Abstract
This paper aimed to evaluate whether human cytomegalovirus (HCMV) infection in extravillous cytotrophoblasts (EVT) could shift the balance between regulatory T (Treg) and T-helper type 17 (Th17) cells in vitro. In this study, primary EVT isolated from first trimester placental tissues were infected with HCMV, and conditional media were harvested after cultivation for 72 h. T lymphocytes were cultured in the presence or absence of HCMV-infected conditional media. The frequencies of Th17 or Treg cells from HCMV group were significantly lower or higher than those from the control group, with the expression of corresponding key cytokines at both messenger ribonucleic acid and secretion levels, respectively. The ratio of Treg to Th17 cells was significantly lower in HCMV group than that in control group (P < 0.01). In conclusion, tiled Th17/Treg balance at maternal–fetal interface exists after HCMV infection.
Keywords: human cytomegalovirus, extravillous cytotrophoblasts, Treg cells, Th17 cells
Introduction
Human cytomegalovirus (HCMV) is the most common cause of intrauterine viral infection in developed countries1. Epidemiologic evidence has shown that in the United States, approximately 27,000 pregnant women experience primary HCMV infection annually2,3. Primary HCMV infection during gestation involves a 40%–50% risk of intrauterine transmission4. Primary HCMV intrauterine infection, especially during early pregnancy, could affect placental development and result in unfavorable fetal outcomes, such as intrauterine growth restriction, abortion, late miscarriage, stillbirths, and fetal abnormalities5.
T lymphocytes are essential for normal pregnancy. T-helper type 17 (Th17) and regulatory T (Treg) cells fall into two subsets of cluster of differentiation (CD) 4+ T cells6, and their aberrant changes are associated with adverse pregnancy, such as unexplained miscarriages and recurrent spontaneous abortions7–9. It has been recognized that the delicate balance between Th17 and Treg cells is controlled by placental trophoblasts, especially extravillous cytotrophoblasts (EVT), during the first trimester10. We previously reported that the invasive capability of HCMV-infected primary EVT was impaired in vitro 11,12. However, the effect of HCMV on the immune-regulation of EVT has not been studied so far.
In this study, we compared T lymphocytes cultured in the supernatants from HCMV-infected and normal EVT to find if Treg and Th17 cells were different in number and what factors caused the difference.
Materials and Methods
Specimen and the Virus
Placental tissues were obtained from healthy pregnant women aged between 25 and 35 y whose peripheral blood was negative for HCMV antibody and who underwent elective termination of pregnancy at gestational age of 6–10 wk at the department of gynecology and obstetrics of Zhongnan Hospital, Wuhan University, from April to August in 2014. Samples of heparinized peripheral blood were also collected from healthy, nonpregnant fertile women who stayed in the same department. The research project was approved by the Ethics Committee of Zhongnan Hospital, Wuhan University (no. 20140312). The HCMV AD169 strain was procured from Hubei Province Institute of Viruses, Wuhan, China.
Isolation and Cultivation of Primary EVT
Primary EVT were isolated from placental tissues freshly collected at the first trimester as described previously with slight modification13. Briefly, villous explants were washed and kept overnight in dulbecco’s modified eagle medium (DMEM)/Ham’s F-12 medium (Gibco, Waltham, MA, USA). Chorionic villi were digested in Hanks’ balance salt solution for 30 min at 37°C without agitation. Subsequently, cells were passed through a cell strainer (pore size 70 μm) and the cell suspension was separated by Percoll density gradient at 1,000 × g for 25 min at 4°C. Cells (between the 35% and 50% Percoll layer) were cultured in DMEM/Ham’s F-12 medium in 6-well plates coated with 10% Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA). At last, cells were immunohistochemically examined with primary antibodies against cytokeratin-7, vimentin, and c-erbB-2 (Boster, Wuhan, China). Most cells were found to be positive for cytokeratin-7 and c-erbB-2 (>95%) and negative for vimentin (<5%), suggesting that the isolated cells consisted predominantly of EVT11.
Experimental Grouping
Primary EVT in HCMV group were infected with HCMV by culturing the cells in a 1:6 (v/v) mixture of 100TCID50 HCMV and culture media in 24-well plates. Primary EVT in control group were cultured with an equal amount of phosphate buffered saline (PBS). The supernatant was removed 2 h after the culture, and then culture media were added. After culture for 72 h, the cells were collected and then immune-fluorescently detected for pp65. The supernatants harvested were exposed to ultraviolet light for 15 min, and then used as conditional media for peripheral blood mononuclear cells (PBMCs) culture.
