Abstract
It has been reported that extracellular vesicles (EVs) derived from human umbilical cord mesenchymal stem cells (HUCMSCs) can promote the proliferative and secretive functions of granulosa cells. In vivo study further demonstrated that EVs derived from HUCMSCs can not only promote the angiogenesis of ovarian tissue but also restore the function of an ovary of chemically induced premature ovarian insufficiency (POI) mice. However, no study investigates the effects of HUCMSCs derived EVs on fertility recovery of POI mice and evaluating their offspring. This study investigates the effects of HUCMSCs derived EVs on fertility recovery and the cognitive function of their offspring. A POI model was established by intraperitoneal injection of cyclophosphamide (CTX) and busulfan (BUS), and randomly divided into EVs-transplantation group (a single injection of 150 µg EVs proteins which suspended in 0.1 ml phosphate buffer saline [PBS] via tail vein), POI group (a single injection of 0.1 ml PBS via tail vein), and normal control group (a single injection of 0.1 ml PBS via tail vein without intraperitoneal injection of CTX and BUS). After EVs treatment, not only the ovarian function of POI mice recovered but also the fertility increased with less time to get pregnant, evaluating by in vitro fertilization and mating test. Cognitive behaviors of the offspring were similar among the three groups through the Y-maze test and novel object recognition task. An anti-apoptotic effect was identified through immunohistochemistry, real-time polymerase chain reaction and western blot. These findings indicate that HUCMSCs derived EVs can improve the fertility of POI mice without adverse effects on the cognitive behavior of their offspring, highlighting the potential value of EVs to be a cell-free therapy for patients suffering from POI.
Keywords: extracellular vesicles, mesenchymal stem cell, premature ovarian insufficiency, fertility, in vitro fertilization, offspring
Introduction
Premature ovarian insufficiency (POI), which defined as the loss of ovarian activity before the age of 40 yr, affects approximately 1% of women with slightly varies among different ethnics1,2. It is characterized by menstrual disturbance (amenorrhea or oligomenorrhea), infertility, hypoestrogenism, and raised gonadotropins, which were associated with increased risks of osteoporosis, metabolic, and cardiovascular disorders2,3. Up to now, there are no effective treatments for women with POI. Although hormone replacement therapy (HRT) can correct the disordered hormone profiles of women with POI, it cannot effectively recover the fertility of them and will increase the risks of developing thrombosis and breast cancer4,5. At the moment, for the women with POI who want to get pregnant, ovum donation (OD) with in vitro fertilization (IVF) may be the only available solution, but there will be no biological lineage between the mother and the child6. In addition, some ethical and legal policies for OD in some countries and regions will confine its practice7.
Over the last decade, extracellular vesicles (EVs), used to be regarded as membrane debris with no real biological significance, are now emerged as important mediators of cell-to-cell communication8. It can interact with target cells through their inherent surface-expressed ligands, deliver its cargo (proteins, microribonucleic acid [mRNA], and bioactive lipids) to the cytosol of the recipient cell, and then affect the phenotype and function of target cells9. The potential therapeutical effects of EVs derived from stem cells on different disease models, including myocardial infarction, central nervous system disorders, hepatic failure, and stroke and graft-versus-host disease, have been investigated by various researchers and achieving encouraging outcome10–13. Furthermore, previous studies evaluate the safety of human umbilical cord mesenchymal stem cells (HUCMSCs) derived EVs on liver or renal function14, but no study investigates whether the treatment of EVs to women of child-bearing age will influence the development of their offspring. Recently, Yang et al.15 found that EVs derived from HUCMSCs could locate in ovarian tissue after transplantation and play essential roles in promoting ovarian angiogenesis and restoring ovarian function of POI mice. However, they neither evaluate the fertility of the POI mice nor the influence on their offspring after EVs transplantation, which is of great concern for a new treatment for women with POI who desire to have a child.
This study aimed to evaluate the effects of EVs derived from HUCMSCs on fertility recovery of POI mice and to assess the cognitive behavior of their offspring. We hypothesized that EVs could recover the fertility of POI mice and would not influence the development of their first generation’s cognition. We use IVF and mating test for POI mice and Y-maze test and novel object recognition task (NORT) for their offspring to investigate this hypothesis.
Materials and Methods
Experimental Animals
Five- to six-week-old specific pathogen-free grade CD1 (ICR) female mice and 8–9 wk old male mice were purchased from Anhui Medical University, Hefei, China. All the mice were fed ad libitum food and water in a 25°C animal facility on a 12-h light-dark cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Anhui Medical University (ID: LLSC20160337).
Culture of MSCs and Isolation of Extracellular Vesicles
The primary generation of HUCMSCs was offered by Cell Therapy Center of 105th Hospital of the People’s Liberation Army (PLA), and cultured in α-minimum essential medium (α-MEM, Gibco, Gaithersburg, MD, USA) with 10% fetal bovine serum (FBS) (Sijiqing, Hangzhou, Zhejiang, China) and 1% penicillin/streptomycin (Gibco) at 37°C in 5% CO2 humidified air. Three to eight passages of HUCMSCs were cultured to produce EVs. When the density of HUCMSCs reached 70%–80% confluency, 10% FBS was replaced by 0.5% of bovine serum albumin (BSA) (Sigma-Aldrich, St Louis, MO, USA) for 24–48 h (Fig. 1A). When HUCMSCs confluence reached 90% or more, the serum-free culture medium was collected and stored at −4°C, and then EVs were isolated within 24 h by differential ultracentrifugation16. Briefly, the cell supernatants were obtained through centrifugation at 300 × g for 10 min and 2,000 × g for 20 min to remove cells and debris, cell-free supernatants were then centrifuged at 100,000 × g (Beckman Coulter Optima L-100XP ultracentrifuge) for 70 min at 4°C, the sediments were resuspended in phosphate buffer saline (PBS) (Hyclone, Logan, UT, USA) and submitted to a second and third 100,000 × g ultracentrifugation at the same conditions.
