Abstract
Recent data suggest gut microbiota dysbiosis as a contributing factor in neurodegenerative diseases, such as Parkinson’s Disease (PD) and Alzheimer’s Disease (AD), and these pathologies may manifest via the microbiota-gut-brain-axis, which comprises bidirectional communication through neuroimmune, neuroendocrine, and direct neural pathways such as the vagus nerve. Preclinical and human clinical trial data reveal exciting potential for novel treatment targets and therapeutic modulation with prebiotics, medicinal herbs, probiotics, and synbiotics in health, aging, and neurodegeneration and are reviewed here. While greater insights and characterization of the microbiota-gut-brain axis have been revealed over the past decade, salient questions related to the pathology, pathogenesis and clinical treatment of the axis in the context of both health and neurodegenerative disease remain and are discussed in this review. Future directions such as additional well-controlled, large scale, longitudinal human clinical trials are urgently needed to further elucidate both mechanism and therapeutic opportunity in health, neurological disease, and disease subpopulations to ensure that the next decade ushers the dawn of targeted therapeutic modulation of the microbiota-gut-brain axis.
Keywords: prebiotic, probiotic, gut-brain axis
Introduction
Many millions of individuals are affected by neurodegenerative disease worldwide, and global health improvements largely driven by clinical research have further augmented the human lifespan potential to increase this disease and economic burden.1,2 Neurodegenerative diseases, such as Parkinson’s Disease (PD) and Alzheimer’s Disease (AD), represent a heterogenous collection of disorders that feature deterioration of the central and/or peripheral nervous systems that affect an estimated 1% and 8% of the population, respectively.3 Predictions by the World Health Organization estimate that by 2040, neurodegenerative diseases will bypass cancer to become the second leading cause of death globally.4 As life expectancy in developed nations continues to increase, the economic burden for the treatment of neurodegenerative conditions is expected to increase substantially beyond the $20 billion currently spent per annum. Thus, the identification of early disease biomarkers and both new drug targets and therapeutics to attenuate the progression of neurodegeneration are greatly needed. In addition, as gastrointestinal comorbidities are common in neurodegenerative diseases such as PD and AD, management of the gut microbiota is perceived to be important in clinical care to both treat and potentially prevent these chronic conditions.
Microbiota-Gut-Brain Axis
Accommodating the highest cell densities documented for any ecosystem, the human gut contains around 1013 to 1014 bacteria5,6 with a diversity of at least 1,000 species.7 Over 50 phyla colonize the planet but the predominant phyla in the mammalian intestine include Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, and Fusobacteria.8 With its 400 m2 of surface area, the gastrointestinal (GI) tract represents one of the largest interfaces for host-microbe interactions.5,9 The microbiota interacts with the host in a dynamic way, contributing to both health and disease. The gut microbiota performs important protective and metabolic functions, such as fermentation of indigestible plant components and endogenous mucous using genes encoding enzymes and biochemical pathways not contained within the host genome.10
The information encoded by the mammalian genome is not sufficient to maintain health and protect against disease. However, some or most gut bacteria are mutualists and after millions of years of co-evolution provide many key health functions to the host such as adequate digestion, metabolism, nutrient biosynthesis, angiogenesis, detoxification, enterocyte growth, defense against colonization by opportunistic pathogens, and immune system development and function.11–15 The gut microbiota provides enzymes for the synthesis of vitamin K, B vitamins, and amino acids.12 A major function of this bacterial population is energy harvest through the metabolism of otherwise indigestible complex polysaccharides from food and the subsequent generation of short-chain fatty acids (SCFA).16 The GI tract is also a site of production for hormones involved in energy homeostasis (e.g. insulin, glucagon, leptin, and ghrelin) and growth (e.g. glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP)).17 Symbiotic bacteria interact with the host in dynamic ways to influence the development and function of the intestinal architecture, immune system, and gut brain axis.
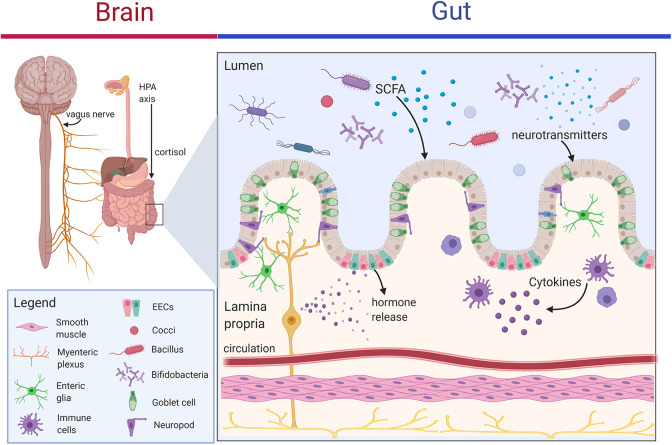
The microbiota-gut-brain axis, or gut-brain axis, refers to interactions between the enteric nervous system (ENS) within the GI tract and the central nervous system (CNS) through a bi-directional communication system that is comprised of neural, immune, and endocrine pathways (Figure 1).18,19 The enteric nervous system, often referred to as the “second brain,” contains as many nerves as the spinal cord, regulates basic gut functions such as gut motility, intestinal permeability, enteroendocrine signaling, and mucosal immune activity and shares similar neurotransmitters and signaling molecules with the brain. The brain communicates with the GI tract through multiple, parallel pathways which include both sympathetic and parasympathetic divisions of the autonomic nervous system (ANS), the hypothalamic pituitary adrenal axis (HPA), and sympathoadrenal axis, which modulates the gut-associated lymphoid tissue.20 Mostly vagal afferents communicate from the intestine to the brain and represent 80–90% of all vagal fibers.21 The vagal afferent fibers also interface with the HPA axis to coordinate the stress response and anti-inflammatory actions.
Figure 1.
Communication pathways of the microbiota-gut-brain axis. Multiple hard-wired or direct (e.g. ENS and vagus nerve) and indirect (e.g. neurotransmitters, SCFAs, cytokines) communication pathways of the gut-brain axis are modulated by gut microbiota. These routes include the neural pathway (e.g. vagus nerve, ENS, neurotransmitters and neuroactive metabolites such as the SCFA butyrate), immune pathway (e.g. cytokines), and neuroendocrine pathways (e.g. gut hormone secretion such as peptide YY, neuropeptide Y, and glucagon-like peptide-1; cortisol secretion via the hypothalamus pituitary adrenal (HPA) axis). Neuroactive dietary and microbially-produced metabolites modulate the microbiome-gut-brain axis to affect gut-barrier function, hormone secretion from enteroendocrine cells (EECs), neurotransmitter production by gut epithelium and microbiota, and enteric glial signaling which are relevant to neurodegenerative disease. Image was generated with BioRender.
The gut has been recognized as the largest endocrine organ in the body, producing myriad hormones and bioactive peptides.22 Gut to brain signaling in the gut is accomplished by highly chemosensitive primary afferent neurons, immune cells and enteroendocrine cells, which contain over 30 different hormones. Immune pathways, such as cytokine signaling stimulated by microbial lipopolysaccharide (LPS) or peptidoglycan, also represent a communication link to the brain. Changes in gut barrier integrity may also lead to translocation of these microbial products in the periphery with downstream microglial activation and neuroinflammation.23
A small set of specialized gut epithelial cells, called enteroendocrine cells (EEC) monitor the contents of the lumen and secrete hormones and signaling mediators such as peptides that bind to vagal afferent receptor sites. Metabolites from gut commensal microbes also regulate the hormone secretion from EECs to affect processes such as intestinal transit time.24 As EECs directly provide signals to the brain via the vagus nerve, this neuroendocrine pathway is a key bidirectional pathway. EECs can interface with enteric glial cells, which are important in barrier and defense functions, to affect hormone secretion and ENS signaling. In addition, about 95% of serotonin is produced by specialized neuroendocrine cells, namely enterochromaffin cells, in the gut mucosa beneath the epithelium with mechanosensory and chemosensory functions that regulate motility and secretion.25 The gut microbiota also provides a key role in tryptophan, the serotonin precursor, metabolism. In addition to enteric nerves, the various hormones, immune mediators, and microbial metabolites represent pathways through which the brain receives information about environmental exposures such as diet and the gut ecosystem functioning.
Role of Gut Microbiota Dysbiosis in Neurodegenerative Disease
Research points to microbiota dysbiosis as a contributing factor in neurological disease such as neurodegeneration and mood disorders.26,27 Some of this pathology may occur via the microbiota-gut-brain-axis. While multiple pathways are currently under investigation, additional research must clarify the mechanisms through which residential microbes impact this axis. Gut microbes affect this bidirectional communication system through neuroimmune, neuroendocrine, and direct neural pathways such as the vagus nerve. Mounting evidence suggests that microbes can produce neurotransmitters, promote serotonin production by gut epithelial cells, produce bioactive constituents, alter epigenetic regulation with fermentation by-products, and release metabolites that may enter circulation and cross the blood brain barrier.28 In addition, both the elderly and those with neurodegenerative disease exhibit reduced gut microbial biodiversity.29–31 During gut dysbiosis, dysfunctional gut brain axis signaling leads to increased oxidative stress and inflammation as well as imbalanced metabolism and immune function.32
Recent burgeoning investigations suggest that altered gut microbiota and microbial metabolites such as butyrate and amyloid are associated with neurodegenerative diseases such as PD, AD, and amyotrophic lateral sclerosis (ALS); however, the precise role, mechanisms and any causal relationships with microbiota have yet to be fully elucidated. A recent study reported gut microbiota dysbiosis in AD and suggested that it may contribute to the pathology of AD.33 In the AD patients compared to age- and gender-matched non-AD controls, Bacteroidaceae, Veillonellaceae, and Lachnospiraceae, which contain several key butyrate producers, decreased while Ruminococcaceae, Enterococcaceae, and Lactobacillaceae increased in relative abundance at the family level. Studies have also indicated reduced abundance of gram-negative species and increased intestinal permeability in AD. In addition, several potentially pathogenic gut residents such as Escherichia coli, Klebsiella pneumoniae, Mycobacterium tuberculosis, Salmonella enterica, Salmonella typhimurium, and Staphylococcus aureus, are known to produce amyloid proteins, which have been proposed as potential seeds in the formation of pathological amyloid protein misfolding.34 In a mouse model of AD, antibiotic exposure lowered the biodiversity of the gut microbiota and reduced both neuroinflammation and amyloidosis thus implicating the microbiota in AD pathology.35 In ALS patient stools, increases in potentially inflammatory Ruminococcaceae, Enterobacteria, and Escherichia coli have been observed compared to matched controls.36,37
Investigations of dysbiosis in PD have revealed some key features of the PD gut microbiome and metagenome. For example, several studies have reported a reduction in the relative abundance of Prevotella,38–40 which may be associated with reduced metabolism of high-fiber foods, mucin production, gut barrier function, and SCFA levels. SCFAs such as butyrate have been observed as lowered in PD stool compared to age-matched controls and represent a relevant clinical consideration in patients given the anti-inflammatory and neuroactive effects of these microbial end products of fermentation.39 Reduced relative abundance of other butyrate producers such as Blautia, Coprococcus, Faecalibacterium and Roseburia species has been observed in PD stool compared to healthy controls and suggests potentially increased intestinal permeability in these patients given that butyrate mediates barrier integrity and neuroimmune mechanisms.41–43 This reduced Lachnospiraceae family abundance is consistent with lowered SCFA levels observed in PD. Other salient changes in the PD gut microbiome include reductions in relative abundances of Bifidobacterium, Bacteroides fragilis, and Clostridium leptum 44 which indicates lowered beneficial anti-inflammatory species. In addition, increases in Enterobacter,38 Ruminococcus,42 and Ralstonia 43 species have been observed in PD and are associated with potential inflammation. A recent microbiome-wide-associated study found 15 genera that are different in PD compared to healthy subjects. The 5 genera Porphyromonas, Prevotella, Corynebacterium, Bifidobacterium, and Lactobacillus were higher in abundance while the 10 genera Faecalibacterium, Agathobacter, Blautia, Roseburia, Fusicatenibacter, Lachnospira, Butyricicoccus were lower in abundance in PD compared to controls. The data from these genera formed 3 distinct clusters and suggest the PD microbiome is associated with an increased abundance of a cluster of opportunistic pathogens, reduced abundance of SCFA-producing microbes, and increased levels of carbohydrate metabolizers.
