Abstract
Background
Progesterone receptor (PR) modulates neuroprotective and regenerative responses in Parkinson's disease and related neurological diseases.
Objectives
The present study was designed to determine whether botanical drug puerarin could exhibit neuroprotective and neurorestorative activities via PR signaling.
Methods
The neuroprotective and neurotrophic activities of puerarin were investigated in MPTP-lesioned mice and MPP+-challenged primary rat midbrain neurons. Rotarod performance test and tail suspension test were used to assess motor functions. Tyrosine hydroxylase (TH) and PR were determined by immunostaining, Western blotting, and luciferase reporter assays. Neurite outgrowth was assessed by fluorescence staining and immunostaining.
Results
Puerarin effectively ameliorated the MPTP-induced motor abnormalities in MPTP-lesioned mice and protected primary rat midbrain neurons against MPP+-induced toxicity via PR signaling although progesterone exhibited the neuroprotection. PR antagonist mifepristone (RU486) diminished the neuroprotection of puerarin in MPTP-lesioned mice and MPP+-induced primary rat midbrain neurons. Moreover, puerarin promoted the differentiation of primary rat midbrain neurons and potentiated NGF to induce neuritogenesis in PC12 cells. RU486 and PR-siRNA could inhibit the effect of puerarin. Puerarin and progesterone could enhance the PR promoter.
Conclusion
Puerarin attenuated MPTP- and MPP+-induced toxicity and potentiated neurite outgrowth via PR. These results suggested that puerarin may become an alternative hormone for suppressing MPTP- and MPP+-induced toxicity in neurodegenerative diseases.
1. Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disease worldwide and characterized by motor dysfunctions (e.g., tremor, rigidity, and dyskinesia) as well as some other nonmotor symptoms (e.g., cognition deficit and depression) [1]. The pathological change in PD patients is hallmarked by the progressive loss of dopaminergic neurons in substantia nigra compacta (SNpc) [2]. Levodopa is a commonly accepted drug for the treatment of PD although the unavoidable side effects and instability are documented after long-term use [3]. Therefore, it is pressingly needed to discover new neuroprotective and neuroregenerative small molecules to meet the clinical demand.
Clinical evidence shows that men suffer from higher prevalence and incidence of PD than women, suggesting the neuroprotective role of sex hormones in the brain [4, 5]. Estrogens (e.g., 17β-estradiol and estradiol) showed neuroprotective potential to diminish the nigrostriatal degeneration in mouse model of PD [6, 7]. However, the feminizing effects of estrogens limit its medical use in the treatment of PD. On the other hand, progesterone is free of feminizing effects and could be available for both men and women [4]. Although progesterone is classically recognized as an endogenous steroid from ovary, Pomata et al. found that progesterone could be synthesized from cholesterol in the central nervous system [8]. Several studies showed that progesterone played the neuromodulatory and neuroprotective roles in PD [9, 10]. Presumably, progesterone exhibits neuroprotective effects via interacting progesterone receptor (PR) [11]. PR exists in two isoforms: PR-A and PR-B, which are transcribed from two different promoter regions of single gene and expressed in the brain [12]. PR-B is a stronger transcriptional activator compared with PR-A [13]. Interestingly, PRs are recognized as therapeutic target for the control of neurodegeneration [14].
Herbal medicines are well documented for the chemical compositions of stilbenoids, flavonoids, catechols, and terpenes and the potential activities against neurodegeneration [15, 16]. Puerarin (Figure 1(a)) is a unique C-glycosylated isoflavone from the herb Radix Puerariae lobatae [17]. We previously demonstrated that puerarin markedly reduced neurotoxicity of 6-hydroxydopamine (6-OHDA) and MPTP, in vitro and in vivo, respectively [18, 19]. As far as the mechanism is concerned, puerarin might mimic estrogen to protect the nigral neurons from apoptosis [20, 21]. On the other hand, puerarin could recover the decrease in progesterone levels in rats that suffered from chronic stress and posttraumatic stress disorder [22, 23]. Puerarin disrupted the implantation of the rat uterus by inhibiting PR [24]. Thus, the present study was designed to determine whether puerarin could exhibit neuroprotective and neurorestorative activities via PR signaling.
Figure 1.

Puerarin ameliorated behavioral impairments in MPTP-lesioned mice. (a) Chemical structure of puerarin. Chemical structure was generated by ChemSketch (http://www.acdlabs.com/home/). (b) Design for animal experiments. C57BL/6N mice were randomly divided into five groups: control, MPTP, MPTP+Puerarin, MPTP+Progesterone, and MPTP+Puerarin+RU486. Following treatments, the mice were assessed for behavioral impairments and biomarker expression. (c) Rotarod performance test. Animals were evaluated for the riding time. (d) Tail suspension test. Animals were assessed for the immobility time. The results of behavioral tests were presented as mean ± SEM (n = 10) and analyzed by one-way ANOVA followed by Dunnett's test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
2. Materials and Methods
2.1. Biochemical Reagents
Antibody against tyrosine hydroxylase (TH) was purchased from Merck Millipore (Billerica, MA, USA). Antibodies against GAPDH and Alexa Fluor 594-conjugate anti-rabbit IgG antibody were purchased from Cell Signaling Technology (Boston, BA, USA). Mouse antigrowth-associated protein 43 (GAP43) antibody and rabbit anti-PR antibody were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Protein assay dye reagent concentrate was purchased from Bio-Rad Labs (Hercules, CA, USA). Enhanced chemiluminescence (ECL) detection reagent was purchased from GE Healthcare (Uppsala, Sweden). 3,3-N-diaminobenzidine tetrahydrochloride (DAB) substrate kit was obtained from Dako Corporation (Carpintera, CA, USA). PR siRNA, HiPerFect transfection reagent, and Effectene Transfection Reagent were purchased from Qiagen (Hilden, Germany). The luciferase reporter vectors (i.e., 2xPRE-TK-Luc) were obtained from Addgene (Cambridge, MA, USA). pRL-TK vector encoding Renilla luciferase and dual-luciferase reporter assay system was purchased from Promega (Madison, USA). The neurite outgrowth staining kit was purchased from Invitrogen (Carlsbad, CA, USA). HRP-conjugated anti-rabbit IgG secondary antibody, MPTP, MPP+ iodide, NGF-2.5S, Hoechst 33342, and propidium iodide (PI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Puerarin and RU486 (mifepristone) were purchased from Yick-Vic Chemicals & Pharmaceuticals (Shatin, Hong Kong).