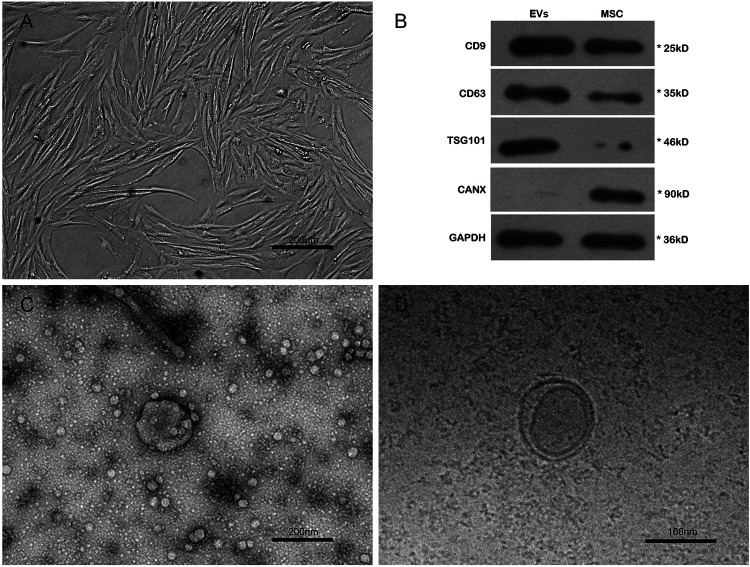
Fig. 1.
Characterizations of HUCMSCs and EVs. (A) Fibroblast-like morphology of the sixth passage HUCMSCs. (B) The protein expressions of CD9, CD63, TSG101, and CANX in the EVs and HUCMSCs by western blot. (C–D) The transmission electron microscope images of EVs. HUCMSCs: human umbilical cord mesenchymal stem cells; EVs: extracellular vesicles.
EVs associated proteins were determined by the Bradford method to estimate the amount of EVs17. About 600 ml serum-free culture medium of mesenchymal stem cells (MSCs) were used for collecting EVs, and 162.7 ± 17.5 µg EVs proteins were isolated in every 106 HUCMSCs (about 10 ml of MSCs’ serum-free culture medium). Western blot (WB) was applied to identify the protein expressions of CD9, CD63, TSG101, and CANX in the HUCMSCs and EVs, and a comparable positive for CD9, CD63, TSG101, and negative for CANX in EVs were identified when compared with HUCMSCs, indicating the origination of EVs were from HUCMSCs (Fig. 1B)18. EVs were suspended in PBS and loaded on copper grids and stained with 1% (w/v) phosphotungstic acid for morphological observation under the transmission electron microscope (TEM, FEI, Oregon, USA) (Fig. 1C,D). Details were described elsewhere15.
Establishment of Mouse POI Model and Grouping
Vaginal smears of mice were conducted every morning at 8:00 am and stained with hematoxylin and eosin, lasting for 2 wk19. Mice that went through at least two consecutive regular estrous cycles were included in this study. To establish the model of POI, 200 mg/kg cyclophosphamide (CTX, Sigma-Aldrich) and 20 mg/kg busulfan (BUS, Sigma-Aldrich) dissolved in dimethyl sulfoxide were intraperitoneally injected at one time. Vaginal smears were applied again after CTX and BUS injection to evaluate the success of the POI model for 2 wk, and mice with consecutive irregular estrous cycles were treated as successful model establishment.
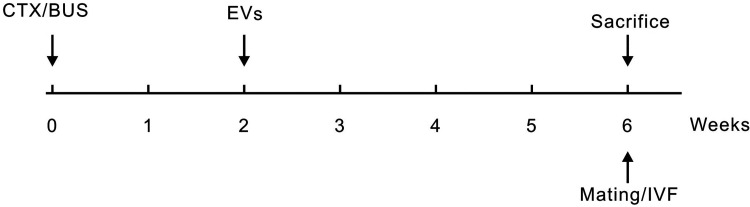
After successful model establishment, 116 POI mice were selected and randomized into the EVs group (received a single injection of 150 µg EVs proteins [suspended in 0.1 ml PBS] via tail vein, n = 58)15 and the POI group (received a single injection of 0.1 ml PBS via tail vein, n = 58), the age- and weight-matched female mice received a single injection of 0.1 ml PBS via tail vein without intraperitoneal injection of CTX and BUS were served as a normal control (NC, n = 58). All the treatment was conducted 2 wk after the injection of CTX and BUS. The protocol of the experiment is presented in Fig. 2.
Fig. 2.
Schematic diagram of the experimental protocol. CTX: cyclophosphamide; BUS: busulfan; EVs: extracellular vesicles; IVF: in vitro fertilization.
Hormones Measurement and Ovarian Morphologic Observation
Two weeks after the injection of CTX and BUS, six mice were sacrificed to evaluate the success of POI model establishment; another 6 mice without the injection of CTX and BUS were sacrificed as a normal control (NC group). After EVs treatment, 6 mice in each group (EVs, POI, and NC groups) were sacrificed weekly until the fourth week after EVs transplantation to evaluate the recovery of the ovarian function.