The PD associated metagenome features increased representation of genes annotated in LPS biosynthesis and type III bacterial secretion systems, which suggests potentially increased inflammation from microbial products.43 These observations support emerging evidence which suggests that neuroinflammation in PD may be induced by circulating inflammatory products in the periphery.45 While gut barrier dysfunction may allow microbial metabolites and antigens, such as LPS, into circulation to potentially activate neuroimmune responses, additional investigation is needed to define pathological mechanisms.
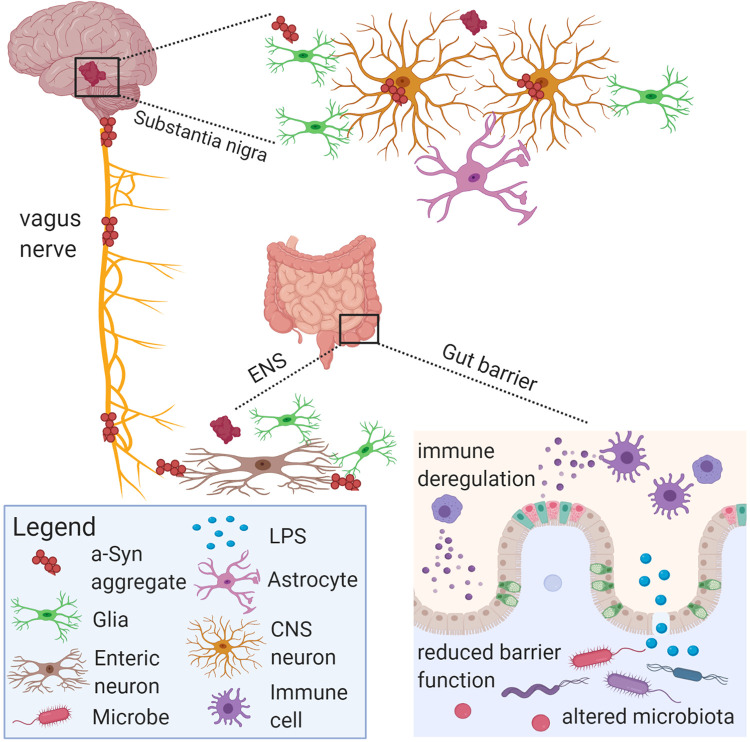
Mounting evidence on gut dysbiosis in PD patients suggests both qualitative and quantitative changes in the gut microbiota are associated with PD and its progression44; however, the precise role, mechanisms and any causal relationships with microbiota have yet to be fully established.46 Gut dysbiosis may lead to lowered or altered SCFA profiles, decreased mucin production, increased bacterial/antigen translocation which contributes to increased intestinal permeability, inflammation, altered gut-brain axis communication, and reduced nutritional status such as B1 and folate deficiency.26 Gut and systemic inflammation as well as activation of microglia or enteric glia are also hypothesized to contribute to alpha synuclein misfolding in PD and represent areas of priority for future investigation (Figure 2).
Figure 2.
Schematic diagram of aspects of PD pathology in the microbiota-gut-brain axis. Gut microbiota and their metabolites may communicate with the vagus nerve through enteroendocrine cells that synapse with vagus nerve terminals through specialized structures called neuropods. Microbial antigens such as LPS may cross the gut epithelium through M cells or areas with increased intestinal permeability to trigger local and systemic inflammation. Pathology may develop within myenteric nerve cell plexus with subsequent inflammation, oxidative stress and thus alpha-synuclein accumulation. The vagus nerve might facilitate the pathological spread of alpha-synuclein aggregates from the ENS to the brain. Image was generated with BioRender.
Key features of PD pathology include loss of dopamine-producing neurons in the substantia nigra and Lewy bodies containing inclusions of alpha-synuclein, which is expressed in neurons and enteroendocrine cells in the gut.47 Alpha synucleinopathy has been observed in the enteric nervous system prior to disease onset and subsequent central nervous system involvement; thus, it is hypothesized that PD pathology may originate in the gut.48,49 In mice overexpressing alpha-synuclein, it was determined that the gut microbiota were required for motor symptoms, Lewy body pathology, microglial cell activation, and neuroinflammation. Germ-free versions of these mice exhibit greater motor dysfunction when fecal microbiota transplantation from PD patient donors were compared to healthy donors. In addition, oral administration of microbial metabolites to germ-free mice promoted motor dysfunction thus providing a functional link between gut microbiota and neurodegenerative disease via gut to brain signaling.49 Similarly, a recent genome-wide-association study found that PD was genetically associated with cholinergic and monoaminergic neurons, including dopaminergic neurons, enteric neurons and oligodendrocytes.50
Dysbiosis and lowered gut biodiversity seen in aging and neurological diseases may contribute to neuroinflammation underlying neurodegenerative diseases.51 Microbiota may contribute to neuroinflammation in several ways including via endotoxic bacterial cell wall constituents such as LPS or microbiota-produced amyloid. Therapeutic modulation of dysbiotic gut microbiotas toward an increased abundance of butyrate-producers may ameliorate neuroimmune activation.
Gut-Related Amyloids in Neurodegenerative Disease
In addition to alterations in gut microbiota, neurodegenerative diseases such as PD and AD exhibit key protein misfolding, aggregation, and accumulation in the brain.52 Proteins hypothesized to be involved in neurodegeneration, such as alpha-synuclein in PD and both amyloid beta (Aβ) and tau in AD, are heavily studied proteins that adopt cross beta-sheet amyloid structures.53,54 Evidence suggests that proteins may misfold in the gut then migrate to the brain via hypothesized routes that include M cells that sample the lumen, goblet cell-associated antigen passages, or enteroendocrine cells. In addition, recent evidence demonstrates that enteroendocrine cells, which express alpha-synuclein, have neuron-like features and synapse with enteric nerves found to contain alpha-synuclein.55 This represents an additional potential route of the spread of pathological alpha-synuclein in addition to the longer established hypothesis of neuron-to-neuron propagation in a prion-like manner which can then seed new oligomers, fibrils and eventually Lewy bodies.56 Misfolded alpha-synuclein has been detected in the brain, peripheral nervous system including the gut, and gastrointestinal mucosa.57 As the substantia nigra also exhibits high mitochondrial oxidative stress, several pathways and endogenous factors likely contribute to the pathology of PD and other neurodegenerative diseases. While the misfolded and aggregated proteins of various neurodegenerative diseases remain discrete, the conserved nature of the misfolded conformation and prion-like features result in neuroinflammation and potentially similar pathways for therapeutic intervention. Indeed, a recent study reported that probiotic treatment inhibited alpha-synuclein aggregation and facilitated aggregate clearance in a Caenorhabditis elegans model of synucleinopathy thus suggesting gut microbiota modulation as a potential therapy in PD.58
The identification of alpha-synuclein gene expression in enteroendocrine cells, enteric neurons and the vagus nerve reveal a potential neuroepithelial pathway through which PD and perhaps other neurodegenerative disease may begin in the gut.59–61 Interestingly, alpha-synuclein can be transported bidirectionally via the vagus nerve and while incompletely understood, such inclusions found in submucosal and myenteric nerves may first arise in the gut prior to potential transport to brain regions.57,62,63 The vagus nerve is thought to be a pathological route for alpha-synuclein given clinical evidence that truncal vagotomy reduced the risk of PD development as well as findings in animal models.64–67 In the gastrointestinal tract, alpha-synuclein is expressed in preganglionic vagal nerves and the submucosal and myenteric plexuses.68 While these nerves do not directly sample the contents of the lumen, alpha-synuclein expressing enteroendocrine cells are part of the gastrointestinal lining and may represent a physical link to the enteric nervous system.
As noted earlier, EECs are chemosensory cells with neuron-like properties within the epithelial layer of the gut lumen that may represent a direct conduit between the lumen and nervous system via enteric nerves.69 Stimuli such as dietary nutrients and bacteria elicit the transmission of signals from the basal side of the EECs, which contain neuronal features such as axon-like processes called neuropods, neurofilaments, neurotrophin receptors, dopamine synthesis potential, and synaptic proteins.70,71 While paracrine transmission of hormonal sensory signals from EECs has been long understood, recently the direct enteroendocrine cell to nerve contact has recently been observed in vivo via the axon-like processes of EECs.59,69,70 Such observations lead to interesting speculation regarding the nature of a neural pathway via EECs. The EECs receive and relay messages from the lumen to the nervous system and may transport pathogens and potentially toxins to such sites.
Given that enteroendocrine cells express alpha-synuclein, exposure to toxins, pathogens, poor diet, or inflammatory conditions could promote misfolding and transport to enteric neurons such as submucosal, myenteric neurons and preganglionic vagal nerves, which also express alpha-synuclein, to trigger a prion-like cascade of events leading to alpha-synucleinopathies.68 Some hypothesize that abnormal alpha-synuclein aggregates in EECs spread to the nervous system through their synapse with alpha-synuclein containing enteric nerves to later enter the vagus nerve and brain.55 Interestingly, alpha-synuclein aggregates are secreted from neurons via exocytosis.72 In addition, neurons cultured in vitro transport alpha-synuclein aggregates via endocytosis.73 The alpha-synuclein aggregates may propagate via endocytosis to adjacent neurons and neuronal precursor cells to form Lewy body type inclusions.56 Amyloid type alpha-synuclein aggregates are the main constituents of Lewy bodies in PD which are related to synaptic dysfunction and neurodegeneration of dopaminergic neurons.74 Given that EECs are exposed to gut microbes and express receptors for microbial metabolites such as SCFAs, the gut microbiota itself may play a currently unknown role in this gut neural circuit. This potential pathway from gut to brain may encourage dietary, probiotic and nutraceutical interventions in neurodegenerative disease. As gut microbes may potentially influence alpha-synuclein morphology or communication through the enteroendocrine neural pathway, probiotics and microbial metabolites represent potential therapeutics that warrant further exploration in this context.
Interestingly, several species of the human microbiota produce curli, which are functional fibers that share biophysical properties with amyloids.75 Such amyloid-like fiber producing microbes include Bacillus, Citrobacter, Escherichia, Klebsiella, Mycobacteria, Pseudomonas, Salmonella, Staphylococcus, and Streptococcus species.76 These proteins enable microbes to form biofilms that are resistant to both physical and chemical eradication. Rats orally treated with an E. coli strain that produced curli had increased production of alpha-synuclein in the gut and brain, increased aggregation of alpha-synuclein in the brain, and increased neuroinflammation compared to rats exposed to a non-curli producing control.77 Both PD patient-derived and E. coli-produced alpha-synuclein were able to seed the formation of Lewy body-like inclusions that spread from the GI tract via the vagus nerve to the brain in a rat model.78
While prions self-seed to trigger the same type of protein to adopt a pathological conformation, amyloids may also cross-seed other proteins.79 Microbially-produced amyloids are released into the extracellular space where they can be internalized by adjacent cells such as enteric neurons to seed the formation of pathological alpha-synuclein through permissive templating.80 The spreading of pathological aggregates may occur molecule-to-molecule, cell-to-cell and nervous system region-to-region. Bacterial amyloid and amyloids derived from other sources may cross-seed the formation of additional neural protein aggregates or may be endocytosed by enteric nerves, although additional investigation into biological mechanisms is necessary.
