86. Halfpenny A, Ferris SP, Grafe M, et al. A case of recurrent epilepsy-associated rosette-forming glioneuronal tumor with anaplastic transformation in the absence of therapy. Neuropathology. 2019;39(5):389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.