2.2. Animals and Drug Treatment
The experimental procedures were approved by the Committee on the Use of Live Animal in Teaching and Research (CULATR No. 4046-16), University of Hong Kong. The animal experiments were outlined in Figure 1(b). C57BL/6N male mice (7-8 weeks old, 21-25 g) were provided by the Laboratory Animal Unit on the campus and housed under a controlled temperature (25 ± 2°C) and humidity (55 ± 10%) condition in a 12-12 h light/dark cycle at the animal unit. For drug treatment, puerarin and progesterone were dissolved in saline containing 50% 1,2-propylene glycol, and the solutions were sterilized via 0.22 μm filters from Pall Corporate (Port Washington, NY, USA). Prior to use, RU486 was freshly prepared in saline containing 1% Tween-80 with 30-second sonication, while MPTP was dissolved in saline. Following the regulation of the university safety office, when MPTP was used, mice were isolated in carcinogen suite for additional 3 days after the final treatment. Thereafter, mice were moved to the conventional facility for behavioral tests and bioassays.
2.3. Animal Experimental Design
The doses and delivery of different drugs were decided based on previous publications [9, 18, 25]. Briefly, mice were firstly randomly divided into five groups (n = 10): control, MPTP, MPTP+Puerarin, MPTP+Progesterone, and MPTP+Puerarin+RU486. Mice were treated with MPTP (25 mg/kg/day via intraperitoneal injection for 7 days while animals in the control group were injected the equal volume of saline. At the time point of 4 hours prior to daily MPTP injection, puerarin (120 mg/kg/day) or progesterone (8 mg/kg/day) was administered to the mice in the indicated group via oral gavage, while mice in the control and MPTP groups were injected the same volume of vehicle. For RU486 administration, mice were intraperitoneally injected with RU486 (3 mg/kg/day) or vehicle at 30 min prior to puerarin treatment for 7 days.
2.4. Rotarod Performance Test
Mice were evaluated for motor impairments with a rotarod apparatus (Harvard apparatus, Holliston, MA, USA) as described [26, 27]. In brief, at 3 days after last dosage of MPTP treatment, mice were subjected to behavioral assessments. On the day for behavioral tests, animals were firstly pretrained for 3 sessions prior to the assessment and placed on the lane (constant 15 rpm) for 5 min. The dropping time was automatically recorded for each mouse. Three independent tests were performed with each mouse at 1 h intervals.
2.5. Tail Suspension Test
Tail suspension test was carried out at 4 hours after rotarod test as described [26]. Briefly, mice were suspended by fixing the tail to the pole with adhesive tape. The distance between the mouse's nose and the bottom was 20-25 cm, and the test time was 6 min. Mice were monitored by video during the process, while the results were analyzed by the observers after the behavior tests. The immobility time of each mouse within 6 min was measured for assessing the motor functions.
2.6. Immunohistochemistry Staining
The expression of TH in the midbrain was detected by immunohistochemical staining as previously described [27]. In brief, mice were transcardially perfused sequentially with saline and 4% paraformaldehyde under anesthetic condition. The brains were collected and fixed in 4% paraformaldehyde overnight at 4°C. Following immersed in a 30% sucrose containing 4% paraformaldehyde, the brains were embedded in Tissue-Tek O.C.T. Compound (Sakura, USA) and stored at -80°C. The cryosections were cut into serial coronal sections at a thickness of 30 μm on a freezing microtome (Model CM-1850, Leica, Germany). After thawed at room temperature for 1 hour, the cryostat sections were heated in antigen retrieval buffer, immersed in 3% hydrogen peroxide solution to quench the endogenous peroxidase, and subsequently blocked with 5% goat serum to avoid nonspecific staining. For TH detection, the brain sections were probed with anti-TH primary antibody overnight at 4°C. After excessive wash, the bound antibodies were detected with HRP-conjugated goat anti-rabbit secondary antibody for 1 hour at room temperature. The horseradish peroxidase was then assayed with 3,3-N-diaminobenzidine tetrahydrochloride (DAB) substrate kit from Dako (Carpintera, CA, USA). The slices were counterstained with hematoxylin and imaged under an Olympus microscope (Olympus Corp, Tokyo, Japan). The number of TH+ neurons in each section was counted within three nonoverlapping views at tenfold magnification.
2.7. Western Blotting Analysis
The expression of indicated biomarkers was examined by Western blotting as described [26]. Briefly, midbrain tissues, primary midbrain neurons, and PC12 cells were lysed in RIPA buffer containing 1x protease inhibitor cocktail. The proteins were recovered by centrifugation at 13,000 rpm for 15 min at 4°C. The concentration of proteins was determined with the protein assay dye reagent from Bio-Rad (Hercules, CA, USA). Proteins (70 μg for tissue, 30 μg for cells) were resolved on 10% SDS-polyacrylamide gels and transferred onto PVDF membranes. After 2-hour incubation in 5% BSA in Tris buffer containing 1% Tween-20 (TBS-T), the membranes were incubated with primary antibodies overnight at 4°C and then detected with HRP-conjugated goat anti-rabbit IgG secondary antibodies for another 3 hour at 4°C. The blots were visualized with Amersham™ ECT™ detection reagent from GE Healthcare (Uppsala, Sweden).
2.8. PC12 Cell Culture
Rat pheochromocytoma PC12 cell line was obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% horse serum (HS), 5% fetal bovine serum (FBS), and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) in incubators with 37°C and 5% CO2 atmosphere. For drug treatment, the cells were cultured to the density of 70-80% and subsequently treated with indicated drugs.