Immunofluorescent Assay
The cells harvested were blocked with 10% bovine serum albumin (BSA) in PBS for 30 min and then incubated with a mouse anti-CMVpp65 monoclonal antibody (Chemicon, Temecula, CA, USA; 1:1,000 dilution) for 24 h at 4°C. Mounting media containing Hoechst were used to stain nuclei. Slides were observed under a fluorescence microscope.
Extraction of Peripheral Blood and Isolation of PBMCs
PBMCs were isolated from heparinized peripheral blood by density gradient centrifugation through Ficoll-Hypaque (HaoYang Biological, Tianjin, China), then resuspended at a density of 2 × 106/ml in 24-well plates with anti-CD3 (2.5 μg/ml) and anti-CD28 (5 μg/ml), and cultured with conditional media supplemented with recombinant human interleukin (IL-2) (100 ng/ml; BioLegend, San Diego, CA, USA) at 37°C in 5% CO2. After 72 h culture, the supernatant and PBMCs were taken and employed for subsequent experiment.
Flow Cytometric Analysis
For detection of Th17 cells, PBMCs (2 × 106 cells/ml) were stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng/ml) containing ionomycin (1 μμ) and Monensin (500 ng/ml). Upon harvesting, the cells were stained for CD4 by incubating the cells with fluorescein isothiocyanate (FITC)-conjugated antihuman CD4 (BioLegend) at 4°C for 30 min. After fixation and permeabilization, the cells were further stained with phycoerythrin (PE)-conjugated antihuman IL-17A (eBioscience, San Diego, CA, USA) for detection of Th17 cells. For detection of Treg cells, PBMCs were incubated with allophycocyanin (APC)-conjugated antihuman CD4, PE-Cy5-conjugated antihuman CD25, and FITC-conjugated antihuman CD127 (eBioscience) in the dark for 30 min at room temperature. Finally, the cells were analyzed by multicolor flow cytometry on a FACSCalibur (Becton Dickinson).
Enzyme-Linked Immunosorbent Assay (ELISA)
IL-17A and transforming growth factor (TGF)-β1 levels in the supernatants were measured by ELISA following the manufacturer’s protocols (R&D Systems, Minneapolis, MN, USA). The results were expressed in picograms per milliliter.
Real-Time Quantitative PCR (qRT-PCR)
qRT-PCR was used to detect the messenger ribonucleic acid (mRNA) expression of Forkhead Box P3 (Foxp3), TGF-β, retinoic acid receptor-related orphan receptor γt (RORγt), IL-17A, and IL-6 in PBMCs. Briefly, total RNA was extracted with Trizol reagent (Invitrogen) and reversely transcribed into complementary DNA (cDNA) with Prime Script RT reagent (Toyobo, Osaka, Japan). The primers were synthesized by Sangon Biotech Co. and their sequences are listed in Table 1. The qRT-polymerase chain reaction (PCR) assay was used to amplify the mRNA and performed in triplicate by employing Fast Start Universal SYBR Green Master (ROX; Roche Diagnostics, Mannheim, Germany) on an ABI 7500 Real-Time PCR system (Thermo Fisher Scientific, Shanghai, China). Data were analyzed and converted into threshold cycle (Ct) values.
Table 1.
Primers, Length of PCR Product, and Annealing Temperatures for Real-Time PCR Assay.
| Gene symbol | Primers (F: forward, R: reverse) | Length of product (bp) | Annealing temperature (°C) |
|---|---|---|---|
| Foxp3 | F: CGCCACAACCTGAGTCTGC | 174 | 60 |
| R: TGTTCGTCCATCCTCCTTTCC | |||
| TGF-β | F:GTGACAGCAGGGATAACACACTG | 242 | 60 |
| R:GTCCTTGCGGAAGTCAATGTAC | |||
| RORγt | F:CTCAAAGCAGGAGCAATGGAA | 164 | 60 |
| R:AGGGAGTGGGAGAAGTCAAAGA | |||
| IL-17A | F:CTACAACCGATCCACCTCACC | 233 | 60 |
| R:AGCCCACGGACACCAGTATC | |||
| IL-6 | F:GCCACTCACCTCTTCAGAACG | 121 | 60 |
| R:CAGTGCCTCTTTGCTGCTTT | |||
| β-actin | F:GTCCACCGCAAATGCTTCTA | 190 | 60 |
| R:TGCTGTCACCTTCACCGTTC |
Statistical Analysis
Data were presented as means with standard error (S.E.). The data were analyzed by Student’s t-test since data were normally distributed as tested by Kolmogorov–Smirnov test. A P value <0.05 was considered statistically significant.