Blood samples (retroorbital puncture) were centrifuged at 1,000 × g for 15 min at 4°C to separate the serum. The concentrations of estrogen (E2) and follicle-stimulating hormone (FSH) were detected with gonadal hormone enzyme-linked immunosorbent assay (ELISA) kits (Cusabio, Wuhan, Hubei, China) according to the manufacturer’s instructions. Ovaries of each mouse were weighed and fixed with 4% paraformaldehyde for 6 h, dehydrated through a graded series of ethanol, vitrified in xylene, embedded in paraffin, and then 3-µm sections were stained with hematoxylin and eosin. The ovarian histological examination and follicle counts (primordia, primary, secondary, and antral follicles) were carried out under light microscopy (Zeiss, Jena, Germany).
For the body weight, we labeled 10 mice of each group and weighed at 0, 3, 7, and 14 days after the injection of CTX and BUS and weekly until the fourth week after treatment, all the mice were weighed in the morning.
In Vitro Fertilization and Embryo Culture
In order to keep the quality of the sperm, male mice were housed with female mice (do not belong to NC, POI, or EVs groups) for 1 wk, then housed alone for 1 wk before sperm collection. Six female mice in each group (NC, POI, and EVs groups) were superovulated via a single intraperitoneal injection of 10 IU pregnant mare serum gonadotropin (PMSG, Zhejiang, China) 4 wk after treatment, followed by injection of 10 IU human chorionic gonadotropin (hCG, Sansheng, Ningbo, Zhejiang, China) 48 h later. The cumulus oocyte complexes were collected from the ampulla portion of the oviduct under stereomicroscope 12–14 h after hCG administration and placed in IVF culture media (Vitrolife, Stockholm, Sweden) at 37°C in 5% CO2 air. Meanwhile, the 12–13-wk-old male mice were sacrificed by cervical dislocation; the sperm in epididymides were collected through 5–7 longitudinal cuts on epididymis with a syringe needle, and then incubated for at least 1 h at 37°C in 5% CO2 air for capacitation in IVF culture media. The metaphase II oocytes were incubated with sperm to facilitate fertilization in IVF culture media for 5–6 h, and fertilized zygotes were subsequently cultured in G1 and G2 culture media (Vitrolife) at 37°C in 5% CO2 air20. The development of the fertilized zygote was observed and recorded.
Mating Test and Cognitive Behavior Tests of Offspring
Four weeks after treatment, 15 female mice in each group (NC, POI, and EVs groups) housed with 12–13 wk-old-male mice for 21 days (two to three female mice and one male mouse were housed in the same cage). Once the presence of a copulatory plug was confirmed, the female mouse was separated. The numbers of neonatal mice per litter in each group was recorded. In each group, 25 neonatal mice were labeled and weighed weekly for 6 wk.
When offspring reached 6-wk-old, 12 offspring mice (six male and six female) were selected randomly for behavioral evaluation by the Y-maze test and NORT. Y-maze and NORT tests were indicators to test the immediate working memory of the rodents, which is based on the spontaneous tendency of rodents to explore a novel object or surroundings more often than a familiar one. The apparatus, performance, and data analysis for Y-maze and NORT are described elsewhere21,22.
Immunohistochemical Analysis
Cleaved caspase 3 in ovarian tissue was detected using immunohistochemistry. All sections (3 µm) were stained using the automated immunostaining system (Ventana Benchmark XT, Roche, Basel, Switzerland). All the procedures were followed by the system instructions. Briefly, sections of the ovarian tissue were dewaxed and pretreated with cell conditioning 1 solution (Roche Diagnostics) for 30 min, then washed with reaction buffer followed by incubation with the rabbit anti-cleaved caspase 3 (1:600, Cell Signaling, China) for 32 min. To detect the location and expression of cleaved caspase 3, 3,3′-Diaminobenzidine tetrahydrochloride (DAB) kit (Roche Diagnostics) was used. The results were expressed as the percentages of apoptotic cells in each section by counting 5 random fields from each sample under the Olympus BX-51 light microscope (Olympus, Tokyo, Japan).
Real-Time Quantitative Polymerase Chain Reaction and Western Blot
Seven mice of each group (NC, POI, and EVs groups) were sacrificed to collect ovaries for polymerase chain reaction (PCR) and WB. For PCR, the total ribonucleic acid (RNA) was extracted from the ovarian tissue of mice with Trizol reagent (Invitrogen, Carlsbad, CA, USA), and reversely transcribed into complementary deoxyribonucleic acid (cDNA) using Random 6 mers and Oligo dT Primer (Promega, Shanghai, China). Primer sequences of Caspase 3, Bcl-2, and Bax were designed using Primer 5 according to its cDNA sequences in the National Center for Biotechnology Information (NCBI) database (Table 1). Real-time quantitative PCR (qPCR) was carried out using SYBR Green Real-time PCR Master Mix (Promega). The reaction conditions were as follows: 95°C, 3 min; 95°C, 10 s; and 60°C, 1 min for 40 cycles. Gene expression level of each sample was evaluated through threshold cycle (Ct) value, and the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Table 1.
Primers Used for qPCR Validation.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Caspase 3 | TGGGACTGATGAGGAGATGGC | AGGGACTGGATGAACCACGAC |
| Bcl-2 | GGAGGATTGTGGCCTTCTTTG | GCAGATGCCGGTTCAGGTAC |
| Bax | TGCTACAGGGTTTCATCCAGG | AGTTCATCTCCAATTCGCCG |
qPCR: quantitative polymerase chain reaction.
