Abstract
Background
Allergic rhinitis (AR) is a type I hypersensitivity mediated by IgE in the nose. Thioredoxin-interacting protein (TXNIP) plays a pivotal role in the process of producing reactive oxygen species (ROS). Resveratrol is a TXNIP inhibitor. Nonetheless, its role and mechanism in AR are still undetermined. The present study aimed to explore the effect and mechanism of resveratrol on an ovalbumin (OVA) induced mouse model of AR.
Methods
AR murine model was established using OVA and administrated intranasally with resveratrol or N-acetylcysteine (NAC). Hematoxylin and eosin (HE) stain was used for evaluating eosinophils. Immunohistochemistry (IHC) staining and real-time PCR were employed to evaluate immunolabeling and mRNA expression of TXNIP in nasal mucosas of mice. Malondialdehyde (MDA) level and superoxide dismutase (SOD) activity in nasal tissue homogenates were measured using MDA and SOD Assay Kit. Concentrations of OVA-specific IgE and histamines in serum, and OVA-specific IgE, PGD2, LTC4, ECP, IL-4, IL-5, IL-6, IL-33 and TNF-α in nasal lavage fluid (NLF) were assayed by ELISA. In vitro studies, western blotting, real-time PCR, ELISA, ROS detecting dye DCFH-DA, MDA, and SOD Assay Kit were performed to evaluate the effects and mechanisms of OVA, resveratrol or NAC on spleen mononuclear cells.
Results
We found significant alternations of sneezing, nasal rubbing, inflammatory cytokines, eosinophil numbers, TXNIP, MDA, and SOD levels in resveratrol or NAC treated mice compared with untreated AR mice. In cultured spleen mononuclear cells, TXNIP, MDA, SOD, ROS and inflammatory cytokines levels were altered by OVA but reversed by resveratrol or NAC.
Conclusions
Resveratrol could effectively alleviate murine AR by inhibiting TXNIP-oxidative stress pathway.
Keywords: Allergic rhinitis, TXNIP, Oxidative stress, Ovalbumin, Reactive oxygen species, Resveratrol
Abbreviations: AR, Allergic rhinitis; TXNIP, Thioredoxin-interacting protein; ROS, Reactive oxygen species; OVA, Ovalbumin; NAC, N-acetylcysteine; IHC, Immunohistochemistry; ELISA, Enzyme-linked immunosorbent assay; MDA, Malondialdehyde; SOD, Superoxide dismutase; NLF, Nasal lavage fluid; Th2, Type 2T helper
Introduction
Allergic rhinitis (AR) is a type I hypersensitivity mediated by IgE in the nose characterised by sneezing, rhinorrhea, and nasal congestion, which increases patients’ economic burden and negatively affects their quality of life.1, 2, 3 Accumulating evidence has shown that AR is a Type 2 T helper (Th2) immune response-mediated nasal allergic and inflammatory disease triggered by allergens in the air,4,5 and antihistamines and corticosteroids are effective in the treatment of AR.6 Whereas, the precise pathogenesis of AR remains undetermined, and some AR patients remain insensitive to antihistamines and corticosteroids.7,8 Thus, a novel drug with better effect on AR is urgently needed to be explored.
Considerable data indicate that excess reactive oxygen species (ROS) accumulation and oxidative damage play crucial roles in the process of allergic and inflammatory response.9,10 Thioredoxin-interacting protein (TXNIP) is a multi-functional protein mediating redox homeostasis by increasing ROS production and inducing oxidative stress.11
Resveratrol (trans-3,5,4′-trihyroxystilbene), an inhibitor of TXNIP,12 is a stilbenoid belonging to the polyphenols, and the sources of resveratrol in food comprise grapes, berries, and peanuts.13,14 Resveratrol can exert antioxidant, anti-inflammatory, antiproliferative, and angioregulatory effects in a variety of diseases.15 Resveratrol could inhibit ROS production and reduce oxidative stress, resulting in the alleviation of inflammatory damage and apoptosis induced by ischemia/reperfusion injury in the brains of rats.16
The present study aimed to explore the effect of resveratrol in an ovalbumin (OVA) induced mouse model of AR. First, AR murine model was established using OVA and administrated intranasally with resveratrol or N-acetylcysteine (NAC, an ROS scavenger). Immunohistochemistry (IHC) staining and real-time PCR were employed to evaluate immunolabeling, and mRNA levels of TXNIP in nasal mucosas of mice. Concentrations of OVA-specific IgE and histamines in serum, and OVA-specific IgE, PGD2, LTC4, ECP, IL-4, IL-5, IL-6, IL-33, and TNF-α in nasal lavage fluid (NLF) were assayed by enzyme-linked immunosorbent assay (ELISA). Second, in vitro studies, western blotting, flow cytometry, real-time PCR, and ELISA were performed to evaluate the effects and mechanisms of OVA and resveratrol on spleen mononuclear cells. We found significant alternations of sneezing, nasal rubbing, inflammatory cytokines, TXNIP, MDA, and SOD levels in resveratrol or NAC treated mice compared with untreated AR mice. In cultured spleen mononuclear cells, significant alternations of TXNIP, MDA, SOD, ROS, and inflammatory cytokines levels were induced by OVA but reversed by resveratrol or NAC.
Materials and methods
Animals
A total of 50 female BALB/c mice (6–8 weeks of age) were included and kept in a specific-pathogen free facility. The mice were divided into 5 groups (n = 10 per group): normal control group, untreated AR group, 200 μg resveratrol treated AR group, 400 μg resveratrol treated AR group, and 100 μg NAC treated AR group.