2.9. Primary Culture of Rat Midbrain Neurons
Primary rat midbrain neurons were isolated from 17-day-old Sprague-Dawley (SD) rat embryos as previously described [27]. Briefly, 1 day before the isolation of the midbrain neurons, 6-well plates were precoated with poly-D-lysine. The midbrain neurons were dissociated by using trypsin and DNase. After centrifugation, freshly isolated neurons were seeded onto six-well plates at a density of 5 or 8 × 100000 cells/ml and cultured in Neurobasal® medium containing 2% B27 supplement from Thermo Fisher Scientific Inc. (Waltham, MA, USA) for 3 or 7 days prior to drug treatment.
2.10. Hoechst 33342/Propidium Iodide Staining
Freshly isolated midbrain neurons were seeded on 6-well-plate at the density of 1 × 106 cells/well and cultured for 7 days. The neurons were treated with puerarin, progesterone, RU486, and MPP+, alone or in combination for 24 h. To stain the dead cells, 5 μM Hoechst 33342 and 5 μM PI were added to each well. The neurons were visualized under a Zeiss fluorescence microscope from Carl Zeiss Company (Jena, Germany).
2.11. Immunofluorescence Staining
The expression of PR in the brains was examined by the immunofluorescence staining as previously described [5, 26, 28]. Following antigen retrieval, the slides were immersed in 0.5% Triton X-100 in PBS for 30 mins and blocked in 5% goat serum for 2 h. The sections were probed with polyclonal antibody against PR overnight at 4°C, washed with PBS for 3 times, and detected with Alexa Fluor 594-conjugated rabbit IgG secondary antibody. The cell nuclei were stained with 4′-6-diamidino-2-phenylindole (DAPI) for 10 min. After the slides were mounted with coverslips, the images were then acquired under a Zeiss LSM 780 confocal microscopy from Carl-Zeiss Company (Jena, Germany) with 20x objective lens.
On the other hand, GAP43 was detected by immunostaining as previously described [29]. After the drug treatment with puerarin and RU486, alone or in combination for 48 h, the neurons were fixed in 4% paraformaldehyde, incubated in 0.5% Triton X-100 for 30 min to permeabilize the cells, and then blocked in 5% normal goat serum for 2 h at room temperature. The cells were then incubated with anti-GAP43 primary antibodies at 4°C overnight, and the bound antibodies were detected with Alexa Fluor 594 anti-rabbit IgG secondary antibody for 2 h at room temperature. After the cells were washed with PBS for three times, the cell nuclei were then stained with DAPI for 10 min. The fluorescence images were visualized under fluorescence microscope (Carl Zeiss, Jena, Germany). The neurite length of GAP-43+ neurons was automatically detected by ImageJ software (http://imagej.nih.gov).
2.12. siRNA-Mediated Silencing of PR Expression
The expression of PR in PC12 cells was transiently silenced with PR-specific siRNAs as previously described [28]. Briefly, PC12 cells were grown on poly-L-lysine precoated coverslips and then transfected with specific siRNAs using HiPerFect Transfection Reagent according to the manufacturer's protocol. After 24 h incubation, the transfected cells were treated with puerarin and NGF, alone or in combination, for 72 h. The cells were then stained with neurite outgrowth staining kit and analyzed for PR expression by Western blotting with specific antibodies.
2.13. Assay of Neurite Outgrowth
Neurite outgrowth was assessed by commercial neurite outgrowth assay kit as described [18]. In practice, PC12 cells were transfected with specific siRNAs and subsequently treated with puerarin and NGF, alone or in combination, for 72 h. The cells were stained by neurite outgrowth assay kit according to the manufacture's instruction. The neurites were imaged under a Zeiss fluorescence microscope. The average length of neurites was determined by Image J software (https://imagej.nih.gov/ij/).
2.14. Transfection and Luciferase Reporter Assays
The promoter activities of PR were assayed using promoter-reporter system as previously described [30]. Briefly, PC12 cells were seeded in 96-well plates at the density of 1 × 105 cells/well, cultured to the cell density of 70-80% and transiently transfected with 0.1 μg 2xPRE-TK-Luc and 0.05 μg pRL-TK. The transfection was continued for 6 h using Transfectene (Qiagen) according to the manufacturer's instruction. At 24 h after transfection, the cells were treated with puerarin and progesterone for 24 h. The promoter activity was then assayed with the Dual-Luciferase Reporter System according to the manufacturer's protocol while the luciferase activity was monitored on a Clariostar microplate reader from BMG Labtech (Ortenberg, Germany). The results were expressed as luciferase activity after normalization to Renilla activity.
2.15. Molecular Docking
The crystal structure (PDB: 3D90) of human PR was downloaded from RCSB PDB website (http://www.rcsb.org/pdb). The chemical structures of the ligand molecules (i.e., puerarin and progesterone) were generated by the ChemBioDraw Ultra 12.0 software. The protein-ligand interactions were simulated by the AutoDock Vina in PyRx-virtual screen tool package. Prior to docking, all water molecules were removed from PR structure. The docking results were analyzed by the Discovery Studio Visualizer software.
2.16. Statistical Analysis
The results were represented as mean ± SEM for behavioral tests and mean ± SD for other experiments. The statistical significance was determined by one-way analysis of variance (ANOVA) followed by Dunnett's test with GraphPad Prism 6 (La Jolla, CA, USA). A p value of <0.05 was considered as statistically significant.
3. Results
3.1. Puerarin Ameliorated Behavioral Impairments in MPTP-Lesioned Mice
To validate the in vivo neuroprotective activity of puerarin, we firstly employed rotarod performance test and tail suspension test to determine the effects of puerarin on the behavioral deficits in MPTP-treated mice. In practice, the animals were sequentially evaluated for motor disability by rotarod performance test and the stress response by tail suspension test while two tests were performed in a 4-hour interval. As shown in Figure 1(c), MPTP reduced the time for mice to stay on the rod compared with control mice. Both puerarin and progesterone effectively helped mice to ride on the rotarod for longer time compared with untreated MPTP mice. PR inhibitor RU486 diminished the beneficial effects of puerarin in rotarod performance test. As shown in Figure 1(d), on the other hand, tail suspension test also confirmed that MPTP prolonged the immobility time of mice whereas puerarin and progesterone markedly reduced the immobility time of mice. RU486 reversed the beneficial effects of puerarin.