For WB, proteins of ovary tissues of the mice were homogenized in Trizol reagent (Invitrogen) and were separated on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels, and then transferred to Millipore® polyvinyldifluoridine (PVDF) membranes. The PVDF membranes were blocked with 5% nonfat milk solution and incubated with rabbit antibodies against Caspase 3 (1: 5000, Abcam, Cambridge, MA, USA), Bcl-2 (1: 5000, Abcam), Bax (1: 5000, Abcam), and GAPDH (1: 5000, Abcam). The coloration bands were visualized on X-ray films with ECL reagents (Pierce, Colorado, USA). The band of GAPDH was used as an internal standard, and the relative intensity of bands was quantified by digital densitometry (Image J software, Bethesda, USA).
Statistical Analysis
Continuous variables were compared by unpaired Student’s t-test or analysis of variance (ANOVA) with Tukey’s multiple comparisons test among the two and three groups. Categorical variables were compared by chi-square tests. The Log-rank (Mantel-Cox) test was used to compare the successful mating rate. A two-tailed P-value of less than 0.05 was considered statistically significant. All analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Evaluation of the POI Model
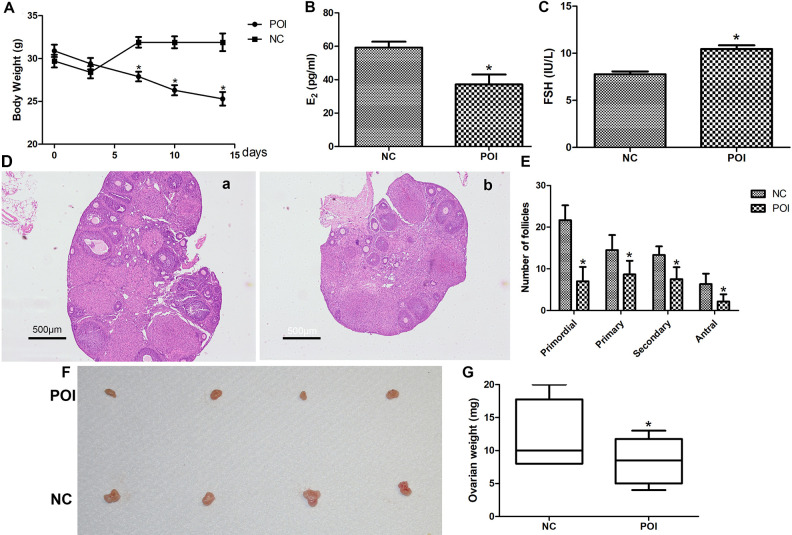
Two weeks after CTX and BUS injection, the body weight of the mice in the POI group decreased persistently and reached statistical difference at the 17th day compared with the mice in the NC group (Fig. 3A). Furthermore, the serum concentrations of E2 lower, and FSH greater significantly for mice in the POI group compared with mice in the NC group (Fig. 3B,C). The numbers of primordial, primary, secondary, and antral follicles for mice in the POI group were significantly fewer compared with mice in the NC group under ovarian histological examination (Fig. 3D,E). Moreover, the ovarian weight also decreased significantly after CTX and BUS injection compared with the mice without injection (Fig. 3F,G).
Fig. 3.
Evaluation of the POI model. (A) The body weight of mice in the NC and POI groups (n = 10, *P < 0.05). (B) The concentrations of E2 in the NC and POI groups (n = 6). (C) The concentrations of FSH in the NC and POI groups (n = 6). (D) Histopathologic images of ovaries in the NC group (a) and POI groups (b). (E) The number of follicles at different development stages. Data represent means ± SEM, n = 6; *P < 0.05. (F) Representative photograph of ovaries in the NC and POI group. (G) The weight of ovaries in the NC and POI groups (n = 6). NC: normal control; POI: premature ovarian insufficiency; FSH: follicle-stimulating hormone.
EVs Transplantation Improves the Ovarian Function of POI Mice
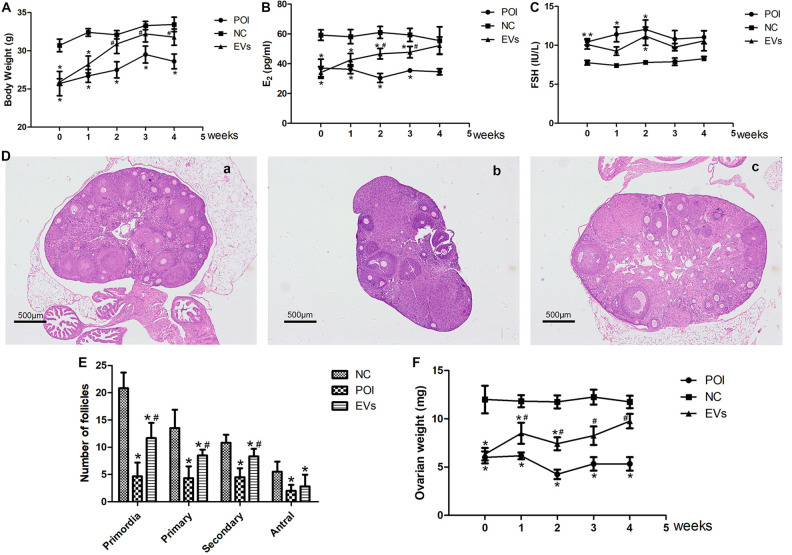
After EVs transplantation, the body weight of mice in the EVs group increasing persistently and reached the statistical difference 2 wk later compared with mice in the POI group, but it did not reach the level of mice in the NC group (Fig. 4A). The concentrations of serum E2 in the EVs group increased significantly 2 wk after EVs transplantation and was similar with mice in the NC group 4 wk after treatment (Fig. 4B). The concentrations of serum FSH for mice in the EVs group dropped 1 wk after EVs transplantation, and fluctuated afterward, but still lower than mice in the POI group (Fig. 4C). Furthermore, a significantly increase in the numbers of primordial, secondary, and antral follicles were found 4 wk after EVs transplantation (Fig. 4D,E), and the ovarian weight of the mice in the EVs group was heavier significantly than those in the POI group since 1 wk after EVs transplantation (Fig. 4F).