Emerging hypotheses regarding the pathogenesis of AD suggest potential microbial infection. A greater relative abundance of proinflammatory microbial species have been reported in cognitively impaired elderly patients with amyloidosis compared to controls.81 Aβ was reported as inhibitory to several pathogens including E. coli, Staphylococcus aureus, and Candida albicans. The study concluded that pathogenic microbes elicit innate immune responses whereby Aβ serves an antimicrobial role through biofilm formation.82 In a follow-up study, a mouse model of AD was used to create a Salmonella model of brain infection. The infection increased the seeding of Aβ plaques in the hippocampus and temporal cortex, which are areas vulnerable to BBB permeability, and Salmonella was recovered from within these plaques.83 Other researchers have identified other potential infection link in AD neuropathology such as Herpes Simplex Virus 1, which was identified within microbial biofilms colocalized with Aβ in AD patient derived brain samples.84 As evidence suggests a potential mechanism of microbial infection involved in the pathogenesis of AD, additional research on immune responses that result in Aβ and plaque formation and the role of amyloid in innate immunity are needed.
Therapeutic Opportunities and the Gut Brain Axis
Prebiotics and the Gut-Brain Axis
The impact of gut dysbiosis in various diseases has fueled investigation into strategies to therapeutically modulate gut microbiomes and the immune system.85 Important areas of therapeutic opportunities include dietary treatment such as probiotics, synbiotics, and prebiotics in the form of isolates or herbal medicines. Prebiotic oligosaccharide isolates are commonly used as dietary supplements. The prebiotic potential of herbal medicines has also recently been established.86–88 The prebiotic definition, while highly debated, most commonly refers to dietary carbohydrates that are selectively fermented by gut microbiota to modulate the composition of microbiota and in turn confer health benefits to the host.89,90 Prebiotics reach the site of action in the colon and are fermented by saccharolytic microbes such as beneficial Bifidobacteria. The end products of microbial carbohydrate fermentation include SCFA such as butyrate which perform several host-specific functions. For example, butyrate can function as a histone deacetylase inhibitor, inhibit gene expression of proinflammatory cytokines, improve gut barrier function, induce T-regulatory cells, and function as a signaling molecule via the gut-brain axis.91,92 SCFAs such as butyrate also mediate neuroimmune mechanisms, inflammatory processes that drive inflammaging, and the integrity of both the gut and blood brain barriers.23,93 The microbe-produced SCFAs may be influencing brain-derived neurotrophic factor (BDNF) expression and thus various brain functions as well as the survival of existing and growth of new neurons.
Several murine studies have demonstrated the psychophysiological effects of prebiotics. A study examining B-GOS, FOS (fructooligosaccharide), or placebo in a rat model reported alterations in pivotal receptors for synaptic plasticity and memory function.94 Specifically, probiotic supplementation increased gene expression of BDNF expression in the hippocampus and dentate gyrus as well as N-methyl-d-aspartate receptor (NMDAR) expression in the hippocampus. In neonatal rats, B-GOS supplementation also increased BDNF and NMDAR expression in the hippocampus compared to placebo, which was detectable 26 days after cessation of treatment.95 Similar effects using a human milk oligosaccharide, 2′-fucosyllactose, have been observed in both mouse and rat models whereby BDNF expression was increased in cortical structures in parallel with increased long-term potentiation.96,97 In neurodegenerative disease models such as an amyloid-β1-42-induced rat model of Alzheimer’s disease, chitosan oligosaccharides (COS) reduced cognitive deficits by inhibiting oxidative stress and neuroinflammatory responses.98 In a SOD1G93A mouse model of amyotrophic lateral sclerosis, GOS decreased activation of astrocytes and microglia as well as motor neuron death.99 While the translation of these findings to humans is promising, additional studies remain necessary.
Very few studies have examined prebiotics targeting the microbiota-gut-brain axis in human interventions (Table 1). One study used B-GOS, FOS or placebo to determine emotional appraisal and the psychophysiological effects of the prebiotics treatment.112 Healthy human participants that consumed B-GOS had a significantly improved cortisol awakening response, which is a marker of emotional disorders such as depression and stress. The B-GOS prebiotic also reduced vigilance, which suggests a reduced reaction to negative emotions and potential anti-depressant and anti-anxiety (anxiolytic) effects. The authors concluded that prebiotics may modulate neural networks associated with emotional attention, such as reduced attention to negative stimuli, to exert the observed beneficial effects. A clinical trial administering oligofructose-enriched inulin reported that treatment improved mood, recognition memory, and recall compared to placebo.113 Taken together, these results also suggest that prebiotics could be personalized to contain mental performance enhancing effects as well as to improve mood and neural functioning. Prebiotic investigations in populations with neurodegenerative disease are lacking.
Table 1.
Selected Clinical Trials Investigating Modulation of the Microbiota-Gut-Brain Axis in Health and Neurodegenerative Diseases With Prebiotic Isolates and Probiotics.
| Disease state | N | Treatment | Duration | Assessment | Effects compared to placebo | Ref. |
|---|---|---|---|---|---|---|
| Prebiotic Isolates | ||||||
| Healthy subjects | 45 | 5.5 g B-GOS, FOS, placebo | 3 weeks | Emotional appraisal and the psychophysiological effects | Prebiotic treatment with B-GOS had a significantly improved cortisol awakening response and reduced vigilance | 100 |
| Healthy subjects | 47 | 5 g oligofructose-enriched inulin or placebo | single dose | Acute effects on mood and cognition | Prebiotic treatment improved mood, recognition memory, and recall | 101 |
| Probiotics | ||||||
| Healthy subjects | 55 | Combination of L. helveticus R0052 and B. longum R0175 or placebo | 4 weeks | Measures on mood and distress | Probiotic treated group self-reported reduced negative mood and distress and decreased urinary cortisol | 103 |
| Healthy subjects | 25 | Combination of L. helveticus R0052 and B. longum R0175 or placebo | 4 weeks | Follow-up to above study on subset of subjects with lowest cortisol | Probiotic treatment improves the same mood scores as in the general population (i.e. same benefits compared to those with higher cortisol concentrations) | 121 |
| Healthy subjects | 20 | Multistrain probiotic (Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, and Lactococcus lactis (W19 and W58) or placebo | 5 weeks | Cognitive reactivity in non-depressed individuals | Probiotic treatment reduced cognitive reactivity to sad mood, rumination and aggressive thoughts | 124 |
| Healthy females | 36 | Multistrain probiotic (Bifidobacterium animalis, Streptococcus thermophiles, Lactobacillus bulgaricus, and Lactococcus lactis), placebo, or passive control (no treatment) | 4 weeks | Attention and vigilance to negative emotional stimuli via fMRI | Probiotic treatment group displayed decreased activity in a functional network associated with emotional, somatosensory, and interoceptive processing associated with the insula and other cortical structures | 115 |
| Parkinson’s Disease | 120 | Fermented milk, containing multistrain probiotic and prebiotic fiber, or placebo | 4 weeks | Bowel movements in patients with constipation | Probiotic and prebiotic treatment combination significantly increased the number of complete bowel movements | 125 |
| Parkinson’s Disease | 50 | Multistrain probiotic containing Lactobacillus acidophilus, Bifidobacterium bifidum, L. reuteri, and Lactobacillus fermentum or placebo | 12 weeks | Inflammation, insulin and lipid profiles | Downregulation of inflammatory IL-1, IL-8 and TNF-α and upregulated TGF-β and PPAR-γ in PBMCs | 126 |
| Parkinson’s Disease | 60 | Multistrain probiotic containing Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum | 12 weeks | Movement and metabolic parameters | Probiotic treatment decreased high-sensitivity CRP and malondialdehyde and increased glutathione levels; Reduced MDS-UPDRS, insulin and insulin resistance | 127 |
| Alzheimer’s Disease | 30 | Multistrain probiotic containing L. acidophilus, L. casei, B. bifidum, and L. fermentum or no treatment in disease matched subjects | 12 weeks | Cognition and metabolic parameters | Probiotic treatment increased MMSE scores and beta cell function; decreased malondialdehyde levels, CRP, insulin resistance, and triglycerides | 157 |
| Alzheimer’s Disease | 79 | Multistrain probiotic (Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum) with 200 ug selenium, 200 ug selenium only, or placebo | 12 weeks | Cognition and metabolic parameters | Probiotic with selenium treatment significantly increased MMSE scores, total antioxidant capacity and glutathione and reduced hs-CRP, serum triglycerides, LDL and total cholesterol, and insulin levels compared with selenium alone and placebo treatment | 158 |
| Alzheimer’s Disease | 13 | Fermented milk containing Acetobacter aceti, Acetobacter sp., Lactobacillus delbrueckii delbrueckii, Lactobacillus fermentum, Lactobacillus fructivorans, Enterococcus faecium, Leuconostoc spp., Lactobacillus kefiranofaciens, Candida famata, and Candida krusei | 12 weeks | Cognition, inflammation, and oxidative stress | Probiotic treatment improved cognitive scores and markers of systemic inflammation, oxidative stress, and blood cell damage | 160 |
Probiotics and the Gut-Brain Axis
While the pathways of the microbiota-gut-brain axis are still being elucidated, evidence suggests that bacterial cell wall sugars, bacterially produced metabolites such as SCFAs and neurotransmitters, vagal activation, and microbial modulation of the immune system are involved. Both direct physical interactions of gut microbes with the host and microbial metabolites exert powerful effects. The SCFAs, for example, affect processes from gut motility to immune system development to hormonal secretion in the gut. Some Lactobacillus species produce immune and nervous system-regulating nitric oxide, while others produce neurotransmitters such as GABA.114
Probiotic supplementation increases fatty acids levels in the brain which are important for brain function, learning, memory, and neurogenesis. In a clinical trial of healthy subjects (Table 1), one multi-strain probiotic (i.e. Bifidobacterium lactis, Lactobacillus bulgaricus, Streptococcus thermophilus, and Lactobacillus lactis) was found to reduce activity in a functional network associated with the insula, which is a region of the brain with a role in the regulation of mood, and reduce attention and vigilance to negative emotional stimuli.115 In addition, the gut microbiota may modulate BDNF expression to promote the survival of existing and growth of new neurons, learning, memory, and cognitive processes. The probiotics B. longum and Bifidobacterium breve 6330 have also promoted increased BDNF levels in animal models.94,116
SCFAs produced by microbial fermentation are neuroactive and activate the vagus nerve. Vagal nerve activation exerts an anti-inflammatory effect and may be required for some probiotics as well as beneficial endogenous gut microbes to exert health-promoting effects. Probiotics, such as Bifidobacterium infantis, may alter levels of kynurenine which is an arm of the tryptophan (serotonin precursor) pathway important in the context of oxidative stress.117,118 Gut microbes can also produce and modulate neurotransmitters and neuromodulating compounds such as GABA, serotonin, dopamine, acetylcholine, and noradrenalin.119 GABA is the primary inhibitory neurotransmitter in the brain and may exert influence on brain activity by acting on the vagus nerve. In mice, Lactobacillus rhamnosus probiotic supplementation significantly increased GABA activity in the brain and changed their response to stress in a vagus nerve-dependent manner.120
Gut microbe-derived metabolites such as SCFAs influence the permeability of the both intestinal and blood–brain barrier. Mounting research in rodents has revealed that probiotic treatment (e.g. Lactobacillus and Bifidobacteria) can reduce intestinal permeability, abdominal pain, and inflammation as well as reduce HPA axis dysfunction due to stress.100 In human studies, intestinal permeability is associated with stress-related psychiatric disorders such as depression which underscores the importance of the microbiota-gut-brain axis in regulating stress responses. In addition, certain species and strains of Lactobacillus and Bifidobacteria increase gut barrier function (i.e. decrease so-called “leaky gut”) and reverse stress-induced changes to the HPA axis. Two studies reported that a combination of L. helveticus R0052 and B. longum R0175 reduced cortisol in healthy human subjects.101,121 Prbiotic treatment in humans also reduces cortisol awakening response and emotional reactivity in healthy individuals. B. infantis has been shown to reduce stress-related behaviors in animals and reduce inflammation in patients with Irritable Bowel Syndrome (IBS), which is recognized to be correlated with disruption in the gut-brain axis.105,122
Probiotic studies in healthy humans with Bifidobacterium and Lactobacillus (e.g. L. helveticus and B. longum) supplementation reveal a decrease in anxiety, depression and stress-related behaviors (Table 1).101 In a follow up study, even in human subjects with low stress and cortisol levels, this probiotic supplementation was associated with reduced cortisol, anxiety and depression levels.121 Healthy brain function in humans has also been associated with the consumption of fermented dairy products.123 Probiotic supplementation increases fatty acid levels in the brain which are important for brain function, learning, memory, and neurogenesis. Data in both animals and humans suggest that probiotics, often through a strain-specific effect, can therapeutically modulate brain function and behavior via the various communication routes of the microbiota-gut-brain axis. A clinical trial using a multi-strain probiotic containing B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, and L. lactis W19 and W58 for 30 days reported reduced reactivity to sad mood, which was associated with reduced rumination and aggressive cognition, in the probiotic treated group compared to placebo group.124 Fully understanding the mechanisms of microbiota-gut-brain axis communication will catalyze the development of microbiome-based therapeutics for the CNS.