AR mouse model and treatment
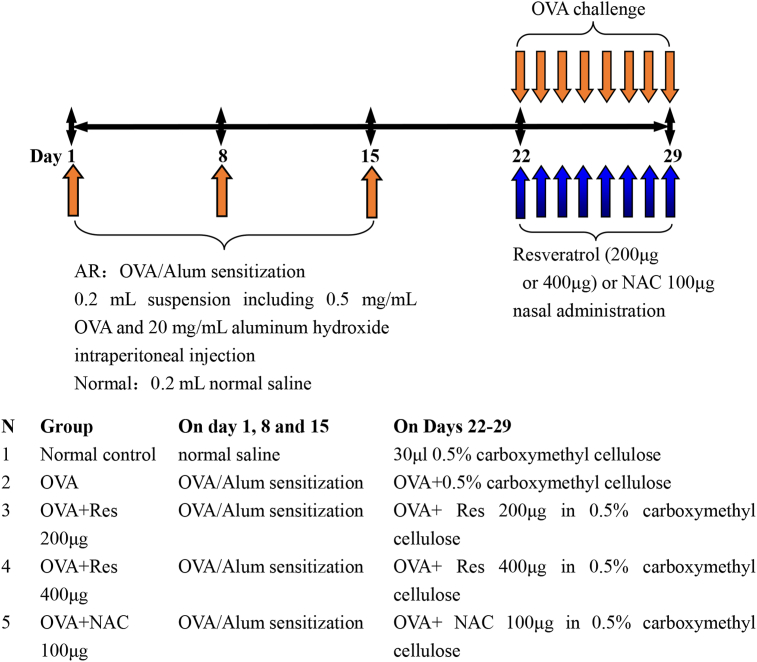
The murine AR model was induced by ovalbumin (OVA) as previously described.17,18 A schematic diagram of AR mouse model establishment and resveratrol or NAC treatment protocol is depicted in Fig. 1. First, to establish AR murine model, mice were sensitized with 0.2 mL suspension including 0.5 mg/mL ovalbumin (OVA, Sigma-Aldrich) and 20 mg/mL aluminum hydroxide (Sinopharm Chemical Reagent Co) by intraperitoneal injection on day 1, 8, and 15, respectively. Furthermore, normal control mice were intraperitoneally administered with 0.2 mL normal saline on day 1, 8, and 15, respectively. On days 22–29, the mice in all AR groups were challenged daily with 20 μl OVA (40 mg/mL) by intranasal instillation; then, normal control mice and untreated AR mice were administrated with 30 μl vehicle of 0.5% carboxymethyl cellulose solution. In addition, on days 22–29, resveratrol treated AR mice were instilled intranasally with resveratrol (200 μg or 400 μg, Sigma-Aldrich) dissolved in 30 μl 0.5% carboxymethyl cellulose solution on days 22–29. Moreover, during this period, NAC treated AR mice were instilled intranasally with NAC (100 μg) dissolved in 30 μl 0.5% carboxymethyl cellulose solution.
Fig. 1.
Schematic diagram demonstrating the process of AR mouse model establishment and resveratrol treatment
Mice nasal symptoms evaluation and sample preparation
Mice nasal symptoms including frequency of sneezes and nasal rubbing were evaluated for 10 min on the 29th day, immediately following the final OVA provocation. After 24 h, the mice were killed, and the blood and NLF samples were collected for ELISA. Nasal mucosas were collected and assayed by IHC staining and real-time PCR methods. More detailed protocols can be found in the Online Supplementary Material.
ELISA assay in the serum and NLF
Serum OVA-specific IgE and histamines levels and concentrations of OVA-specific IgE, PGD2, LTC4, ECP, IL-4, IL-5, IL-6, IL-33 and TNF-α in NLF were assayed with specific ELISA kits. Each sample was analyzed in triplicate. More detailed protocols can be found in the Online Supplementary Material.
HE and IHC staining
Nasal mucosas of mice were paraffin-embedded, serially sectioned (4 μm). Following deparaffinization and rehydration, HE staining was used for evaluating eosinophils counts and IHC staining was employed to evaluate TXNIP immunolabeling. More detailed protocols can be found in the Online Supplementary Material.
Quantitative real-time reverse transcriptions PCR
Quantitative real-time PCR was employed to assess TXNIP expression in nasal mucosas of mice. Total RNA in the collected tissues was extracted utilizing RNeasy commercial kit (Qiagen, Chatsworth, CA, USA). More detailed protocols can be found in the Online Supplementary Material.
Measurement of MDA, and SOD in nasal tissue homogenates
MDA level and SOD activity in nasal tissue homogenates were detected using MDA Assay Kit and SOD Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively, according to the manufacturer's protocol. Additional information can be found in this article's Online Supplementary Material.
Spleen mononuclear cells culture and stimulation
Spleen mononuclear cells were isolated from 6 untreated AR mice and cultured as previously described.19 Briefly, spleen mononuclear cells were stimulated with control PBS, OVA (10 μg/mL) alone or with resveratrol (10 μM or 20 μM) or NAC (10 μM) for 24 h. Following stimulation, the cells and supernatants were collected for western blotting, real-time PCR, flow cytometry, and ELISA. More detailed protocols can be found in the Online Supporting Information.
Western blotting
TXNIP protein levels in the spleen mononuclear cells were assayed with western blotting methods. Additional information can be found in this article's Online Supplementary Material.
Quantitative real-time reverse transcriptions PCR
TXNIP mRNA levels in the spleen mononuclear cells were assayed with quantitative real-time PCR methods. Additional information can be found in this article's Online Supplementary Material.