3.2. Puerarin Enhanced the Survival of Dopaminergic Neurons against MPTP Neurotoxicity
To evaluate the in vivo neuroprotective activity of puerarin, dopaminergic neurons was examined by detecting the expression of TH in MPTP-treated mice. As shown in Figures 2(a) and 2(b), MPTP dramatically reduced TH expression as the indicator for TH-positive dopaminergic neurons in SNpc, whereas both puerarin and progesterone effectively halted the loss of dopaminergic neuron. RU486 attenuated the beneficial effects of puerarin. On the other hand, the expression of TH was also assessed by Western blotting with specific antibody. The results in Figures 2(c) and 2(d) essentially verified the neuroprotective effects of puerarin on dopaminergic neurons against MPTP neurotoxicity and the inhibitory effects of RU486 on the activity of puerarin.
Figure 2.
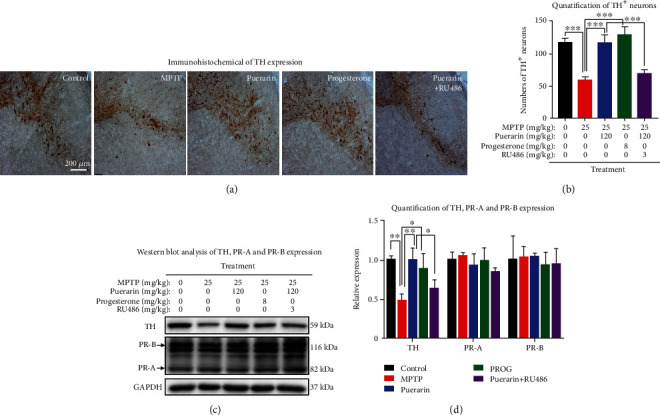
Puerarin protected dopaminergic neurons from MPTP-induced damage in mice. (a) Immunohistochemical staining of TH expression. The frozen brain sections were analyzed by immunohistochemical staining with anti-TH antibody. The images were captured under an Olympus microscope (Tokyo, Japan). (b) Quantification for TH+ neurons. The number of TH+ neurons in each group (n = 3) were counted. ∗∗∗p < 0.001. (c) Western blot analysis of TH, PR-A, and PR-B expression. Midbrain tissues were collected and analyzed by Western blotting with antibodies against TH, PR-A, and PR-B, whereas GAPDH was analyzed as control. Representative blots for each group were shown. (d) Quantification of TH, PR-A, and PR-B expression. Western blots (n = 3) in (c) were determined by a densitometric method. ∗p < 0.05, ∗∗p < 0.01.
3.3. Puerarin Did Not Change the Expression of PR in MPTP-Lesioned Mice
To clarify why RU486 could antagonize puerarin, we employed Western blotting and fluorescence immunostaining techniques to determine the effect of puerarin on the expression of PR in the MPTP-treated mice. Western blotting results in Figures 2(c) and 2(d) did not show much difference in the levels of PR expression between different treatment groups. The immunofluorescence staining results in Figure 3 further confirmed that the levels of PR expression in the brain were not changed under the experimental conditions.
Figure 3.

Immunofluorescence analysis of PR expression. (a) Detection of PR expression. The model of MPTP-induced parkinsonism was treated with puerarin (120 mg/kg/day), progesterone (8 mg/kg/day), or RU486 (3 mg/kg/day), as indicated. Midbrain cryosections were probed with primary anti-PR antibody and visualized with Alexa Fluor 594-conjugated secondary antibody, whereas the cell nuclei were stained with DAPI. The substantia nigra areas were examined under a Zeiss LSM 780 confocal microscopy. Representative images with 20x amplification were shown. Scale bar, 100 μm. (b) Quantitative analysis of PR levels. Fluorescence intensity of PR staining in the midbrain was quantified by a densitometric method and expressed in mean ± SD (n = 3).
3.4. Puerarin Exerted Neuroprotective Properties in Primary Midbrain Neurons
To verify the in vitro neuroprotective activities of puerarin, we employed fluorescence dye PI to detect MPP+-induced cell death in primary rat midbrain neurons. Primary rat midbrain neurons were freshly isolated for each experiment. Following 7-day culture in complete growth medium, the neurons were challenged with MPP+, and the neuronal viability was assessed by PI/Hoechst 33342 staining. As shown in Figures 4(a) and 4(b), 100 μM MPP+ dramatically caused cell death, whereas puerarin (25 μM) and progesterone (10 nM or 100 nM) effectively enhanced the survival of neurons against MPP+-induced neurotoxicity. RU486 effectively prohibited the neuroprotective activity of puerarin and, to a lesser extent, affected the activity of progesterone. On the other hand, the expression of PR in primary neurons was also determined by Western blot analysis. As shown in Figures 4(c) and 4(d), the expression of PR did not vary much between the treatment groups.
Figure 4.