Results
HCMV in Primary EVT
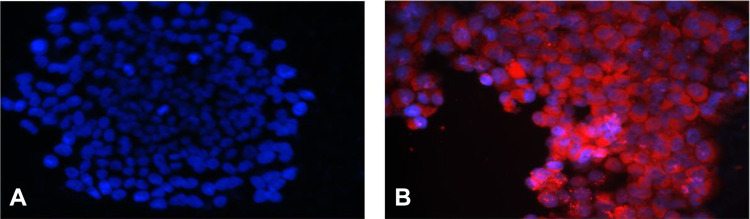
Results of immunofluorescence assay are shown in Fig. 1. In HCMV group, a great number of red-stained HCMV pp65 antigen signals were observed in the cytoplasm of primary EVT (Fig. 1B), while no such red signals were found in the primary EVT of control group (Fig. 1A).
Figure 1.
Immunofluorescence detection of HCMV pp65 antigen in primary EVT (×400). (A) Control group. Nuclei are stained blue; (B) HCMV group. Red-stained HCMV pp65 antigen signals are localized in the cytoplasm of primary EVT. EVT: extravillous cytotrophoblasts; HCMV: human cytomegalovirus.
Frequency of Th17 Cells in PMBCs After HCMV Infection
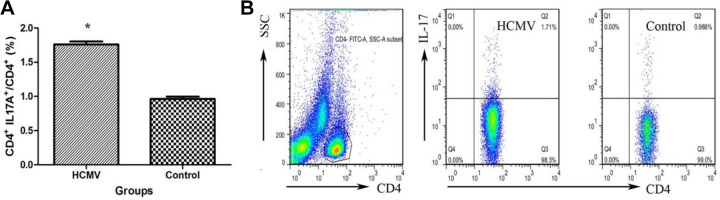
As shown in Fig. 2A, Th17 frequency (CD4+ IL-17A+/CD4+ T cells) was significantly increased in PBMCs cultured in conditional media from HCMV group (1.76% ± 0.04%) than those from control group (0.96% ± 0.03%, P < 0.01). Figure 2B shows a representative flow cytometric finding of increased Th17 frequency in HCMV group as compared to those of the control.
Figure 2.
Increased Th17 cell frequency in PBMCs after HCMV infection. (A) CD4+ IL-17A+ cell frequency in PBMCs from two groups is shown. (* indicates P < 0.05 vs. control group). Data are presented as mean ± S.E. (B) Representative dot plots illustrate the regions and gates under immune phenotypic analysis. Cells stained for surface expression of CD4 and for intracellular expression of IL-17A were flow cytometrically and gated on CD4+ T cells. CD: cluster of differentiation; HCMV: human cytomegalovirus; IL: interleukin; PBMC: peripheral blood mononuclear cell; SSC: side scatter; Th17: T-helper type 17.
Frequency of Treg Cells in PBMCs After HCMV Infection
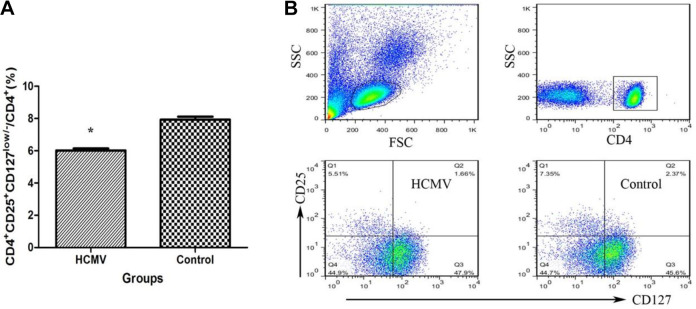
Treg cells were identified according to the expression of CD4, CD25, and the transcription factor, Foxp3. It has been reported that CD127 expression could be a surrogate surface marker for Foxp3 because these two proteins were inversely correlated, with Foxp3+ cells being CD127low/– 14. In this study, Treg cells were stained with CD4+CD25+CD127low/– T cells. Figure 3A shows that the frequency of Treg (CD4+CD25+CD127low/–/CD4+ T cells) was significantly lower in PBMCs from HCMV group (6.01% ± 0.13%) than in those from control group (7.93% ± 0.18%, P < 0.01).