Fig. 4.
EVs treatment improves the ovarian function of POI mice. (A) Body weight in 3 groups during the experimental period (n = 10, *P < 0.05 vs. NC; # P < 0.05 vs. POI). (B) E2 concentrations in the NC, POI and EVs groups during the experimental period (n = 6, *P < 0.05 vs. NC; # P< 0.05 vs. POI). (C) FSH concentrations in the NC, POI and EVs groups during the experimental period (n = 6, *P < 0.05 vs. NC; # P < 0.05 vs. POI). (D) Histopathologic images of ovarian tissue 28 days after interventions in the NC (a), POI (b), and EVs (c) groups. (E) The follicles of different development stages in three groups (n = 6). (F) Ovarian weight in three groups during the experimental period (n = 6, *P < 0.05 vs. NC; # P < 0.05 vs. POI). NC: normal control; POI: premature ovarian insufficiency; EVs: extracellular vesicles; FSH: follicle-stimulating hormone.
EVs Transplantation Improves the Number of Oocytes and Embryos of POI Mice
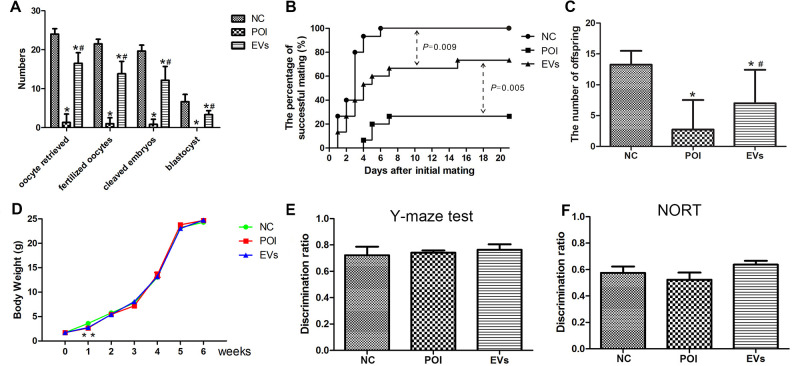
Four weeks after EVs transplantation, the total numbers of oocytes retrieved, fertilized zygotes, cleaved embryos, and blastocysts of mice in the EVs group were significantly elevated than those in the POI group (Fig. 5A). As shown in Table 2, the rates of fertilization, cleavage, and blastulation in the NC, POI, and EVs group were similar.
Fig. 5.
EVs transplantation improves the outcomes of in vitro fertilization and natural fertility of POI mice with no adverse effects on their offspring. (A) The numbers of oocytes retrieved, fertilized zygotes, cleaved embryos, and blastocysts in the NC, POI, and EVs groups (n = 6). (B) The time to get pregnancy in three groups evaluated by Log-rank (Mantel-Cox) test (P < 0.05 vs. POI, n = 15). (C) The average numbers of offspring in the NC, POI, and EVs groups (*P < 0.05 vs. NC; # P < 0.05 vs. POI). (D) The body weight of offspring in three groups (n = 25). (E, F) The Y-maze test and NORT results of offspring in three groups (n = 12). NC: normal control; POI: premature ovarian insufficiency; EVs: extracellular vesicles; NORT: novel object recognition task.
Table 2.
In Vitro Fertilization Outcomes of Three Groups.
| NC group (n = 6) | POI group (n = 6) | EVs group (n = 6) | P | |
|---|---|---|---|---|
| Oocytes retrieved, mean (SD) | 23.5 (3.0) | 2.3 (2.9) | 14.8 (2.6) | <0.001 |
| Fertilization rate, % (n) | 92.2 (130) | 71.4 (10) | 88.8 (79) | 0.702 |
| Cleavage rate, % (n) | 92.3 (120) | 70.0 (7) | 90.0 (71) | 0.375 |
| Blastulation rate, % (n) | 21.7 (26) | 14.3 (1) | 15.5 (11) | 0.239 |
NC: normal control; POI: premature ovarian insufficiency; EVs: extracellular vesicles.
EVs Transplantation Improves the Natural Fertility of POI Mice and the Effects on Offspring
During the 21-day mating test, 4, 11, and 15 female mice in the POI, EVs, and NC group gave birth, respectively. Furthermore, the female mice in the EVs group presented less time to get pregnant with a greater number of neonatal mice than mice in the POI group but did not reached the level of mice in the NC group (Fig. 5B,C). The body weight of their offspring in the three groups increased gradually after birth and showed no significant difference 2 wk after birth (Fig. 5D).