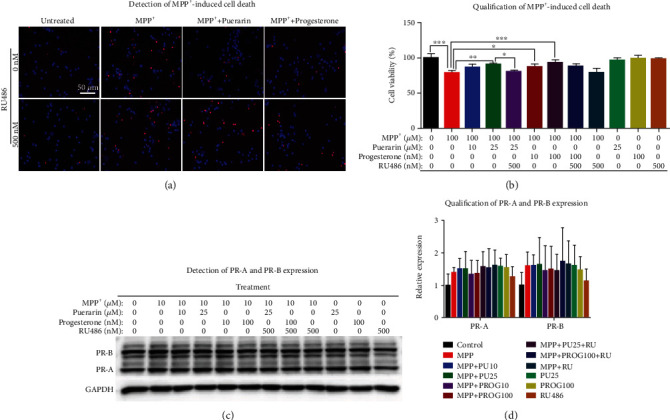
Puerarin enhanced the survival of primary midbrain neurons against MPP+-induced neurotoxicity. (a) Detection of MPP+-induced cell death. Primary midbrain neurons were treated with MPP+, puerarin, progeresterone, and RU486 as indicated. Cell viability was assessed by Hoechst 33342 and PI staining. The representative images were shown. (b) Quantification of neuronal viability. Hoechst- and PI-positive cells were counted under a Zeiss fluorescence microscope (Carl Zeiss, Jena, Germany). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. (c) Western blot analysis of PR-A and PR-B expression. Primary midbrain neurons were treated with MPP+, puerarin, progeresterone, and RU486 as indicated. The expression of PRs was analyzed by Western blot analysis with specific antibodies, while GAPDH was analyzed as the loading control. Representative blot was shown. (d) Quantification of PR-A and PR-B expression. Western blots (n = 3) in (c) were determined by a densitometric method. ∗p < 0.05.
3.5. Puerarin Promoted Neurite Outgrowth via PR Signaling
To further determine whether puerarin exhibits neurotrophic activities in a PR-dependent manner, we evaluated the effects of puerarin on neurite outgrowth in primary midbrain neurons and PC12 cells. As shown in Figures 5(a) and 5(b), puerarin greatly promoted the neurite extension in primary midbrain neurons. RU486 abolished the neurotrophic effect of puerarin so that puerarin did not increase the neurite length in the presence of RU486 relative to the control group.
Figure 5.
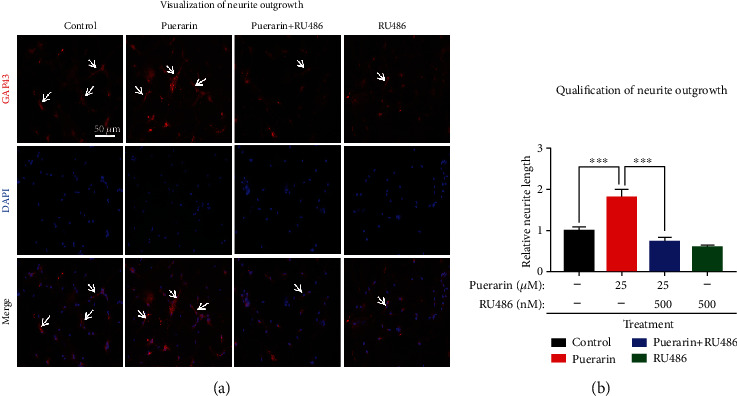
Puerarin promoted neurite outgrowth in primary midbrain neurons. (a) Visualization of neurite outgrowth. Primary midbrain neurons were treated with puearin and RU486 as indicated. Following immunofluorescence staining with anti-GAP43 antibody and DAPI, the cells were examined under a fluorescence microscope from Carl Zeiss (Jena, Germany). Scale bar, 50 μm. (b) Quantification of neurite outgrowth. The neurite length was quantified by using ImageJ (http://imageJ.nih.gov). Neurites from three nonoverlapping fields for each slice were recorded and analyzed for (a). ∗∗∗p < 0.001.
To determine the role of PR in the neurotrophic activities of puerarin, on the other hand, we successfully generated a PR-silenced PC12 cell model by transiently transfecting specific siRNAs for evaluating the effects of puerarin on neurite outgrowth. As shown in Figure 6(a), PR-specific siRNAs effectively downregulated PR expression whereas the negative control siRNAs did not alter PR expression. Upon transfection with PR siRNAs, as shown in Figures 6(b) and 6(c), the transfected cells failed to generate neurites in response to puerarin (10, 25, and 50 μM) whereas PC12 cell transfected with the negative control siRNA group responded to puerarin for neurite outgrowth in a highly similar fashion compared with the untransfected cells.
Figure 6.
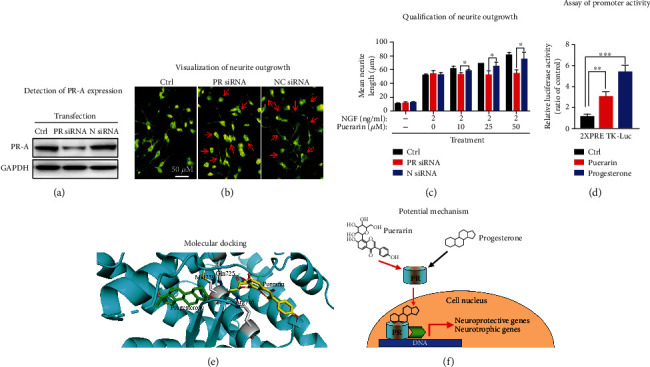
Puerarin promoted neurite outgrowth via PR signaling. (a) siRNA-mediated silencing of PR in PC12 cells. PC12 cells were transiently silenced by PR siRNAs and negative control siRNAs. The expression of PR was determined by Western blot analysis with anti-PR antibody. Representative blots were shown. (b) Visualization of neurite outgrowth. After siRNA transfection, PC12 cells were treated with puerarin and NGF for 72 h. Neurite outgrowth was determined by a commercial neurite staining kit and analyzed under a fluorescence microscope from Carl Zeiss (Jena, Germany). The representative images were shown. Scale bar, 50 μm. (c) Quantification of neurite outgrowth. Neurites from three nonoverlapping fields for each slice were recorded and analyzed. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (d) Luciferase assay for promoter activity. PC12 cells were transiently transfected with 2xPRE-TK-Luc and pRL-TK and cultured for 24 h. The cells were subsequently treated with puerarin and progesterone for 24 h. The promoter activity was assayed with the Dual-Luciferase Reporter System under a Clariostar microplate reader from BMG Labtech (Ortenberg, Germany). Results were expressed as luciferase activity and normalized to Renilla activity for three independent experiments. ∗∗p < 0.01, ∗∗∗p < 0.001. (e) Molecular docking of puerarin and progesterone. Puerarin and progesterone were docked into PR structure (PDB: 3D90) by AutoDock Vina. (f) Potential mechanism. Puerarin may interact with PR and activate the transcriptional factor activity of PR to induce neuroprotective genes and neurotrophic factors.