Measurement of MDA, and SOD in spleen mononuclear cells
Following exposure, MDA level and SOD activity in spleen mononuclear cells were detected using MDA Assay Kit and SOD Assay Kit (Nanjing Jiancheng Bioengineering Institute), respectively, according to the manufacturer's protocol. Additional information can be found in this article's Online Supplementary Material.
Intracellular reactive oxygen species (ROS) assay
Following stimulation, intracellular ROS in spleen mononuclear cells was assayed using DCFH-DA (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer's protocol. Additional information can be found in this article's Online Supplementary Material.
ELISA assay in the supernatants
Briefly, following treatment, the supernatants were collected for assaying IL-4, IL-5, IL-6, IL-33, and TNF-α protein levels using ELISA methods. Additional information can be found in this article's Online Supplementary Material.
Statistical analysis
Values were expressed as mean ± standard error of mean (SEM). A P value of less than 0.05 was considered statistically significant. One-way ANOVA with Bonferroni post hoc test was employed for intergroup comparison. Correlations were assessed by Spearman's test. Statistical analyses were performed with SPSS Software (Version 22.0, Chicago, IL, USA) and GraphPad Prism 7 software (GraphPad Software, San Diego, Calif, USA).
Results
Effects of resveratrol on nasal symptoms in AR mice
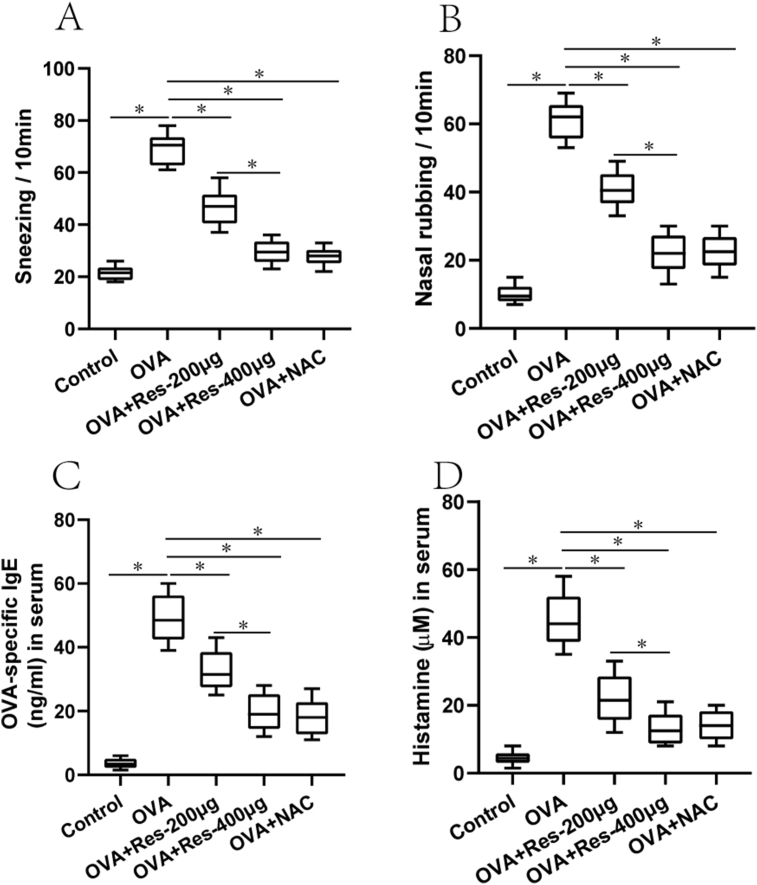
Notably, significant upregulation of frequencies of sneezes and nasal rubbing were found in the untreated AR group compared with the normal control group (Fig. 2A and B), and these effects were attenuated in the resveratrol (200 μg or 400 μg) treated AR group and NAC treated AR group compared with the untreated AR group (Fig. 2A and B). In addition, the resveratrol (400 μg) treated AR group exhibited significant downregulation of frequencies of sneezes and nasal rubbing in comparison to the resveratrol treated AR (200 μg) group (Fig. 2A and B).
Fig. 2.