As shown in Fig. 3E,F, no significant differences were identified in the discrimination ratios of the Y-maze test (time spent in the novel arm object)/(time spent in the novel and other arms) and NORT (time spent exploring the novel object)/(time spent exploring two objects) among the three groups.
Anti-Apoptotic Effects of EVs
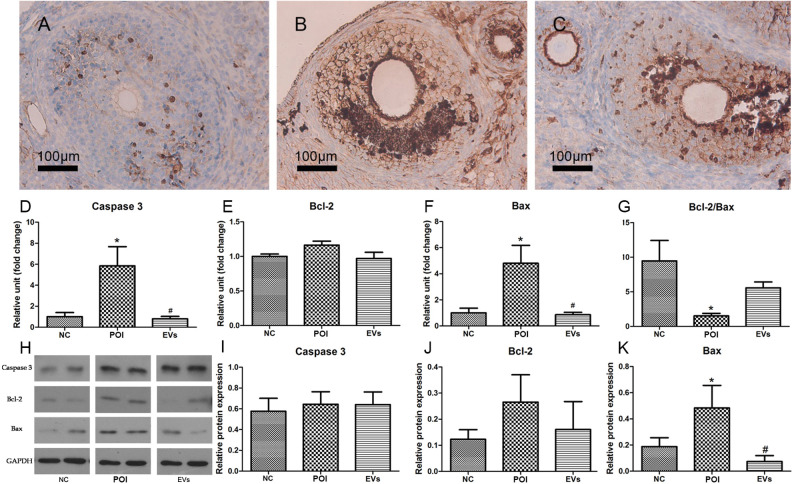
The result of immunohistochemistry indicated that cleaved caspase 3 positive cells in the EVs group was significantly lower than those in the POI group (35.11% vs. 44.84%) (Fig. 6A–C). The mRNA levels of Caspase 3 and Bax in the EVs group were significantly lower than those in the POI group, while the ratio of Bcl-2 to Bax (Bcl-2/Bax) in the EVs group was higher than those in the POI group (Fig. 6D–G). WB assay also demonstrated that the protein expressions of Bax in EVs group decreased significantly (Fig. 6K), but no differences were found in the protein expressions of caspase 3 and Bcl-2 among the three groups (Fig. 6H–J).
Fig. 6.
Anti-apoptotic effect of EVs. (A–C) The cleaved caspase 3 positive cells in ovarian tissue of the NC (A), POI (B), and EVs (C) group (n = 6). The mRNA expressions of caspase 3 (D), Bcl-2 (E), Bax (F), and Bcl-2/Bax (G) in ovarian tissue of three groups 28 days after interventions. Data represent means ± SEM; n = 7, *P < 0.05 vs. NC. (H) Western blotting detected protein expressions of caspase 3 (I), Bcl-2 (J) and Bax (K) in ovarian tissue of three groups 28 days after interventions. Data represent means ± SEM; n = 7, *P < 0.05 vs. NC; # P < 0.05 vs. POI. NC: normal control; POI: premature ovarian insufficiency; EVs: extracellular vesicles; mRNA: microribonucleic acid.
Discussion
The present study suggested that EVs derived from HUCMSCs can not only restore ovarian function by improving hormone profiles and the number of ovarian follicles, but also restore fertility by increasing the number of oocytes retrieved, shortening the time to get pregnant, and increasing the number of newborns. Furthermore, the cognitive behavior of the first generation was not damaged by the treatment of EVs for their mothers. This is the first study using IVF and mating test to examine the fertility of POI mice and evaluate their offspring after the treatment of HUCMSCs derived EVs.
A previous study demonstrated that co-culture EVs derived from HUCMSCs with granulosa cells (GCs) could proliferate GCs and enhance the secretion of E2 23. After using cisplatin to induce GCs damage, Sun et al.24 found a higher percentage of living cells in EVs co-culture group compared with cisplatin only group (80.1% vs. 71.4%, P < 0.05). In vivo study of POI mice further suggested that HUCMSCs derived EVs can recover the disturbed estrous cycle, increase the body weight, and the number of ovarian follicles of POI mice15, but they did not monitor the change of hormone profile regularly. In our study, although a greater number of ovarian follicles and better hormones profiles were identified for mice in the EVs group compared with those in the POI group, the concentrations of serum FSH kept on fluctuating throughout the study period, which indicates that serum FSH concentrations might not be validated enough to evaluate the recovery of ovarian function of POI mice.
There is growing evidence that EVs plays an essential role in oocyte and sperm maturation, fertilization, prevention of polyspermy, and embryo implantation25. Several in vitro studies have demonstrated that EVs derived from MSCs can be used as a culture supplement to improve embryo quality and recover the embryo developmental competence26–29. In this study, we found HUCMSCs derived EVs can increase the numbers of oocytes retrieved, fertilized zygotes, cleaved embryos, and blastocysts of mice in the EVs group compared with mice in the POI group. This finding was supported by Marinaro et al.30 who demonstrated that the expanded blastocyst rate of aged female mice (aged 24 wk old) could be increased when EVs from human endometrial MSCs (range from 10 µg/ml to 80 µg/ml) were added to the culture medium. Their further study suggested that not only developmental competence, but also the quality of the embryos could be enhanced by EVs transplantation29. Inconsistent with their study, there were no significant differences in the rates of fertilization, cleavage, and blastulation among the three groups in our study, suggesting that HUCMSCs-EVs might not improve the quality of embryos. However, it is important to note that the oocytes retrieved in our study were from young female mice (aged 11–12 wks) which have a better developmental competence than age advanced mice used in the previous studies, and the effects of EVs in vivo and in vitro as well as different breed of mice may result in this different conclusion. In addition, the results of the mating test (natural fertility) in our study indicated that EVs transplantation could shorten the time to get pregnant for POI mice and increase the number of their newborns, enhancing the conclusion that EVs can restore the fertility of POI mice.