To clarify whether puerarin could activate PR-mediated transcriptional activity, we introduced the promoter-reporter construct carrying PR-binding DNA sequence into PC12 cells. In the experiments, PC12 cells were transfected with plasmid DNA construct 2xPRE-TK-Luc. As shown in Figure 6(d), puerarin and progesterone markedly increased the PR-mediated transcriptional activity. Based on molecular docking as shown in Figure 6(e), puerarin and progesterone bind to the different sites of PR with the binding energy of -8.2 kcal/mol for puerarin and -10.1 kcal/mol for progesterone, respectively. Puerarin appeared to be a moderate to strong binder, whereas progesterone is a high affinity ligand.
4. Discussion
Early diagnosis and early intervention are equally important to maintain the life quality of PD patients [31, 32]. While the efficacy of the existing clinical drugs is controversial, natural product flavonoids hold promise for halting the progression of the disease and restoring the neuronal functions in PD patients [17]. As a key example, puerarin is extensively evaluated for the neuroprotective effects in different PD models [33–35]. We previously demonstrated several indirect mechanisms underlying the neuroprotective and neurotrophic effects of puerarin [18, 19]. The present study focused on the question of whether puerarin could modulate PR signaling to exhibit neuroprotective and neurotrophic activity.
The progesterone-PR signaling pathway is initially known for its important regulatory role in female reproduction and the link to the progression of breast cancer [13]. Meanwhile, a few earlier studies reported that endogenous progesterone and PR could exhibit cerebroprotection in stroke [36]. Progesterone is now known as an important neurosteroid for its capacity of protecting neurons and promoting neuronal repair in PD and traumatic brain injury [9, 37]. The neuroprotective potential of progesterone was validated to be PR-dependent in MPTP-induced PD models [4, 38]. These results highlight that PR signaling is a potential therapeutic target for the treatment of neurodegeneration [14]. Progesterone induces genomic and nongenomic signals through classical intracellular PRs, the seven transmembrane domain 7TMPRb, and the membrane-associated PR component 1 (PGRMC1) [39]. Progesterone directly interacts with PR and promotes the translocation of PR into cell nuclei, whereas PR functions as a transcriptional factor to regulate the expression of genes for various functions including neuroprotection and neuronal restoration [40].
On the other hand, puerarin is classically tested for its estrogenic activity in the animal models [41]. In the present study, we found that puerarin (120 mg/kg) could reduce MPTP-induced motor dysfunctions to the similar extent to progesterone (8 mg/kg) (Figures 1(c) and 1(d)). RU486 effectively antagonized the beneficial effect of puerarin on MPTP-induced behavioral impairments. Based on the immunohistochemical analysis for TH expression, puerarin (120 mg/kg) and progesterone (8 mg/kg) enhanced the survival of dopaminergic neurons against MPTP-induced neurotoxicity to a similar extent. Again, RU486 abolished the neuropotective effect of puerarin (Figures 2(a) and 2(b)). The assay of neuronal viability also confirmed the neuroprotective activity of puerarin and the antagonizing effect of RU486 (Figures 4(a) and 4(b)). Furthermore, puerarin effectively promoted neurite growth in primary rat midbrain neurons, whereas RU486 abolished the neurotrophic activity of puerarin. Importantly, puerarin did not alter the in vivo and in vitro expression of PR (Figures 2(c), 2(d), 3, 4(c), and 4(d)). Taken together, these results suggested that puerarin might be able to interact with PR or some signaling molecules in PR signaling pathway. In the present study, therefore, we examined the role of PR in the beneficial effects of puerarin. Our strategy was to transiently silence PR-A expression in dopaminergic PC12 cells by specific siRNAs and examine the neurite outgrowth in response to puerarin. Indeed, puerarin failed to induce neurite outgrowth in PR siRNA-transfected cells (Figures 6(a)–6(c)). These results suggest that PR is essential for puerarin to exhibit neurotrophic activity, suggesting that puerarin could potentiate neurite outgrowth via PR signaling. To clarify the dependence of PR, we employed the promoter-reporter system to assay the effects of puerarin on the transcriptional activities of PR. As a result, puerarin markedly induced the transcriptional activity of PR, whereas progesterone specifically induced the transcriptional activity of PR (Figure 6(d)). Moreover, the molecular docking software AutoDock Vina was employed to simulate the binding of the puerarin and progesterone to human PR structure (PDB: 3D90). As shown in Figure 6(e), puerarin and progesterone appeared to bind to the different sites of PR while the residues such as Gln725, Met759, and Arg766 might contribute to the binding. Puerarin and progesterone bind to PR at the binding energy of -8.2 kcal/mol and -10.1 kcal/mol, respectively. Collectively, these results suggested that puerarin attenuated MPTP- and MPP+-induced neurotoxicity and potentiated neurite outgrowth via PR signaling.
5. Conclusion
The present study demonstrated that puerarin ameliorated MPTP- or MPP+-induced neurotoxicity and promoted neurite outgrowth in mouse PD model and cultured primary rat midbrain neurons via PR-dependent mechanism. Our results for the first time suggest that puerarin may mimic progesterone to interact with PR at the cell surface, cytosol, or cell nucleus although the involvement of PGRMC1 is not excluded. Ultimately, puerarin could exhibit neuroprotective and neurotrophic activities via PR signaling (Figure 6(f)). We believe that puerarin may serve as lead compounds for the development of novel PR agonists to treat PD and other neurodegenerative diseases.