Probiotics and Herbal Medicines in Neurodegenerative Disease
Parkinson’s disease
Probiotic interventions in neurodegenerative diseases such as PD have recently emerged (Table 1). A 12-week randomized, double-blind, controlled trial in 120 patients with PD and constipation revealed that treatment with both a probiotic and prebiotic significantly increased the number of complete bowel movements compared to placebo.125 In a randomized, double-blind, placebo-controlled clinical trial in 50 patients with PD, probiotic containing Bifidobacterium bifidum, Lactobacillus acidophilus, L. reuteri, and L. fermentum or placebo was provided for 12 weeks.126 Probiotic supplementation downregulated gene expression of the pro-inflammatory cytokines interleukin-1 (IL-1), IL-8 and tumor necrosis factor alpha (TNF-α) and upregulated transforming growth factor beta (TGF-β) and peroxisome proliferator activated receptor gamma (PPAR-γ) in peripheral blood mononuclear cells (PBMCs) compared to placebo in PD subjects. In another randomized, double-blind, placebo-controlled clinical trial in 60 patients with PD, a multistrain probiotic supplement containing Bifidobacterium bifidum, Lactobacillus acidophilus, L. reuteri, and L. fermentum or placebo was provided.127 In the probiotic treatment group, significantly decreased hs-CRP, decreased malondialdehyde, and increased glutathione levels were observed relative to the placebo group. Reduced insulin levels and insulin resistance were also observed in the probiotic compared to control group. Additional well-controlled, longitudinal controlled trials in larger cohorts of patients with AD and PD, including more focused designs targeting key features of the gut-brain axis, are needed.
Myriad medicinal herbs have been used in traditional medicine as both nootropics and to treat neurological disease. Bacopa monnieri (common name: brahmi, bacopa or water hyssop) is an important nootropic and treatment for neurodegenerative diseases in Ayurveda and other traditional medicine. Bacosides A and B are high abundance dammarane-type triterpenoid saponins implicated in some neuropharmacological effects of bacopa and cross the blood-brain barrier.102,103 In addition, several clinical trials (Table 2) using bacopa in patients with age-associated memory impairment and healthy elderly subjects suggest that treatment improves cognition, focus, and memory outcomes.104,109,110,142–145 Both in vitro and animal studies have indicated the anti-amyloidogenic and neuroprotective potential of bacopa.106,111 While additional studies are needed, the putative mechanisms of action in bacopa include antioxidant mediated neuroprotection, acetylcholinesterase and/or choline acetyltransferase modulation, cerebral blood flow stimulation, reduced amyloid formation, and neurotransmitter modulation of acetylcholine, 5-hydroxytryptamine, and dopamine.107,108 However, clinical trials investigating the efficacy and safety in neurodegenerative diseases are still lacking. Several animal studies have demonstrated that Bacopa modulates serotonergic pathways to enhance cognition.146–149 In models of PD, a study in humanized transgenic C. elegans with PD-type neurodegeneration reported that Bacopa treatment reduced alpha-synuclein aggregation, prevented dopaminergic neurodegeneration and restored proper lipid levels.150 In MPTP-induced PD mouse models, Bacopa treatment provided neuroprotection in the nigrostriatal dopaminergic neurons and modulated oxidative stress and apoptotic pathways.151,152
Table 2.
Clinical Trials Investigating Modulation of the Microbiota-Gut-Brain Axis in Health and Neurodegenerative Diseases With Selected Medicinal Herbs.
| Disease state | N | Treatment | Duration | Assessment | Effects compared to placebo | Ref. |
|---|---|---|---|---|---|---|
| Memory impaired elderly | 40 | 250 mg Bacopa monnieri extract or placebo | 12 weeks | Cognition and memory | Treatment increased mental control, logical memory and paired associated learning | 128 |
| Healthy elderly | 98 | 300 mg B. monnieri extract or placebo | 12 weeks | Memory and learning | Treatment improved verbal learning, memory acquisition, and recall. | 130 |
| Healthy elderly | 60 | 300 mg B. monnieri extract or placebo | 12 weeks | Cognition, memory, and neurotransmitter activity | Treatment improved attention, cognitive processing, working memory, and reduced acetylhydrolase activity | 106 |
| Healthy elderly | 48 | 300 mg B. monnieri extract or placebo | 12 weeks | Cognition, memory, and mood | Treatment improved memory and attention; scores of anxiety, depression and heart rate decreased | 131 |
| Senile dementia of Alzheimer’s type patients | 201 | 1,000 mg B. monnieri (whole plant), Hippophae rhamnoides (leaves and fruits) and Dioscorea bulbifera (bulbils) or 20 mg donepezil drug (Aricept) | 12 months | Cognition, memory, and mood | Treatment improved markers of inflammation, oxidative stress and cognition which included MMSE, word recall, attention span, functional activity and depression scores | 166 |
| Mild cognitive impairment | 50 | 600 mg/day Withania somnifera root extract or placebo | 8 weeks | Cognition and memory | Treatment improved memory, executive function, focused attention, and information-processing speed | 174 |
| Cognitive dysfunction in bipolar disorder | 53 | 500 mg/ day W. somnifera root extract or placebo | 8 weeks | Cognition and memory | Treatment improved auditory-verbal working memory, reaction time, and social cognition | 175 |
| Parkinson’s Disease | 23 | 40-50 g Mucuna pruriens (4.5-5.5% L-Dopa) per day | 4 weeks | Efficacy and tolerability of M. pruriens | Treatment improved motor scores and treatment was tolerated | 142 |
| Parkinson’s Disease | 60 | 7.5 g HP-200 (4% L-Dopa) from M. pruriens | 12 weeks | Efficacy and tolerability of HP-200 derived from M. pruriens | Treatment reduced Hoehn and Yahr stage and UPDRS scores; mild adverse events | 143 |
| Parkinson’s Disease | 8 | 15 g or 30 g M. pruriens, or levodopa/carbidopa control | 1 dose | Motor symptoms and pharmacokinetics | Compared to conventional drug therapy, 30 g mucuna led to faster onset of effect with shorter latencies to peak L-dopa plasma concentrations, longer on time, and higher peak L-dopa plasma concentration | 154 |
| Parkinson’s Disease | 2 | 200 mg M. pruriens extract after conventional drug washout | 2 doses | Kinetic-dynamic comparison case study | Impaired L-dopa bioavailability after M. pruriens administration | 156 |
| Parkinson’s Disease | 18 | 17.5 mg/kg M. pruriens, 12.5 mg/kg M. pruriens, Mucuna with DCCI, levodopa control, levodopa plus DCCI control or placebo | 1 dose | Motor and cardiovascular parameters | Low dose M. pruriens promoted fewer dyskinesias and adverse events compared to drug control; high dose M. pruriens promoted better motor response, longer on time, fewer dyskinesias, and fewer adverse events compared to drug control; cardiovascular parameters were unchanged | 155 |
| Parkinson’s Disease | 14 | M. pruriens dosage based on patients’ levodopa dosage | 16 weeks | Efficacy and tolerability of M. pruriens | Treatment response was similar to drug response on all efficacy measures | 153 |
| Healthy elderly subjects | 28 | 250 to 750 mg Centella asiatica extract/day or placebo | 8 weeks | Memory and mood | Treatment improved working memory and mood | 176 |
Bacopa has also been shown to enhance predicted butyrate, which is reported as reduced in PD, potential in human gut microbiota.39,128 In addition, the effects of medicinal herbs on gut microbiota has been scarcely studied. An in vitro study of 10 nervine herbal medicines used in neurodegenerative disease such as PD reported that the greatest overall microbiota modulatory capacity was observed in cultures supplemented with bacopa supplementation.128 Compared to glucose-supplemented cultures, bacopa selected for an increase in butyrate producers. The gut microbiota provides an important role in the efficacy of bacopa through these mechanisms. While previous human studies in non-clinical populations have elucidated some of the beneficial effects of bacopa on memory and cognitive impairment,129,130,153 human clinical trials using bacopa in neurodegenerative disease are lacking and warranted.
Mucuna pruriens var. utilis (common names: kapikacchu, velvet bean, cowhage) seeds are a natural source of levodopa and cornerstone treatment for Parkinson’s disease in Ayurvedic medicine.154 The seeds contain L-dopa, various alkaloids, and trace 5-hydroxytryptamine. The neuroprotective action of kapikacchu may be mediated by the restoration of the endogenous monoamine contents in the CNS and degenerating dopaminergic neurons within the substantia nigra.155 One of the first human investigations, an open label clinical trial using kapikacchu powder in 23 PD patients, reported improvement in motor symptoms (Table 2).131 Later, a standardized extract of kapikacchu containing 4% L-dopa was provided to 60 patients, including 34 levodopa naïve patients, in an open label clinical trial and reported as effective for motor score improvement with mild adverse events.132 Similar effects have been reported in clinical trials (see Table 2) in which kapikacchu promoted similar motor scores compared to drug treatment control in patients that tolerated the herbal medicine.132,156 A randomized, controlled, double-blind trial in patients with short-duration L-dopa response examined 2 doses of kapikacchu compared to levodopa/carbidopa drug as control. Compared to conventional drug therapy, 30 g kapikacchu led to faster onset of effect with shorter latencies to peak L-dopa plasma concentrations, longer on time, and higher peak L-dopa plasma concentration without concomitant increases in dyskinesias.157
A double-blind, randomized, controlled, crossover study in patients with advanced PD also examined a low (12.5 mg/kg) and high (17.5 mg/kg) dose of kapikacchu compared to levodopa and levodopa/carbidopa controls. Both high and low dose led to improved motor symptoms with the high dose promoting the greatest motor response, longer on time, fewer dyskinesias, and fewer adverse events compared to drug controls.158 A case control study in 2 patients treated with conventional drug therapy provided 200 mg kapikacchu seed extract after a 12-hour washout reported impaired L-dopa bioavailability after kapikacchu administration.159 The authors concluded this may explain the lower dyskinetic potential of kapikacchu compared to standard drug therapy. The rapid onset of action, longer motor-on times and reduced dyskinetic outcomes observed in these patient studies warrant larger clinical trials focused on long term efficacy and safety.