Figure 3.
Decreased Treg cell frequency in PBMCs after HCMV infection. (A) The percentages of Treg cells in the CD4+ cells in PBMCs from the two groups are shown (*: P < 0.05 vs. control group). Data are presented as mean ± S.E. (B) Representative dot plots illustrate the regions and gates for immune phenotypic analysis. Cells are stained with CD4, CD25, and CD127, and flow cytometrically detected. CD: cluster of differentiation; FSC: forward sactter; HCMV: human cytomegalovirus; IL: interleukin; PBMC: peripheral blood mononuclear cell; SSC: side scatter; Treg: regulatory T cell.
Th17/ Treg Imbalance in PBMCs After HCMV Infection
Figure 4 shows the ratio of Treg to Th17 in PMBCs from HCMV group (3.36 ± 0.08) was significantly lower than those from control group (8.30 ± 0.41, P < 0.01).
Figure 4.
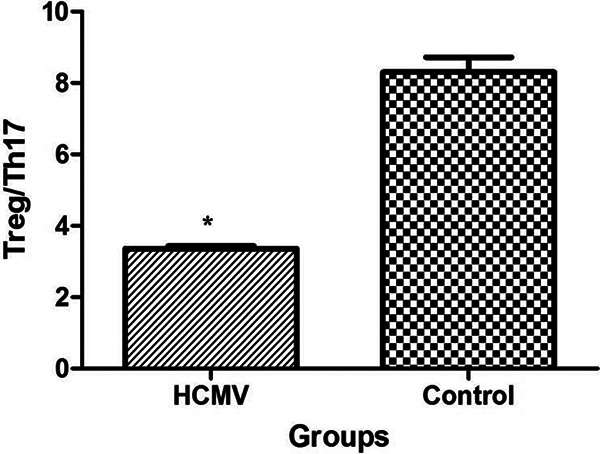
Imbalance between Treg and Th17 in PBMCs after HCMV infection. The ratio of Treg/Th17 was significantly decreased in PBMCs after HCMV infection (*: P < 0.01 vs. control group). Data are presented as mean ± S.E. HCMV: human cytomegalovirus; PBMC: peripheral blood mononuclear cell; Th17: T-helper type 17; Treg: regulatory T cell.
Soluble Cytokine IL-17A and TGF-β1 in Cell Culture Supernatants After HCMV Infection
As shown in Fig. 5, the concentration of soluble IL-17 in HCMV group (1,494.44 ± 55.56) was significantly higher compared to control group (533.33 ± 33.33, P = 0.002), while TGF-β1 concentrations were lower in HCMV group (230 ± 11.67) than in control group (1,277.78 ± 89.41, P < 0.001).
Figure 5.
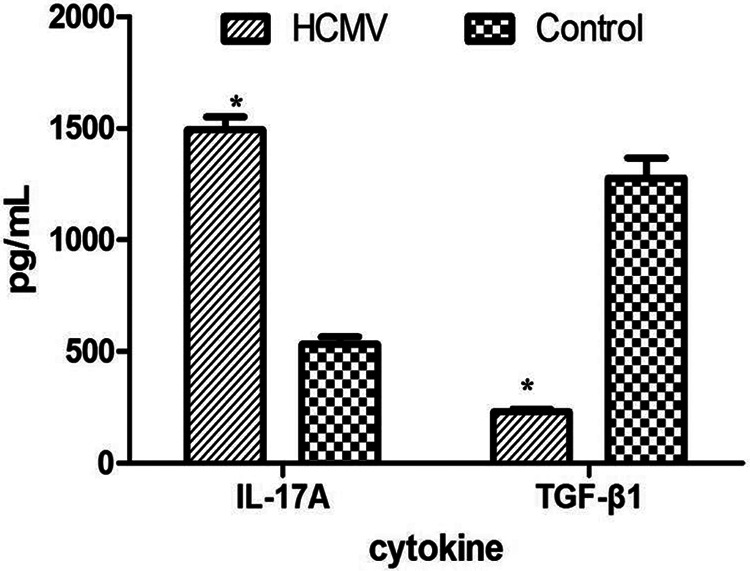
Soluble TGF-β1 and IL-17A in the supernatants of PBMCs. The concentration of TGF-β1 was significantly decreased, while the IL-17A expression was increased in the supernatants from HCMV group as compared with control group (*: P < 0.05 vs. control group). Data are presented as mean ± S.E. HCMV: human cytomegalovirus; IL: interleukin; PBMC: peripheral blood mononuclear cell; TGF: transforming growth factor.