Acknowledgments
The authors are grateful to Mr. Wong Hei Kiu, Ms. Lee Wai Sin, and Mr. Shek Chun Shum for their technical assistance. This work was supported by General Research Fund (GRF) grants (17120915, 17146216, 17100317, and 17119619) from the Research Grants Council of Hong Kong, National Natural Science Foundation of China (Nos. 81703726 and 21778046), Health and Medical Research Fund (15161731, 16171751, and 17181231), Science, Technology and Innovation Commission of Shenzhen Municipality (Basic Research Program, Free Exploration Project JCYJ20180306173835901), Research and Cultivation Plan of High-Level Hospital Construction (HKUSZH201902040), Midstream Research Programme for Universities (MRP) (053/18X), and the Seed Funding (201611159156) for Basic Research Programme from University of Hong Kong.
Data Availability
The data were available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there is no competing interest.
Authors' Contributions
Yingke Zhao, Jia Zhao, Xiuying Zhang, Yuanyuan Cheng, and Dan Luo performed the experiments. Simon Ming-Yuen Lee provided the instrument and reagents. Lixing Lao supervised the data analysis. Jianhui Rong designed the research and revised the article. Yingke Zhao and Jia Zhao contributed equally to this work.
Supplementary Materials
Figure S1: simplification of Figure 4. Table S1: list of antibodies.
References
- 1.Feigin V. L., Abajobir A. A., Abate K. H., et al. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Neurology. 2017;16(11):877–897. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Przedborski S. The two-century journey of Parkinson disease research. Nature Reviews Neuroscience. 2017;18(4):251–259. doi: 10.1038/nrn.2017.25. [DOI] [PubMed] [Google Scholar]
- 3.Kataoka H., Ueno S. Can postural abnormality really respond to levodopa in Parkinson's disease? Journal of the Neurological Sciences. 2017;377:179–184. doi: 10.1016/j.jns.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Bourque M., Morissette M., Di Paolo T. Repurposing sex steroids and related drugs as potential treatment for Parkinson's disease. Neuropharmacology. 2019;147:37–54. doi: 10.1016/j.neuropharm.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Tenkorang M. A., Snyder B., Cunningham R. L. Sex-related differences in oxidative stress and neurodegeneration. Steroids. 2018;133:21–27. doi: 10.1016/j.steroids.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siani F., Greco R., Levandis G., et al. Influence of estrogen modulation on glia activation in a murine model of Parkinson's disease. Frontiers in Neuroscience. 2017;11:p. 306. doi: 10.3389/fnins.2017.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi H., Bao X., Tang X., Fan X., Xu H. Estrogen modulation of calretinin and BDNF expression in midbrain dopaminergic neurons of ovariectomised mice. Journal of Chemical Neuroanatomy. 2016;77:60–67. doi: 10.1016/j.jchemneu.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Pomata P. E., Colman-Lerner A. A., Baranao J. L., Fiszman M. L. In vivo evidences of early neurosteroid synthesis in the developing rat central nervous system and placenta. Developmental Brain Research. 2000;120(1):83–86. doi: 10.1016/S0165-3806(99)00181-9. [DOI] [PubMed] [Google Scholar]
- 9.Bourque M., Morissette M., al Sweidi S., Caruso D., Melcangi R. C., di Paolo T. Neuroprotective effect of progesterone in MPTP-treated male mice. Neuroendocrinology. 2016;103(3-4):300–314. doi: 10.1159/000438789. [DOI] [PubMed] [Google Scholar]
- 10.Casas S., Giuliani F., Cremaschi F., Yunes R., Cabrera R. Neuromodulatory effect of progesterone on the dopaminergic, glutamatergic, and GABAergic activities in a male rat model of Parkinson's disease. Neurological Research. 2013;35(7):719–725. doi: 10.1179/1743132812Y.0000000142. [DOI] [PubMed] [Google Scholar]
- 11.Rudolph L. M., Cornil C. A., Mittelman-Smith M. A., et al. Actions of steroids: new neurotransmitters. The Journal of Neuroscience. 2016;36(45):11449–11458. doi: 10.1523/JNEUROSCI.2473-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schumacher M., Mattern C., Ghoumari A., et al. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Progress in Neurobiology. 2014;113:6–39. doi: 10.1016/j.pneurobio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Grimm S. L., Hartig S. M., Edwards D. P. Progesterone receptor signaling mechanisms. Journal of Molecular Biology. 2016;428(19):3831–3849. doi: 10.1016/j.jmb.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 14.de Nicola A. F., Gonzalez Deniselle M. C., Garay L., et al. Progesterone protective effects in neurodegeneration and neuroinflammation. Journal of Neuroendocrinology. 2013;25(11):1095–1103. doi: 10.1111/jne.12043. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava P., Yadav R. S. Efficacy of natural compounds in neurodegenerative disorders. Advances in Neurobiology. 2016;12:p. 107. doi: 10.1007/978-3-319-28383-8_7. [DOI] [PubMed] [Google Scholar]
- 16.Solanki I., Parihar P., Parihar M. S. Neurodegenerative diseases: from available treatments to prospective herbal therapy. Neurochemistry International. 2016;95:100–108. doi: 10.1016/j.neuint.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J., Zhu M., Kumar M., et al. A pharmacological appraisal of neuroprotective and neurorestorative flavonoids against neurodegenerative diseases. CNS & Neurological Disorders Drug Targets. 2019;18(2):103–114. doi: 10.2174/1871527317666181105093834. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J., Cheng Y. Y., Fan W., et al. Botanical drug puerarin coordinates with nerve growth factor in the regulation of neuronal survival and neuritogenesis via activating ERK1/2 and PI3K/Akt signaling pathways in the neurite extension process. CNS Neuroscience & Therapeutics. 2015;21(1):61–70. doi: 10.1111/cns.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J., Cheng Y., Yang C., et al. Botanical drug puerarin attenuates 6-hydroxydopamine (6-OHDA)-induced neurotoxicity via upregulating mitochondrial enzyme arginase-2. Molecular Neurobiology. 2016;53(4):2200–2211. doi: 10.1007/s12035-015-9195-1. [DOI] [PubMed] [Google Scholar]