Alzheimer’s disease
Probiotic interventions in neurodegenerative diseases such as AD have recently emerged (Table 1). In a double-blind, randomized, placebo-controlled trial in 30 patients with AD, subjects were provided a probiotic containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum and Lactobacillus fermentum or no treatment in disease-matched control subjects for 12 weeks.160 A significant increase in learning and memory as measured with the Mini-Mini-Mental State Examination (MMSE) test and decrease in malondialdehyde, a marker of oxidative stress, was observed in the probiotic compared to placebo group. Probiotic treatment also improved Beta cell function and reduced serum high-sensitivity C-reactive protein (hs-CRP), insulin resistance, and serum triglycerides in the probiotic group. Similar results were reported in another 12-week randomized, double-blind, controlled trial in 79 AD patients that received a probiotic containing selenium (200 ug/day), probiotic plus selenium, or placebo as treatment.161
Probiotic with selenium treatment significantly increased MMSE scores, total antioxidant capacity and glutathione compared with selenium only and placebo. In addition, subjects who received probiotic plus selenium supplements displayed reduced hs-CRP, serum triglycerides, LDL and total cholesterol, and insulin levels and higher insulin sensitivity compared with selenium alone and placebo treatment. Selenium has been shown to alter the composition of gut microbiota and increase microbial diversity, while increasing levels and activity of glutathione peroxidase 1 and methionine-R-sulfoxide reductase 1 in mice.162
An uncontrolled clinical trial examining cognition, inflammation and oxidative stress in 13 AD patients with cognitive deficit provided kefir fermented milk containing Acetobacter sp., A. aceti, Lactobacillus delbrueckii, Lactobacillus fermentum, L. fructivorans, Enterococcus faecium, Leuconostoc spp., L. kefiranofaciens, Candida famata, and C. krusei for 12 weeks.163 The authors reported that probiotic treatment improved memory, visual-spatial/abstraction, and executive/language functions. In addition, treatment increased nitric oxide bioavailability and decreased markers of inflammation, oxidative stress, serum protein oxidation, mitochondrial dysfunction, and DNA damage. Thus, probiotic treatment may improve cognitive function, inflammation, oxidative stress and some metabolic parameters in AD although additional clinical studies are required to fully understand the therapeutic benefits.
Many studies in animal models have demonstrated the antioxidant free radical scavenging potential of bacopa.164–166 In AD, animal models have demonstrated protection of the cholinergic neurons, which are classically affected in AD, and amelioration of memory deficits with bacopa treatment.167,168 Clinical trials (Table 2) in patients with age-associated memory impairment and healthy elderly subjects suggest that treatment improves focus, memory and learning outcomes.104,109,110,142–145 One clinical trial examined bacopa, although in a polyherbal blend with 2 other herbs, in senile dementia of Alzheimer type.169 Treatment improved markers of inflammation, oxidative stress and cognition which included MMSE, word recall, attention span, functional activity and depression scores; however, while promising for furthering the understanding of Bacopa in neurodegeneration, the intervention is confounded by the other herbal medicines in this context.
Withania somnifera (common name: ashwagandha) is an important Ayurvedic medical herb for many therapeutic purposes including immunity and the nervous system in health and disease.170 Active chemical constituents include various alkaloids, steroidal lactones including withanolides and withaferins, and distinct saponins.171 Observed biological effects in the CNS include attenuation of oxidative stress and modulation of key proteins for the growth, differentiation as well as communication of neural cells. These putative biological mechanisms include modulation of neurotrophic factors, cell adhesion molecules and synaptic proteins.172
Oral administration of withanoside IV, a bioactive compound derived from ashwagandha, in Aβ-injected mice improved memory and prevented loss of neuronal cells and connections.173 Wistar rats orally fed ashwagandha extract displayed reduced acetylcholinesterase activity, inhibition of Aβ formation, and attenuated proinflammatory cytokine levels which alleviated cognitive dysfunction.174 Ashwagandha may also restore proper levels of neurotrophin BDNF, its receptor tropomyosin receptor kinase B, and other synaptic regulators vital for synaptic plasticity.135 Several other animal studies report cognitive improvement with ashwagandha treatment in murine models of memory deficiency, neurodegeneration and cognitive dysfunction.136–140,175,176
In a double-blind, randomized, placebo-controlled pilot trial (Table 2) in adults with mild cognitive impairment, treatment with ashwagandha root extract (600 mg/day) was associated with improvement in memory, executive function, focused attention, and information-processing speed.133 A double-blind, randomized, placebo-controlled trial administered ashwagandha root extract (500 mg/day) to patients with cognitive dysfunction in bipolar disorder and reported improved auditory-verbal working memory, reaction time, and social cognition.134 Human clinical trials administering ashwagandha in neurodegenerative diseases are lacking. Preclinical and clinical data regarding the herbal medicines described here warrant further study in clinical trials. Indeed, several herbal medicines for neurodegenerative disease show promise and should be further studied such as Centella asiatica (common names: gotu kola, pennywort) which improved working memory and mood in healthy elderly subjects.141
Gut Microbiota-Modulatory Potential of Nervine Herbal Medicines
Medicinal herbs possess substantial prebiotic effects on gut microbial communities suggesting that the activities of gut microbes may play an important role in the medicinal properties of medicinal herbs.86,128 Research on the prebiotic potential and nervous system effects of medicinal herbs, including those described in this section, is scarce. Fecal cultures supplemented with bacopa, ashwagandha and kapikacchu all significantly altered the gut microbiota composition and were among the largest modulators in 10 nootropic herbal medicines analyzed in vitro. 128 Interestingly, microbial communities selected by ashwagandha and bacopa were similar and did not separate based on β-diversity, which was a measure of the diversity of species between fecal culture environments. Ashwagandha and kapikacchu-supplementation strongly selected for members of Bifidobacterium and a similar complement of Bacteroides spp. including B. vulgatus and B. uniformis. Kapikacchu uniquely selected for Ruminococcus bromii, an organism with resistant starch degradation potential.177 All 3 medicinal herbs selected for B. thetaiotaomicron as a dominant member of the gastrointestinal community. B. thetaiotaomicron produces polysaccharide A which stimulates inflammation-suppressing regulatory T cell expansion in the gut.178 Bacopa also selected for a number of dominant beneficial species including Bacteroides xylanolyticus, B. uniformis, and Butyrivibrio crossotus.
Medicinal herbs are predicted to alter the relative abundance of taxa encoding SCFA pathways which may impact luminal butyrate production potential and thus levels of neuroactive butyrate. Bacopa selected for an overall increase in butyrate producers in vitro. 128 Ashwagandha supplemented fecal co-cultures had increased Faecalibacterium prausnitzii and Eubacterium rectale whereas E. rectale was the primary butyrate producer in kapikacchu-supplemented cultures. Clostridium symbiosum, a butyrate producer via amino acid fermentation, was dominant in response to bacopa supplementation. Finally, Roseburia faecis was increased significantly by Kapikacchu supplementation.
Analysis of these nervine herbs demonstrates both a strong microbiota modulatory capacity and concomitant restructure in metabolic preferences and products. It is anticipated that changes in gut microbial community metabolism induced by these nervine herbs will accordingly alter signaling via the ENS. However, further work is required to verify these predictions and their implications on host health.
Future Directions
Several positive reviews on the subject of therapeutic modulation of the gut brain axis in health and disease have been published; however, strong translational value of this preclinical work relies on thorough elucidation of pertinent mechanisms and further study of both prebiotic and probiotic treatment in relevant, appropriately sized human cohorts. Examining these currently unknown variables will facilitate the emergence of efficacious prebiotic and probiotic therapies; however, many open questions remain. For example, what is the relevance of post-biotics (e.g. SCFAs) in the mechanism of action of probiotics and prebiotics? Do post-biotics modulate prebiotic and probiotic therapy outcomes? How do these treatments differ in terms of their quantitative and qualitative impacts on gut microbiome structure and function? Is there a difference in the effects of a prebiotic isolate versus whole herbs? What is the kinetic-dynamic relationship associated with prebiotic and probiotics administered to patients with neurodegenerative disease? How do gut produced or modulated neurotransmitters effect signaling in the ENS and gut brain axis with and without dietary supplementation with probiotics, prebiotics, and medicinal herbs? How do host factors such as diet, genetic susceptibility, drug medication, and age modulate the therapeutic efficacy of dietary supplements targeting the gut-brain axis? How are the prebiotic effects of isolates versus polymolecular herbs different and is one more efficacious in the context of specific neurodegenerative disease? Are specific target populations most appropriate to assess these treatments in the context of disease? What are the ideal dosages and long-term safety considerations of treatment in target populations? These and numerous other unknown questions must be rigorously examined to both temper enthusiasm for the field and gain a more comprehensive scientific understanding.
Conclusions
While greater insights and characterization of the microbiota-gut-brain axis have been revealed over the past decade, salient questions related to the pathology, pathogenesis and treatment of the axis in the context of both health and neurodegenerative disease remain. While robust translation of many rodent to human findings has emerged, an abundance of caution is required when extrapolating from such findings and early clinical data. Additional human studies using targeted therapeutic modulation of the microbiota-gut-brain axis with prebiotic, medicinal herb, probiotic, and synbiotic interventions are needed. Well-controlled, large scale, longitudinal clinical trials are urgently needed to further elucidate both mechanism and therapeutic opportunity in both health and neurological disease, including disease subpopulations. This strategy coupled with a multi-omic approach will ensure that the next decade ushers in the dawn of targeted therapeutic modulation of the microbiota-gut-brain axis.
Footnotes
Authors’ Note: CTP conceived the manuscript, wrote the manuscript, revised the manuscript, and generated tables and figures.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a fellowship grant from the Chopra Foundation.