Expression of Transcription Factors in PBMCs After HCMV Infection
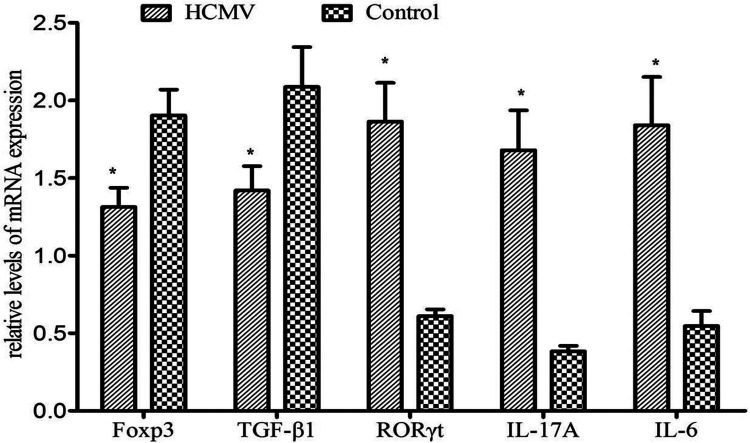
The IL-17A, IL-6, and RORγt mRNA expressions were all significantly higher (P < 0.01, =0.001, <0.01, respectively), while Foxp3 and TGF-β1 mRNA expressions were significantly lower (P = 0.006 and 0.012, respectively) when PBMCs were cultured in conditional media from HCMV group as compared with control group (Fig. 6).
Figure 6.
The expression of transcription factors in PBMCs after HCMV infection. The expressions of Foxp3 and TGF-β1 are significantly decreased while the expressions of RORγt, IL-17A, and IL-6 were increased in PBMCs from HCMV group when compared with control group (*: P < 0.05 vs. control group). Data are presented as mean ± S.E. Foxp3: forkhead box p3; HCMV: human cytomegalovirus; IL: interleukin; PBMC: peripheral blood mononuclear cell; RORγt: retinoic acid receptor-related orphan receptor γt; TGF: transforming growth factor.
Discussion
Treg and Th17 cells differentiate from naïve CD4+ T cell precursors, and IL-6 and TGF-β are key cytokines involved in the differentiation of Th17 and Treg cells. CD4+ T cells differentiate to Treg cells when exposed to high levels of TGF-β8. However, when CD4+ T cells are exposed to both IL-6 and TGF-β, they differentiate into Th17 cells15. The equilibrium between Treg and Th17 cells at maternal–fetal interface is a prerequisite for a successful pregnancy, and an upset balance between the two cells lines may result in pregnancy failure16. The replication cycle of HCMV is characterized by the expression of immediate-early, early, and late gene regions17. The early–late gene product, pp65, was detected in EVT after infection with cell-free strain AD169 at 72hpi. The results showed that EVT allowed a complete HCMV replication in vitro. To assess the effect of HCMV on the EVT’s immunoregulation, conditional media from the cells were subjected to ultraviolet light exposure to inactivate infective HCMV but retain the relevant soluble trophoblast-derived factors. We demonstrated that the percentage of Th17 cells was increased and that of Treg cells was decreased in PBMCs from HCMV group. Furthermore, the Treg/Th17 ratio was also altered in PBMCs from HCMV group.
At maternal–fetal interface, EVT could effectively inhibit immunity of Th17 cells and enhance the immunity of Treg cells18,19. Th17 cells are involved in the initiation or progression of inflammation, which is essential for vascular remodeling and placental invasion, but excessive inflammation can cause embryo resorption20. The uterine cavity is not completely sterile, and therefore the number of Th17 cells in the decidua of normal pregnant women is slightly higher compared to peripheral blood, which may induce a protective immune response against extracellular microbes. It has been documented that the conditional media derived from placental trophoblasts reduce IL-17 production in T lymphocytes18,21. Th17 cells in the decidua of pregnant women suffering from spontaneous abortion or preeclampsia were significantly higher as compared to their nonpregnant or normal pregnant counterparts, suggesting that increased Th17 cells in decidua may be disadvantageous for pregnancy9,16,22,23. We found that the percentage of Th17 cells among CD4+ T cells was significantly increased in HCMV group, with the secretion of IL-17 elevated and the transcription level of both RORγt and IL-17 also increased. It suggested that the increased Th17 may be involved in the pathogenesis of HCMV intrauterine infection.