(A–D) Sneezes, nasal rubbing and OVA-specific IgE and histamines levels in the serum from normal control group (n = 10), untreated AR group (OVA, n = 10), 200 μg resveratrol treated AR group (OVA + Res-200μg, n = 10), 400 μg resveratrol treated AR group (OVA + Res-400μg, n = 10) and NAC treated AR group (OVA + NAC-100μg, n = 10). ﹡P < 0.05. One-way ANOVA with Bonferroni post hoc test was employed for intergroup comparison. Each experiment was repeated three times at short time intervals
Effects of resveratrol on OVA-specific IgE and cytokines levels in the serum and NLF
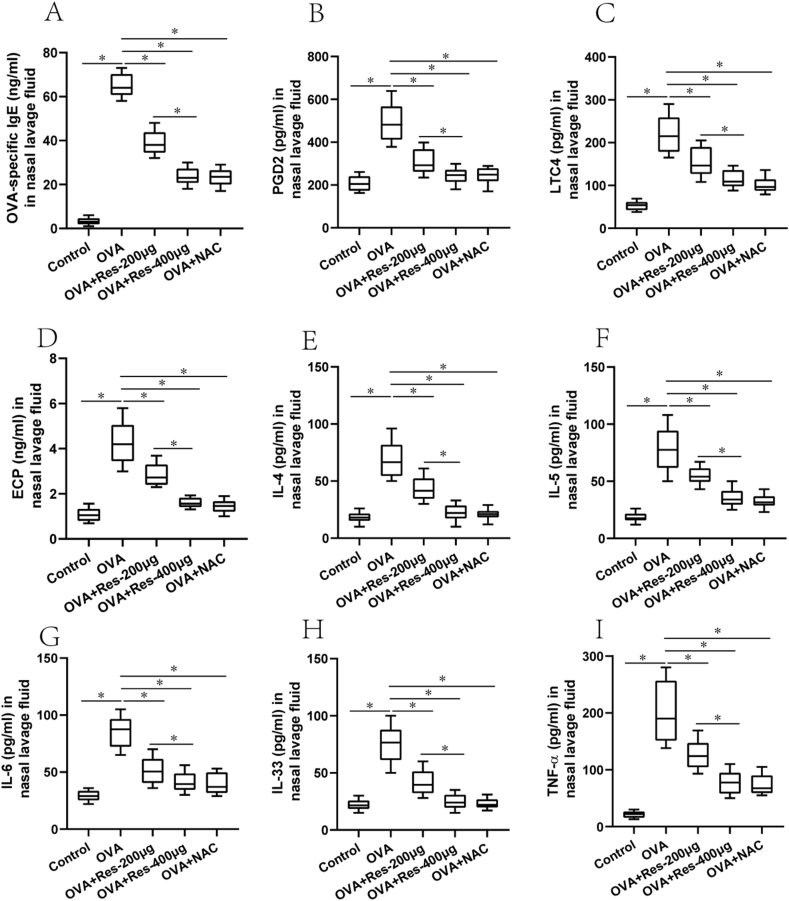
Consistent with above findings, OVA-specific IgE and histamines levels in the serum, and OVA-specific IgE, PGD2, LTC4, ECP, IL-4, IL-5, IL-6, IL-33, and TNF-α levels in the NLF from the untreated AR group were significantly elevated compared with the normal control group (Fig. 2C and D, and Fig. 3A–I). Furthermore, OVA-specific IgE and cytokine levels in the serum and NLF were diminished in the resveratrol (200 μg or 400 μg) treated AR group and NAC treated AR group compared with the untreated AR group (Fig. 2C and D, and Fig. 3A–I). In addition, these effects were more obvious in the resveratrol (400 μg) treated AR group in comparison to the resveratrol (200 μg) treated AR group (Fig. 2C and D, and Fig. 3A–I).
Fig. 3.
(A–I) OVA-specific IgE, PGD2, LTC4, ECP, IL-4, IL-5, IL-6, IL-33 and TNF-α levels in the NLF from normal control group (n = 10), untreated AR group (OVA, n = 10), 200 μg resveratrol treated AR group (OVA + Res-200μg, n = 10), 400 μg resveratrol treated AR group (OVA + Res-400μg, n = 10) and NAC treated AR group (OVA + NAC-100μg, n = 10).﹡P < 0.05. One-way ANOVA with Bonferroni post hoc test was employed for intergroup comparison. Each experiment was repeated three times at short time intervals
Effects of resveratrol on eosinophil numbers in nasal tissues from AR mice
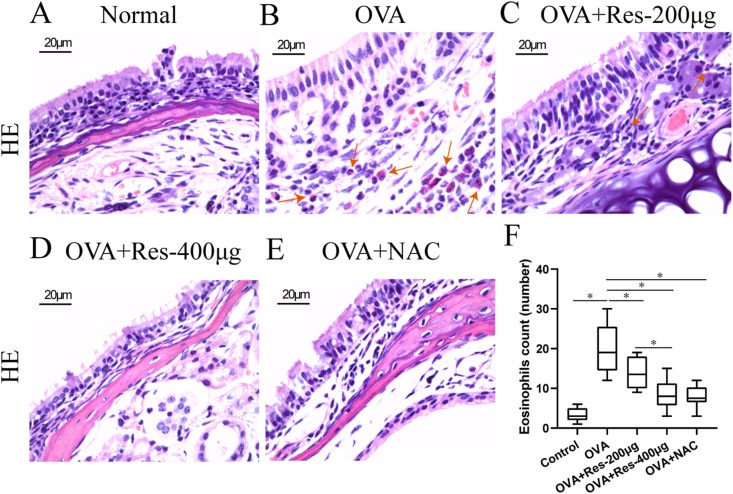
Notably, eosinophil numbers were significantly elevated in the untreated AR group compared with the normal control group and resveratrol (200 μg or 400 μg) or NAC treatment significantly diminished the effects in comparison to the untreated AR group (Fig. 4A–F). Of note, these effects were more obvious in resveratrol (400 μg) treated AR group in comparison to the resveratrol (200 μg) treated AR group (Fig. 4A–F).
Fig. 4.
(A–F) HE staining for eosinophils in nasal tissues from normal control group (n = 10), untreated AR group (OVA, n = 10), 200 μg resveratrol treated AR group (OVA + Res-200μg, n = 10), 400 μg resveratrol treated AR group (OVA + Res-400μg, n = 10) and NAC treated AR group (OVA + NAC-100μg, n = 10). Red arrows indicate eosinophils. Scale bar = 20 μm ﹡P < 0.05. One-way ANOVA with Bonferroni post hoc test was employed for intergroup comparison. Each experiment was repeated three times at short time intervals.