ORCID iD: Christine Tara Peterson, PhD  https://orcid.org/0000-0002-6951-5753
https://orcid.org/0000-0002-6951-5753
References
- 1. Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10 doi:10.1101/Cshperspect.A033118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–897. doi:10.1016/S1474-4422(17)30299-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Endres K, Schafer KH. Influence of commensal microbiota on the enteric nervous system and its role in neurodegenerative diseases. J Innate Immun. 2018;10(3):171–180. doi:10.1159/000488629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gammon K. Neurodegenerative disease: brain windfall. Nature. 2014;515(7526):299–300. [DOI] [PubMed] [Google Scholar]
- 5. Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15(13):1546–1558. [DOI] [PubMed] [Google Scholar]
- 6. Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. [DOI] [PubMed] [Google Scholar]
- 7. Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi:10.1016/J.Cell.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 8. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi:10.1126/Science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307(5717):1920–1925. [DOI] [PubMed] [Google Scholar]
- 10. Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dibaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc. 2008;83(4):460–469. doi:S0025-6196(11)60702-7[Pii]10.4065/83.4.460 [DOI] [PubMed] [Google Scholar]
- 12. Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi:10.1146/Annurev.Nutr.22.011602.092259011602.092259 [DOI] [PubMed] [Google Scholar]
- 13. Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8(6):411–420. doi:10.1038/Nri2316 [DOI] [PubMed] [Google Scholar]
- 14. Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002;99(24):15451–15455. doi:10.1073/Pnas.202604299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10(10):735–744. doi:10.1038/Nri2850 [DOI] [PubMed] [Google Scholar]
- 16. Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hansotia T, Drucker DJ. Gip and Glp-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept. 2005;128(2):125–134. doi:10.1016/J.Regpep.2004.07.019 [DOI] [PubMed] [Google Scholar]
- 18. Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24(5):405–413. doi:10.1111/J.1365-2982.2012.01906.X [DOI] [PubMed] [Google Scholar]
- 19. Burokas A, Moloney RD, Dinan TG, Cryan JF. Microbiota regulation of the mammalian gut-brain axis. Adv Appl Microbiol. 2015;91:1–62. doi:10.1016/Bs.Aambs.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 20. Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–466. doi:10.1038/Nrn3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44 doi:10.3389/Fpsyt.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahlman H. Nilsson. The gut as the largest endocrine organ in the body. Ann Oncol. 2001;12(suppl 2):S63–S68. [DOI] [PubMed] [Google Scholar]
- 23. Erny D, de Angelis ALH, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–977. doi:10.1038/Nn.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cani PD, Everard A, Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Curr Opin Pharmacol. 2013;13(6):935–940. doi:10.1016/J.Coph.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 25. Alcaino C, Knutson KR, Treichel AJ, et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires piezo2 to convert force into serotonin release. Proc Natl Acad Sci U S A. 2018;115(32):E7632–E7641. doi:10.1073/Pnas.1804938115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;21(37):10609–10620. doi:10.3748/Wjg.V21.I37.10609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595(2):489–503. doi:10.1113/Jp273106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56–63. doi:10.1016/J.Neulet.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi:10.1038/Nature11319 [DOI] [PubMed] [Google Scholar]
- 30. Kinross J, Nicholson JK. Gut microbiota: dietary and social modulation of gut microbiota in the elderly. Nat Rev Gastroenterol Hepatol. 2012;9(10):563–564. doi:10.1038/Nrgastro.2012.169 [DOI] [PubMed] [Google Scholar]
- 31. Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(suppl 1):4586–4591. doi:10.1073/Pnas.1000097107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noble EE, Hsu TM, Kanoski SE. Gut to brain dysbiosis: mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci. 2017;11:9 doi:10.3389/Fnbeh.2017.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhuang ZQ, Shen L-L, Li W-W, et al. Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis. 2018;63(4):1337–1346. doi:10.3233/Jad-180176 [DOI] [PubMed] [Google Scholar]
- 34. Friedland RP. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J Alzheimers Dis. 2015;45(2):349–362. doi:10.3233/Jad-142841 [DOI] [PubMed] [Google Scholar]
- 35. Minter MR, Zhang C, Leone V, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016;6:30028 doi:10.1038/Srep30028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brenner D, Hiergeist A, Adis C, et al. The fecal microbiome of ALS patients. Neurobiol Aging. 2018;61:132–137. doi:10.1016/J.Neurobiolaging.2017.09.023 [DOI] [PubMed] [Google Scholar]
- 37. Mazzini L, Mogna L, De Marchi F, et al. Potential role of gut microbiota in ALS pathogenesis and possible novel therapeutic strategies. J Clin Gastroenterol. 2018;52(suppl 1). doi:10.1097/Mcg.0000000000001042 [DOI] [PubMed] [Google Scholar]
- 38. Scheperjans F, Aho V, Pereira PAB, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–358. doi:10.1002/Mds.26069 [DOI] [PubMed] [Google Scholar]
- 39. Unger MM, Spiegel J, Dillmann K-U, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi:10.1016/J.Parkreldis.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 40. Petrov VA, Saltykova IV, Zhukova IA, et al. Analysis of gut microbiota in patients with Parkinson’s disease. Bull Exp Biol Med. 2017;162(6):734–737. doi:10.1007/S10517-017-3700-7 [DOI] [PubMed] [Google Scholar]
- 41. Perez-Pardo P, Kliest T, Dodiya HB, et al. The gut-brain axis in Parkinson’s disease: possibilities for food-based therapies. Eur J Pharmacol. 2017;817:86–95. doi:10.1016/J.Ejphar.2017.05.042 [DOI] [PubMed] [Google Scholar]
- 42. Hill-Burns EM, Debelius JW, Morton JT, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32(5):739–749. doi:10.1002/Mds.26942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30(10):1351–1360. doi:10.1002/Mds.26307 [DOI] [PubMed] [Google Scholar]
- 44. Minato T, Maeda T, Fujisawa Y, et al. Progression of Parkinson’s disease is associated with gut dysbiosis: two-year follow-up study. Plos One. 2017;12(11):E0187307 doi:10.1371/Journal.Pone.0187307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kozina E, Sadasivan S, Jiao Y, et al. Mutant Lrrk2 mediates peripheral and central immune responses leading to neurodegeneration in vivo. Brain. 2018;141(6):1753–1769. doi:10.1093/Brain/Awy077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Felice VD, Quigley EM, Sullivan AM, O’keeffe GW, O’mahony SM. Microbiota-gut-brain signalling in Parkinson’s disease: implications for non-motor symptoms. Parkinsonism Relat Disord. 2016;27:1–8. doi:10.1016/J.Parkreldis.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 47. Angot E, Brundin P. Dissecting the potential molecular mechanisms underlying alpha-synuclein cell-to-cell transfer in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(suppl 3):S143–147. doi:10.1016/S1353-8020(09)70802-8 [DOI] [PubMed] [Google Scholar]
- 48. Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna). 2003;110(5):517–536. doi:10.1007/S00702-002-0808-2 [DOI] [PubMed] [Google Scholar]
- 49. Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480. E1412 doi:10.1016/J.Cell.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bryois J, Skene NG, Hansen TF, et al. Genetic identification of cell types underlying brain complex traits yields insights into the etiology of Parkinson’s disease. Nat Genet. 2020;52(5):482–493. doi:10.1038/S41588-020-0610-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walton GE, van den Heuvel EG, Kosters MH, Rastall RA, Tuohy KM, Gibson GR. A randomised crossover study investigating the effects of galacto-oligosaccharides on the faecal microbiota in men and women over 50 years of age. Br J Nutr. 2012;107(10):1466–1475. doi:10.1017/S0007114511004697 [DOI] [PubMed] [Google Scholar]
- 52. Tjernberg LO, Rising A, Johansson J, Jaudzems K, Westermark P. Transmissible amyloid. J Intern Med. 2016;280(2):153–163. doi:10.1111/Joim.12499 [DOI] [PubMed] [Google Scholar]
- 53. Kaufman SK, Sanders DW, Thomas TL, et al. Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron. 2016;92(4):796–812. doi:10.1016/J.Neuron.2016.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prusiner SB. Cell biology. A unifying role for prions in neurodegenerative diseases. Science. 2012;336(6088):1511–1513. doi:10.1126/Science.1222951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liddle RA. Parkinson’s disease from the gut. Brain Res. 2018;1693(pt B):201–206. doi:10.1016/J.Brainres.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Desplats P, Lee HJ, Bae EJ, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106(31):13010–13015. doi:10.1073/Pnas.0903691106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Braak H, De Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. doi:10.1016/J.Neulet.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 58. Goya ME, Xue F, Sampedro-Torres-Quevedo C, et al. Probiotic bacillus subtilis protects against alpha-synuclein aggregation in C. elegans. Cell Rep. 2020;30(2):367–380. E367 doi:10.1016/J.Celrep.2019.12.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bohorquez DV, Shahid RA, Erdmann A, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125(2):782–786. doi:10.1172/Jci78361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Borghammer P. How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord. 2018;33(1):48–57. doi:10.1002/Mds.27138 [DOI] [PubMed] [Google Scholar]
- 61. Santos SF, De Oliveira HL, Yamada ES, Neves BC, Pereira A., Jr The gut and Parkinson’s disease-a bidirectional pathway. Front Neurol. 2019;10:574 doi:10.3389/Fneur.2019.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ulusoy A, Phillips RJ, Helwig M, Klinkenberg M, Powley TL, Di Monte DA. Brain-to-stomach transfer of alpha-synuclein via vagal preganglionic projections. Acta Neuropathol. 2017;133(3):381–393. doi:10.1007/S00401-016-1661-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pouclet H, Lebouvier T, Coron E, Des Varannes SB, Neunlist M, Derkinderen P. A comparison between colonic submucosa and mucosa to detect Lewy pathology in Parkinson’s disease. Neurogastroenterol Motil. 2012;24(4):E202–205. doi:10.1111/J.1365-2982.2012.01887.X [DOI] [PubMed] [Google Scholar]
- 64. Lin SY, Lin CL, Wang IK, et al. Dementia and vagotomy in Taiwan: a population-based cohort study. BMJ Open. 2018;8(3):E019582 doi:10.1136/Bmjopen-2017-019582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Svensson E, Horváth-Puhó E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–529. doi:10.1002/Ana.24448 [DOI] [PubMed] [Google Scholar]
- 66. Liu B, Fang F, Pedersen NL, et al. Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology. 2017;88(21):1996–2002. doi:10.1212/Wnl.0000000000003961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim S, Kwon SH, Kam TI, et al. Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson’s disease. Neuron. 2019;103:627–641. E627 doi:10.1016/J.Neuron.2019.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Phillips RJ, Walter GC, Wilder SL, Baronowsky EA, Powley TL. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson’s disease? Neuroscience. 2008;153(3):733–750. doi:10.1016/J.Neuroscience.2008.02.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chandra R, Hiniker A, Kuo YM, Nussbaum RL, Liddle RA. Alpha-synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight. 2017;2(12). doi:10.1172/Jci.Insight.92295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bohorquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, Liddle RA. An enteroendocrine cell-enteric glia connection revealed by 3d electron microscopy. Plos One. 2014;9(2):E89881 doi:10.1371/Journal.Pone.0089881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Perry T, Greig NH. The glucagon-like peptides: a new genre in therapeutic targets for intervention in Alzheimer’s disease. J Alzheimers Dis. 2002;4(6):487–496. [DOI] [PubMed] [Google Scholar]