Although transplantation of EVs derived from MSCs is an attractive agent for cell-free therapy, safety concerns restrain its clinical application. Sun et al.14 administrated HUCMSCs derived EVs to rabbits, guinea pigs, and rats, and found no adverse effects on liver or renal function. Another toxicology study indicated that EVs derived from MSCs were safer than those from bovine milk31. Recently, a review demonstrated that EVs transplantation was safe in mammals with the advantage of avoiding some inherent side effects of stem cell transplantation, such as malignant stem cell transformation32. However, no study investigated the impact of EVs treatment for the female on their offspring. In this study, we found that the body weight of the offspring mice were similar to those without EVs treatment to the mothers. The immediate working memory and recognition memory tested by Y-maze test and NORT of the offspring were similar in each group, suggesting that EVs treatment for female mice will not cause cognitive deficits of the offspring.
The mechanisms of EVs on restoring ovarian function and fertility remain unclear. Except for angiogenesis function of EVs for ovarian tissue through upregulating the expression of the vascular endothelial growth factor, insulin-like growth factor and angiogenin15, anti-apoptotic effect of EVs on GCs may also contribute to their ability to protect the fertility of POI mice. The study of Xiao et al.33 demonstrated that amniotic fluid stem cells derived EVs can prevent ovarian follicular atresia in CTX induced POI mice via the delivery of mRNAs in which both miR-146a and miR-10a were highly enriched and their potential target genes were critical to apoptosis. In this study, we found a higher percentage of cleaved caspase 3 positive cells in ovarian tissue of the mice in the POI group compared with those in the EVs group. Moreover, the mRNA expression of apoptosis-related genes (caspase 3 and Bax) in EVs group down-regulated significantly, indicating an antiapoptotic effect of EVs transplantation, which might be a potential mechanism related to recovery of ovarian function and fertility. Although WB assay also showed that the protein expressions of Bax in EVs group decreased significantly compared with the POI group, no difference was found in the protein expressions of caspase 3 among the three groups, which may attribute to posttranscriptional or posttranslational modifications or protein degradation or the different sensitivity between WB and qPCR.
There are several limitations to the study. Firstly, we do not distinguish larger vesicles (microvesicles) and small vesicles (exosomes) from EVs, so it cannot be sure which one plays a more critical role in restoring the fertility of POI mice. Secondly, the sample of this study used for IVF is limited; some results remain to be verified in future study with a large sample size. Thirdly, the model of POI mice in this study was established by the intraperitoneal injection of CTX/BUS which means the effect of the EVs could be specific to the chemically induced POI. Finally, we only use cognitive behavior tests to evaluate offspring, which is not enough. More comprehensive systematic behavior tests with an extended duration of follow-up are needed for further evaluation of the safety of HUCMSCs derived EVs on offspring before it is applied to the clinic.
Conclusion
The present study demonstrated that EVs derived from HUCMSCs can recover the fertility of POI mice and have no adverse influences on the cognitive behavior of their offspring, which may be severed as a prospective cell-free therapy for patients suffering from POI.
Acknowledgments
The authors thank all the staffs in Reproductive Center, Liwei Liu in Cell Therapy Center, and Jian Wang of the Department of Pathology of 105th Hospital of PLA.
Footnotes
Authors’ Contribution: CL contributed to designing the study, data collection and analysis, writing of the original paper. HY contributed to conceiving and designing the study. HJ contributed to conceiving and designing the study, paper reviewing and editing. XD, CW, YL, YL, and ZY contributed to data collection.
Additional Information: The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Data Availability Statement: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval: This study was approved by the Institutional Animal Care and Use Committee of Anhui Medical University (No. LLSC20160337).
Statement of Human and Animal Rights: All experimental procedures in this study were approved by the Institutional Animal Care and Use Committee of Anhui Medical University.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science and Technology Innovation Project of Nanjing Military Region (grant number 14ZX06 and 11Z010).