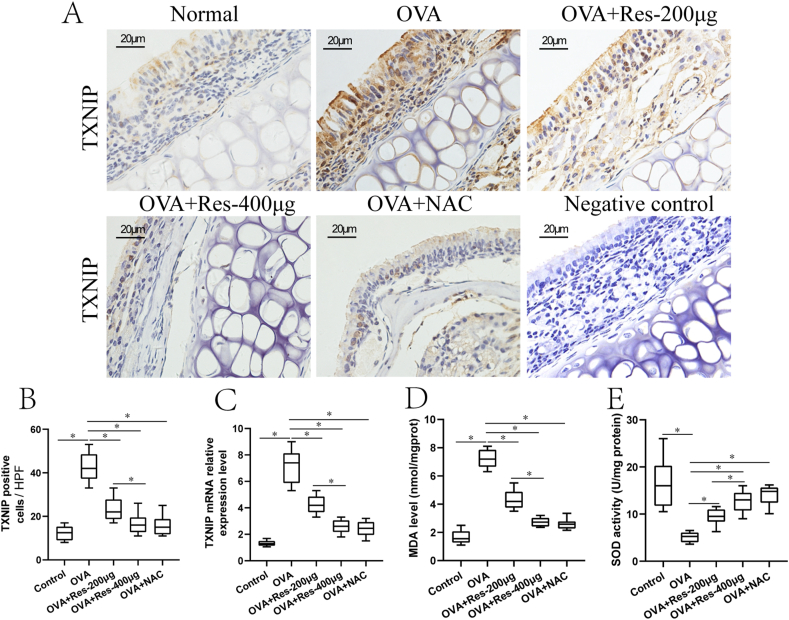
Effects of resveratrol on TXNIP immunolabeling and mRNA in nasal tissues from AR mice
Notably, TXNIP positive cells were mainly epithelial cells and inflammatory cells in the nasal mucosa (Fig. 5A). TXNIP positive cells were significantly elevated in the untreated AR group compared with the normal control group and resveratrol (200 μg or 400 μg) or NAC treatment significantly diminished the effects in comparison to the untreated AR group (Fig. 5A and B). Of note, these effects were more obvious in the resveratrol (400 μg) treated AR group in comparison to the resveratrol (200 μg) treated AR group (Fig. 5A and B).
Fig. 5.
(A–E) Immunolabeling and mRNA expression of TXNIP, MDA levels and SOD activities in nasal tissues from normal control group (n = 10), untreated AR group (OVA, n = 10), 200 μg resveratrol treated AR group (OVA + Res-200μg, n = 10), 400 μg resveratrol treated AR group (OVA + Res-400μg, n = 10) and NAC treated AR group (OVA + NAC-100μg, n = 10). Scale bar = 20 μm ﹡P < 0.05. One-way ANOVA with Bonferroni post hoc test was employed for intergroup comparison. Each experiment was repeated three times at short time intervals
As indicated in Fig. 5C, TXNIP mRNA levels were significantly increased in nasal tissues from the untreated AR group compared with the normal control group and TXNIP mRNA levels were diminished in the resveratrol (200 μg or 400 μg) or NAC treated group compared with the untreated AR group. Of note, these effects were more obvious in the resveratrol (400 μg) treated AR group in comparison to the resveratrol (200 μg) treated AR group (Fig. 5C).
Effects of resveratrol on MDA level and SOD activities in nasal tissue homogenates from AR mice
Significantly increased MDA levels and decreased SOD activities were found in nasal tissue homogenates from the untreated AR group compared with the normal control group, and these alternations were reversed in the resveratrol (200 μg or 400 μg) or NAC treated group compared with the untreated AR group (Fig. 5D and E). In addition, these effects were more obvious in the resveratrol (400 μg) treated AR group in comparison to the resveratrol (200 μg) treated AR group (Fig. 5D and E).
Interestingly, MDA levels were positively corelated with TXNIP protein levels (Spearman's test, r = 0.689, P < 0.001), whereas, SOD activities were negatively corelated with TXNIP protein levels (Spearman's test, r = −0.730, P < 0.001).
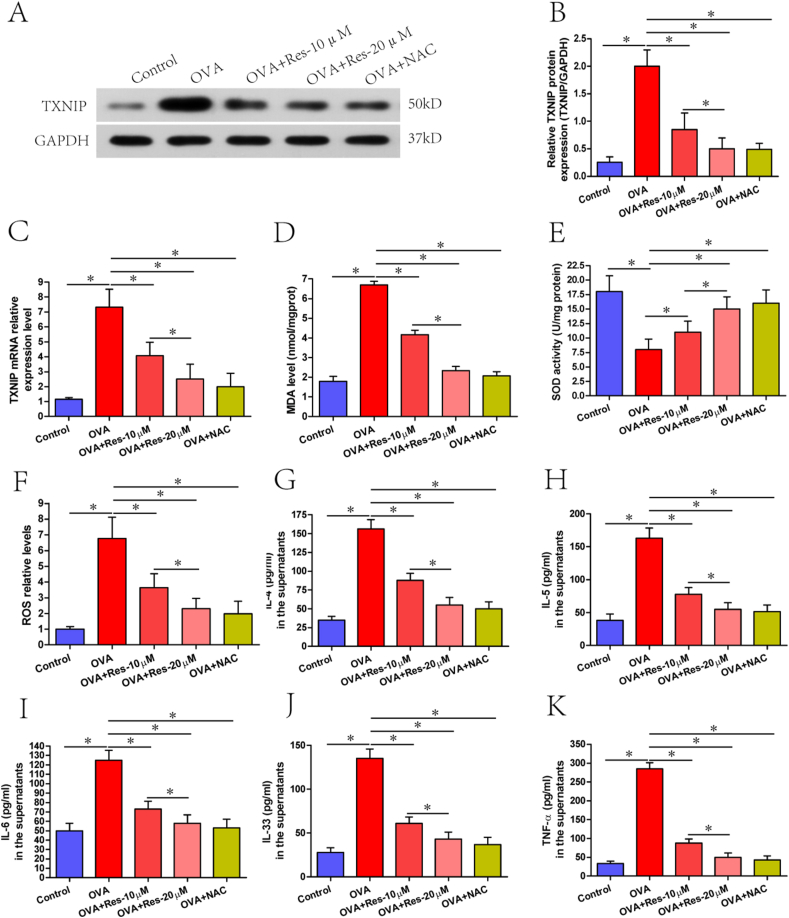
TXNIP protein and mRNA levels in spleen mononuclear cells
Notably, stronger bands and upregulated mRNA levels of TXNIP were found in OVA treated cells in comparison to control cells, and these effects were attenuated in resveratrol (10 μM or 20 μM) or NAC treated cells (Fig. 6A–C). Of note, these effects were more obvious in resveratrol (20 μM) treated cells in comparison to resveratrol (10 μM) treated cells (Fig. 6A–C).