- 72. Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25(25):6016–6024. doi:10.1523/Jneurosci.0692-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40(9):1835–1849. doi:10.1016/J.Biocel.2008.01.017 [DOI] [PubMed] [Google Scholar]
- 74. Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120(2):131–143. doi:10.1007/S00401-010-0711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chapman MR, Robinson LS, Pinkner JS, et al. Role of Escherichia coli Curli Operons in directing amyloid fiber formation. Science. 2002;295(5556):851–855. doi:10.1126/Science.1067484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Erskine EE, Macphee CE, Stanley-Wall NR. Functional amyloid and other protein fibres in the biofilm matrix. J Mol Biol. doi:10.1016/J.Jmb.2018.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen SG, Stribinskis V, Rane MJ, et al. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged fischer 344 rats and caenorhabditis elegans. Sci Rep. 2016;6(1):34477 doi:10.1038/Srep34477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128(6):805–820. doi:10.1007/S00401-014-1343-6 [DOI] [PubMed] [Google Scholar]
- 79. Morales R, Estrada LD, Diaz-Espinoza R, et al. Molecular cross talk between misfolded proteins in animal models of Alzheimer’s and prion diseases. J Neurosci. 2010;30(13):4528–4535. doi:10.1523/Jneurosci.5924-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Soto C, Pritzkow S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci. 2018;21(10):1332–1340. doi:10.1038/S41593-018-0235-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cattaneo A, Cattane N, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi:10.1016/J.Neurobiolaging.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 82. Soscia SJ, Kirby JE, Washicosky KJ, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLos One. 2010;5(3):E9505 doi:10.1371/Journal.Pone.0009505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kumar DK, Choi SH, Washicosky KJ, et al. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8(340):340ra372 doi:10.1126/Scitranslmed.Aaf1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cheng SB, Ferland P, Webster P, Bearer EL. Herpes simplex virus dances with amyloid precursor protein while exiting the cell. Plos One. 2011;6(3):e17966 doi:10.1371/Journal.Pone.0017966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Peterson CT, Sharma V, Elmen L, Peterson SN. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol. 2015;179(3):363–377. doi:10.1111/Cei.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Peterson CT, Sharma V, Uchitel S, et al. Prebiotic potential of herbal medicines used in digestive health and disease. J Altern Complement Med. 2018;24(7):656–665. doi:10.1089/Acm.2017.0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Peterson CT, Vaughn AR, Sharma V, et al. Effects of turmeric and curcumin dietary supplementation on human gut microbiota: a double-blind, randomized, placebo-controlled pilot study. J Evid Based Integr Med. 2018;23:2515690x18790725 doi:10.1177/2515690x18790725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zwickey H, Lipski L. Expanding our view of herbal medicine. J Altern Complement Med. 2018;24(7):619–620. doi:10.1089/Acm.2018.0123 [DOI] [PubMed] [Google Scholar]
- 89. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–1412. [DOI] [PubMed] [Google Scholar]
- 90. Hutkins RW, Krumbeck JA, Bindels LB, et al. Prebiotics: why definitions matter. Curr Opin Biotechnol. 2016;37:1–7. doi:10.1016/J.Copbio.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014;14(6):277–288. doi:10.4110/In.2014.14.6.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi:10.1038/Nature12721 [DOI] [PubMed] [Google Scholar]
- 93. Braniste V, Al-Asmakh M, Kowal C, et al. The Gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158 doi:10.1126/Scitranslmed.3009759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Savignac HM, Corona G, Mills H, et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-Serine. Neurochem Int. 2013;63(8):756–764. doi:10.1016/J.Neuint.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Williams S, Chen L, Savignac HM, Tzortzis G, Anthony DC, Burnet PW. Neonatal prebiotic (Bgos) supplementation increases the levels of synaptophysin, Glun2a-Subunits and Bdnf proteins in the adult rat hippocampus. Synapse. 2016;70(3):121–124. doi:10.1002/Syn.21880 [DOI] [PubMed] [Google Scholar]
- 96. Vazquez E, Ramírez M, Gruart A, et al. Effects of a human milk oligosaccharide, 2’-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J Nutr Biochem. 2015;26(5):455–465. doi:10.1016/J.Jnutbio.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 97. Oliveros E, Ramirez M, Vazquez E, et al. Oral supplementation of 2’-fucosyllactose during lactation improves memory and learning in rats. J Nutr Biochem. 2016;31:20–27. doi:10.1016/J.Jnutbio.2015.12.014 [DOI] [PubMed] [Google Scholar]
- 98. Jia S, Lu Z, Gao Z, et al. Chitosan oligosaccharides alleviate cognitive deficits in an Amyloid-Beta1-42-induced rat model of Alzheimer’s disease. Int J Biol Macromol. 2016;83:416–425. doi:10.1016/J.Ijbiomac.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 99. Song L, Gao Y, Zhang X, Le W. Galactooligosaccharide improves the animal survival and alleviates motor neuron death in Sod1g93a mouse model of amyotrophic lateral sclerosis. Neuroscience. 2013;246:281–290. doi:10.1016/J.Neuroscience.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 100. Schmidt K, et al. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl). 2015;232:1793–1801. doi:10.1007/S00213-014-3810-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Smith AP, Sutherland D, Hewlett P. An investigation of the acute effects of oligofructose-enriched inulin on subjective wellbeing, mood and cognitive performance. Nutrients. 2015;7(11):8887–8896. doi:10.3390/Nu7115441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PW. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39(11):763–781. doi:10.1016/J.Tins.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105(5):755–764. doi:10.1017/S0007114510004319 [DOI] [PubMed] [Google Scholar]
- 104. Chakravarty AK, Sarkar T, Masuda K, Shiojima K, Nakane T, Kawahara N. Bacopaside I And Ii: two pseudojujubogenin glycosides from Bacopa monniera. Phytochemistry. 2001;58(4):553–556. [DOI] [PubMed] [Google Scholar]
- 105. Thomas RB, Joy S, Ajayan MS, Paulose CS. Neuroprotective potential of Bacopa monnieri and Bacoside A against dopamine receptor dysfunction in the cerebral cortex of neonatal hypoglycaemic rats. Cell Mol Neurobiol. 2013;33(8):1065–1074. doi:10.1007/S10571-013-9973-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Peth-Nui T, Wattanathorn J, Muchimapura S, et al. Effects of 12-week Bacopa monnieri consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers. Evid Based Complement Alternat Med. 2012:606424 doi:10.1155/2012/606424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. O’mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541–551. doi:10.1053/J.Gastro.2004.11.050 [DOI] [PubMed] [Google Scholar]
- 108. Limpeanchob N, Jaipan S, Rattanakaruna S, Phrompittayarat W, Ingkaninan K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J Ethnopharmacol. 2008;120(1):112–117. doi:10.1016/J.Jep.2008.07.039 [DOI] [PubMed] [Google Scholar]
- 109. Aguiar S, Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013;16(4):313–326. doi:10.1089/Rej.2013.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nemetchek MD, Stierle AA, Stierle DB, Lurie DI. The Ayurvedic plant Bacopa monnieri inhibits inflammatory pathways in the brain. J Ethnopharmacol. 2017;197:92–100. doi:10.1016/J.Jep.2016.07.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Downey LA, Kean J, Nemeh F, et al. An acute, double-blind, placebo-controlled crossover study of 320 mg and 640 mg doses of a special extract of Bacopa monnieri (Cdri 08) on sustained cognitive performance. Phytother Res. 2013;27(9):1407–1413. doi:10.1002/Ptr.4864 [DOI] [PubMed] [Google Scholar]
- 112. Stough C, Downey LA, Lloyd J, et al. Examining the nootropic effects of a special extract of Bacopa monniera on human cognitive functioning: 90 day double-blind placebo-controlled randomized trial. Phytother Res. 2008;22(12):1629–1634. doi:10.1002/Ptr.2537 [DOI] [PubMed] [Google Scholar]
- 113. Holcomb LA, Dhanasekaran M, Hitt AR, Young KA, Riggs M, Manyam BV. Bacopa monniera extract reduces amyloid levels in psapp mice. J Alzheimers Dis. 2006;9(3):243–251. [DOI] [PubMed] [Google Scholar]
- 114. Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi:10.1016/J.Brainres.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tillisch K, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–1401. doi:10.1053/J.Gastro.2013.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. O’sullivan E, Barrett E, Grenham S, et al. Bdnf expression in the hippocampus of maternally separated rats: does bifidobacterium breve 6330 alter Bdnf levels? Benef Microbes. 2011;2(3):199–207. doi:10.3920/Bm2011.0015 [DOI] [PubMed] [Google Scholar]
- 117. Strasser B, Geiger D, Schauer M, et al. Probiotic supplements beneficially affect tryptophan-kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: a randomized, double-blinded, placebo-controlled trial. Nutrients. 2016;8(11):752 doi:10.3390/Nu8110752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dehhaghi M, Kazemi Shariat Panahi H, Guillemin GJ. Microorganisms, tryptophan metabolism, and kynurenine pathway: a complex interconnected loop influencing human health status. Int J Tryptophan Res. 2019;12:1178646919852996 doi:10.1177/1178646919852996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Roy Sarkar S, Banerjee S. Gut microbiota in neurodegenerative disorders. J Neuroimmunol. 2019;328:98–104. doi:10.1016/J.Jneuroim.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 120. Bravo JA, Forsythe P, Chew MV, et al. Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–16055. doi:10.1073/Pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2(4):256–261. doi:10.4161/Gmic.2.4.16108 [DOI] [PubMed] [Google Scholar]
- 122. Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179–1188. doi:10.1016/J.Neuroscience.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 123. Kato-Kataoka A, Nishida K, Takada M, et al. Fermented milk containing lactobacillus casei strain shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl Environ Microbiol. 2016;82(12):3649–3658. doi:10.1128/Aem.04134-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Steenbergen L, Sellaro R, Van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–264. doi:10.1016/J.Bbi.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 125. Barichella M, Pacchetti C, Bolliri C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology. 2016;87(12):1274–1280. doi:10.1212/Wnl.0000000000003127 [DOI] [PubMed] [Google Scholar]
- 126. Borzabadi S, Oryan S, Eidi A, et al. The effects of probiotic supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Arch Iran Med. 2018;21(7):289–295. [PubMed] [Google Scholar]
- 127. Tamtaji OR, Taghizadeh M, Kakhaki RD, et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(3):1031–1035. doi:10.1016/J.Clnu.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 128. Raghav S, Singh H, Dalal PK, Srivastava JS, Asthana OP. Randomized controlled trial of standardized Bacopa monniera extract in age-associated memory impairment. Indian J Psychiatry. 2006;48(4):238–242. doi:10.4103/0019-5545.31555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Stough C, et al. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology (Berl). 2001;156(4):481–484. [DOI] [PubMed] [Google Scholar]