ORCID iD: Hong Jiang  https://orcid.org/0000-0002-4690-7906
https://orcid.org/0000-0002-4690-7906
References
- 1. Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. 2003;18(1):199–206. [DOI] [PubMed] [Google Scholar]
- 2. Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck Keizer- Schrama S, Hogervorst E, Janse F. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–937. [DOI] [PubMed] [Google Scholar]
- 3. Panay N, Fenton A. Premature ovarian failure: a growing concern. Climacteric. 2008;11(1):1–3. [DOI] [PubMed] [Google Scholar]
- 4. Cancer CGoHFiB. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52 705 women with breast cancer and 108 411 women without breast cancer. Lancet. 1997;350(9084):1047–1059. [PubMed] [Google Scholar]
- 5. Canonico M, Plu-Bureau G, Lowe GD, Scarabin P-Y. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frith L. Gamete donation and anonymity: the ethical and legal debate. Hum Reprod. 2001;16(5):818–824. [DOI] [PubMed] [Google Scholar]
- 7. Hudson N. Egg donation imaginaries: embodiment, ethics and future family formation. Sociology. 2020; 54(2):346–362. [Google Scholar]
- 8. ELA S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. [DOI] [PubMed] [Google Scholar]
- 9. Maas SL, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular vesicles: novel mediators of cell communication In metabolic disease. Trends Endocrinol Metab. 2017;28(1):3–18. [DOI] [PubMed] [Google Scholar]
- 11. Ohno SI, Drummen G, Kuroda M. Focus on extracellular vesicles: development of extracellular vesicle-based therapeutic systems. Int J Mol Sci. 2016;17(2):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang J, Cen P, Chen J, Fan L, Li J, Cao H, Li L. Role of mesenchymal stem cells, their derived factors, and extracellular vesicles in liver failure. Stem Cell Res Ther. 2017;8(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L, Gu Z, Zhao X, Yang N, Wang F, Deng A, Zhao S, Luo L, Wei H, Guan L. Extracellular vesicles released from human umbilical cord-derived mesenchymal stromal cells prevent life-threatening acute graft-versus-host disease in a mouse model of allogeneic hematopoietic stem cell transplantation. Stem Cells Dev. 2016; 25(24):1874–1883. [DOI] [PubMed] [Google Scholar]
- 14. Sun L, Xu R, Sun X, Duan Y, Han Y, Zhao Y, Qian H, Zhu W, Xu W. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy 2016;18(3):413–422. [DOI] [PubMed] [Google Scholar]
- 15. Yang Z, Du X, Wang C, Zhang J, Liu C, Li Y, Jiang H. Therapeutic effects of human umbilical cord mesenchymal stem cell-derived microvesicles on premature ovarian insufficiency in mice. Stem Cell Res Ther. 2019;10(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;3(22):1–29. [DOI] [PubMed] [Google Scholar]
- 17. Kruger N.J. The Bradford method for protein quantitation In: Walker JM. (ed.), The protein protocols handbook. Springer Protocols Handbooks Totowa (NJ): Humana Press; 2009:17–24. [Google Scholar]
- 18. Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3(1):26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009, Appendix 4(48):Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takeo T, Nakagata N. In vitro fertilization in mice. Cold Spring Harb Protoc. 2018, 2018(6):1–7. [DOI] [PubMed] [Google Scholar]
- 21. Alkam T, Kim HC, Mamiya T, Yamada K, Hiramatsu M, Nabeshima T. Evaluation of cognitive behaviors in young offspring of C57BL/6 J mice after gestational nicotine exposure during different time-windows. Psychopharmacology. 2013;230(3):451–463. [DOI] [PubMed] [Google Scholar]
- 22. Reger ML, Hovda DA, Giza CC. Ontogeny of rat recognition memory measured by the novel object recognition task. Dev Psychobiol. 2009;51(8):672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan LI, Yin HQ, Chen JJ, Liu YC, Jiang H. Effect of mesenchymal stem cell-derived microvesicles on ovarian granular cells. J Reprod Med. 2016; 25(12):1089–1094. [Google Scholar]
- 24. Sun L, Li D, Song K, Wei J, Yao S, Li Z, Su X, Ju X, Chao L, Deng X. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Sci Rep. 2017;7(1):2552–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update. 2016;22(2):182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopera-Vasquez R, Hamdi M, Maillo V, Gutierrez-Adan A, Bermejo-Alvarez P, Ramirez MA, Yanez-Mo M, Rizos D. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction. 2017;153(4):461–470. [DOI] [PubMed] [Google Scholar]
- 27. Blazquez R, Sanchez-Margallo FM, Alvarez V, Matilla E, Hernandez N, Marinaro F, Gomez-Serrano M, Jorge I, Casado JG, Macias-Garcia B. Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates. PLoS One 2018;13(4):e0196080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lopera-Vasquez R, Hamdi M, Fernandez-Fuertes B, Maillo V, Beltran-Brena P, Calle A, Redruello A, Lopez-Martin S, Gutierrez-Adan A, Yanez-Mo M. Extracellular vesicles from BOEC in in vitro embryo development and quality. PLoS One 2016;11(2):e0148083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marinaro F, Macias-Garcia B, Sanchez-Margallo FM, Blazquez R, Alvarez V, Matilla E, Hernandez N, Gomez-Serrano M, Jorge I, Vazquez J, et al. Extracellular vesicles derived from endometrial human mesenchymal stem cells enhance embryo yield and quality in an aged murine modeldagger. Biol Reprod. 2019;100(5):1180–1192. [DOI] [PubMed] [Google Scholar]
- 30. Marinaro F, Pericuesta E, Sanchez-Margallo FM, Casado JG, Alvarez V, Matilla E, Hernandez N, Blazquez R, Gonzalez-Fernandez L, Gutierrez- A. Extracellular vesicles derived from endometrial human mesenchymal stem cells improve IVF outcome in an aged murine model. Rep Domest Anim. 2018;3(Suppl 2):46–49. [DOI] [PubMed] [Google Scholar]
- 31. Maji S, Yan IK, Parasramka M, Mohankumar S, Matsuda A, Patel T. In vitro toxicology studies of extracellular vesicles. J Appl Toxicol. 2017;37(3):310–318. [DOI] [PubMed] [Google Scholar]
- 32. Doeppner TR, Bahr M, Hermann DM, Giebel B. Concise review: extracellular vesicles overcoming limitations of cell therapies in ischemic stroke. Stem Cells Transl Med. 2017;6(11):2044–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao G-Y, Cheng C-C, Chiang Y-S, Cheng WT-K, Liu IH, Wu S-C. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep. 2016;6:23120. [DOI] [PMC free article] [PubMed] [Google Scholar]