- 20.Li L., Xue Z., Chen L., Chen X., Wang H., Wang X. Puerarin suppression of Aβ 1–42 -induced primary cortical neuron death is largely dependent on ERβ. Brain Research. 2017;1657:87–94. doi: 10.1016/j.brainres.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y. F., Zhu G. Q., Wu Q. W., et al. GPR30 activation contributes to the puerarin-mediated neuroprotection in MPP+-induced SH-SY5Y cell death. Journal of Molecular Neuroscience. 2017;61(2):227–234. doi: 10.1007/s12031-016-0856-y. [DOI] [PubMed] [Google Scholar]
- 22.Qiu Z. K., Zhang G. H., Zhong D. S., et al. Puerarin ameliorated the behavioral deficits induced by chronic stress in rats. Scientific Reports. 2017;7(1, article 6266) doi: 10.1038/s41598-017-06552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su A. S., Zhang J. W., Zou J. The anxiolytic-like effects of puerarin on an animal model of PTSD. Biomedicine & Pharmacotherapy. 2019;115, article 108978 doi: 10.1016/j.biopha.2019.108978. [DOI] [PubMed] [Google Scholar]
- 24.Saha P., Saraswat G., Chakraborty P., Banerjee S., Pal B. C., Kabir S. N. Puerarin, a selective oestrogen receptor modulator, disrupts pregnancy in rats at pre-implantation stage. Reproduction. 2012;144(5):633–645. doi: 10.1530/REP-11-0423. [DOI] [PubMed] [Google Scholar]
- 25.Wulsin A. C., Herman J. P., Danzer S. C. RU486 mitigates hippocampal pathology following status epilepticus. Frontiers in Neurology. 2016;7 doi: 10.3389/fneur.2016.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y., Luo D., Ning Z., Rong J., Lao L. Electro-acupuncture ameliorated MPTP-induced parkinsonism in mice via TrkB neurotrophic signaling. Frontiers in Neuroscience. 2019;13:p. 496. doi: 10.3389/fnins.2019.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo D., Zhao J., Cheng Y. Y., Lee S. M. Y., Rong J. H. N-propargyl caffeamide (PACA) ameliorates dopaminergic neuronal loss and motor dysfunctions in MPTP mouse model of Parkinson's disease and in MPP+-induced neurons via promoting the conversion of proNGF to NGF. Molecular Neurobiology. 2018;55(3):2258–2267. doi: 10.1007/s12035-017-0486-6. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y., Yang C., Zhao J., Tse H. F., Rong J. Proteomic identification of calcium-binding chaperone calreticulin as a potential mediator for the neuroprotective and neuritogenic activities of fruit-derived glycoside amygdalin. The Journal of Nutritional Biochemistry. 2015;26(2):146–154. doi: 10.1016/j.jnutbio.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Luo D., Guo Y., Cheng Y., Zhao J., Wang Y., Rong J. Natural product celastrol suppressed macrophage M1 polarization against inflammation in diet-induced obese mice via regulating Nrf2/HO-1, MAP kinase and NF-κB pathways. Aging. 2017;9(10):2069–2082. doi: 10.18632/aging.101302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purandare N., Somayajulu M., Huttemann M., Grossman L. I., Aras S. The cellular stress proteins CHCHD10 and MNRR1 (CHCHD2): partners in mitochondrial and nuclear function and dysfunction. Journal of Biological Chemistry. 2018;293(17):6517–6529. doi: 10.1074/jbc.RA117.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalia L. V., Lang A. E. Parkinson's disease. The Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 32.Burbulla L. F., Jeon S., Zheng J., Song P., Silverman R. B., Krainc D. A modulator of wild-type glucocerebrosidase improves pathogenic phenotypes in dopaminergic neuronal models of Parkinson's disease. Science Translational Medicine. 2019;11(514, article eaau6870) doi: 10.1126/scitranslmed.aau6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang M., Yun Q., Niu G., Gao Y., Shi F., Yu S. Puerarin prevents inflammation and apoptosis in the neurocytes of a murine Parkinson's disease model. Genetics and Molecular Research. 2016;15(4) doi: 10.4238/gmr.15047501. [DOI] [PubMed] [Google Scholar]
- 34.Li R., Liang T., Xu L., Zheng N., Zhang K., Duan X. Puerarin attenuates neuronal degeneration in the substantia nigra of 6-OHDA-lesioned rats through regulating BDNF expression and activating the Nrf2/ARE signaling pathway. Brain Research. 2013;1523:1–9. doi: 10.1016/j.brainres.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 35.Zhu G., Wang X., Wu S., Li X., Li Q. Neuroprotective effects of puerarin on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's disease model in mice. Phytotherapy Research. 2014;28(2):179–186. doi: 10.1002/ptr.4975. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X., Frechou M., Liere P., et al. A role of endogenous progesterone in stroke cerebroprotection revealed by the neural-specific deletion of its intracellular receptors. The Journal of Neuroscience. 2017;37(45):10998–11020. doi: 10.1523/JNEUROSCI.3874-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei J., Xiao G. M. The neuroprotective effects of progesterone on traumatic brain injury: current status and future prospects. Acta Pharmacologica Sinica. 2013;34(12):1485–1490. doi: 10.1038/aps.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brotfain E., Gruenbaum S. E., Boyko M., Kutz R., Zlotnik A., Klein M. Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Current Neuropharmacology. 2016;14(6):641–653. doi: 10.2174/1570159X14666160309123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg D., Ng S. S. M., Baig K. M., Driggers P., Segars J. Progesterone-mediated non-classical signaling. Trends in Endocrinology and Metabolism. 2017;28(9):656–668. doi: 10.1016/j.tem.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez S. L., Coronel M. F., Raggio M. C., Labombarda F. Progesterone receptor-mediated actions and the treatment of central nervous system disorders: an up-date of the known and the challenge of the unknown. Steroids. 2019;153, article 108525 doi: 10.1016/j.steroids.2019.108525. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y. X., Zhang H., Peng C. Puerarin: a review of pharmacological effects. Phytotherapy Research. 2014;28(7):961–975. doi: 10.1002/ptr.5083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: simplification of Figure 4. Table S1: list of antibodies.
Data Availability Statement
The data were available from the corresponding author upon request.