Increased Treg cells may confer a strong maternal tolerant state during early pregnancy24. Treg cells exert their suppressive effect on conceptus antigens partially by secreting the anti-inflammatory cytokine TGF-β1 and expressing Foxp325,26. We found that conditional media from HCMV group could decrease the frequency of Treg cells. Moreover, soluble TGF-β1 was also decreased in supernatants from HCMV group. The decrease in both the number and function of Treg cells suggested that the HCMV work on Treg cells not only quantitatively, but also functionally. The mechanisms by which HCMV infection reduces Treg cells are multiple. Trophoblasts could contribute to the recruitment and differentiation of Treg through the secretion of cytokines and chemokines, such as histocompatibility antigen G, indoleamine 2,3-dioxygenae (IDO), and vasoactive intestinal peptide (VIP). Experts demonstrated that HCMV infection inhibited IDO activity in early-stage placenta5, and it is possible that the decreased expression of Foxp3 and TGF-β1 may impair the suppressive ability of Treg cells at PMBCs. Moreover, our further study also showed that VIP expression was decreased in HCMV-infected EVT, which would be one of the mechanisms for the Treg/Th17 imbalance after HCMV infection27.
Conclusion
From the perspective of immunology, successful pregnancy depends on establishment of maternal immune tolerance to accommodate antigens expressed by the conceptus. T lymphocytes and secreted cytokines play a crucial role in reproductive immune tolerance, and the balance of Th17/Treg, as a newly identified CD4+ effector T cell lineage, has been widely studied. The finding that the Th17/Treg balance at maternal–fetal interface is upset after HCMV infection provides a new perspective to the mechanism by which HCMV causes adverse pregnancy outcomes during early pregnancy.
Acknowledgments
We thank the clinical laboratory of Zhongnan Hospital of Wuhan University for the pathologic diagnosis and MeiJiaYouKe Co. Ltd. (Wuhan, China) for English editing.
Footnotes
Authors’ Contributions: QY was responsible for the conception, study design, date analysis, and manuscript writing. HC, JG, and DX were responsible for completing the experimental part of the study. TL and MY analyzed the date. HK revised the manuscript.
Ethical Approval: This study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University of China (no. 20140312).
Statement of Human and Animal Rights: Human and animal experiments contained in this article were reviewed and approved by the Ethics Committee of Zhongnan Hospital of Wuhan University of China (no. 20140312).
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article. This study made use of placental tissues from healthy pregnant women at gestational age of 6–10 wk. These tissues were provided to Zhongnan Hospital of Wuhan University for research use according to the ethical approval. Heparinized peripheral blood from healthy, nonpregnant fertile women were also used for research according to the ethical approval.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported by grants from the project of the Young Teachers funded by Wuhan University of China (2042015kf0154).