Fig. 6.
(A–K) TXNIP protein and mRNA levels, MDA levels, SOD activities, ROS levels and inflammatory cytokines levels in five groups of spleen mononuclear cells classified as control cells, OVA treated cells, OVA + resveratrol (10 μM) treated cells, OVA + resveratrol (20 μM) treated cells and NAC treated cells.﹡P < 0.05. One-way ANOVA with Bonferroni post hoc test was employed for intergroup comparison. Each experiment was repeated six times at short time intervals
MDA, SOD and ROS levels in spleen mononuclear cells and inflammatory cytokines levels in the supernatants
Significantly, increased levels of MDA and ROS, and decreased SOD activities in spleen mononuclear cells, were found in OVA treated cells in comparison to control cells, and these effects were attenuated in resveratrol (10 μM or 20 μM) or NAC treated cells (Fig. 6D–F). In addition, inflammatory cytokines including IL-4, IL-5, IL-6, IL-33 and TNF-α levels in the supernatants were significantly upregulated following OVA treatment in comparison to control cells, and these effects were attenuated following resveratrol (10 μM or 20 μM) or NAC treatment (Fig. 6G–K). Furthermore, these effects were more obvious in resveratrol (20 μM) treated cells in comparison to resveratrol (10 μM) treated cells (Fig. 6D–K).
Discussion
Considerable data indicate that TXNIP is essential for regulating redox homeostasis.20 Activation of TXNIP could enhance ROS generation and trigger oxidative stress, leading to inflammatory response exacerbation,20 and these cell activities could be inhibited by TXNIP inhibitor resveratrol.13
Accumulating evidence indicates that antioxidant supplementation with resveratrol is capable of attenuating asthma symptoms in patients with asthma,21 and resveratrol could reduce asthma-induced airway inflammation and remodeling in a mouse or rat model of asthma.22,23 In addition, intranasal resveratrol is effective for improving nasal symptoms in adults and children with AR.24,25 Nonetheless, its precise role and mechanism in AR are still undetermined.
In the present study, frequencies of sneezes and nasal rubbing, OVA-specific IgE and histamines levels in the serum, and OVA-specific IgE, PGD2, LTC4, ECP, IL-4, IL-5, IL-6, IL-33 and TNF-α levels in the NLF were significantly upregulated in untreated AR group compared with normal control group, and these effects were attenuated by resveratrol or NAC treated AR group compared with untreated AR group. These results indicate that AR murine model is successfully established, and resveratrol treatment could ameliorate nasal symptoms and attenuate allergic and inflammatory response in AR mice.
In the nasal tissue samples of mice, significant alternations of eosinophil numbers, TXNIP positive cells and mRNA levels, MDA levels, and SOD activities were found in the untreated AR group compared with the normal control group and resveratrol or NAC treatment significantly diminished the effects in comparison to the untreated AR group. Furthermore, TXNIP level was positively correlated with MDA level, and negatively correlated with SOD activities respectively, indicating that resveratrol treatment could decrease TXNIP and oxidative stress levels in nasal tissue samples from AR mice, which are consistent with a previous report indicating that resveratrol could ameliorate obesity-associated allergic airway inflammation in mice by elevating SOD levels and reducing ROS production in lung tissues of obese mice.26
In the in vitro studies, TXNIP protein and mRNA levels, MDA, SOD, ROS, and inflammatory cytokines levels were significantly altered following OVA treatment compared with control cells, and these effects were reversed by resveratrol or NAC. Consistent with the above findings, resveratrol treatment could alter TXNIP, MDA, SOD, ROS, and inflammatory cytokine levels in spleen mononuclear cells. These results indicate that resveratrol could suppress TXNIP and SOD activities, and then reduce MDA and ROS generation, resulting in the downregulation of inflammatory mediators in the spleen mononuclear cells, which are consistent with previous reports that resveratrol could exert antioxidant and anti-inflammatory effects by inhibiting TXNIP and oxidative stress in a variety of cells.27, 28, 29
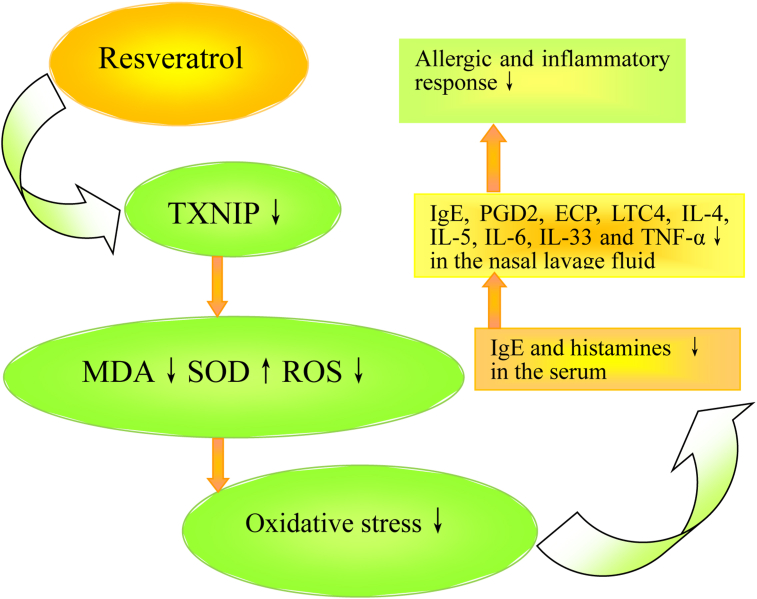
As depicted in Fig. 7, resveratrol could suppress TXNIP and increase SOD activity, leads to reduction of MDA and ROS levels; thus, oxidative stress are reduced, and then OVA-specific IgE and histamine levels in the serum, and OVA-specific IgE, PGD2, LTC4, ECP, IL-4, IL-5, IL-6, IL-33, and TNF-α levels in the NLF are attenuated, resulting in the reduction of allergic and inflammatory responses.