- 130. Morgan A, Stevens J. Does Bacopa monnieri improve memory performance in older persons? Results of a randomized, placebo-controlled, double-blind trial. J Altern Complement Med. 2010;16(7):753–759. doi:10.1089/Acm.2009.0342 [DOI] [PubMed] [Google Scholar]
- 131. Calabrese C, Gregory WL, Leo M, Kraemer D, Bone K, Oken B. Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2008;14(6):707–713. doi:10.1089/Acm.2008.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sheikh N, Ahmad A, Siripurapu KB, Kuchibhotla VK, Singh S, Palit G. Effect of Bacopa monniera on stress induced changes in plasma corticosterone and brain monoamines in rats. J Ethnopharmacol. 2007;111(3):671–676. doi:10.1016/J.Jep.2007.01.025 [DOI] [PubMed] [Google Scholar]
- 133. Charles PD, Ambigapathy G, Geraldine P, Akbarsha MA, Rajan KE. Bacopa monniera leaf extract up-regulates tryptophan hydroxylase (Tph2) and serotonin transporter (Sert) expression: implications in memory formation. J Ethnopharmacol. 2011;134(1):55–61. doi:10.1016/J.Jep.2010.11.045 [DOI] [PubMed] [Google Scholar]
- 134. Joshi H, Parle M. Brahmi rasayana improves learning and memory in mice. Evid Based Complement Alternat Med. 2006;3:79–85. doi:10.1093/Ecam/Nek014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rajan KE, Singh HK, Parkavi A, Charles PD. Attenuation of 1-(M-Chlorophenyl)-biguanide induced hippocampus-dependent memory impairment by a standardised extract of bacopa monniera (Beseb Cdri-08). Neurochem Res. 2011;36(11):2136–2144. doi:10.1007/S11064-011-0538-7 [DOI] [PubMed] [Google Scholar]
- 136. Jadiya P, Khan A, Sammi SR, Kaur S, Mir SS, Nazir A. Anti-parkinsonian effects of Bacopa monnieri: insights from transgenic and pharmacological Caenorhabditis elegans models of Parkinson’s disease. Biochem Biophys Res Commun. 2011;413(4):605–610. doi:10.1016/J.Bbrc.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 137. Singh B, Pandey S, Yadav SK, Verma R, Singh SP, Mahdi AA. Role of ethanolic extract of Bacopa monnieri against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced mice model via inhibition of apoptotic pathways of dopaminergic neurons. Brain Res Bull. 2017;135:120–128. doi:10.1016/J.Brainresbull.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 138. Singh B, Pandey S, Verma R, Ansari JA, Mahdi AA. Comparative evaluation of extract of Bacopa monnieri and Mucuna pruriens as neuroprotectant in MPTP model of Parkinson’s disease. Indian J Exp Biol. 2016;54(11):758–766. [PubMed] [Google Scholar]
- 139. Peterson CT, Sharma V, Iablokov SN, et al. 16 S Rrna gene profiling and genome reconstruction reveal community metabolic interactions and prebiotic potential of medicinal herbs used in neurodegenerative disease and as nootropics. Plos One. 2019;14(3):E0213869 doi:10.1371/Journal.Pone.0213869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pase MP, Kean J, Sarris J, Neale C, Scholey AB, Stough C. The cognitive-enhancing effects of Bacopa monnieri: a systematic review of randomized, controlled human clinical trials. J Altern Complement Med. 2012;18(7):647–652. doi:10.1089/Acm.2011.0367 [DOI] [PubMed] [Google Scholar]
- 141. Dhanasekaran M, Tharakan B, Holcomb LA, Hitt AR, Young KA, Manyam BV. Neuroprotective mechanisms of Ayurvedic antidementia botanical Bacopa monniera. Phytother Res. 2007;21(10):965–969. doi:10.1002/Ptr.2195 [DOI] [PubMed] [Google Scholar]
- 142. Vaidya AB, Rajagopalan TG, Mankodi NA, et al. Treatment of Parkinson’s disease with the cowhage plant-mucuna pruriens bak. Neurol India. 1978;26(4):171–176. [PubMed] [Google Scholar]
- 143. Manyam BV. An alternative medicine treatment for Parkinson’s disease: results of a multicenter clinical trial. Hp-200 in Parkinson’s Disease Study Group. J Altern Complement Med. 1995;1:249–255. doi:10.1089/Acm.1995.1.249 [DOI] [PubMed] [Google Scholar]
- 144. Sangiovanni E, Brivio P, Dell’agli M, Calabrese F. Botanicals as modulators of neuroplasticity: focus on BDNF. Neural Plast. 2017;5965371 doi:10.1155/2017/5965371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Naidu PS, Singh A, Kulkarni SK. Effect of Withania somnifera root extract on reserpine-induced orofacial dyskinesia and cognitive dysfunction. Phytother Res. 2006;20(2):140–146. doi:10.1002/Ptr.1823 [DOI] [PubMed] [Google Scholar]
- 146. Kuboyama T, Tohda C, Komatsu K. Withanoside Iv and its active metabolite, sominone, attenuate abeta(25-35)-induced neurodegeneration. Eur J Neurosci. 2006;23(6):1417–1426. doi:10.1111/J.1460-9568.2006.04664.X [DOI] [PubMed] [Google Scholar]
- 147. Rajasankar S, Manivasagam T, Sankar V, et al. Withania somnifera root extract improves catecholamines and physiological abnormalities seen in a Parkinson’s disease model mouse. J Ethnopharmacol. 2009;125(3):369–373. doi:10.1016/J.Jep.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 148. Tohda C, Joyashiki E. Sominone enhances neurite outgrowth and spatial memory mediated by the neurotrophic factor receptor, ret. Br J Pharmacol. 2009;157(8):1427–1440. doi:10.1111/J.1476-5381.2009.00313.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kuboyama T, Tohda C, Komatsu K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol. 2005;144(7):961–971. doi:10.1038/Sj.Bjp.0706122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kongkeaw C, Dilokthornsakul P, Thanarangsarit P, Limpeanchob N, Norman Scholfield C. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. J Ethnopharmacol. 2014;151(1):528–535. doi:10.1016/J.Jep.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 151. Pathak-Gandhi N, Vaidya AD. Management of Parkinson’s disease in Ayurveda: medicinal plants and adjuvant measures. J Ethnopharmacol. 2017;197:46–51. doi:10.1016/J.Jep.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 152. Pathania R, Chawla P, Khan H, Kaushik R, Khan MA. An assessment of potential nutritive and medicinal properties of Mucuna pruriens: a natural food legume. 3 Biotech. 2020;10(6):261 doi:10.1007/S13205-020-02253-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Cilia R, Laguna J, Cassani E, et al. Daily intake of Mucuna pruriens in advanced Parkinson’s disease: a 16-week, noninferiority, randomized, crossover, pilot study. Parkinsonism Relat Disord. 2018;49:60–66. doi:10.1016/J.Parkreldis.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 154. Katzenschlager R, Evans A, Manson A, et al. Mucuna pruriens in Parkinson’s disease: a double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry. 2004;75(12):1672–1677. doi:10.1136/Jnnp.2003.028761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cilia R, Laguna J, Cassani E, et al. Mucuna pruriens in Parkinson disease: a double-blind, randomized, controlled, crossover study. Neurology. 2017;89(5):432–438. doi:10.1212/Wnl.0000000000004175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Contin M, Lopane G, Passini A, Poli F, Iannello C, Guarino M. Mucuna pruriens in Parkinson disease: a kinetic-dynamic comparison with levodopa standard formulations. Clin Neuropharmacol. 2015;38(5):201–203. doi:10.1097/Wnf.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 157. Akbari E, Asemi Z, Daneshvar Kakhaki R, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256 doi:10.3389/Fnagi.2016.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr. 2019;38(6):2569–2575. doi:10.1016/J.Clnu.2018.11.034 [DOI] [PubMed] [Google Scholar]
- 159. Kasaikina MV, Kravtsova MA, Lee BC, et al. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. Faseb J. 2011;25(7):2492–2499. doi:10.1096/Fj.11-181990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ton EMM, Campagnaro BP, Alves GA, et al. Oxidative stress and dementia in Alzheimer’s patients: effects of synbiotic supplementation. Oxid Med Cell Longev. 2020;14 doi:10.1155/2020/2638703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res. 2000;14(3):174–179. [DOI] [PubMed] [Google Scholar]
- 162. Falangola MF, Lee SP, Nixon RA, Duff K, Helpern JA. Histological co-localization of iron in abeta plaques of Ps/App transgenic mice. Neurochem Res. 2005;30(2):201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kapoor R, Srivastava S, Kakkar P. Bacopa monnieri modulates antioxidant responses in brain and kidney of diabetic rats. Environ Toxicol Pharmacol. 2009;27(1):62–69. doi:10.1016/J.Etap.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 164. Le XT, Pham HTN, Do PT, et al. Bacopa monnieri ameliorates memory deficits in olfactory bulbectomized mice: possible involvement of glutamatergic and cholinergic systems. Neurochem Res. 2013;38(10):2201–2215. doi:10.1007/S11064-013-1129-6 [DOI] [PubMed] [Google Scholar]
- 165. Uabundit N, Wattanathorn J, Mucimapura S, Ingkaninan K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer’s disease model. J Ethnopharmacol. 2010;127(1):26–31. doi:10.1016/J.Jep.2009.09.056 [DOI] [PubMed] [Google Scholar]
- 166. Sadhu A, Upadhyay P, Agrawal A, et al. Management of cognitive determinants in senile dementia of Alzheimer’s type: therapeutic potential of a novel polyherbal drug product. Clin Drug Investig. 2014;34(12):857–869. doi:10.1007/S40261-014-0235-9 [DOI] [PubMed] [Google Scholar]
- 167. Srivastav S, Fatima M, Mondal AC. Important medicinal herbs in Parkinson’s disease pharmacotherapy. Biomed Pharmacother. 2017;92:856–863. doi:10.1016/J.Biopha.2017.05.137 [DOI] [PubMed] [Google Scholar]
- 168. Tandon N, Yadav SS. Safety and clinical effectiveness of Withania somnifera (Linn.) dunal root in human ailments. J Ethnopharmacol. 2020;255:112768 doi:10.1016/J.Jep.2020.112768 [DOI] [PubMed] [Google Scholar]
- 169. Kuboyama T, Tohda C, Komatsu K. Effects of ashwagandha (roots of Withania somnifera) on neurodegenerative diseases. Biol Pharm Bull. 2014;37(6):892–897. doi:10.1248/Bpb.B14-00022 [DOI] [PubMed] [Google Scholar]
- 170. Zhao J, Nakamura N, Hattori M, Kuboyama T, Tohda C, Komatsu K. Withanolide derivatives from the roots of Withania somnifera and their neurite outgrowth activities. Chem Pharm Bull (Tokyo). 2002;50(6):760–765. doi:10.1248/Cpb.50.760 [DOI] [PubMed] [Google Scholar]
- 171. Pandey A, Bani S, Dutt P, Satti NK, Suri KA, Qazi GN. Multifunctional neuroprotective effect of withanone, a compound from Withania somnifera roots in alleviating cognitive dysfunction. Cytokine. 2018;102:211–221. doi:10.1016/J.Cyto.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 172. Prakash J, Yadav SK, Chouhan S, Singh SP. Neuroprotective role of Withania somnifera root extract in maneb-paraquat induced mouse model of parkinsonism. Neurochem Res. 2013;38(5):972–980. doi:10.1007/S11064-013-1005-4 [DOI] [PubMed] [Google Scholar]
- 173. Sehgal N, Gupta A, Valli RK, et al. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci U S A. 2012;109(9):3510–3515. doi:10.1073/Pnas.1112209109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of Ashwagandha (Withania somnifera (L.) dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14(6):599–612. doi:10.1080/19390211.2017.1284970 [DOI] [PubMed] [Google Scholar]
- 175. Chengappa KN, Bowie CR, Schlicht PJ, et al. Randomized placebo-controlled adjunctive study of an extract of Withania somnifera for cognitive dysfunction in bipolar disorder. J Clin Psychiatry. 2013;74(11):1076–1083. doi:10.4088/Jcp.13m08413 [DOI] [PubMed] [Google Scholar]
- 176. Wattanathorn J, Mator L, Muchimapura S, et al. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J Ethnopharmacol. 2008;116(2):325–332. doi:10.1016/J.Jep.2007.11.038 [DOI] [PubMed] [Google Scholar]
- 177. Crost EH, Le Gall G, Laverde-Gomez JA, et al. Mechanistic insights into the cross-feeding of Ruminococcus gnavus and Ruminococcus bromii on host and dietary carbohydrates. Front Microbiol. 2018;9:2558 doi:10.3389/Fmicb.2018.02558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hoffmann TW, Pham HP, Bridonneau C, et al. Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. Isme J. 2016;10(2):460–477. doi:10.1038/Ismej.2015.127 [DOI] [PMC free article] [PubMed] [Google Scholar]