ORCID iD: Yuan Qiao  https://orcid.org/0000-0002-5099-4424
https://orcid.org/0000-0002-5099-4424
References
- 1. Marsico C, Kimberlin DW. Congenital cytomegalovirus infection: advances and challenges in diagnosis, prevention and treatment. Ital J Pediatr. 2017;43(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–213. [DOI] [PubMed] [Google Scholar]
- 3. Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tabata T, Petitt M, Fang-Hoover J, Zydek M, Pereira L. Persistent cytomegalovirus infection in amniotic membranes of the human placenta. Am J Pathol. 2016;186(11):2970–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopez H, Benard M, Saint-Aubert E, Baron M, Martin H, Al Saati T, Plantavid M, Duga-Neulat I, Berrebi A, Cristini C, Arnaud C, et al. Novel model of placental tissue explants infected by cytomegalovirus reveals different permissiveness in early and term placentae and inhibition of indoleamine 2,3-dioxygenase activity. Placenta. 2011;32(7):522–530. [DOI] [PubMed] [Google Scholar]
- 6. Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326(5955):986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang H, Hu X, Liu X, Zhang R, Fu Q, Xu X. The Treg/Th17 imbalance in Toxoplasma gondii-infected pregnant mice. Am J Reprod Immunol. 2012;67(2):112–121. [DOI] [PubMed] [Google Scholar]
- 8. Warning JC, McCracken SA, Morris JM. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction. 2011;141(6):715–724. [DOI] [PubMed] [Google Scholar]
- 9. Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH, Lin QD. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. 2010;84(2):164–170. [DOI] [PubMed] [Google Scholar]
- 10. Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–610. [DOI] [PubMed] [Google Scholar]
- 11. Liu T, Zheng X, Chen J, Wang N, Xiao J, Zhang D, Yin Z, Li W, Chen S. Effect of human cytomegalovirus on invasive capability of early pregnant extravillous cytotrophoblasts. J Huazhong Univ Sci Technolog Med Sci. 2011;31(6):819–823. [DOI] [PubMed] [Google Scholar]
- 12. Tao L, Suhua C, Juanjuan C, Zongzhi Y, Juan X, Dandan Z. In vitro study on human cytomegalovirus affecting early pregnancy villous EVT’s invasion function. Virol J. 2011;8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. James JL, Hurley DG, Gamage TK, Zhang T, Vather R, Pantham P, Murthi P, Chamley LW. Isolation and characterisation of a novel trophoblast side-population from first trimester placentae. Reproduction. 2015;150(5):449–462. [DOI] [PubMed] [Google Scholar]
- 14. Mjosberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod. 2010;82(4):698–705. [DOI] [PubMed] [Google Scholar]
- 15. Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29(4):628–636. [DOI] [PubMed] [Google Scholar]
- 16. Liu F, Guo J, Tian T, Wang H, Dong F, Huang H, Dong M. Placental trophoblasts shifted Th1/Th2 balance toward Th2 and inhibited Th17 immunity at fetomaternal interface. APMIS. 2011;119(9):597–604. [DOI] [PubMed] [Google Scholar]
- 17. Mercorelli B, Luganini A, Muratore G, Massari S, Terlizzi ME, Tabarrini O, Gribaudo G, Palu G, Loregian A. The 6-Aminoquinolone WC5 inhibits different functions of the immediate-early 2 (IE2) protein of human cytomegalovirus that are essential for viral replication. Antimicrob Agents Chemother. 2014;58(11):6615–6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou GX, Luo LH, Luan HB. Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2011;65(5):503–511. [DOI] [PubMed] [Google Scholar]
- 19. Fraccaroli L, Alfieri J, Larocca L, Calafat M, Roca V, Lombardi E, Ramhorst R, Leiros CP. VIP modulates the pro-inflammatory maternal response, inducing tolerance to trophoblast cells. Br J Pharmacol. 2009;156(1):116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY. Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol. 2012;228(2):260. [PubMed] [Google Scholar]
- 21. Xu WM, Xiao ZN, Wang XB, Huang Y. IL-17 Induces fetal loss in a CBA/JxBALB/c mouse model, and an Anti-IL-17 antibody prevents fetal loss in a CBA/JxDBA/2 mouse model. Am J Reprod Immunol. 2016;75(1):51–58. [DOI] [PubMed] [Google Scholar]
- 22. Santner-Nanan B, Peek MJ, Khanam R, 0harts L, Zhu E, Fazekas de St Groth B, Nanan R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183(11):7023–7030. [DOI] [PubMed] [Google Scholar]
- 23. Nakashima A, Ito M, Shima T, Bac ND, Hidaka T, Saito S. Accumulation of IL-17-positive cells in decidua of inevitable abortion cases. Am J Reprod Immunol. 2010;64(1):4–11. [DOI] [PubMed] [Google Scholar]
- 24. Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016;148(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta. 2014;35(4):241–248. [DOI] [PubMed] [Google Scholar]
- 26. Jones K, Ballesteros A, Mentink-Kane M, Warren J, Rattila S, Malech H, Kang E, Dveksler G. PSG9 stimulates increase in FoxP3+ regulatory T-Cells through the TGF-beta1 Pathway. PLoS One. 2016;11(7):e0158050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiao Y, Fang JG, Xiao J, Liu T, Liu J, Zhang YL, Chen SH. Effect of baicalein on the expression of VIP in extravillous cytotrophoblasts infected with human cytomegalovirus in vitro. J Huazhong Univ Sci Technolog Med Sci. 2013;33(3):406–411. [DOI] [PubMed] [Google Scholar]