Fig. 7.
Schematic diagram displaying a hypothetical chain of events in which resveratrol treatment could attenuate the allergic and inflammatory response via inhibiting TXNIP-oxidative stress signaling pathway in a murine AR model
Some limitations of the present study should be interpreted. First, with respect to the studies on guandose-dependent effects of resveratrol on AR murine model, only 2 doses were used, and studies with more doses are warranted to further clarify this finding. Second, in splenocytes culture and stimulation experiment, we only isolated splenocytes from untreated AR mice, and splenocytes should be isolated from different mice groups and splenocyte response should be compared among these groups in the future. In conclusion, resveratrol could effectively alleviate murine AR by inhibiting TXNIP-oxidative stress pathway. Resveratrol may be a promising therapeutic strategy for AR.
Funding sources
This work was supported by the National Natural Science Foundation of China (No. 81870700), Shanghai Natural Science Foundation of China (No. 16ZR1426100), Science and Technology Innovation Action Plan of Science and Technology Commission of Shanghai Municipality (No. 19411950700) and the Key Laboratory Funding of Science and Technology Commission of Shanghai Municipality (No. 18DZ2260200).
Ethics statement
All animal procedures and experimental protocols were approved by the Animal Ethics Committee of Shanghai Sixth People's Hospital.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional information fles. Any dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
All authors contributed to design this study, read and approved the final version of manuscript. HL designed the study. WTZ, RT and HL carried out the experiments, analyzed data, prepared figures, prepared the manuscript and edited the manuscript. RT and GYB carried out the conduction of animal experiments and edited the manuscript. MXL contributed to the nose histopathology, captured the images and prepared the manuscript. WTZ, RT and HL contributed to edit the manuscript.
Consent for publication
All authors agreed to publication of the work.
Declaration of competing interest
The authors declare that they have no relevant conflicts of interest.
Acknowledgements
Not applicable.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100473.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hemmati S., Rahimi N., Dabiri S., Alaeddini M., Etemad-Moghadam S., Dehpour A.R. Inhibition of ovalbumin-induced allergic rhinitis by sumatriptan through the nitric oxide pathway in mice. Life Sci. 2019;236:116901. doi: 10.1016/j.lfs.2019.116901. [DOI] [PubMed] [Google Scholar]
- 2.Kim H.Y., Kim J., Jeong H.J., Kim H.M. Potential anti-inflammatory effect of Madi-Ryuk and its active ingredient tannic acid on allergic rhinitis. Mol Immunol. 2019;114:362–368. doi: 10.1016/j.molimm.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Zeng X.H., Yang G., Liu J.Q. Nasal instillation of probiotic extracts inhibits experimental allergic rhinitis. Immunotherapy. 2019;11(15):1315–1323. doi: 10.2217/imt-2019-0119. [DOI] [PubMed] [Google Scholar]
- 4.Zeng Q., Luo X., Han M., Liu W., Li H. Leptin/Osteopontin Axis regulated type 2T helper cell response in allergic rhinitis with obesity. EBioMedicine. 2018;32:43–49. doi: 10.1016/j.ebiom.2018.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y., Tao Q., Wu J., Liu H. DMBT1 has a protective effect on allergic rhinitis. Biomed Pharmacother. 2020;121:109675. doi: 10.1016/j.biopha.2019.109675. [DOI] [PubMed] [Google Scholar]
- 6.Wallace D.V., Dykewicz M.S., Oppenheimer J., Portnoy J.M., Lang D.M. Pharmacologic treatment of seasonal allergic rhinitis: synopsis of guidance from the 2017 joint task force on practice parameters. Ann Intern Med. 2017;167(12):876–881. doi: 10.7326/M17-2203. [DOI] [PubMed] [Google Scholar]
- 7.Lin H., Zheng C., Li J., Yang C., Hu L. Ca2+ -activated K+ channel-3.1 blocker TRAM-34 alleviates murine allergic rhinitis. Int Immunopharm. 2014;23(2):642–648. doi: 10.1016/j.intimp.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Shin J.H., Kim D.H., Kim B.Y. Anti-Interleukin-9 antibody increases the effect of allergen-specific immunotherapy in murine allergic rhinitis. Allergy Asthma Immunol Res. 2017;9(3):237–246. doi: 10.4168/aair.2017.9.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahiner U.M., Birben E., Erzurum S., Sackesen C., Kalayci O. Oxidative stress in asthma: Part of the puzzle. Pediatr Allergy Immunol. 2018;29(8):789–800. doi: 10.1111/pai.12965. [DOI] [PubMed] [Google Scholar]
- 10.Kim H.J., Lee S.H., Jeong S., Hong S.J. Protease-Activated receptors 2-antagonist suppresses asthma by inhibiting reactive oxygen species-thymic stromal lymphopoietin inflammation and epithelial tight junction degradation. Allergy Asthma Immunol Res. 2019;11(4):560–571. doi: 10.4168/aair.2019.11.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar A., Mittal R. Mapping Txnip: Key connexions in progression of diabetic nephropathy. Pharmacol Rep. 2018;70(3):614–622. doi: 10.1016/j.pharep.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Bedarida T., Baron S., Vibert F. Resveratrol decreases TXNIP mRNA and protein nuclear expressions with an arterial function improvement in old mice. J Gerontol A Biol Sci Med Sci. 2016;71(6):720–729. doi: 10.1093/gerona/glv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. 2019;11(5) doi: 10.3390/nu11050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao X., Zhang Y., Pan W., Chen F. miR-139-mediated NOTCH1 regulation is crucial for the inhibition of osteosarcoma progression caused by resveratrol. Life Sci. 2019:117215. doi: 10.1016/j.lfs.2019.117215. [DOI] [PubMed] [Google Scholar]
- 15.Cheng C.K., Luo J.Y., Lau C.W., Chen Z.Y., Tian X.Y., Huang Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br J Pharmacol. 2020;177(6):1258–1277. doi: 10.1111/bph.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan Y., Tong F., Chen J. Endogenous BMP-4/ROS/COX-2 mediated IPC and resveratrol alleviated brain damage. Curr Pharmaceut Des. 2019;25(9):1030–1039. doi: 10.2174/1381612825666190506120611. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda Y., Kaneko A., Yamamoto M., Ishige A., Sasaki H. Possible involvement of suppression of Th2 differentiation in the anti-allergic effect of Sho-seiryu-to in mice. Jpn J Pharmacol. 2002;90(4):328–336. doi: 10.1254/jjp.90.328. [DOI] [PubMed] [Google Scholar]
- 18.Lin H., Zheng C., Li J., Yang C., Hu L. Lentiviral shRNA against KCa3.1 inhibits allergic response in allergic rhinitis and suppresses mast cell activity via PI3K/AKT signaling pathway. Sci Rep. 2015;5:13127. doi: 10.1038/srep13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Lu X., Yu H.J. The expression of osteopontin and its association with Clara cell 10 kDa protein in allergic rhinitis. Clin Exp Allergy. 2010;40(11):1632–1641. doi: 10.1111/j.1365-2222.2010.03549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devi T.S., Yumnamcha T., Yao F., Somayajulu M., Kowluru R.A., Singh L.P. TXNIP mediates high glucose-induced mitophagic flux and lysosome enlargement in human retinal pigment epithelial cells. Biol Open. 2019;8(4) doi: 10.1242/bio.038521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenero L., Piazza M., Zanoni L., Bodini A., Peroni D., Piacentini G.L. Antioxidant supplementation and exhaled nitric oxide in children with asthma. Allergy Asthma Proc. 2016;37(1):13. doi: 10.2500/aap.2016.37.3920. e8. [DOI] [PubMed] [Google Scholar]
- 22.Alharris E., Alghetaa H., Seth R. Resveratrol attenuates allergic asthma and associated inflammation in the lungs through regulation of miRNA-34a that targets FoxP3 in mice. Front Immunol. 2018;9:2992. doi: 10.3389/fimmu.2018.02992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H., Duan J., Xu K., Zhang W. Resveratrol protects against asthma-induced airway inflammation and remodeling by inhibiting the HMGB1/TLR4/NF-kappaB pathway. Exp Ther Med. 2019;18(1):459–466. doi: 10.3892/etm.2019.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv C., Zhang Y., Shen L. Preliminary clinical effect evaluation of resveratrol in adults with allergic rhinitis. Int Arch Allergy Immunol. 2018;175(4):231–236. doi: 10.1159/000486959. [DOI] [PubMed] [Google Scholar]
- 25.Miraglia Del Giudice M., Maiello N., Decimo F. Resveratrol plus carboxymethyl-beta-glucan may affect respiratory infections in children with allergic rhinitis. Pediatr Allergy Immunol. 2014;25(7):724–728. doi: 10.1111/pai.12279. [DOI] [PubMed] [Google Scholar]
- 26.Andre D.M., Calixto M.C., Sollon C. Therapy with resveratrol attenuates obesity-associated allergic airway inflammation in mice. Int Immunopharm. 2016;38:298–305. doi: 10.1016/j.intimp.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Zhou D.Y., Su Y., Gao P., Yang Q.H., Wang Z., Xu Q. Resveratrol ameliorates high glucose-induced oxidative stress injury in human umbilical vein endothelial cells by activating AMPK. Life Sci. 2015;136:94–99. doi: 10.1016/j.lfs.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Feng Y., Wang Y. Resveratrol ameliorates disorders of mitochondrial biogenesis and dynamics in a rat chronic ocular hypertension model. Life Sci. 2018;207:234–245. doi: 10.1016/j.lfs.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Feng L., Zhang L. Resveratrol suppresses abeta-induced microglial activation through the TXNIP/TRX/NLRP3 signaling pathway. DNA Cell Biol. 2019;38(8):874–879. doi: 10.1089/dna.2018.4308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its additional information fles. Any dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.